Abstract
Purpose
Chemotherapeutic agents are known to produce persistent cognitive deficits in cancer patients. However, little progress has been made in developing animal models to explore underlying mechanisms and potential therapeutic interventions. We report an electrophysiological model of chemotherapy-induced cognitive deficits using a sensory gating paradigm, to correspond with performance in two behavioral tasks.
Experimental Design
Mice received four weekly injections of methotrexate and 5-fluorouracil. Whole-brain event-related potentials (ERPs) were recorded throughout using a paired-click paradigm. Mice underwent contextual fear conditioning (CFC) and novel-object recognition testing (NOR).
Results
Chemotherapy treated animals showed significantly impaired gating five weeks after drug treatments began, as measured by the ratio of P1-N1 between first and second auditory stimuli. There was no effect of drug on the amplitude of P1-N1 or latency of P1. The drug treated animals also showed significantly increased freezing during fear conditioning and increased exploration without memory impairment during novel object recognition.
Conclusions
Chemotherapy causes decreased ability to gate incoming auditory stimuli, which may underlie associated cognitive impairments. These gating deficits were associated with a hyperactive response to fear conditioning and reduced adaptation to novel objects, suggesting an additional component of emotional dysregulation. However, amplitudes and latencies of ERP components were unaffected, as was NOR performance, highlighting the subtle nature of these deficits.
Keywords: chemotherapy, cognitive impairment, sensory gating of evoked-potentials, methotrexate, 5-fluorouracil, fear conditioning, novel object recognition, post-traumatic stress disorder
Recent data suggest that there may be a greater degree of neurotoxicity and cognitive decline following treatment of non-CNS tumors with systemic chemotherapy than was previously recognized. Over the last decade, several longitudinal clinical studies have established a strong connection between cancer chemotherapy and cognitive deficits, even when controlling for differences in gender, cancer diagnosis, drug treatment regimen, dosage administered, and related psychological comorbidities such as depression or anxiety (Schagen et al., 1999, Dietrich et al., 2006). These findings, colloquially referred to as ‘chemo-brain’, have received much concern of late, with recent reviews and a 2003 conference (Tannock et al., 2004, Ahles and Saykin, 2007) stressing the need to develop an appropriate animal model, to create a sensitive neuropsychological paradigm to detect these deficits, and to probe involved neural circuitry with neuroimaging.
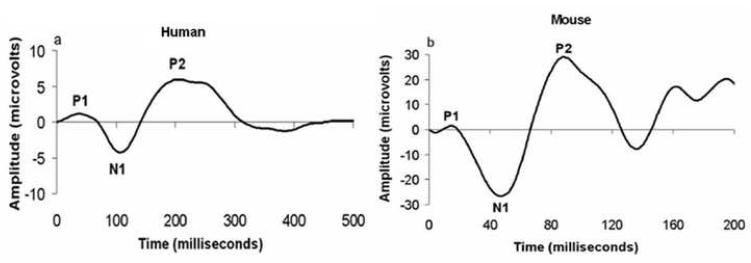
We addressed these gaps in the literature by investigating the effects of chemotherapy on sensory information processing and cognitive-behavioral functioning in a mouse model. We employed a sensory gating paradigm using auditory event-related potentials (ERPs) in which whole-brain electrical activity is recorded while animals are presented with pairs of identical auditory stimuli (termed S1 and S2). Neural responses are correlated with the onset of each stimulus such that extraneous electrical activity is averaged out and the remaining waveform reflects coordinated activity of the neural generators in the auditory information processing pathway. Previously, we developed and utilized mouse models for measuring auditory event related potentials following either pharmacological or genetic manipulations that are relevant to psychiatric conditions (Connolly et al., 2003, Siegel et al., 2003, Maxwell et al., 2006a, Maxwell et al., 2006b, Metzger et al., 2006). In humans, early ERPs (1–8ms) represent activation of brainstem structures such as the cochlear nucleus; mid-latency ERPs (8–40ms) indicate forebrain activity including thalamus and hippocampus and long-latency ERPs (50–300ms) represent processing at the level of the cortex, involving primary and association cortices (Picton et al., 1974, Reite et al., 1988). Early, middle, and long-latency ERPs recorded from mice resemble corresponding human components in topography and response properties (Connolly et al., 2003, Umbricht et al., 2004). Rodents share many similarities with humans for specific portions of the ERP, including the mouse P1, N1, P2 and P3 which share stimulus and pharmacologic response properties with the human P50, N100, P200 and P300 respectively (Figure 1) (Siegel et al., 2003, Connolly et al., 2004). The latencies of ERP peaks from mice have been shown to be consistently 40% of those recorded from human subjects (Umbricht et al., 2004). Thus, ERPs are an especially attractive means of evaluating sensory processing in animals as there are direct human correlates for these measures.
Figure 1.
Correspondence between auditory evoked potentials recorded from humans in the Cz configuration (panel A) and mice (panel B). Morphology of the waveforms is largely homologous between species with the main peaks labeled P1, N1, and P2. The latencies of ERP peaks from mice are approximately 40% of those recorded from humans. The amplitudes are smaller in humans reflecting the use of scalp EEG rather than depth electrodes in mice.
We specifically focused on sensory gating of ERPs, in which the amplitude of the neural response to S2 is normally diminished compared to that of S1, reflecting basic sensory habituation. Sensory gating is a fundamental aspect of pre-attentional processing characterized by habituation of brain responses to a repetitive sensory stimulus, which provides individuals with the ability to filter out irrelevant or redundant information from the environment and to attend to more salient stimuli. Abnormalities in sensory gating have become an attractive target for animal models of impaired cognitive function, linked to disorders such as schizophrenia, in which gating deficits are largely accepted as an endophenotypic marker of the disease (Adler et al., 1993). This has facilitated the development of animal models which have led to an enhanced mechanistic understanding of the neural circuitry involved and identification of new targets for therapeutic interventions (Braff and Light, 2004, Ellenbroek, 2004).
In the present study, we administered a combination of methotrexate (MTX) and 5-fluorouracil (5-FU) to adult mice, which is a standard clinical regimen for treatment of human breast cancers. We employed a dosing strategy that has already been shown to produce mild cognitive-behavioral impairments in mice (Winocur et al., 2006) in order to elicit electrophysiological correlates of those deficits. In addition, we extend previous animal behavioral studies by employing novel object recognition and contextual fear conditioning tasks, which are sensitive to various stages of cognitive and emotional processing that may be altered with chemotherapy. Novel object recognition (NOR) is a recognition memory task that has been shown to require both the hippocampus and the cortex (Carpenter and Grossberg, 1993), while contextual fear conditioning (CFC) is also hippocampally-dependent as well as sensitive to modulation by the amygdala (Phillips and LeDoux, 1992). These tasks are especially relevant in light of recently published data which demonstrate that chemotherapeutic agents disrupt hippocampal neurogenesis and that these cellular changes may underlie the observed cognitive impairments (Seigers et al., 2008).
