Abstract
Dioxins are nearly ubiquitous environmental contaminants that are produced as byproducts during industrial processes, including the bleaching of paper and textiles. Contamination of animal bedding material with dioxins has been a concern for both laboratory and farm animals. The objective of this study was to determine whether the presence of cotton balls, provided to mice for enrichment, caused induction of the cytochrome P450 1A1 gene (Cyp1A1), which typically is stimulated through activation of the aryl hydrocarbon receptor (AhR) by dioxins and dioxin-like compounds. Cyp1A1 transcripts and protein in the liver were increased significantly by either exposure to cotton balls or treatment with a single dose of 2,3,7,8-tetrachlorodibenzo-para-dioxin. Unexposed controls displayed low levels of Cyp1A1 transcript and undetectable levels of CYP1A1 protein. These results suggest that cotton balls are potentially contaminated with dioxins and/or dioxin-like compounds that act as potent inducers of Cyp1a1 in laboratory animals if used as nesting material. This study underscores the necessity of considering dioxin content in products used for enrichment in animal facilities.
Abbreviation: TCDD, 2, 3, 7, 8-tetrachlorodibenzo-para-dioxin; AhR, aryl hydrocarbon receptor; ARNT, aryl hydrocarbon nuclear transporter; Cyp1A1, cytochrome P450 1A1
The primary function of the cytochrome P450 1 (Cyp1) family of hemoproteins is the metabolism of foreign compounds including drugs, food additives, and environmental pollutants,41 although more recent evidence suggests a role in homeostatic functions. Induction of Cyp1A1/2 occurs in response to increased activity of the aryl hydrocarbon receptor (AhR). AhR is activated by numerous, structurally diverse chemical substances as well as a variety of endogenous ligands. Endogenous ligands include indoles, tetrapyroles, arachidonic acid metabolites, retinoids, and oxysterols, as well as heme and tryptophan metabolites.14,44 These weak AhR agonists likely cause transient induction of Cyp1A1/2, which leads rapidly to their degradation.14 A number of naturally occurring, dietary compounds are also AhR agonists; flavonoids and indoles, or gut-derived metabolites of these compounds, are the most common. Like the endogenous ligands, these substances are weak AhR agonists and substrates for degradation by Cyp1A enzymes.14 Anthropogenic chemicals, produced largely as byproducts of industrial processes, are the most well-studied ligands of AhR. Polycyclic aromatic hydrocarbons, such as benzo[a]pyrene and 3-methylcholanthrene, are biotransformed by Cyp1A enzymes into carcinogens.44 Toxic effects linked to AhR activation occur after exposure to high levels of polychlorinated biphenyls, which are chemically stable constituents of many commercial products. However, the most potent of the known AhR agonists are the dioxins.
The term ‘dioxins’ refers to polyhalogenated aromatic hydrocarbons that are structurally similar and share a common mechanism of toxicity. The dioxin family includes several polychlorinated dibenzofurans, polychlorinated biphenyls, and polychlorinated dibenzo-dioxins. Dioxins are among the most toxic chemicals known to man.5,44 The detrimental human health effects of the most toxic of the dioxins, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) were recently highlighted in the popular press after the deliberate poisoning of Ukrainian president Victor Yushchenko during his 2004 election campaign.8,18 Accidental human exposures at high concentrations in Italy,48 Japan,1 and Vietnam36 have resulted in a plethora of health problems, including dermal and ocular lesions, altered immune responses, irregular menstrual cycles, and multisite carcinogenesis.
Dioxins and other AhR agonists are responsible for a variety of health related effects in biologic systems.55 Very small doses of dioxins can cause wasting syndrome and ultimately death in some laboratory animals.6 Dioxins cause developmental defects and immune deficiency34 and are linked to pathologies of the central and peripheral nervous system.12 Dioxins are well-known endocrine disruptors, interfering with reproductive19,20 and thyroid hormone homeostasis59 and are associated with the development of diabetes.62 TCDD and other AhR agonists, benzo[a]pyrene, and 3-methylcholanthrene are carcinogenic.44 Mechanistically, dioxins exert their biologic effects through the AhR, a ligand-activated basic helix–loop–helix transcription factor that belongs to the Per–Arnt–Sim domain family of chemosensors. Ligand binding promotes translocation of the AhR–ligand complex to the nucleus, where it associates with the aryl hydrocarbon nuclear transporter (ARNT), which directs the complex to dioxin response elements on target genes. Important target genes regulated by AhR–ARNT include the xenobiotic metabolizing enzymes, such as cytochrome P450 1A1 (Cyp1A1). Induction of Cyp1A1 is commonly used as an indicator of AhR activation.25 Recent studies indicate that activated AhR also may interact directly with proteins involved in cell cycle control,28 elements of the apoptotic machinery,12 and other cellular kinases.51 Therefore, the vast array of effects attributable to AhR agonists should raise concern about their presence to a broad spectrum of researchers. Clearly, the presence of dioxins or dioxin-like compounds has the potential to create havoc in numerous research studies.
