Abstract
mTOR complex 1 (mTORC1) plays a central role in cell growth and cellular responses to metabolic stress. Although mTORC1 has been shown to be activated after Toll-like receptor (TLR)-4 engagement, there is little information concerning the role that mTORC1 may play in modulating neutrophil function and neutrophil-dependent inflammatory events, such as acute lung injury. To examine these issues, we determined the effects of rapamycin-induced inhibition of mTORC1 on TLR2- and TLR4-induced neutrophil activation. mTORC1 was dose- and time-dependently activated in murine bone marrow neutrophils cultured with the TLR4 ligand, LPS, or the TLR2 ligand, Pam3 Cys-Ser-(Lys)4 (PAM). Incubation of PAM- or LPS-stimulated neutrophils with rapamycin inhibited expression of TNF-α and IL-6, but not IκB-α degradation or nuclear translocation of NF-κB. Exposure of PAM or LPS-stimulated neutrophils to rapamycin inhibited phosphorylation of serine 276 in the NF-κB p65 subunit, a phosphorylation event required for optimal transcriptional activity of NF-κB. Rapamycin pretreatment inhibited PAM- or LPS-induced mTORC1 activation in the lungs. Administration of rapamycin also decreased the severity of lung injury after intratracheal LPS or PAM administration, as determined by diminished neutrophil accumulation in the lungs, reduced interstitial pulmonary edema, and diminished levels of TNF-α and IL-6 in bronchoalveolar lavage fluid. These results indicate that mTORC1 activation is essential in TLR2- and TLR4-induced neutrophil activation, as well as in the development and severity of acute lung injury.
Keywords: rapamycin, neutrophil, NF-κB, cytokine, mTOR
CLINICAL RELEVANCE
These studies show that mammalian target of rapamycin participates in Toll-like receptor (TLR) 2– and TLR4-induced neutrophil activation as well as in the development and severity of acute lung injury.
There are two functionally distinct mammalian target of rapamycin (mTOR) complexes (mTORCs), mTORC1 and mTORC2 (1). mTORC1 acts downstream of the tuberous sclerosis complex (TSC) 1 and 2–Rheb signaling pathway to activate S6K1, which phosphorylates ribosomal protein S6 (rpS6), as well as to inhibit the eukaryotic transcriptional initiation factor, 4E-binding protein 1 (4E-BP1) in a rapamycin-sensitive fashion (1, 2). mTORC1 participates in the regulation of transcriptional as well as translational events for genes involved in biosynthetic and metabolism pathways (3–5). In contrast, mTORC2 controls the organization of the actin cytoskeleton and is rapamycin insensitive (6).
Rapamycin is a fungicide that forms a complex with the immunophilin, FKBP12, which then binds to and inhibits the activity of mTORC1 (3). Rapamycin is clinically used as an immunosuppressant to prevent the rejection of transplanted organs and to inhibit endothelial proliferation and vessel restenosis after angioplasty (7–10). However, the ability of rapamycin to modulate activation of neutrophils, particularly after Toll-like receptor (TLR)-2 or -4 stimulation, or to ameliorate the severity of inflammatory processes, such as acute lung injury (ALI), in which neutrophils play a major role, has not been described.
In recent work, we have shown that AMP-activated protein kinase (AMPK) activation prevents TLR4-induced activation of neutrophils and diminishes the severity of LPS-induced ALI (11). Because AMPK is upstream of Rheb phosphorylation, AMPK activation is postulated to inhibit the activities of both mTORC1 and mTORC2 (3). Therefore, given the antiinflammatory properties of AMPK activation, we hypothesized that direct inhibition of mTORC1 with rapamycin would also decrease TLR4- as well as TLR2-induced activation of neutrophils, and reduce the severity of TLR2- or TLR4-associated ALI.
MATERIALS AND METHODS
Mice
Male C57BL/6 mice, 8 to 12 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME). The mice were kept on a 12 hour:12 hour light:dark cycle, with free access to food and water. All experiments were conducted in accordance with institutional review board–approved protocols (Institutional Animal Care and Use Committee, University of Alabama, Birmingham, Alabama).
Materials
RPMI 1,640, L-glutamine, penicillin–streptomycin, and Escherichia coli 0111:B4 endotoxin highly purified with phenol extraction (LPS) were purchased from Sigma-Aldrich (St. Louis, MO). Pam3Cys-Ser-(Lys)4 was obtained from Alexis Biochemicals (San Diego, CA). FBS was purchased from Atlanta Biologicals (Norcross, GA). Rapamycin was purchased from Calbiochem (San Diego, CA). Antibodies against nonphosphorylated rpS6, phosphorylated rpS6 (Ser240/244), 4E-BP1, and p-p65 (Ser276) were purchased from Cell Signaling Technology (Beverly, MA). Anti-actin antibody was purchased from Sigma-Aldrich. Antibody against total p65 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-rabbit IgG (H+L)–horseradish peroxidase conjugate was obtained from Bio-Rad (Hercules, CA). Custom antibody mixtures and negative selection columns for neutrophil isolation were purchased from Stem Cell Technologies (Vancouver, BC, Canada). Cytokine ELISA kits were obtained from R&D Systems (Minneapolis, MN).
Neutrophil Isolation and Culture
Bone marrow neutrophils were isolated as previously described (12–14). Neutrophil purity was consistently greater than 97%, as determined by Wright-Giemsa–stained cytospin preparations. Neutrophils were cultured in RPMI 1,640 medium containing 0.5% FBS and treated as indicated in the figure legends. Neutrophil viability under experimental conditions was determined using trypan blue staining, and was consistently greater than 95%.