The significance of this work is that it furthers the development of a much-needed animal model and, for the first time, probes involved neural-circuitry with electrophysiological recording. Any observed ERP changes can be easily corroborated in the clinical populations using analogous scalp EEG techniques. Finally, ERP animal models are ideally suited for investigation into relevant genetic and pharmacological manipulations to develop an adjuvant therapy and could prove to be a pre-clinical screening technique for new chemotherapeutic agents to evaluate their neurotoxic potential.
Experimental Procedures
2.1 Animals
Twenty-four male C57BL/6Hsd (B6) mice were obtained at 7-8 weeks of age from Harlan (Indianapolis, IN). All testing was conducted between 10 and 18 weeks of age. Mice acclimated to the Animal Facility for seven days before experimentation began. Mice were housed 4-5 per cage until surgeries, after which they were single-housed for the remainder of the study. They were maintained in a standard 12-h light-dark cycle with free access to food and water. Experiments were performed during the light phase between 9:00 AM and 4:00 PM. All protocols were conducted in accordance with University Laboratory Animal Resources (ULAR) guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania.
2.2 Surgery
Animals underwent stereotaxic implantation of tripolar electrode assemblies (PlasticsOne Inc., Roanoke, VA) for non-anesthetized recording of ERPs, as previously described (Connolly et al., 2003, Siegel et al., 2003, Connolly et al., 2004, Maxwell et al., 2004a, Maxwell et al., 2004b). Animals were anesthetized with isoflurane and recording electrodes were placed unilaterally in CA3 of the hippocampus (1.4 mm posterior, 2.65 mm lateral, and 2.75 mm deep relative to bregma) and referenced to the ipsilateral frontal sinus to reflect whole brain electrical activity. ERPs recorded from this electrode configuration are characteristically similar to human recordings from the Cz scalp location as described previously (Siegel et al., 2003). The electrode pedestal was secured to the skull using dental cement and super glue. Electrode placement has been verified to be in the target region using the Perl’s iron reaction (Labossiere and Glickstein, 1976).
2.3 Drugs
One week after electrode placement, mice were randomly assigned to high-dose drug (n=8), low-dose drug (n=8), and control (n=8) groups. Mice in each group received one i.p. injection each week for four consecutive weeks. The high-dose group received a combination of Methotrexate (37.5 mg/kg, MP Biomedicals, Solon, OH, USA) and 5-Fluorouracil (75 mg/kg, MP Biomedicals, Solon, OH, USA) dissolved in DMSO such that each animal received an injection of approximately 0.1 mL of solution. The low-dose groups received drug at a 1:2 dilution at the same injection volume of 0.1 mL. Control animals received equal volumes of DMSO. Drug treatments were always given three days before ERP testing such that animals had time to recover from any acute toxicity. Weight, appearance, and locomotor activity were assessed every 3 days during the drug treatment phase to monitor for signs of toxicity. No animals died during the study.
2.4 EEG Recordings
Recoding of brain activity for gating of evoked potentials occurred at weeks 1, 3, and 5 after implantation of electrodes. Mice concurrently received chemotherapy up to the 4th week after electrode placement. EEGs were recorded during the presentation of a paired-click task. All raw EEG was inline bandpass filtered between 1 and 500 Hz during collection. Stimuli were generated by Micro1401 hardware and Spike2 version 6 software (Cambridge Electronic Design, Cambridge UK) and were delivered through speakers attached to the cage top. All recordings were performed in the home cage environment, which was placed in a Faraday electrical isolation cage 15 minutes before stimulus onset. A series of 150 pairs of white noise clicks (10 ms in duration) were presented with a 500 ms interstimulus interval and a 9 second intertrial interval at 85 dB compared with background white noise of 63 dB. Waveforms were baseline corrected at stimulus onset and individual sweeps were rejected for movement artifact based on a criterion of 2 times the root mean square (RMS) amplitude per mouse. Average waves were created from 50 ms pre-stimulus to 500 ms post stimulus.
2.5 Auditory Gating Analysis
The amplitude and latency of P1 (defined as the most positive deflection between 10 and 30 ms) and N1 (defined as most negative deflection between 25 and 60 ms) components were analyzed. The amplitude from the peak of the P1 to the trough of the N1 was then calculated, as it is reported to be a more stable measure than either component alone and to facilitate comparison with previous studies (Stevens et al., 1996). The amplitudes of response to the first (S1) and second stimuli (S2) as calculated above were named A1 and A2, respectively. The ratio of response following the second tone to the first (A2/A1) was calculated as this has been used as a measure of sensory gating in multiple previous studies (Stevens et al., 1996, de Bruin et al., 1999, Connolly et al., 2003). The latency of P1 following the first stimulus was also assessed. Data were analyzed using repeated measures ANOVAs (rmANOVA), with drug condition as the independent variable and week of recording as the repeated measure. These were used to assess the main effect of drug [F(2,16)] as well as the interaction between drug and week of recording [F(4,32)]. Significant interactions were followed with Fisher LSD post-hoc analyses.
2.6 Contextual Fear Conditioning Task
Assessment of performance in the contextual fear conditioning paradigm began at week 7 of the experiment. The apparatus for contextual fear conditioning was a chamber (10×10×15 in.) that delivers an inescapable electrical shock through a 24-bar grid on the floor. The walls of the chamber were transparent so that freezing responses could be recorded. One mouse was brought into testing room at a time and the apparatus was cleaned with 70% ethanol after each trial to remove odorant cues. Before training, animals were individually habituated for two minutes to the experimenter and the testing room for two consecutive days. During training, mice were placed into the conditioning chamber for a total of 3 min, receiving a 2-sec 1.5 mA scrambled footshock at 2 min and 2.5 min after placement. During testing, mice received a 5-min exposure to the same conditioned context in the absence of shock. Mice were tested on two separate occasions, the first at 24 hours after training and the second 14 days later.