Human and animal exposure to dioxins typically occurs subsequent to their incorporation in food, water, soil, and air. Dioxins are produced as unwanted byproducts of industrial processes; they are formed during the combustion of organic substances in the presence of chlorine, such as during the process of paper and cotton bleaching, or from industrial chemical synthesis, such as the manufacture of certain pesticides, herbicides, and fungicides. Food of animal origin is a leading source of dioxin exposure in animals and humans.31 Previous investigations have demonstrated dioxin contamination of commercially available rodent chow.56 Various types of laboratory animal and farm animal bedding have been found also to contain dioxins.26,47 Relevant to the present study, dioxin contamination occurs in pulp-based products and textiles, including tampons, diapers, cotton balls, and textiles, as a result of bleaching in the presence of chlorine.2,16,22 Dioxins are highly lipophilic compounds that demonstrate remarkable resistance to metabolism in vertebrate species: the half life of the most potent dioxin, TCDD, is estimated to be 2 to 4 wk in rodents53 but can be as long as 11 y in humans.49 These properties promote bioaccumulation throughout the food chain.
The Guide for the Care and Use of Laboratory Animals24 states that the availability of cage enrichments should be considered as 1 of several factors to provide an adequate environment for the appropriate care of rodent species. Enrichment materials often are provided in the form of a rigid shelter or some sort of nesting material. Nesting materials, which allow animals to exert some degree of control over their environment and permit expression of natural ‘nesting’ behaviors, are preferred by mice over rigid shelters.61 Paper-derived nesting materials are preferred over wood-derived materials.60 Therefore, the use of paper products, including paper towels, and cotton balls has become a common, inexpensive source for enrichment provided in animal care facilities. However, the potential effect of dioxin contamination in these products used for enrichment has not been explored. Because the bleaching process often generates dioxins as byproducts, potential contamination of enrichment materials is of great concern. After the Division of Animal Resources at our university instituted a policy of providing cotton balls to rodents for enrichment, we conducted the present study to determine whether the presence of cotton balls can sufficiently activate aryl hydrocarbon receptor signaling. We found that transcripts of the AhR target gene Cyp1A1 were increased in the livers of mice provided cotton balls for enrichment.
Materials and Methods
Animals.
Male C57BL/6J mice (age, 6 wk) from Jackson laboratory (Bar Harbor, ME). This strain is highly sensitive to dioxin, is the background strain for the AhR null mouse, and is frequently used for dioxin studies.27,30,32,37,39,45 Mice were entrained for at least 2 wk under controlled lighting (12:12-h light:dark cycle), temperature (22 °C), and humidity (39%) in light-tight chambers before initiation of experimentation. Rodent chow (8604, Harlan, Indianapolis, IN) and water were provided ad libitum. Animals were group-housed (2 to 4 animals per cage) in standard polycarbonate rodent cages (18 × 28 × 13 cm; Allentown Caging Equipment, Allentown, NJ) on 1/8-in. corncob bedding (Mt Pulaski Products, Mt Pulaski, IL). Bedding was not tested for dioxin content. Mice were divided into control (n = 6), TCDD exposure (n = 6), and cotton ball exposure (n = 6) groups. The TCDD exposure group was given a single dose of TCDD (1 μg/kg body weight, 98% pure, Cambridge Isotope Laboratories, Woburn, MA) by gavage. The cotton ball exposure group was provided with a new cotton ball at each cage change, which occurred once weekly. All animals were euthanized by decapitation 4 wk after cotton ball exposure and 1 wk after TCDD treatment. These exposure times were chosen to reflect the typical amount of time animals might be exposed to cotton balls or TCDD in our laboratory. A liver sample was placed in RNAlater (Ambion, Foster City, CA) for preservation prior to RNA isolation and transcript analysis. Another liver sample was fixed in neutral buffered formalin prior to paraffin embedding and immunohistochemical analysis. All animal use was approved by the Institutional Animal Care and Use Committee of the University of Illinois at Urbana-Champaign, and experiments were conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.24
Quantitative real-time PCR.