Nuclear Extract Purification
Purification of nuclei was performed as previously described (11, 13, 14). Briefly, bone marrow neutrophils (8 × 106) were collected in PBS and centrifuged. The cell pellet was resuspended with 50 μl of hypotonic buffer (10 mM Tris [pH 7.5], 10 mM NaCl, 3 mM MgCl2, 0.05% Nonidet P-40, 1 mM EGTA, and protease/phosphatase inhibitors), then centrifuged at 2,700 × g for 5 minutes at 4°C. The nuclei were lysed in 50 μl NP-40 lysis buffer (50 mM Tris [pH 7.5], 137 mM NaCl, 0.1% NP-40, 2 mM EDTA, and protease/phosphatase inhibitors). After a 1-hour incubation on ice, enriched nuclei fractions were collected by centrifugation (10,000 × g) for 20 minutes at 4°C. The protein concentration of the supernatant was determined using Bradford reagent (Bio-Rad) with BSA as a standard.
Western Blot Analysis
Western blot analysis was performed as previously described (12–14). In brief, samples obtained from neutrophils or lung homogenates were mixed with Laemmli sample buffer and boiled for 5 minutes. Equal amounts of protein were resolved by 10–15% SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Immobilon P; Millipore, Billerica, MA). The membranes were probed with specific antibodies, followed by detection with horseradish peroxidase–conjugated anti-goat IgG. Bands were visualized by enhanced chemiluminescence (Super Signal; Pierce Biotechnology, Rockford, IL) and quantified by using AlphaEaseFCTM software (Alpha Innotech, San Leandro, CA). Each experiment was performed three or more times using cell populations or lung homogenates obtained from separate groups of mice.
Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assays performed as reported previously (12, 14–16). In brief, the κB DNA sequence of the Ig gene was used. Synthetic double-stranded sequences (with enhancer motifs underlined) were filled in and labeled with [32P]dATP (GE Healthcare, Piscataway, NJ) using Sequenase DNA polymerase: B sequence, 5′-GCCATGGGGGGATCCCCGAAGTCC-3′ (Geneka Biotechnology, Montreal, Canada).
ALI Model
ALI was induced by intratracheal administration of 1 mg/kg phenol-extracted LPS or 10 mg/kg Pam3 Cys-Ser-(Lys)4 (PAM) in 50 μl of PBS. Briefly, mice were anesthetized with isoflurane. The tongue was then gently extended, and the LPS solution deposited into the pharynx (12, 17). Mice were pretreated with saline or rapamycin (5 mg/kg body weight dissolved in 0.9% saline, intraperitoneally). After 24 hours, the mice received a second injection of rapamycin or saline, followed by intratracheal administration of either PAM (10 mg/kg) or LPS (1 mg/kg). There were no deaths associated with rapamycin and PAM or LPS administration.
Harvest of Lungs and Bronchoalveolar Lavage
Lungs were harvested 24 hours after PAM or LPS administration. As described previously (12, 17), bronchoalveolar lavage (BAL) was obtained by cannulating the trachea with a blunt 20-gauge needle and then lavaging the lungs three times with 1 ml of ice-cold PBS. Total cell counts were measured in the BAL fluid with a hemocytometer (Hausser Scientific, Horsham, PA).
The BAL fluid cells were collected and cytospin slides were prepared and stained with HEMA 3 (Fisher Scientific, Kalamazoo, MI). The number of total cells and neutrophils were counted.
Wet-to-Dry Lung Weight Ratios
Wet-to-dry ratios were determined as reported previously (17, 18). In brief, lungs were excised, rinsed with PBS, blotted, and then weighed for the wet weight. Lungs were then dried in an oven for 7 days at 80°C and the dry weight measured.
Myeloperoxidase Assay
Myeloperoxidase (MPO) activity was quantified as reported previously with minor modifications (12, 19). In brief, lung homogenates, collected in 1 ml of 50-mM potassium phosphate buffer (pH 6.0) and 10-mM N-ethylmaleimide, were centrifuged at 12,000 × g for 30 minutes at 4°C. The pellet was then washed twice and sonicated on ice for 90 seconds in hexadecyltrimethylammonium bromide buffer (0.5% hexadecyltrimethylammonium bromide, 50 mM potassium phosphate [pH 6.0]). The samples were incubated in a water bath for 2 hours at 56°C and then centrifuged at 12,000 × g for 10 minutes. The supernatant was collected for assay of MPO activity by measuring the H2O2-dependent oxidation of 3,3′-dimethoxybenzidine dihydrochloride (λ = 460 nm).
Cytokine ELISA
ELISA were used to measure cytokines in BAL fluid or in cell culture supernatants from PAM- or LPS-treated neutrophils, as previously described (12, 14, 17). Levels of TNF-α and IL-6 were determined using commercially available ELISA kits (R&D Systems), according to the manufacturer's instructions.
Real-Time RT-PCR
Total RNA was extracted using Trizol reagent (Sigma-Aldrich) according to the manufacturer's instructions. cDNA was synthesized using Taqman reverse transcription reagents (Roche Diagnostics, Mannheim, Germany). Real-time PCR was performed using a Lightcycler 480 SYBR Green I Master system (Roche Diagnostics), as previously reported by us (20, 21). The following primers (Roche Diagnostics) were used to amplify the mouse TNF-α transcript: forward, 5′- CCTCCCTCTCATCAGTTCTA-3′; and reverse, 5′-CTTTGAGATCCATGCCG-3′. The mouse GAPDH transcript was used as an internal control (forward, 5′-TCACTGGCATGGCCTTCC- 3′; reverse, 5′-GGCGGCACGTCAGATCC-3′).
Statistical Analyses
For each experiment, neutrophils were isolated and pooled from groups of mice (n = 3–6), and all conditions were studied simultaneously. In studies examining LPS-induced ALI, each experimental group consisted of at least six mice. One-way ANOVA, the Tukey-Kramer multiple comparisons test (for multiple groups), or Student's t test (for comparisons between two groups) were used. A P value of less than 0.05 was considered to be statistically significant.