Throughout the procedures, freezing responses were recorded with a 5-s interval sampling. Freezing was judged as complete immobility of any part of the body except for respiratory movements. Freezing response during the 2 min before any shock was recorded as baseline, while freezing response during the 30 sec after each shock was recorded as the post-shock training response. All freezing responses during testing and training sessions were assessed manually by a single blinded experimenter. The percent freezing for each mouse was calculated by dividing the amount of time frozen by the total time in each session (time frozen/total time × 100 = % freezing). Repeated measures ANOVAs and post-hoc Fisher LSD tests were used to determine statistical significance.
2.7 Novel Object Recognition Task
Novel object recognition was assessed during week 6 of the experiment. The apparatus for this task consisted of a white open rectangular field box (60×50×26 cm). Mice were individually handled for 2 min by the experimenter in the testing room for three consecutive days before training. The day before training, mice were allowed to explore the experimental apparatus for 5 min in the absence of objects. During the training phase, mice were placed in the experimental apparatus for 15 min with two identical objects positioned in specific locations. After a retention interval of 24 hours, mice were placed back into the rectangular environment in which one of the familiar objects was replaced with a novel one. Mice were allowed to freely explore the environment and the objects for 15 min. Animal behavior during testing and training sessions was manually scored by a single, blinded experimenter. A mouse was scored as exploring an object whenever it was within 1 cm of the object and facing it. Measurement of the time spent exploring each object was recorded and expressed as the percent time spent exploring the novel object relative to the total time spent exploring both of the objects. Total exploration time of both objects was also calculated. The identity of the objects and the spatial location in which the novel and familiar objects were located were counterbalanced between subjects. Objects were cleaned with 70% ethanol between each animal. Repeated measures ANOVAs were used to determine statistical significance. Animals that did not explore the objects for more than 5 seconds over the course of the 15 min training and testing sessions were excluded from analysis.
2.8 Correlation Analysis
Pearson’s r correlations were performed to explore potential relationships between sensory gating ratios, total exploration time in NOR, and freezing response in CFC. Sensory gating ratios were used from week 5 and were calculated as described previously. Total exploration time for NOR was calculated as described previously. Exploration time for each animal was divided by the mean of the exploration times from control group from the same session. These ratios were averaged over testing and training days yielding a single total exploration time ratio for each animal. Similarly, freezing response was expressed as a ratio to the mean of the control group’s response during the same session, which was then averaged over all testing sessions to yield a single number for each animal.
Results
3.1 Chemotherapy Toxicity
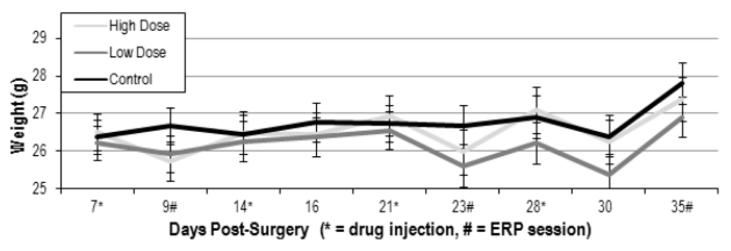
Animals were carefully monitored throughout for signs of acute toxicity, which were largely absent. A rmANOVA showed no main effect (F(2,17) = 0.387, p=0.685) of drug on animal weight and no interaction of drug and day (F(16,136)=1.411, p=0.145) (Figure 5). In addition, no animals died during treatment and there were no other signs of acute toxicity, such as altered physical appearance or grossly reduced locomotor activity.
Figure 5.
Animals were weighed throughout to assess acute toxicity. Average weights (+/- SEM) are shown for high dose, low dose, and control groups. Drug injection and ERP recording days are indicated. There was no significant effect of drug on animal weight.
3.2 ERP Auditory Gating
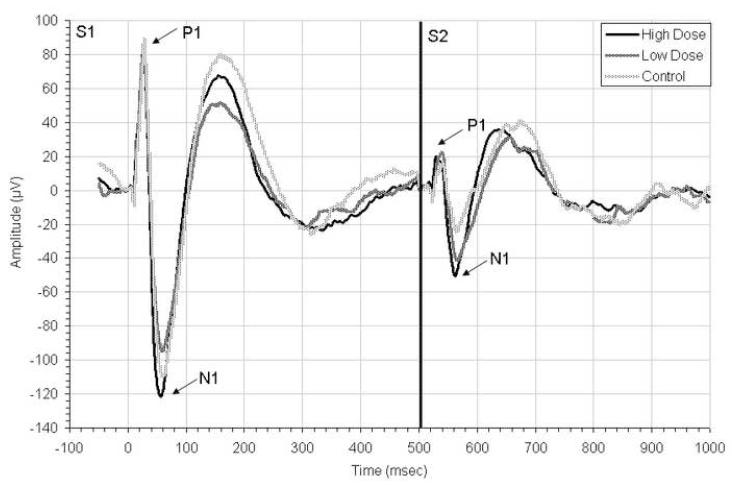
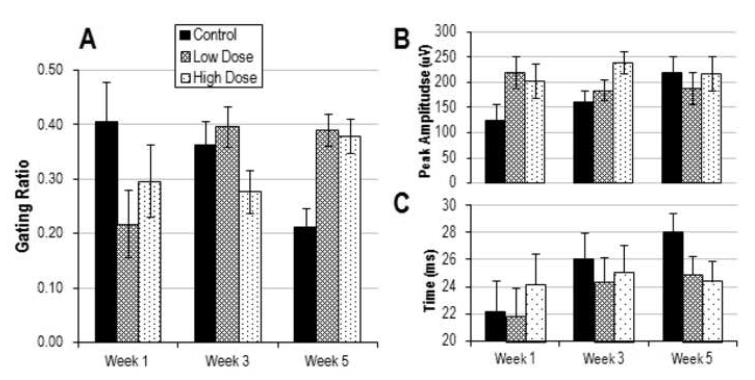
Average ERP waveforms at week 5 are shown in Figure 2. Statistical analyses showed no main effect of drug on sensory gating ratios (F(2,16)=0.225, p=0.801). However, there was a significant interaction between drug condition and week (F(4, 32) = 4.99, p = 0.003) (Figure 3). Post-hoc analyses revealed significantly increased gating ratios at week 5 between high dose and control groups (p=0.036) and between low dose and control groups (p = 0.022), suggesting that chemotherapy impairs sensory habituation. An ANOVA run on data only from week 5 revealed a main effect of drug (F(2,17)=8.143, p=0.003), with impaired gating in both high dose (p=0.004) and low dose (p=0.002) groups compared to control on post-hoc.
Figure 2.