Total RNA was isolated by using TRIzol (Gibco BRL, Carlsbad, CA), and quantitative PCR was performed as previously described.39 Primers used for Cyp1a1 are previously published.39 The threshold cycle (Ct) value was obtained, and relative RNA amount was calculated by using the relative standard curve method according to the manufacturer's instructions. Serial dilutions of pooled liver RNA, representative of all treatment groups, were used to generate standard curves. Linear regression analysis produced an equation for determining doubling efficiency and expression of each transcript.
Immunohistochemistry.
Paraffin-embedded liver samples were sectioned (7 mm) and mounted on slides (Superfrost Plus, Fisher Scientific, Pittsburgh, PA). After deparaffination and hydration of slides, endogenous peroxidase was quenched by incubating in 1% hydrogen peroxide for 30 min. Sections were blocked by using 1% normal goat serum and then incubated at 4 °C overnight with rabbit antihuman CYP1A1 primary antibody (1:500, Santa Cruz Biotechnology, Santa Cruz, CA). Antibody staining was visualized by using 3-amino-9-ethylcarbazole (AEC, Sigma, St Louis, MO) as a chromagen after amplification of the signal with the ABC kit (Vector Laboratories, Burlingame, CA). Slides were counterstained with hematoxylin, dehydrated, and cleared with xylene, and cover slips were secured (Permount, Fisher Scientific).
Statistical analysis.
PCR results were analyzed by 1-way ANOVA using SYSTAT (SSI, Richmond, CA). Tukey posthoc analysis was applied when ANOVA returned a P value less than 0.05.
Results
Cotton balls and TCDD increase Cyp1A1 transcripts.
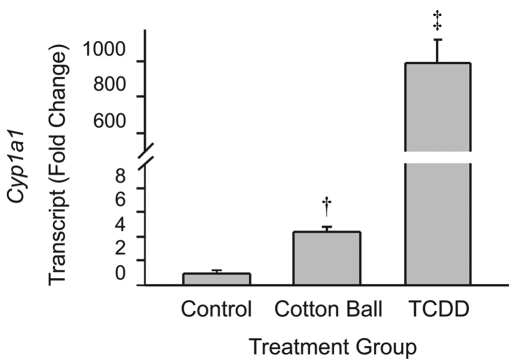
Transcript levels of the AhR target gene, Cyp1a1, in mice provided cotton balls for enrichment and animals treated with TCDD were compared with those of control mice that had no cotton balls or TCDD treatment. Cyp1a1 transcripts in liver were increased (P < 0.001, ANOVA) approximately 1000-fold 7 d after TCDD treatment (Figure 1) compared with levels in mice that were not exposed to cotton balls. Cyp1a1 transcripts in liver were increased (P < 0.01, ANOVA) approximately 4.5-fold in mice exposed to cotton balls for enrichment. Similar to those in control mice, Cyp1a1 transcripts were also very low in liver from mice provided pads of dioxin-free nesting material for enrichment (data not shown). These data suggest that the presence of cotton balls led to activation of AhR signaling.
Figure 1.
Quantitative real-time RT-PCR revealed that the quantity of Cyp1a1 transcripts in liver is increased after exposure to cotton balls or TCDD. †, P < 0.01; ‡, P < 0.001 (ANOVA with Tukey posthoc analysis) compared with control value.
Cotton balls and TCDD increase Cyp1A1 immunoreactivity.
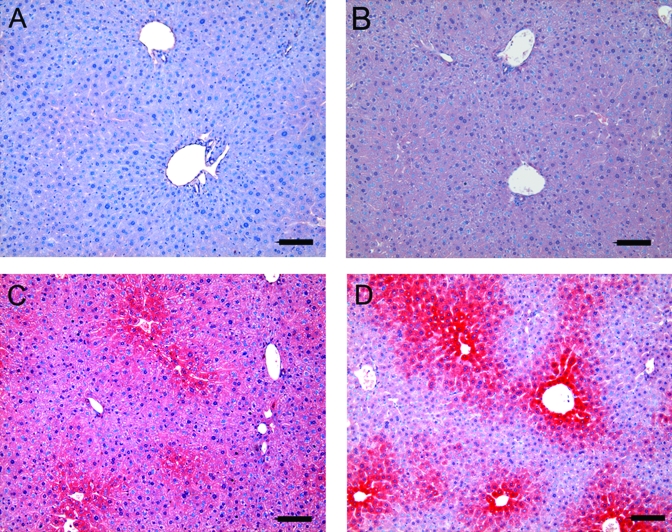
We used immunohistochemistry to determine whether cotton balls and TCDD altered CYP1A1 protein. Immunostaining of CYP1A1 was increased in the livers of animals treated with TCDD compared with control animals that were not exposed to cotton balls (Figure 2). CYP1A1 immunoreactivity in animals exposed to cotton balls was increased also. A characteristic centrilobular pattern of CYP1A1 expression was observed in the livers of mice that were either treated with TCDD or exposed to cotton balls. These data support the hypothesis that exposure to cotton balls led to activation of AhR signaling.