RESULTS
TLR2 and TLR4 Engagement Induces Phosphorylation of the mTORC1 Downstream Effectors, rpS6 and 4EBP1, in Neutrophils
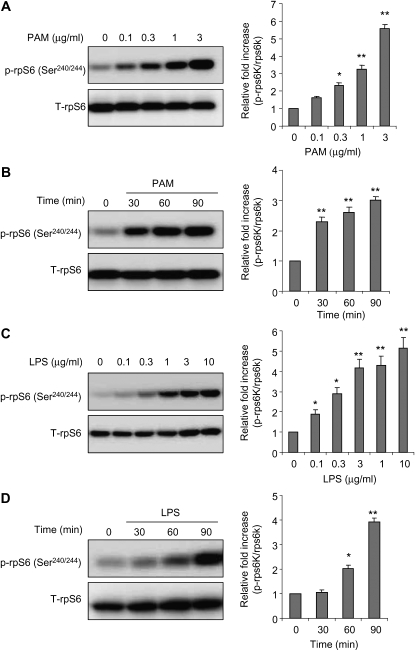
In macrophages, LPS stimulation induces S6K1 phosphorylation, showing that mTORC1 is activated after TLR4 engagement (22). To examine whether TLR2 or TLR4 stimulation activates mTORC1 signaling in neutrophils, we determined the phosphorylation status of rpS6, a major downstream target of mTORC1, after culture with PAM or LPS. As shown in Figure 1, exposure of bone marrow neutrophils to the TLR2 ligand, PAM, or to the TLR4 ligand, LPS, increased the levels of phosphorylated rpS6 (Ser240/244) in a dose- and time-dependent manner.
Figure 1.
Incubation of neutrophils with Toll-like receptor (TLR) 2 and TLR4 ligands induces mammalian target of rapamycin (mTOR) complex 1 (mTORC1) activation in a dose- and time-dependent manner. Bone marrow neutrophils were cultured with the indicated concentrations of Pam3 Cys-Ser-(Lys)4 (PAM) and LPS for 90 minutes (A and C) or for the indicated times with 1 μg/ml PAM or LPS (B and D). Cell extracts were then subjected to SDS-PAGE Western blot analysis with antibodies specific for total and phosphorylated ribosomal protein S6 (rpS6) (Ser240/244). Representative Western blots are shown, as well as densitometry determinations from three independent experiments (mean ± SEM; *P < 0.05 or **P < 0.01 compared with untreated).
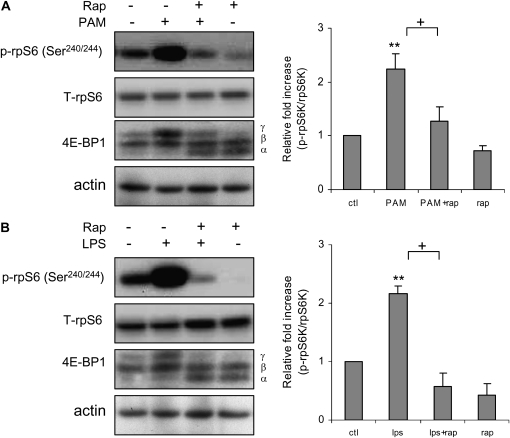
To test the specificity of mTORC1-induced phosphorylation of rpS6 in neutrophils after TLR2 or TLR4 engagement, we included rapamycin, a specific inhibitor of mTORC1 (23, 24), in the cultures. In these experiments, bone marrow neutrophils were left untreated or preincubated with rapamycin, then were stimulated or not with the TLR2 ligand, PAM, or the TLR4 ligand, LPS. The activity of mTORC1 was determined by examining rpS6 (Ser240/244) phosphorylation status, as well as shift of the 4E-BP1 α and β isoforms to the highly phosphorylated γ isoform (25).
The increase in rpS6 phosphorylation and shift of 4E-BP1α and β isoforms to the γ isoform were present in neutrophils stimulated with either PAM or LPS, indicating activation of mTORC1 by TLR2 or TLR4 engagement; these events were inhibited by inclusion of rapamycin in the neutrophil cultures. These results indicate that mTORC1 activation was responsible for rpS6 phosphorylation and appearance of the higher molecular weight form of 4E-BP1 after activation of neutrophils with PAM or LPS (Figures 2A and 2B).
Figure 2.
Rapamycin inhibits PAM- or LPS-induced mTORC1 activation. Bone marrow neutrophils were cultured with rapamycin (0 or 30 nM) for 30 minutes, followed by addition of PAM (0 or 1 μg/ml) or LPS (0 or 1 μg/ml) to the cultures for 60 minutes. Cell extracts were then subjected to SDS-PAGE Western blot analysis with antibodies specific for total and phosphorylated rpS6 (Ser240/244), 4E-binding protein 1 (4E-BP1), and actin. Representative Western blots show inhibitory effects of rapamycin on (A) PAM- or (B) LPS-induced rpS6 phosphorylation, and the appearance of shifted 4E-BP1 γ isoform. Representative Western blots are shown, as well as densitometry determinations (mean ± SEM) of phosphorylated rpS6 (Ser240/244) from three independent experiments. Mean ± SEM, n = 3, **P < 0.01 compared to control, or +P < 0.05 compared to cells treated with PAM or LPS.
Rapamycin Decreases TLR2- and TLR4-Induced Proinflammatory Cytokine Expression by Neutrophils
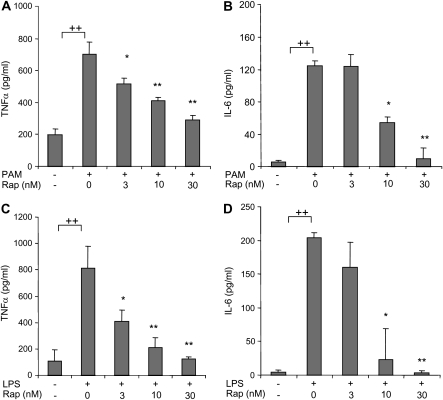
To determine the importance of mTOR in modulating TLR2- or TLR4-induced neutrophil activation, we examined the effects of rapamycin on the production of proinflammatory cytokines after LPS or PAM stimulation. As shown in Figure 3, the addition of rapamycin to neutrophil cultures resulted in a dose-dependent decrease in production of TNF-α and IL-6 by neutrophils stimulated with either PAM or LPS. In these experiments, neutrophil viability was consistently greater than 95% after 5 hours of culture with all concentrations of rapamycin, PAM, and LPS (data not shown).