Grand average auditory event-related potentials recorded to paired click stimuli at week 5 of the experiment, one week after chemotherapy injections had been completed. Responses to the first stimuli (S1) and second (S2) are shown, with P1 and N1 peaks labeled. Across all groups, responses to S2 are significantly dimished compared to S1 reflecting sensory gating. See Figure 3 for analysis of ERP gating, peaks, and latencies.
Figure 3.
Results from ERP experiments across week of recording for chemotherapy treated and control mice. A. Average gating ratios (+/- SEM) defined as S2/S1 B. Mean amplitude (+/- SEM) of response (in μV) to the first stimulus (A1, defined as P1-N1) C. Average latency (+/- SEM, in ms) of the P1 following S1. For gating ratios (A), there was a significant interaction between drug condition and week (p=0.003) with higher ratios (suppressed auditory gating) in low (p=0.022) and high-dose (p=0.036) groups compared to control at week 5. There was no effect of drug on the mean amplitude of P1-N1 or latency of P1.
3.3 ERP Amplitude
We assessed the ability of the mice to generate a coordinated neural response to auditory stimuli by analyzing ERP peak amplitude. A rmANOVA on the A1 amplitude (defined as P1-N1 for S1) showed no main effect of drug (F(2,16)=1.325, p=0.293) and no significant interaction across week (F(4,32)=2.237, p=0.087) (Figure 3).
There is previous data to suggest that chemotherapy may affect peak latency in ERP paradigms, which could correspond to deficits in processing speed. However, rmANOVA of P1 latencies showed no main effect of drug (F(2,16)=0.282, p=0.758) and no significant interaction across week (F(4,32)=1.29, p=0.294), arguing against differences in pre-attentive processing speed.
3.4 Contextual Fear Conditioning
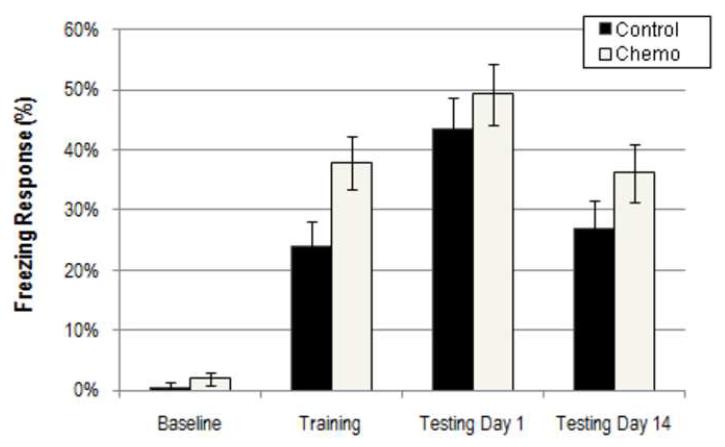
A rmANOVA of percentage freezing during baseline, training, testing day 1, and testing day 14 sessions revealed significantly increased freezing in chemotherapy treated animals (F(3, 42) = 0.913, p = 0.045) which was present at all time-points during the behavioral paradigm (Figure 4). CFC was assessed in high dose and control group animals. There was no interaction between drug treatment and session (F(3,42) = 0.913, p=0.442). These results demonstrate that the chemotherapy treated mice are more reactive to the acute footshock stress but are not impaired in their ability to associate aversive stimuli with a complex spatial environment.
Figure 4.
Results from the contextual fear conditioning paradigm for chemotherapy treated and control mice. Average percentage freezing responses (+/- SEM) are shown during baseline, training, and testing phases. Testing was conducted at 1 day and 14 days after training. There was a main effect of drug on CFC (p=0.045) with chemotherapy exposure leading to increased fearful freezing.
3.5 Novel Object Recognition Paradigm
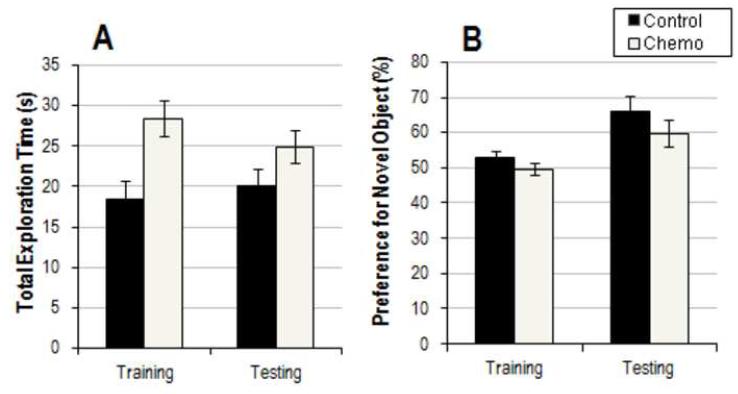
A rmANOVA was performed to assess novel object preference in high dose and control groups (Figure 6). Results showed no main effect of chemotherapy on object preference (F(1,12) = 2.353, p = 0.151) and no significant interaction of GROUP × SESSION (F(1,12) = 0.209, p = 0.656). However, a rmANOVA of total exploration time indicated a main effect of drug (F(1,14)=8.621, p=0.011) with the chemotherapy treated animals showing longer total exploration over both training and testing sessions.
Figure 6.
Results from the novel object recognition paradigm. A. Average total exploration time (+/- SEM) in seconds. B. Average percentage preference for the novel object (+/- SEM). There was a main effect of drug on total exploration time (p=0.011) during NOR but not on novel object preference (p = 0.151), with chemotherapy treated mice showing increased exploratory behavior.
3.6 Correlation between Sensory Gating, NOR, and CFC
We employed a within-animal analysis to assess if any significant correlations exist between sensory gating ratios at week 5 and performance in novel object and contextual fear conditioning paradigms. We calculated single values for total NOR exploration and CFC freezing time for each animal. Results showed no significant correlation between week 5 gating ratios and average NOR exploration time ratios (p = 0.326, r = 0.296). Likewise, there was no significant correlation between week 5 gating ratios and average CFC freezing time ratios (p = 0.476, r = 0.168). However, there was a significant positive correlation between NOR exploration time ratios and CFC freezing time ratios (p = 0.021, r = 0.571). These results demonstrate that performance in the novel object and contextual fear conditioning paradigms may be linked, such that animals which show increased freezing response to footshock also exhibit increased total exploration in novel environments.