Figure 2.
Representative images of immunohistochemistry for hepatic expression of CYP1A1 protein (red). (A) Negative control (omission of primary antibody). (B) Control animal (no exposure to TCDD or cotton balls). (C) Animal exposed to cotton balls. (D) Animal exposed to TCDD. Panels C and D both show a characteristic centrilobular pattern of CYP1A1 expression. Scale bar, 20 μm.
Discussion
The validity of scientific results derived from animal studies depends on the health and well-being of the animals used for investigation. Over that past few decades, standardization of housing in animal facilities has greatly enhanced experimental repeatability across laboratories. However, the lack of complexity in the environment of laboratory rodents raises questions regarding both animal welfare and the degree to which they represent ‘normal’ animals. Animals housed in complex environments are clearly different from their counterparts housed in barren surroundings (for review, see reference 4). To enhance animal welfare, many facilities now provide enrichment for the animals. Sources of enrichment may include addition of (1) rigid structures that create microenvironments for hiding; (2) manipulada that allow chewing or engaging in fine motor movements; (3) novel foods with different tastes and textures; (4) increased social contact through group housing; and (5) olfactory or auditory stimuli.23 Although few studies3,4,54 address the effects of enrichment on experimental outcomes, it is clear that rodents prefer enriched environments. Furthermore, rodents favor environments enriched with paper- or textile-based nesting material.46 However, as the results of the present study indicate, care must be taken to provide nesting materials that will not compromise animal health or the validity of experimental measures.
During an ongoing project designed to investigate the role of AhR in regulation of circadian rhythms,37,39 the Division of Animal Resources at our university initiated the use of standard (not intended for laboratory animal use) cotton balls as environmental enrichment for all mice in the animal facility. Shortly thereafter, we noticed increased Cyp1A1 levels in a control group that was part of a TCDD-treatment study, raising concerns regarding the presence of dioxins or dioxin-like compounds in the facility and thus prompting this investigation. Although the cotton balls we used were not tested for dioxins, the increased Cyp1A1 transcript and protein levels strongly suggest that dioxins or other dioxin-like compounds were present. Cyp1A1 is a sensitive indicator of AhR activation; Cyp1A1 levels in the liver are negligible in animals not exposed to AhR agonists but are increased in rat liver in response to a single dose of TCDD at 1 ng/kg body weight;62 the 1-μg/kg dose used in the present study is a typical dose used in C57BL/6J mice.7 Induction of Cyp1A1 in response to AhR agonists varies among species29,35,50 and even among strains of mice.42 Inbred mouse strains exhibit as much as 10-fold differences in sensitivity;63 C57BL/6J mice are considered sensitive, whereas DBA/2 mice are resistant. Differences in sensitivity are attributable to specific structural differences in AhR.21,29,43 Increased Cyp1A1 transcripts can be detected in mouse liver as early as 1 h after exposure to AhR agonists.38 Maximal induction of Cyp1A1 occurs by 24 h after a single dose of TCDD;58 however, Cyp1A1 activity remains increased for as long as 35 d.17 Furthermore, Cyp1A1 is likely to remain increased with continued exposure to AhR agonists.