Figure 3.
Effects of rapamycin on TLR2- and TLR4-induced proinflammatory cytokine production by neutrophils. (A and B) Rapamycin inhibits PAM (1 μg/ml)-mediated TNF-α and IL-6 production by neutrophils. (C and D) Rapamycin inhibits LPS (1 μg/ml)-mediated TNF-α and IL-6 production by neutrophils. Bone marrow neutrophils were cultured with the noted concentrations of rapamycin for 30 minutes, followed by addition of 0 or 1 μg/ml PAM or LPS to the cultures for 5 hours. TNF-α and IL-6 protein concentrations in culture supernatants were determined using ELISA (mean ± SEM; n = 3; *P < 0.05 or **P < 0.01 compared with cells treated with PAM or LPS, ++P < 0.01 compared to control). Similar results were obtained from two additional experiments using neutrophils from separate groups of mice.
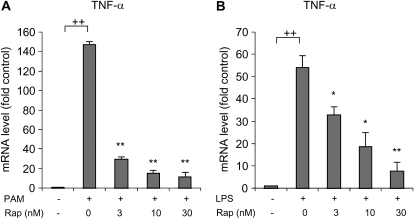
Because of the involvement of mTORC1 in the regulation of transcriptional and translational events (3–5), we examined if rapamycin modified TNF-α mRNA levels in TLR2- or TLR4-stimulated neutrophils. As shown in Figure 4, the addition of rapamycin to neutrophil cultures resulted in a dose-dependent decrease in TNF-α mRNA after TLR2 or TLR4 engagement.
Figure 4.
Effects of rapamycin on TLR2- or TLR4-induced alterations in TNF-α mRNA levels in neutrophils. Rapamycin inhibited (A) PAM- or (B) LPS-mediated increases in TNF-α mRNA levels. Bone marrow neutrophils were cultured with the noted concentrations of rapamycin for 30 minutes, followed by addition of 0 or 1 μg/ml PAM or LPS to the cultures for 2 hours. TNF-α mRNA concentrations in the cell extracts were determined using real-time PCR (mean ± SEM; n = 3; *P < 0.05 or **P < 0.01 compared with cells treated with PAM or LPS only, ++P < 0.01 compared to control). Similar results were obtained from two additional experiments using neutrophils from separate groups of mice.
Rapamycin Inhibits TLR2- and TLR4-Induced Phosphorylation of Serine 276 on the NF-κB p65 Subunit
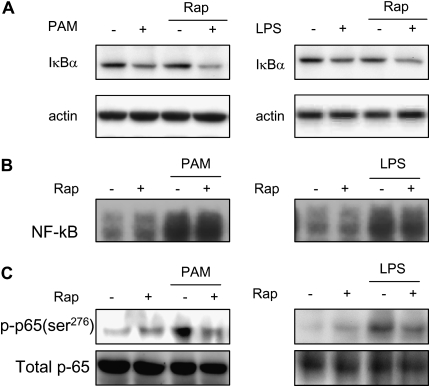
Because NF-κB plays a major role in regulating the transcription of TNF-α and other proinflammatory cytokines, we examined the effect of rapamycin on major intracellular events that regulate nuclear translocation and transcriptional activity of NF-κB. In initial experiments, we determined if rapamycin treatment affected the degradation of IκB-α that normally occurs after TLR2 or TLR4 engagement. IκB-α plays a central role in maintaining cytoplasmic sequestration of NF-κB. Activation of TLR2 or TLR4 signaling cascades leads to IκB-α phosphorylation, ubiquitination, and proteasomal degradation, which result in nuclear translocation of NF-κB (26, 27).
As shown in Figure 5A, rapamycin, in dose ranges that inhibited the expression of TNF-α mRNA and protein, did not affect PAM- and LPS-mediated IκB-α degradation. Given that IκB-α degradation is not affected in rapamycin-exposed neutrophils, we next investigated the effect of mTORC1 inhibition on LPS- or PAM-induced nuclear translocation of NF-κB. As shown in Figure 5B, rapamycin did not affect nuclear translocation of NF-κB in neutrophils treated with either PAM or LPS.
Figure 5.
Effects of rapamycin on TLR2- and TLR4-induced IκBα degradation and NF-κB activation in neutrophils. (A) PAM- or LPS-induced IκBα degradation was not inhibited by addition of rapamycin to the cell cultures. Cell extracts were prepared from bone marrow neutrophils incubated with rapamycin (0 or 30 nM) for 45 minutes, followed by addition of 0 or 1 μg/ml PAM or LPS to the cultures for 60 minutes. Representative Western blots are shown. Two additional experiments using neutrophils from separate groups of mice provided similar results. (B) PAM- or LPS-induced NF-κB nuclear translocation was not inhibited by culture of neutrophils with rapamycin. Nuclear extracts were prepared from bone marrow neutrophils incubated with rapamycin (0 or 30 nM) for 45 minutes, followed by addition of 0 or 1 μg/ml of PAM or LPS to the cultures for 90 minutes. A representative electrophoretic mobility shift assay is shown. A second experiment, using neutrophils from a separate group of mice, provided similar results. (C) PAM- or LPS-induced phosphorylation of Ser276 in the NF-κB p65 subunit is inhibited by exposure to rapamycin. Cell extracts were prepared from bone marrow neutrophils incubated with rapamycin (0 or 30 nM) for 45 minutes, followed by addition of 0 or 1 μg/ml PAM or LPS to the cultures for 60 minutes. A representative Western blot is shown. Two additional experiments with separate groups of mice provided similar results.