Discussion
To our knowledge, this study marks the first attempt to characterize event-related potential changes with chronic chemotherapy administration in an animal model, as well as the first time that sensory gating has been assessed as a potential marker of chemotherapy-induced cognitive decline in humans or animals. Male mice were given four, weekly injections of a combination of MTX and 5-FU, common components of the CMF regimen frequently used to treat human cancers. ERPs were recorded to paired-click stimuli during the study. Behavioral testing began two weeks after chemotherapy treatment was finished and continued for an additional two weeks. Drug-treated animals experienced mild weight loss for 2-3 days after each injection, but overall weight change was not significantly different between groups (Figure 5). No animals died during the experiment and there were no other signs of overt drug toxicity. Therefore, we propose that doses used have good face validity for clinically relevant exposures.
Results demonstrate lasting alterations in sensory encoding of auditory stimuli, indicating that anti-neoplastic agents are capable of inducing long-term changes in CNS function. Chronic effects were characterized by decreased ability to filter auditory stimuli, as measured by gating ratios of the P1-N1 component (Figures 2, 3). No alterations were found in the amplitude of the P1-N1 component or the latency of P1, further suggesting that the chemotherapy-induced defects in information processing are subtle and involve disruption of inhibitory processes rather than generators of the ERP. Gating deficits seen at week 5 occurred seven days after the last dose of drug, indicating that the deficits were lasting, since the half-life of i.p. MTX in mice is about 30 minutes (Lobo and Balthasar, 2003) and the half-life of i.p. 5-FU in mice is about 25 min (Kamm et al., 2003). In the auditory gating paradigm, it is generally accepted that there is an 80% decrement in the amplitude of the response to the second stimulus, representing activation of an inhibitory neural cascade by the first stimulus (Braff and Light, 2004). Here we report that the control animals gate their response to S2 by an average of 77%, whereas the chemotherapy-treated animals do so by only about 60%.
Gating deficits reported above were only present at week 5 of the experiment and were not evident during the earlier ERP recording sessions during weeks 1 and 3. There are several possible reasons why the ERPs recorded during the earlier weeks did not show similar deficits in sensory gating. First, during the earlier sessions, animals were being exposed to chemotherapy acutely which could cause multiple acute and chronic effects, confounding the results. Second, because the doses of drug were set low enough to induce only minimal or no signs of acute toxicity, it is possible that the gating deficits seen at week 5 were a result of the additive effects of repeated low doses of chemotherapy. This cumulative effect is consistent with the clinical observation that repeated sub-lethal doses are needed for efficacy.
Deficits in sensory gating have been explored in a number of conditions with associated cognitive impairments, most notably in schizophrenia, in which patients and approximately half of their first-degree relatives show P1, N1 non-suppression phenotypes (Siegel et al., 1984). Gating abnormalities have also been linked to cognitive changes in patients with post-traumatic stress disorder (PTSD) (Karl et al., 2006), traumatic brain injury (TBI) (Arciniegas and Topkoff, 2004), Alzheimer’s disease (Jessen et al., 2001), Parkinson’s disease (Teo et al., 1997), and in cocaine abusers (Fein et al., 1996), among others. Each tends to show a distinct, characteristic pattern of alterations in P1 and/or N1 latency, amplitude, and habituation (gating) suggesting that they are not all caused by the same underlying neurobiological substrates. For example, PTSD has been linked to a number of ERP changes, including an impairment in P1 gating as well as alterations in the slope of P2 and a decreased amplitude of P3 (Gillette et al., 1997). The level of sensory gating deficit has been correlated with the intensity of symptomatic reoccurrence in patients with PTSD, suggesting a connection between gating deficits and hyperarousal symptoms, both of which have been linked to central noradrenergic hyperactivity (Southwick et al., 1993, Fein et al., 1996).
There is some debate regarding the generators of P1 and the network which mediates its sensory habituation. Candidates include the hippocampus, Heschel’s gyrus, prefrontal cortex, and superior temporal gyrus (Potter et al., 2006). Likewise, several neurotransmitter systems have been implicated in sensory gating. Evidence from the schizophrenia literature suggests that gating is at least in part mediated by cholinergic afferents to inhibitory neurons in the hippocampus and reticular activating system (RAS), as nicotinic agonists affecting those regions are thought to play a role in normalizing gating in schizophrenic patients (Adler et al., 1993, Braff and Light, 2004, Potter et al., 2006). Dopaminergic and noradrenergic systems are also important modulators (Fein et al., 1996, Gillette et al., 1997, Potter et al., 2006). In particular, noradrenergic hyperactivity has been posited as the underlying etiology of the gating deficits seen in patients with PTSD (Gillette et al., 1997).
In the present situation, gating deficits may underlie the qualitative observations of neurocognitive dysfunction reported in the clinical literature. An inability to filter out extraneous sensory stimuli in the pre-attentive stages of sensory processing could overwhelm an individual with a flood of incoming stimuli and interfere with the ability to mount a selective attentive response to the most salient information. Evidence from multiple investigators using complimentary approaches indicates that the inability to interpret complex stimuli may begin with abnormalities in early detection and encoding of stimulus characteristics. Disruption of early neural processes likely degrades the building blocks of sensory interpretation and is consistent with abnormalities in cognitive domains that require qualitative assessment of stimulus parameters on which to base decisions. If a signal is misallocated with respect to its relevance, its encoding in memory will be altered and decisions will be based on distorted information.
Clinical studies have shown that various forms of cancer, including breast cancer and acute lymphoblastic leukemia (ALL), as well as chemotherapy and other cancer therapies can alter cognitive performance and physiological measures including ERPs (Parageorgiou et al., 2000, Schagen et al., 2001, Kreukels et al., 2005). For example, Kreukels et al. retrospectively correlated Cyclophosphamide-Methotrexate-5-Fluorouracil therapy with reduced P300 amplitude and latency during information processing and reaction-time tasks in breast-cancer patients, at an average of 5-years post-treatment. The study compared ERP responses in a cohort of breast cancer patients who had received surgery, radiotherapy, and systemic chemotherapy with another cohort of patients who received surgery and radiotherapy alone (Kreukels et al., 2005). Most clinical studies that employed ERPs focused on the P300 and reported significant, but sometimes contradictory results, perhaps due to differences in patient populations, treatment modalities, or the complexity of the ERP task. Our results bypass these inconsistencies by controlling population differences with a standardized animal model and by assessing the earlier, more robust components of information processing with a sensory gating task. Using an animal model helps overcome several limitations in the clinical studies, including small sample sizes, different drug treatment regimens and doses, variable patient populations, additional health and psychiatric problems, direct effects from the cancer, and non-standardized cognitive assessments.