Cyp1A1/2 are critical mediators of the biotransformation of environmental toxicants, steroid hormones, pharmaceuticals, and carcinogens.10,11,33 Detoxification and maintenance of chemical homeostasis relies on the conversion of chemicals in the liver by Cyp1A1/2 into polar metabolites that can be excreted. Demethylation of caffeine and metabolism of theophylline occurs through Cyp1A1/2 in the liver.9 Endobiotics, including 17β-estradiol, retinoids, and thyroxine, as well as pharmaceuticals such as clazapine, imipramine, and propranolol are high-affinity substrates for Cyp1A1/2 (for review, see reference 57). Anthropogenic contaminants classified as dioxins are, however, the most potent AhR agonists. Cyp1A1/2 act on polyaromatic hydrocarbons to cause formation of DNA and protein adducts that ultimately lead to tumor formation and toxicity. Therefore, persistent exposure to AhR agonists, leading to chronic Cyp1A1/263 activity, ultimately contributes to detrimental health effects. Because Cyp1A activity in liver is central to the conversion of chemicals into ultimate carcinogens and because our previous studies identified the liver as an important target site for the effects of AhR agonists on circadian rhythms,37,39 we examined Cyp1A1 levels in liver during the present study. We observed an approximately 4.5-fold increase in Cyp1A1 transcripts in liver after exposure of mice to cotton balls (Figure 1). This level of induction of Cyp1A1 in the liver is sufficient to cause immunosuppression as measured by a decreased splenic antibody response to immunologic challenge.40 In that study, a single dose of TCDD produced a sustained 6-fold increase in Cyp1A1 that was associated with significant accumulation of TCDD in multiple tissues and suggested that sensitivity to TCDD was higher in the immune system, kidney, and uterus as compared with liver.40 Other studies15,52 also have indicated differences in sensitivity to AhR agonists among organs systems. In pigs, low level exposure to polycyclic aromatic hydrocarbons in soil leads to increased Cyp1A1, most prominently in the duodenum.52 Organ-specific responses are likely dependent on the route of exposure and the chemical nature of the AhR agonist.13,15
The results presented here indicate that activation of AhR signaling can occur in the presence of contaminants found in products intended to provide enrichment for mice housed in laboratory animal facilities. The cotton balls used in our facility were not manufactured for use in laboratory animals and thus information regarding chemical residues was unavailable. A variety of paper-based products for enrichment are available from various laboratory animal suppliers, some of which are similar in consistency to cotton balls; these vendors routinely disclose information on residual pesticide and chemical concentrations in their enrichment and bedding products. Therefore, products that provide the desired enrichment of rodent environments yet do not compromise animal health are readily available.
This study underscores the necessity for awareness of potential sources of contamination in control groups of mice, especially those used for investigation of AhR activation. Given the critical role of Cyp1A enzymes in steroid and drug metabolism and chemical detoxification and their roles in chemical toxicity and carcinogenesis, the current study raises important questions regarding how the presence of low levels of AhR agonists might influence animal well-being and experimental outcomes. At the very least, the presence of dioxins or other AhR agonists potentially adds an additional scientific variable that is not accounted for in experimental results. This potential confound, however, can easily be avoided by the use of dioxin-free enrichment materials.
Acknowledgments
We thank Kara Escutia for assistance with immunohistochemistry and Kathy Bottum and Stacey Krager for reviewing the manuscript. The work was supported by grant ES012948 from the National Institute of Environmental Health Sciences (to SAT) and an Eli Lilly Predoctoral Fellowship (to MM).
References
- 1.Aoki Y. 2001. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters—what we have learned from Yusho disease. Environ Res 86:2–11 [DOI] [PubMed] [Google Scholar]