Although TLR2- or TLR4-induced nuclear accumulation of NF-κB is not modified in rapamycin-exposed neutrophils, additional NF-κB phosphorylation events regulate its transcriptional activity, and may be responsible for the inhibitory effects of rapamycin on cytokine production. In particular, the transcriptional activity of NF-κB is enhanced by phosphorylation of Ser276 in the p65 subunit, and NF-κB Ser276 phosphorylation status had no effects on nuclear translocation or binding to DNA of NF-κB (28, 29). As shown in Figure 5C, rapamycin prevented Ser276 phosphorylation of p65 in TLR2- or TLR4-stimulated neutrophils.
Rapamycin Attenuates the Severity of TLR2- or TLR4-Induced ALI
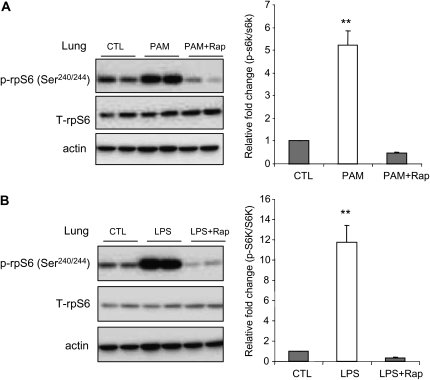
Intratracheal administration of PAM or LPS resulted in increased phosphorylation of rpS6 in lung homogenates, consistent with in vivo activation of mTORC1 (Figure 6A). Rapamycin administration significantly prevented this effect (Figure 6B).
Figure 6.
Effects of rapamycin on mTORC1 activation in PAM- or LPS-induced acute lung injury (ALI). Mice were pretreated with saline or saline plus rapamycin (5 mg/kg, intraperitoneal). After 24 hours, the mice received a second injection of rapamycin or saline, followed by intratracheal administration of either PAM (10 mg/kg) or LPS (1 mg/kg). The lungs were harvested 24 hours after PAM or LPS administration. Whole-lung extracts were subjected to SDS-PAGE Western blot analysis with antibodies specific for total and phosphorylated rpS6 (Ser240/244) and actin. Representative Western blots are shown. Densitometry determinations are from three independent experiments (mean ± SEM; n = 6 mice in each group; **P < 0.01 compared with control).
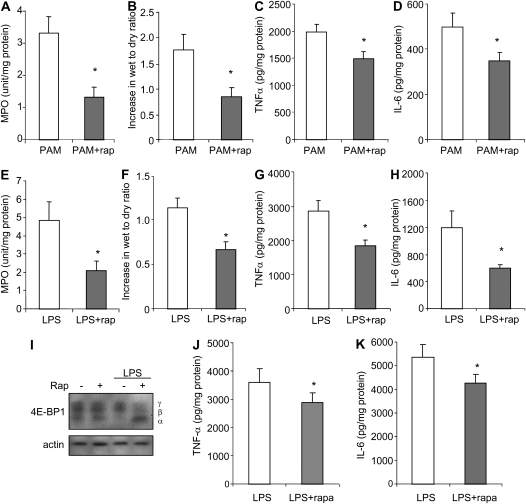
Given the ability of TLR2 or TLR4 engagement to activate mTORC1 in vivo in the lungs, and the inhibitory effects of rapamycin on proinflammatory cytokine production by TLR2- or TLR4-stimulated neutrophils, we hypothesized that such therapy would reduce the severity of ALI, an inflammatory process in which neutrophils play a major role (16, 18, 30). As shown in Figure 7, rapamycin-treated mice had less severe lung injury than did mice given vehicle alone before LPS or PAM. In particular, lung wet-to-dry ratios and pulmonary MPO concentrations were significantly decreased after rapamycin administration (Figures 7A–7H). The inhibitory effects of rapamycin on LPS-induced mTORC1 activation and cytokine expression were apparent as early as 4 hours after LPS administration. As shown Figures 7I–7K, treatment with rapamycin was associated with diminished phosphorylation of 4E-BP1 as well as expression of TNF-α and IL-6 in the lungs 4 hours after LPS administration. There were no significant differences in pulmonary wet-to-dry ratios or MPO concentrations between control mice given vehicle or rapamycin alone, without LPS or PAM administration (data not shown). Similarly, there were no apparent changes in behavior and no significant alteration in the weight of mice after two injections of rapamycin as compared with unmanipulated mice. Plasma levels of IL-6 and TNF-α were less than 10 pg/ml in mice that were unmanipulated, given vehicle 48 and 24 hours previously, or given rapamycin 48 and 24 hours previously (data not shown).
Figure 7.
Rapamycin attenuates PAM- or LPS-induced ALI in vivo. Mice were pretreated with saline or saline plus rapamycin (5 mg/kg, intraperitoneal). After 24 hours, the mice received a second injection of rapamycin or saline, followed by intratracheal administration of either PAM (10 mg/kg) or LPS (1 mg/kg). Lungs were collected 4 or 24 hours after PAM or LPS administration. (A–D) Effects of rapamycin treatment on PAM-induced increases in lung wet-to-dry ratios, lung myeloperoxidase (MPO) levels, and TNF-α and IL-6 levels in whole-lung homogenates at 24 hours. (E–H) Effects of rapamycin treatment on LPS-induced increases in lung wet-to-dry ratios, lung MPO levels, and TNF-α and IL-6 levels in whole-lung homogenate at 24 hours. (I–K) Rapamycin treatment abrogates LPS-induced 4E-BP1 γ expression and diminishes TNF-α and IL-6 levels in whole-lung homogenates at 4 hours after LPS administration. (I) Whole-lung extracts were subjected to SDS-PAGE Western blot analysis with antibodies specific for 4E-BP1 and actin. Means (±SEM) are shown (n = 6 mice per group; *P < 0.05 when compared the rapamycin/LPS or PAM group to mice treated with saline/LPS or PAM).