In addition to ERPs, we explored the effects of chemotherapy on animal performance in NOR and CFC paradigms. Surprisingly, there have only been a few published studies investigating cognitive-behavioral changes associated with chemotherapy drugs in adult animals. Winocur et al. found mild deficits in spatial memory and conditional learning in mice treated with a combination regimen of i.p. MTX and 5-FU, but no alterations in cued memory or discrimination learning, suggesting that susceptibility to the neurotoxic effects of chemotherapy is prominent in the hippocampus and frontal lobe structures (Winocur et al., 2006). Lee et al. investigated electrophysiological changes in hippocampal slices from rats treated chronically with i.p. cyclophosphamide, reporting deficits in long-term potentiation (LTP) in animals undergoing concurrent drug treatment but remarkably LTP enhancement in rats who had sufficiently recovered from the chemotherapy (Lee et al., 2006). In addition, they reported no chronic deficits in spatial learning and memory. Seigers et al. administered a single i.v. dose of MTX to rats and found lasting impairments in spatial memory and novel object recognition memory, as well as a dose-dependent decrease in hippocampal neurogenesis (Seigers et al., 2008).
We demonstrate that chemotherapy affects performance in contextual fear conditioning (Figure 4) which is sensitive to hippocampal and amygdalar function (Phillips and LeDoux, 1992). Animals treated with high dose MTX + 5-FU showed an increase in freezing to a footshock paired with a context. The drug-treated mice continued to respond with increased freezing when placed in the context two weeks later, arguing against overt impairments in learning or memory of the context. This indicates that the memory deficits reported in the previous animal models (including spatial, recognition, and conditional memory) do not reflect global disruption in hippocampal function. The increase in immediate freezing to shock suggests that the chemotherapy treated mice have some degree of emotional dysregulation. Increasing the intensity of the shock causes exaggerated freezing responses (Siegmund and Wotjak, 2007) and so it is possible that the chemotherapy treated animals have heightened sensitivity to sensory input or a decreased nociceptive threshold. However, direct measurement of neural responses to auditory stimuli noted above (as reflected by ERP amplitudes) indicates no change in sensory registration, which argues against an increased overall neural response to the nociceptive inputs. Another possibility is that modulation of the neural response to emotionally arousing stimuli is disrupted by chemotherapy. Extensive work has established that the stress-related neurotransmitter norepinephrine (NE) plays a key role in the formation of aversive memories and is also integrally involved in the behavioral response to fearful stimuli (Davies et al., 2003, Mueller et al., 2008). Blockade of central noradrenergic beta-receptor signaling inhibits formation of fear conditioned memories (Davies et al., 2003). Enhanced noradrenergic release facilitates formation of aversive memories, also through beta-receptor signaling (Mueller et al., 2008). Of note, increased NE release during the training phase of fear conditioning leads to a heightened freezing response as well as enhanced sensitivity to the learning paradigm, suggesting memory augmentation (Davies et al., 2003). Thus, our results could be explained by underlying noradrenergic hyperactivity, which has also been shown to cause gating deficits in PTSD. On a broader level, these findings demonstrate that cancer patients may be more vulnerable to noxious stimuli that would otherwise bother them less. This is conceptually consistent with the gating results which demonstrate an impaired ability to filter out sensory information and decreased inhibitory control in the brain’s ability to modulate its response to incoming stimuli.
We also assessed the effects of chemotherapy on performance in novel object recognition (Figure 6), which is sensitive to cortico-hippocampal functioning (Carpenter and Grossberg, 1993). There were no significant differences between groups as assessed by percentage preference for the novel object, although the chemotherapy-treated mice trended toward having a deficit in this area. The drug-treated mice did show significantly more total object exploration time, during both training and testing sessions. Since all animals were habituated to the novel object apparatus (without objects) prior to the training and testing sessions, it is possible that memory of this phase of the experiment was impaired in the drug-treated mice, which caused them to react to the apparatus with more novelty. A second hypothesis is that the drug-treated mice are hyperaroused by the novel environment and thus it takes them longer to habituate to their surroundings, an idea that has been previously suggested (Okuda et al., 2004). This hyperarousal could be consistent with the increased freezing seen on fear conditioning and could be explained by a basic deficiency in habituation and sensory inhibitory control as indicated by the gating deficits. Our NOR results differ from those of a recently published study which showed impairment in novel-object preference in rats treated with a single dose of MTX but no difference in total exploration time (Seigers et al., 2008). The two studies do differ in several ways — including animal species, drug dose, and single drug vs combination regimen— which could explain the inconsistent findings. It is also possible that our study was underpowered (n=8 in each group) to pick up a significant group differences, as our data does qualitatively trend in the same direction. This possibility is inconsistent with the observation that we had sufficient power to detect increased exploration, increased freezing, and decreased gating of ERPs. Thus, we conclude that if there are deficits in hippocampally mediated learning and memory of the novel object, these deficits are subtle, in accordance with the clinical literature.
We present evidence that the behavioral and cognitive deficits associated with chemotherapy treatment could be linked to an underlying state of hyperarousal. This furthers the connection between the cognitive deficits affecting cancer survivors and those of patients with PTSD, suggesting similar mechanisms may be at work in both patient populations (Metzger et al., 1997, Karl et al., 2006). In addition to the ERP similarities reported in this study, reports have demonstrated that patients with PTSD have smaller P3 amplitudes and increased reaction times on auditory discrimination tasks (McFarlane et al., 1993, Metzger et al., 1997) which correspond to the findings from Kreukels et al. in a population of breast cancer survivors (Kreukels et al., 2005). In accordance with our behavioral data, physiological studies in patients with PTSD have demonstrated an increased responsiveness to neutral or stressful stimuli, as well as a hypersensitive startle response, which corresponds with the enhanced freezing response to the acute footshock stressor in our fear conditioning paradigm (Skinner et al., 1999).