- 2.Archer JC, Mabry-Smith R, Shojaee S, Threet J, Eckert JJ, Litman VE. 2005. Dioxin and furan levels found in tampons. J Womens Health (Larchmt) 14:311–315 [DOI] [PubMed] [Google Scholar]
- 3.Augustsson H, van de Weerd A, Kruitwagen CL, Boumans V. 2003. Effects of enrichment on variation and results in the light/dark test. Lab Anim 37:328–340 [DOI] [PubMed] [Google Scholar]
- 4.Benefiel AC, Dong WK, Greenough WT. 2005. Mandatory ‘enriched’ housing of laboratory animals: the need for evidence-based evaluation. ILAR J 46:95–105 [DOI] [PubMed] [Google Scholar]
- 5.Birnbaum LS. 1994. The mechanism of dioxin toxicity: relationship to risk assessment. Environ Health Perspect 102:157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birnbaum LS, Tuomisto J. 2000. Noncarcinogenic effects of TCDD in animals. Food Addit Contam 17:275–288 [DOI] [PubMed] [Google Scholar]
- 7.Celius T, Roblin S, Harper PA, Matthews J, Boutros PC, Pohjanvirta R, Okey AB. 2008. Aryl hydrocarbon receptor-dependent induction of flavin-containing monooxygenase mRNAs in mouse liver. Drug Metab Dispos 36:2499–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chivers CJ. 2004. A dinner in Ukraine made for Agatha Christie. New York Times; December 20 [Google Scholar]
- 9.Chung NG, Roh HK, Kim HM, Cha YN. 1998. Involvement of CYP3A1, 2B1 and 2E1 in C-8 hydroxylation and CYP1A2 and flavin-containing monooxygenase in N-demethylation of caffeine identified by using inducer treated rat liver microsomes that are characterized with testosterone metabolic patterns. Chem Biol Interact 113:1–14 [DOI] [PubMed] [Google Scholar]
- 10.Conney AH. 2003. Induction of drug-metabolizing enzymes: a path to the discovery of multiple cytochromes P450. Annu Rev Pharmacol Toxicol 43:1–30 [DOI] [PubMed] [Google Scholar]
- 11.Coon MJ. 2005. Cytochrome P450: nature's most versatile biological catalyst. Annu Rev Pharmacol Toxicol 45:1–25 [DOI] [PubMed] [Google Scholar]
- 12.Davis JW, 2nd, Lauer FT, Burdick AD, Hudson LG, Burchiel SW. 2001. Prevention of apoptosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the MCF-10A cell line: correlation with increased transforming growth factor αproduction. Cancer Res 61:3314–3320 [PubMed] [Google Scholar]
- 13.De Vito MJ, Maier WE, Diliberto JJ, Birnbaum LS. 1993. Comparative ability of various PCBs, PCDFs, and TCDD to induce cytochrome P450 1A1 and 1A2 activity following 4 weeks of treatment. Fundam Appl Toxicol 20:125–130 [PubMed] [Google Scholar]
- 14.Denison MS, Nagy SR. 2003. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43:309–334 [DOI] [PubMed] [Google Scholar]
- 15.DeVito MJ, Menache MG, Diliberto JJ, Ross DG, Birnbaum LS. 2000. Dose–response relationships for induction of CYP1A1 and CYP1A2 enzyme activity in liver, lung, and skin in female mice following subchronic exposure to polychlorinated biphenyls. Toxicol Appl Pharmacol 167:157–172 [DOI] [PubMed] [Google Scholar]
- 16.DeVito MJ, Schecter A. 2002. Exposure assessment to dioxins from the use of tampons and diapers. Environ Health Perspect 110:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diliberto JJ, Akubue PI, Luebke RW, Birnbaum LS. 1995. Dose–response relationships of tissue distribution and induction of CYP1A1 and CYP1A2 enzymatic activities following acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice. Toxicol Appl Pharmacol 130:197–208 [DOI] [PubMed] [Google Scholar]
- 18.Fackelmann K. 2004. Ukrainian opposition candidate was poisoned. USA Today; December 11 [Google Scholar]
- 19.Gray LE, Ostby JS, Kelce WR. 1997. A dose–response analysis of the reproductive effects of a single gestational dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin in male Long Evans Hooded rat offspring. Toxicol Appl Pharmacol 146:11–20 [DOI] [PubMed] [Google Scholar]
- 20.Gray LE, Jr, Kelce WR, Monosson E, Ostby JS, Birnbaum LS. 1995. Exposure to TCDD during development permanently alters reproductive function in male Long Evans rats and hamsters: reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicol Appl Pharmacol 131:108–118 [DOI] [PubMed] [Google Scholar]
- 21.Hahn ME. 2002. Aryl hydrocarbon receptors: diversity and evolution. Chem Biol Interact 141:131–160 [DOI] [PubMed] [Google Scholar]