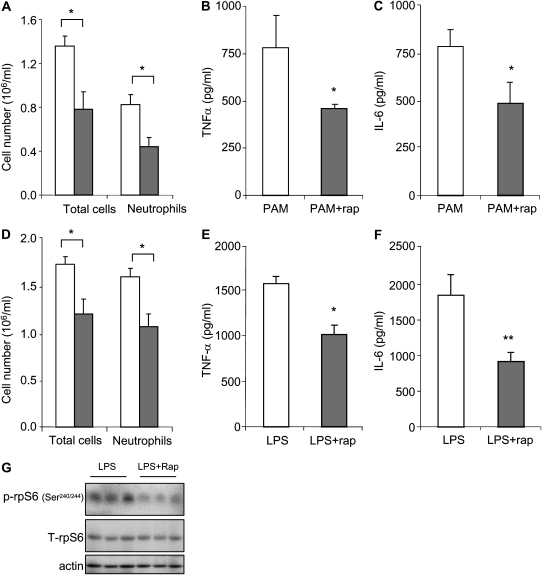
Neutrophil numbers in BAL fluid were significantly reduced in mice that received rapamycin before PAM or LPS administration (Figures 8A and 8D). Levels of TNF-α and IL-6 were also significantly decreased in BAL fluid from rapamycin-treated mice (Figures 8B, 8C, 8E, and 8F). Airway neutrophils that were isolated from BAL fluid of the rapamycin-treated mice after LPS administration demonstrated less phosphorylated rpS6 (Ser240/244) than that found in the control group treated with saline before LPS exposure (Figure 8G).
Figure 8.
Rapamycin decreases PAM- or LPS-induced accumulation of neutrophils and increases in cytokine levels in bronchoalveolar lavage (BAL) fluid. Mice were pretreated with saline or saline plus rapamycin (5 mg/kg, intraperitoneal). After 24 hours, the mice received a second injection of rapamycin or saline, followed by intratracheal administration of either PAM (10 mg/kg) or LPS (1 mg/kg). Total cell and neutrophil numbers in BAL fluid were determined 24 hours after intratracheal injection of PAM (A) or LPS (D). (A) Open bars, PAM; shaded bars, PAM plus rapamycin. (D) Open bars, LPS; shaded bars, LPS plus Rap. TNF-α and IL-6 levels in BAL fluid collected 24 hours after administration of PAM (B and C) or LPS (E and F). (G) Rapamycin decreases LPS-induced phosphorylation of rpS6 in BAL fluid neutrophils. Airway neutrophils were collected 24 hours after intratracheal administration of LPS. Cell extracts were then subjected to SDS-PAGE Western blot analysis with antibodies specific for total and phosphorylated rpS6 (Ser240/244) and actin. Means (±SEM) are shown (n = 6 mice in each group; *P < 0.05 and **P < 0.01 comparing the rapamycin/LPS or rapamycin/PAM group to mice treated with PBS/LPS or PBS/PAM).
DISCUSSION
In the present studies, we investigated the potential role of mTORC1 signaling on TLR2- and TLR4-induced proinflammatory responses in neutrophils and ALI, an inflammatory process in which neutrophils play an important role (16, 30, 31). Rapamycin, a specific inhibitor of mTORC1, potently reduced the production of proinflammatory cytokines in neutrophils as well as the severity of ALI in vivo. The mechanism through which mTORC1 decreased the activation of neutrophils appears to be through inhibiting phosphorylation of Ser276 within the p65 subunit of NF-κB, an event required for optimal transcriptional activity (28, 29).
Our results show that TLR2 and TLR4 stimulation of neutrophils results in significant increases in phosphorylation of rpS6 and 4EBP1, two downstream targets of mTORC1, demonstrating activation of mTORC1 in this setting. These findings are consistent with those of previous studies showing that LPS exposure activated mTORC1 in macrophages (22), and that mTORC1 participates in modulating translation of IL-6 receptor mRNA in platelet-activating factor–stimulated neutrophils (32).
In these experiments, we found that rapamycin exposure reduced TNF-α and IL-6 production among TLR2- or TLR4-activated neutrophils. The ability of TLR2 and TLR4 to enhance TNF-α mRNA accumulation was also diminished by rapamycin treatment, suggesting that rapamycin inhibited TLR2- and TLR4-induced activation pathways upstream of protein translation.
The steps at which mTORC1 is involved in NF-κB activation have not been completely delineated. Although previous studies found that the antiinflammatory effects of rapamycin occur, at least in part, by affecting the activity of NF-κB, there appear to be several possible mechanisms for such actions. For example, rapamycin has been reported to diminish TLR4-mediated IκB-α degradation and nuclear localization of NF-κB in colon cancer cells and hepatocytes (33, 34). Vinciguerra and colleagues (35) found that rapamycin diminished phosphorylation of Ser276 in the NF-κB p65 subunit and, in addition, also blocked nuclear localization of p65 in HepG2 cells stimulated with oleic acid. However, in the studies demonstrating inhibition of nuclear translocation of NF-κB by rapamycin, the concentrations used, from 200 nM to 10 μM, are much higher than those present in vivo after rapamycin administration (36, 37). In our studies, exposure of neutrophils to rapamycin at lower concentrations that are similar to those found in vivo (30 nM) inhibited proinflammatory cytokine production, but did not affect TLR2- or TLR4-induced IκB-α degradation or nuclear translocation of NF-κB. These findings are consistent with those from previous studies showing that rapamycin in the nanomolar range (5 nM) did not decrease NF-κB translocation in thrombin-activated endothelial cells (38).