Chemotherapy treatment and cancer diagnosis have long been epidemiologically associated with PTSD. Recent studies have reported that between 3-18% of breast-cancer survivors go on to develop full-blown PSTD, while as many as 41% experience symptoms of PTSD (Hakamata et al., 2007). One study reported significantly increased prevalence of PTSD symptoms, especially hyperarousal, in breast-cancer patients who had been disease free for at least three-years (Amir and Ramati, 2002). Taken together, our data would seem to argue that this increase in PTSD symptomatology in cancer-survivors may be as attributable to the neurotoxic potential of chemotherapeutic agents as it is to the psychological trauma caused by battling cancer. In addition, this study would suggest that the cognitive impairments associated with both PTSD and chemotherapy administration may be linked to similar neurobiological substrates, such as hyperactive noradrenergic tone and/or potentiated limbic circuitry. Importantly, in the PTSD literature, appropriate treatment with medication has been shown to reverse ERP abnormalities and possibly cognitive deficits, suggesting that this may be possible in cancer survivors as well (Metzger et al., 1997). An advantage of our animal ERP model is that further studies could easily be performed to assess the therapeutic potential of beta-blockers and selective-serotonin reuptake inhibitors (SSRIs), in addition to any other classes of drugs, in reversing the sensory gating and behavioral abnormalities associated with chemotherapy exposures.
There are a number of limitations to this study that must be addressed before proceeding in the direction implicated by our findings. First, the ERP results presented here must be replicated in human subjects using analogous techniques to assess predictive validity for the clinical syndrome. Also, while ERPs and cognitive behavioral paradigms are useful measures, neither is diagnostically specific to disease populations. While the connections between chemotherapy and PTSD like symptoms implied by our data are a promising direction for understanding the lasting effects of cancer treatment, further analysis must be performed to solidify these links.
Future studies should employ this animal model to probe underlying mechanisms of chemotherapy induced cognitive impairments. While definitive mechanisms for these cognitive changes have yet to be established, there are several potential hypotheses. Leading candidates include disruption of the blood brain barrier, cytokine upregulation and neuroinflammation, DNA damage, oxidative stress, and dysfunction of the neurohormonal axis (Ahles and Saykin, 2007). Using the model described in this study, we hope to probe each of these putative areas in the future with relevant pharmacologic and genetic manipulations to further elucidate neurological mechanisms underlying these cognitive deficits.
Acknowledgment
This work was supported by a pilot grant from the Abramson Cancer Center of the University of Pennsylvania (SJS) and support for MG is provided by the Medical Scientist Training Program at the University of Pennsylvania. Additional support was provided by P50-CA-084718 and P50 MH064045.
Abbreviations:
- 5-FU
5-fluorouracil
- ALL
acute lymphoblastic leukemia
- ANOVA
analysis of variance
- B6
C57BL/6Hsd
- CNS
central nervous system
- CFC
contextual fear conditioning
- r
correlation coefficient
- CMF
cyclophosphamide-methotrexate-5-fluorouracil
- DMSO
dimethyl sulfoxide
- EEG
electroencephalogram
- ERPs
event-related potentials
- N1
first negative peak in the ERP
- P1
first positive peak in the ERP
- S1
first stimulus
- IACUC
Institutional Animal Care and Use Committee
- LTP
long-term potentiation
- MTX
methotrexate
- NE
norepinephrine
- NOR
novel-object recognition testing
- PTSD
post-traumatic stress disorder
- p
probability
- A2/A1
ratio of response following the second tone to the first
- rmANOVA
repeated measures ANOVA
- RAS
reticular activating system
- RMS
root mean square
- P2
second positive peak in the ERP
- S2
second stimulus
- SSRIs
selective-serotonin reuptake inhibitors
- SEM
standard error of the mean
- P3
third positive peak in the ERP
- TBI
traumatic brain injury
- ULAR
University Laboratory Animal Resources
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature reviews. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir M, Ramati A. Post-traumatic symptoms, emotional distress and quality of life in long-term survivors of breast cancer: a preliminary research. J Anxiety Disord. 2002;16:195–206. doi: 10.1016/s0887-6185(02)00095-6. [DOI] [PubMed] [Google Scholar]
- Arciniegas DB, Topkoff JL. Applications of the P50 evoked response to the evaluation of cognitive impairments after traumatic brain injury. Phys Med Rehabil Clin N Am. 2004;15:177–203. viii. doi: 10.1016/s1047-9651(03)00104-9. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology (Berl) 2004;174:75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- Carpenter GA, Grossberg S. Normal and amnesic learning, recognition and memory by a neural model of cortico-hippocampal interactions. Trends Neurosci. 1993;16:131–137. doi: 10.1016/0166-2236(93)90118-6. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem Res. 2004;29:1179–1188. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell CR, Kanes SJ, Abel T, Liang Y, Tokarczyk J, Bilker WB, Turetsky BI, Gur RE, Siegel SJ. Inhibition of auditory evoked potentials and prepulse inhibition of startle in DBA/2J and DBA/2Hsd inbred mouse substrains. Brain Res. 2003;992:85–95. doi: 10.1016/j.brainres.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Davies MF, Tsui JY, Flannery JA, Li X, DeLorey TM, Hoffman BB. Augmentation of the noradrenergic system in alpha-2 adrenergic receptor deficient mice: anatomical changes associated with enhanced fear memory. Brain research. 2003;986:157–165. doi: 10.1016/s0006-8993(03)03248-7. [DOI] [PubMed] [Google Scholar]
- de Bruin NM, Ellenbroek BA, Cools AR, Coenen AM, van Luijtelaar EL. Differential effects of ketamine on gating of auditory evoked potentials and prepulse inhibition in rats. Psychopharmacology (Berl) 1999;142:9–17. doi: 10.1007/s002130050856. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang M, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitroand in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA. Pre-attentive processing and schizophrenia: animal studies. Psychopharmacology (Berl) . 2004;174:65–74. doi: 10.1007/s00213-003-1684-7. [DOI] [PubMed] [Google Scholar]
- Fein G, Biggins C, MacKay S. Cocaine abusers have reduced auditory P50 amplitude and suppression compared to both normal controls and alcoholics. Biol Psychiatry. 1996;39:955–965. doi: 10.1016/0006-3223(95)00299-5. [DOI] [PubMed] [Google Scholar]