- 22.Horstmann M, McLachlan MS. 1995. Results of an initial survey of polychlorinated dibenzo-p-dioxins (PCDD) and dibenzofurans (PCDF) in textiles. Chemosphere 31:2579–2589 [Google Scholar]
- 23.Hutchinson E, Avery A, Vandewoude S. 2005. Environmental enrichment for laboratory rodents. ILAR J 46:148–161 [DOI] [PubMed] [Google Scholar]
- 24.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 25.Kawajiri K, Fujii-Kuriyama Y. 2007. Cytochrome P450 gene regulation and physiological functions mediated by the aryl hydrocarbon receptor. Arch Biochem Biophys 464:207–212 [DOI] [PubMed] [Google Scholar]
- 26.Kerkvliet NI, Wagner SL, Schmotzer WB, Hackett M, Schrader WK, Hultgren B. 1992. Dioxin intoxication from chronic exposure of horses to pentachlorophenol-contaminated wood shavings. J Am Vet Med Assoc 201:296–302 [PubMed] [Google Scholar]
- 27.Kociba RJ, Schwetz BA. 1982. Toxicity of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD). Drug Metab Rev 13:387–406 [DOI] [PubMed] [Google Scholar]
- 28.Kolluri SK, Weiss C, Koff A, Gottlicher M. 1999. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev 13:1742–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korkalainen M, Tuomisto J, Pohjanvirta R. 2001. The Ah receptor of the most dioxin-sensitive species, guinea pig, is highly homologous to the human AH receptor. Biochem Biophys Res Commun 285:1121–1129 [DOI] [PubMed] [Google Scholar]
- 30.Lahvis GP, Bradfield CA. 1998. Ahr null alleles: distinctive or different? Biochem Pharmacol 56:781–787 [DOI] [PubMed] [Google Scholar]
- 31.Larsen JC. 2006. Risk assessments of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and dioxin-like polychlorinated biphenyls in food. Mol Nutr Food Res 50:885–896 [DOI] [PubMed] [Google Scholar]
- 32.Lin TM, Simanainen U, Moore RW, Peterson RE. 2002. Critical windows of vulnerability for effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci 69:202–209 [DOI] [PubMed] [Google Scholar]
- 33.Ma Q, Lu AY. 2007. CYP1A induction and human risk assessment: an evolving tale of in vitro and in vivo studies. Drug Metab Dispos 35:1009–1016 [DOI] [PubMed] [Google Scholar]
- 34.Mandal PK. 2005. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol [B] 175:221–230 [DOI] [PubMed] [Google Scholar]
- 35.Moffat ID, Roblin S, Harper PA, Okey AB, Pohjanvirta R. 2007. Aryl hydrocarbon receptor splice variants in the dioxin-resistant rat: tissue expression and transactivational activity. Mol Pharmacol 72:956–966 [DOI] [PubMed] [Google Scholar]
- 36.Moretz CB., 3rd 2004. Viet Nam's second cry for help—why the US should answer again. Curr Surg 61:567–568 [DOI] [PubMed] [Google Scholar]
- 37.Mukai M, Lin TM, Peterson RE, Cooke PS, Tischkau SA. 2008. Behavioral rhythmicity of mice lacking AhR and attenuation of light-induced phase shift by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Rhythms 23:200–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukai M, Tischkau SA.2006. Unpublished results.
- 39.Mukai M, Tischkau SA. 2007. Effects of tryptophan photoproducts in the circadian timing system: searching for a physiological role for aryl hydrocarbon receptor. Toxicol Sci 95:172–181 [DOI] [PubMed] [Google Scholar]
- 40.Narasimhan TR, Craig A, Arellano L, Harper N, Howie L, Menache M, Birnbaum L, Safe S. 1994. Relative sensitivities of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced Cyp1a1 and Cyp1a2 gene expression and immunotoxicity in female B6C3F1 mice. Fundam Appl Toxicol 23:598–607 [DOI] [PubMed] [Google Scholar]
- 41.Nebert DW, Dalton TP. 2006. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 6:947–960 [DOI] [PubMed] [Google Scholar]
- 42.Nebert DW, Gelboin HV. 1969. The in vivo and in vitro induction of aryl hydrocarbon hydroxylase in mammalian cells of different species, tissues, strains, and developmental and hormonal states. Arch Biochem Biophys 134:76–89 [DOI] [PubMed] [Google Scholar]
- 43.Nebert DW, McKinnon RA, Puga A. 1996. Human drug-metabolizing enzyme polymorphisms: effects on risk of toxicity and cancer. DNA Cell Biol 15:273–280 [DOI] [PubMed] [Google Scholar]