A potential mechanism for the proinflammatory effects of mTORC1 activation is through enhancing phosphorylation of the Ser276 residue in the NF-κB p65 subunit, an event that is required for optimal transcriptional activity of NF-κB (28, 29). In the present studies, we found that this phosphorylation event is inhibited by rapamycin in TLR2- or TLR4-stimulated neutrophils. These results are consistent with those of reports that Ser276 phosphorylation on p65, although necessary for transcriptional activity of NF-κB, does not affect its nuclear translocation or DNA binding (28, 29). Previous studies have shown that phosphorylation of serine 276 is critical for recruitment of cofactor CREB binding protein (CBP) to nuclear NF-κB after cellular stimulation, thereby enhancing transcription through facilitating chromatin unwinding and remodeling (39, 40). Mutation of serine 276 to alanine in p65 leads to embryonic lethality, demonstrating the importance of this residue in development (28). Embryonic fibroblasts containing the mutated S276A p65 showed markedly diminished transcription of a transfected NF-κB luciferase reporter in response to LPS, IL-1, and TNF-α. In addition, LPS-induced expression of NF-κB target genes was altered in S276A p65 embryonic fibroblasts as compared with that found in wild-type cells. In particular, mRNA levels for macrophage inhibitory protein (MIP)-2, TNF-α, and IL-6 were markedly decreased in the LPS-stimulated S276A p65 cells, whereas expression of IκB-α and Cox-2 was unaffected by this mutation (28).
In previous studies, intraperitoneal injection of rapamycin at a dose of 0.16 mg/mouse produced a whole-blood level of approximately 60 ng/ml (66 nM) 24 hours after administration (36). In the present in vivo studies, we administered a lower dose of rapamycin (i.e., 5 mg/kg or ∼ 0.125 mg/mouse). This dose of rapamycin was sufficent to inhibit mTORC1 activity 24 hours later in PAM- or LPS-exposed mice. In humans, a single dose of 5–20 mg/m2 body surface area of rapamycin resulted in whole-blood concentrations 24 hours later of 8–30 ng/ml (i.e., 8.75–32.00 nM) (37), consistent with the 0–30 nM dose range that we used for in vitro experiments in neutrophils.
The present studies demonstrate that mTORC1 activation contributes to the development and severity of TLR2- or TLR4-induced ALI. Because pretreatment with rapamycin was used, the potential clinical efficacy of mTORC1 inhibition in treating established lung injury remains presently unknown, and will need to be investigated in future studies. Nevertheless, these experiments do suggest that inhibition of mTORC1 may be useful in clinical settings, such as in the presence of severe sepsis accompanied by nonpulmonary organ dysfunction, where there is increased risk of developing ALI (41, 42).
These studies provide new insights into the mechanisms by which mTORC1 affects TLR2- or TLR4-induced neutrophil activation, as well as the development of ALI. The findings suggest that mTORC1 activation enhances expression of proinflammatory mediators, and contributes to the development of lung injury by inducing the phosphorylation of serine 276 in the p65 subunit of NF-κB and enhancing the transcriptional activity of NF-κB. Nevertheless, despite the evidence provided in this study that mTORC1 inhibition decreases TLR2- and TLR4-dependent activation of neutrophils through modulating phosphorylation of NF-κB, additional studies will be necessary to more completely delineate the mechanisms through which mTORC1 activation results in phosphorylation of serine 276 in the NF-κB p65 subunit, and to determine if this is the primary pathway by which mTORC1 affects NF-κB transcriptional activity.
This work was supported in part by National Institutes of Health grants HL62221, HL76206, and HL068743 (E.A.), and by the Société Française d'Anesthésie et de Réanimation and the University Hospital of Amiens (Amiens, France) (E.L.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0290OC on January 8, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006;124:471–484. [DOI] [PubMed] [Google Scholar]
- 2.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood 2008;112:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 2004;18:1926–1945. [DOI] [PubMed] [Google Scholar]
- 4.Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol 2005;16:29–37. [DOI] [PubMed] [Google Scholar]
- 5.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol 2002;22:5575–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 2004;6:1122–1128. [DOI] [PubMed] [Google Scholar]
- 7.McAlister VC, Gao Z, Peltekian K, Domingues J, Mahalati K, MacDonald AS. Sirolimus-tacrolimus combination immunosuppression. Lancet 2000;355:376–377. [DOI] [PubMed] [Google Scholar]
- 8.Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 2000;356:194–202. [DOI] [PubMed] [Google Scholar]
- 9.Spaulding C, Henry P, Teiger E, Beatt K, Bramucci E, Carrie D, Slama MS, Merkely B, Erglis A, Margheri M, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med 2006;355:1093–1104. [DOI] [PubMed] [Google Scholar]
- 10.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315–1323. [DOI] [PubMed] [Google Scholar]
- 11.Zhao, X., J. W. Zmijewski, E. Lorne, G. Liu, Y. J. Park, Y. Tsuruta, and E. Abraham. 2008. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L497–L504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuruta Y, Park YJ, Siegal GP, Liu G, Abraham E. Involvement of vitronectin in lipopolysaccaride-induced acute lung injury. J Immunol 2007;179:7079–7086. [DOI] [PubMed] [Google Scholar]
- 13.Zmijewski JW, Zhao X, Xu Z, Abraham E. Exposure to hydrogen peroxide diminishes NF-kappaB activation, IkappaB-alpha degradation, and proteasome activity in neutrophils. Am J Physiol Cell Physiol 2007;293:C255–C266. [DOI] [PubMed] [Google Scholar]