- Gillette GM, Skinner RD, Rasco LM, Fielstein EM, Davis DH, Pawelak JE, Freeman TW, Karson CN, Boop FA, Garcia-Rill E. Combat veterans with posttraumatic stress disorder exhibit decreased habituation of the P1 midlatency auditory evoked potential. Life Sci. 1997;61:1421–1434. doi: 10.1016/s0024-3205(97)00688-7. [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Matsuoka Y, Inagaki M, Nagamine M, Hara E, Imoto S, Murakami K, Kim Y, Uchitomi Y. Structure of orbitofrontal cortex and its longitudinal course in cancer-related post-traumatic stress disorder. Neurosci Res. 2007;59:383–389. doi: 10.1016/j.neures.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature reviews. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Kucharski C, Fries T, Papassotiropoulos A, Hoenig K, Maier W, Heun R. Sensory gating deficit expressed by a disturbed suppression of the P50 event-related potential in patients with Alzheimer’s disease. Am J Psychiatry. 2001;158:1319–1321. doi: 10.1176/appi.ajp.158.8.1319. [DOI] [PubMed] [Google Scholar]
- Kamm YJ, Peters GJ, Hull WE, Punt CJ, Heerschap A. Correlation between 5-fluorouracil metabolism and treatment response in two variants of C26 murine colon carcinoma. Br J Cancer. 2003;89:754–762. doi: 10.1038/sj.bjc.6601162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Malta LS, Maercker A. Meta-analytic review of event-related potential studies in post-traumatic stress disorder. Biol Psychol. 2006;71:123–147. doi: 10.1016/j.biopsycho.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kreukels BP, Schagen SB, Ridderinkhof KR, Boogerd W, Hamburger HL, van Dam FS. Electrophysiological correlates of information processing in breast-cancer patients treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2005;94:53–61. doi: 10.1007/s10549-005-7093-3. [DOI] [PubMed] [Google Scholar]
- Labossiere E, Glickstein ME. Histological processing for the neural sciences. Thomas; Springfield, Ill.: 1976. [Google Scholar]
- Lee GD, Longo DL, Wang Y, Rifkind JM, Abdul-Raman L, Mamczarz JA, Duffy KB, Spangler EL, Taub DD, Mattson MP, Ingram DK. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin Cancer Res. 2006;12:198–205. doi: 10.1158/1078-0432.CCR-05-1286. [DOI] [PubMed] [Google Scholar]
- Lobo ED, Balthasar JP. Pharmacokinetic-pharmacodynamic modeling of methotrexate-induced toxicity in mice. J Pharm Sci. 2003;92:1654–1664. doi: 10.1002/jps.10431. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Gettes DR, Evans DL, Kanes SJ, Abel T, Karp J, Siegel SJ. Corticosterone modulates auditory gating in mouse. Neuropsychopharmacology. 2006a;31:897–903. doi: 10.1038/sj.npp.1300879. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006b;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Kanes SJ, Abel T, Siegel SJ. Phosphodiesterase inhibitors: a novel mechanism for receptor-independent antipsychotic medications. Neuroscience. 2004a;129:101–107. doi: 10.1016/j.neuroscience.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Liang Y, Weightman BD, Kanes SJ, Abel T, Gur RE, Turetsky BI, Bilker WB, Lenox RH, Siegel SJ. Effects of chronic olanzapine and haloperidol differ on the mouse N1 auditory evoked potential. Neuropsychopharmacology. 2004b;29:739–746. doi: 10.1038/sj.npp.1300376. [DOI] [PubMed] [Google Scholar]
- McFarlane AC, Weber DL, Clark CR. Abnormal stimulus processing in posttraumatic stress disorder. Biol Psychiatry. 1993;34:311–320. doi: 10.1016/0006-3223(93)90088-u. [DOI] [PubMed] [Google Scholar]
- Metzger KL, Maxwell CR, Liang Y, Siegel SJ. Effects of Nicotine Vary Across Two Auditory Evoked Potentials in the Mouse. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Orr SP, Lasko NB, Pitman RK. Auditory event-related potentials to tone stimuli in combat-related posttraumatic stress disorder. Biol Psychiatry. 1997;42:1006–1015. doi: 10.1016/s0006-3223(97)00138-8. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parageorgiou C, Dardoufas C, Kouloulias V, Ventouras E, Uzunoglu N, Vlahos L, Rambavilas A, Christodoulou G. Psychophysiological evaluation of short-term neurotoxicity after prophylactic brain irradiation in patients with small cell lung cancer: a study of event related potentials. J Neurooncol. 2000;50:275–285. doi: 10.1023/a:1006447624574. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA, Krausz HI, Galambos R. Human auditory evoked potentials. I. Evaluation of components. Electroencephalogr Clin Neurophysiol. 1974;36:179–190. doi: 10.1016/0013-4694(74)90155-2. [DOI] [PubMed] [Google Scholar]
- Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reite M, Teale P, Zimmerman J, Davis K, Whalen J. Source location of a 50 msec latency auditory evoked field component. Electroencephalography and clinical neurophysiology. 1988;70:490–498. doi: 10.1016/0013-4694(88)90147-2. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Hamburger HL, Muller MJ, Boogerd W, van Dam FS. Neurophysiological evaluation of late effects of adjuvant high-dose chemotherapy on cognitive function. J Neurooncol. 2001;51:159–165. doi: 10.1023/a:1010635229762. [DOI] [PubMed] [Google Scholar]
- Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, Beerling W, Boogerd W, van Tellingen O, van Dam FS, Koolhaas JM, Buwalda B. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behavioural brain research. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Siegel C, Waldo M, Mizner G, Adler LE, Freedman R. Deficits in sensory gating in schizophrenic patients and their relatives. Evidence obtained with auditory evoked responses. Arch Gen Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, Kanes SJ, Turetsky BI. Effects of Strain, Novelty, and NMDA Blockade on Auditory-Evoked Potentials in Mice. Neuropsychopharmacology. 2003;28:675–682. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- Siegmund A, Wotjak CT. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Rasco LM, Fitzgerald J, Karson CN, Matthew M, Williams DK, Garcia-Rill E. Reduced sensory gating of the P1 potential in rape victims and combat veterans with posttraumatic stress disorder. Depression and anxiety. 1999;9:122–130. doi: 10.1002/(sici)1520-6394(1999)9:3<122::aid-da4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, Heninger GR, Charney DS. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and alpha-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22:2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- Teo C, Rasco L, al-Mefty K, Skinner RD, Boop FA, Garcia-Rill E. Decreased habituation of midlatency auditory evoked responses in Parkinson’s disease. Mov Disord. 1997;12:655–664. doi: 10.1002/mds.870120506. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vyssotky D, Latanov A, Nitsch R, Brambilla R, D’Adamo P, Lipp HP. Midlatency auditory event-related potentials in mice: comparison to midlatency auditory ERPs in humans. Brain Res. 2004;1019:189–200. doi: 10.1016/j.brainres.2004.05.097. [DOI] [PubMed] [Google Scholar]
- Winocur G, Vardy J, Binns MA, Kerr L, Tannock I. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacol Biochem Behav. 2006;85:66–75. doi: 10.1016/j.pbb.2006.07.010. [DOI] [PubMed] [Google Scholar]