- 44.Nguyen LP, Bradfield CA. 2008. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 21:102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okey AB, Franc MA, Moffat ID, Tijet N, Boutros PC, Korkalainen M, Tuomisto J, Pohjanvirta R. 2005. Toxicological implications of polymorphisms in receptors for xenobiotic chemicals: the case of the aryl hydrocarbon receptor. Toxicol Appl Pharmacol 207:43–51 [DOI] [PubMed] [Google Scholar]
- 46.Olsson IAS, Dahlborn K. 2002. Improving housing conditions for laboratory mice: a review of ‘environmental enrichment’. Lab Anim 36:243–270 [DOI] [PubMed] [Google Scholar]
- 47.Pelkonen KH, Hänninen OO. 1997. Cytotoxicity and biotransformation inducing activity of rodent beddings A global survey using the Hepa1 assay. Toxicology 122:73–80 [DOI] [PubMed] [Google Scholar]
- 48.Pesatori AC, Consonni D, Tironi A, Zocchetti C, Fini A, Bertazzi PA. 1993. Cancer in a young population in a dioxin-contaminated area. Int J Epidemiol 22:1010–1013 [DOI] [PubMed] [Google Scholar]
- 49.Pirkle JL, Wolfe WH, Patterson DG, Needham LL, Michalek JE, Miner JC, Peterson MR, Phillips DL. 1989. Estimates of the half-life of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Vietnam Veterans of Operation Ranch Hand. J Toxicol Environ Health 27:165–171 [DOI] [PubMed] [Google Scholar]
- 50.Pohjanvirta R, Viluksela M, Tuomisto JT, Unkila M, Karasinska J, Franc MA, Holowenko M, Giannone JV, Harper PA, Tuomisto J, Okey AB. 1999. Physicochemical differences in the Ah receptors of the most TCDD-susceptible and the most TCDD-resistant rat strains. Toxicol Appl Pharmacol 155:82–95 [DOI] [PubMed] [Google Scholar]
- 51.Randi AS, Sanchez MS, Alvarez L, Cardozo J, Pontillo C, Kleiman de Pisarev DL. 2008. Hexachlorobenzene triggers AhR translocation to the nucleus, c-Src activation and EGFR transactivation in rat liver. Toxicol Lett 177:116–122 [DOI] [PubMed] [Google Scholar]
- 52.Roos PH. 2002. Differential induction of CYP1A1 in duodenum, liver and kidney of rats after oral intake of soil containing polycyclic aromatic hydrocarbons. Arch Toxicol 76:75–82 [DOI] [PubMed] [Google Scholar]
- 53.Rose JQ, Ramsey JC, Wentzler TH, Hummel RA, Gehring PJ. 1976. The fate of 2,3,7,8-tetrachlorodibenzo-p-dioxin following single and repeated oral doses to the rat. Toxicol Appl Pharmacol 36:209–226 [DOI] [PubMed] [Google Scholar]
- 54.Schallert T, Wallee MT, Flemming SM. 2003. Experimental focal ischemic injury: behavior-brain interactions and issues of animal handling and housing. ILAR J 44:130–143 [DOI] [PubMed] [Google Scholar]
- 55.Schecter A, Birnbaum L, Ryan J, Constable J. 2006. Dioxins: an overview. Environ Res 101:419–428 [DOI] [PubMed] [Google Scholar]
- 56.Schecter AJ, Olson J, Papke O. 1996. Exposure of laboratory animals to polychlorinated dibenzodioxins and polychlorinated dibenzofurans from commercial rodent chow. Chemosphere 32:501–508 [DOI] [PubMed] [Google Scholar]
- 57.Schrenk D. 1998. Impact of dioxin-type induction of drug-metabolizing enzymes on the metabolism of endo- and xenobiotics. Biochem Pharmacol 55:1155–1162 [DOI] [PubMed] [Google Scholar]
- 58.Tukey RH, Hannah RR, Negishi M, Nebert DW, Eisen HJ. 1982. The Ah locus: correlation of intranuclear appearance of inducer–receptor complex with induction of cytochrome P1-450 mRNA. Cell 31:275–284 [DOI] [PubMed] [Google Scholar]
- 59.Turyk ME, Anderson HA, Persky VW. 2007. Relationships of thyroid hormones with polychlorinated biphenyls, dioxins, furans, and DDE in adults. Environ Health Perspect 115:1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van deWeerd HA, Van Loo PL, Van Zutphen LF, Koolhaas JM, Baumans V. 1997. Preferences for nesting material as environmental enrichment for laboratory mice. Lab Anim 31:133–143 [DOI] [PubMed] [Google Scholar]
- 61.Van Loo PL, Mol JA, Koolhaas JM, Van Zutphen BF, Baumans V. 2001. Modulation of aggression in male mice: influence of group size and cage size. Physiol Behav 72:675–683 [DOI] [PubMed] [Google Scholar]
- 62.Vanden Heuvel JP, Clark GC, Kohn MC, Tritscher AM, Greenlee WF, Lucier GW, Bell DA. 1994. Dioxin-responsive genes: examination of dose–response relationships using quantitative reverse transcriptase-polymerase chain reaction. Cancer Res 54:62–68 [PubMed] [Google Scholar]
- 63.Whitlock JP., Jr 1999. Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol 39:103–125 [DOI] [PubMed] [Google Scholar]