- 14.Lorne E, Zmijewski JW, Zhao X, Liu G, Tsuruta Y, Park YJ, Dupont H, Abraham E. Role of extracellular superoxide in neutrophil activation: interactions between xanthine oxidase and TLR4 induce proinflammatory cytokine production. Am J Physiol Cell Physiol 2008;294:C985–C993. [DOI] [PubMed] [Google Scholar]
- 15.Shenkar R, Schwartz MD, Terada LS, Repine JE, McCord J, Abraham E. Hemorrhage activates NF-kappa B in murine lung mononuclear cells in vivo. Am J Physiol 1996;270:L729–L735. [DOI] [PubMed] [Google Scholar]
- 16.Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med 2003;167:1567–1574. [DOI] [PubMed] [Google Scholar]
- 17.Zmijewski JW, Lorne E, Zhao X, Tsuruta Y, Sha Y, Liu G, Siegal GP, Abraham E. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am J Respir Crit Care Med 2008;178:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2000;279:L1137–L1145. [DOI] [PubMed] [Google Scholar]
- 19.Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol 1985;59:1978–1985. [DOI] [PubMed] [Google Scholar]
- 20.Liu G, Park YJ, Abraham E. Interleukin-1 receptor–associated kinase (IRAK)-1–mediated NF-kappaB activation requires cytosolic and nuclear activity. FASEB J 2008;22:2285–2296. [DOI] [PubMed] [Google Scholar]
- 21.Liu G, Tsuruta Y, Gao Z, Park YJ, Abraham E. Variant IL-1 receptor–associated kinase-1 mediates increased NF-kappa B activity. J Immunol 2007;179:4125–4134. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein SL, Finn AJ, Dave SH, Meng F, Lowell CA, Sanghera JS, DeFranco AL. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-beta. J Leukoc Biol 2000;67:405–414. [DOI] [PubMed] [Google Scholar]
- 23.Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 1997;272:6653–6662. [DOI] [PubMed] [Google Scholar]
- 24.Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem 1999;274:4341–4346. [DOI] [PubMed] [Google Scholar]
- 25.Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J 2007;403:217–234. [DOI] [PubMed] [Google Scholar]
- 26.Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system. Nat Immunol 2002;3:20–26. [DOI] [PubMed] [Google Scholar]
- 27.Alkalay I, Yaron A, Hatzubai A, Jung S, Avraham A, Gerlitz O, Pashut-Lavon I, Ben-Neriah Y. In vivo stimulation of I kappa B phosphorylation is not sufficient to activate NF-kappa B. Mol Cell Biol 1995;15:1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong J, Jimi E, Zhong H, Hayden MS, Ghosh S. Repression of gene expression by unphosphorylated NF-kappaB p65 through epigenetic mechanisms. Genes Dev 2008;22:1159–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowak DE, Tian B, Jamaluddin M, Boldogh I, Vergara LA, Choudhary S, Brasier AR. RelA Ser276 phosphorylation is required for activation of a subset of NF-kappaB–dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol Cell Biol 2008;28:3623–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003; 31(4 suppl)S195–S199. [DOI] [PubMed] [Google Scholar]
- 31.Togbe D, Schnyder-Candrian S, Schnyder B, Doz E, Noulin N, Janot L, Secher T, Gasse P, Lima C, Coelho FR, et al. Toll-like receptor and tumour necrosis factor dependent endotoxin-induced acute lung injury. Int J Exp Pathol 2007;88:387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindemann SW, Yost CC, Denis MM, McIntyre TM, Weyrich AS, Zimmerman GA. Neutrophils alter the inflammatory milieu by signal-dependent translation of constitutive messenger RNAs. Proc Natl Acad Sci USA 2004;101:7076–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omura T, Yoshiyama M, Izumi Y, Kim S, Matsumoto R, Enomoto S, Kusuyama T, Nishiya D, Nakamura Y, Akioka K, et al. Involvement of c-Jun NH2 terminal kinase and p38MAPK in rapamycin-mediated inhibition of neointimal formation in rat carotid arteries. J Cardiovasc Pharmacol 2005;46:519–525. [DOI] [PubMed] [Google Scholar]
- 34.Sun Q, Liu Q, Zheng Y, Cao X. Rapamycin suppresses TLR4-triggered IL-6 and PGE(2) production of colon cancer cells by inhibiting TLR4 expression and NF-kappaB activation. Mol Immunol 2008;45:2929–2936. [DOI] [PubMed] [Google Scholar]
- 35.Vinciguerra M, Veyrat-Durebex C, Moukil MA, Rubbia-Brandt L, Rohner-Jeanrenaud F, Foti M. PTEN down-regulation by unsaturated fatty acids triggers hepatic steatosis via an NF-kappaBp65/mTOR–dependent mechanism. Gastroenterology 2008;134:268–280. [DOI] [PubMed] [Google Scholar]
- 36.Rauktys A, Lee N, Lee L, Dabora SL. Topical rapamycin inhibits tuberous sclerosis tumor growth in a nude mouse model. BMC Dermatol 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brattstrom C, Sawe J, Tyden G, Herlenius G, Claesson K, Zimmerman J, Groth CG. Kinetics and dynamics of single oral doses of sirolimus in sixteen renal transplant recipients. Ther Drug Monit 1997;19:397–406. [DOI] [PubMed] [Google Scholar]
- 38.Minhajuddin M, Fazal F, Bijli KM, Amin MR, Rahman A. Inhibition of mammalian target of rapamycin potentiates thrombin-induced intercellular adhesion molecule-1 expression by accelerating and stabilizing NF-kappa B activation in endothelial cells. J Immunol 2005;174:5823–5829. [DOI] [PubMed] [Google Scholar]
- 39.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1998;1:661–671. [DOI] [PubMed] [Google Scholar]
- 40.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell 2002;9:625–636. [DOI] [PubMed] [Google Scholar]
- 41.Jia X, Malhotra A, Saeed M, Mark RG, Talmor D. Risk factors for ARDS in patients receiving mechanical ventilation for > 48 h. Chest 2008;133:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubenfeld GD, Herridge MS. Epidemiology and outcomes of acute lung injury. Chest 2007;131:554–562. [DOI] [PubMed] [Google Scholar]