Abstract
Excessive noise is well known to impair rodent health. To better understand the effect of construction noise and to establish effective noise limits during a planned expansion of our vivarium, we analyzed the effects of construction noise on mouse gestation and neonatal growth. Our hypothesis was that high levels of construction noise would reduce the number of live births and retard neonatal growth. Female Swiss Webster mice were individually implanted with 15 B6CBAF1/J embryos and then exposed to 70- and 90-dBA concrete saw cutting noise samples at defined time points during gestation. In addition, groups of mice with litters were exposed to noise at 70, 80, or 90 dBA for 1 h daily during the first week after parturition. Litter size, birth weight, incidence of stillborn pups, and rate of neonatal weight gain were analyzed. Noise decreased reproductive efficiency by decreasing live birth rates and increasing the number of stillborn pups.
Abbreviation: dB, decibel; dBA, A-weighted noise level; Leq, energy-equivalent sound level; Ln, energy-equivalent sound level where n represents the measurement duration in minutes; SPL, sound pressure level
The Guide for the Care and Use of Laboratory Animals notes that researchers and personnel should take noise into consideration when creating and maintaining an environment for laboratory animals.4,15,27 The effects of excessive noise can range from inadvertent triggering of audiogenic seizures to behavioral changes that could confound phenotyping or other behavioral tests.2,3,4,6,26, 27 Studies have linked noise to stimulation of the neuroendocrine stress response system.27 Through chronic or chronic–intermittent stimulation of the stress response system, audiogenic stressors have been linked with physiologic changes such as hypertension, cardiac hypertrophy, altered electrolyte metabolism, changes in immune responses, altered estrus cycles, decreased fertility, and increases in the number of prematurely terminated pregnancies.17,18,20,24,26,27
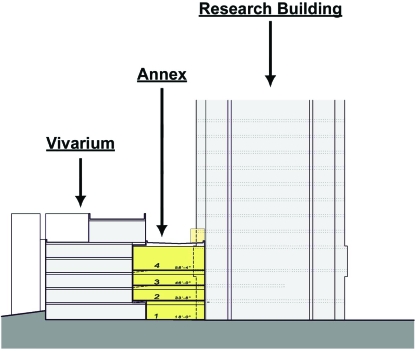
Although noise is generally considered deleterious to rodent health, the effects of noise from facility construction on rodent reproductive efficiency have not been characterized thoroughly. We sought to quantify the effects of construction noise on rodent fetal viability and neonatal growth before expansion of our institution's animal facility. Because the expansion involved building immediately adjacent to the existing vivarium and connecting the 2 buildings into a single contiguous structure (Figure 1), investigators and laboratory animal health personnel were concerned that noise from construction would decrease mouse reproductive efficiency and have deleterious effects on research. Our specific goals were to characterize ambient noise within our animal facility and noise associated with construction activities and to determine the effects of this noise on fetal viability and neonatal growth.
Figure 1.
Expansion of the vivarium. Illustration of the future annex abridging the existing vivarium and an adjacent research building (East–West section). The annex expansion is a 4-story building designed to be contiguous with the existing vivarium.
Sound is characterized primarily based on amplitude and frequency.1,14,27 Amplitude refers to the ‘intensity’ of a sound and is measured on a decibel (dB) scale.1,14,27 A decibel measurement is determined by taking the unit measure for sound pressure amplitude, the pascal (Pa), and converting this number to a decibel scale by using the sound pressure level (SPL). The SPL is a logarithmic scale that allows for the measurement of a large range of pressure variations detectable by the human ear.1,14 More specifically, the intensity of sound measured in this manner is referred to as ‘dB SPL.’ Pitch, or perceived frequency, is a measure of how many sinusoidal oscillations occur during 1 s and is measured in hertz (Hz).1,14,27 Factors affecting the perception of sound include loudness and pitch as well as whether the sound is transmitted through a structure or is airborne, the distance of the sound from its source, and whether any background noise (which may mask the original sound) is present. Vibration conducts groundborne noise. When interior surfaces are excited into motion by vibration, they can radiate sound.
Noise levels that are disturbing to people may not necessarily be disruptive for mice; the converse is also true. The range of frequencies readily detected by humans is between 20 Hz to 20 kHz.13 Sound frequencies too high for human perception are defined as ultrasonic.13 Much of the hearing range of mice (1 to 91 kHz at 60 dB SPL) is ultrasonic.13,23 This difference in hearing ranges places much of a mouse's range above what is audible to people and renders sounds at the lower end of the human range inaudible to mice.
The A-weighted noise level, abbreviated as dBA, is a single number representing the energy sum of the noise (sound), adjusted by frequency (that is, taking into account spectral content). The frequency weighting curve (A weighting) closely represents the frequency response of the human ear to environmental noise. Even though mouse hearing is more sensitive to ultrasound than is human, most construction noise sources lack energy in the ultrasonic range (data not published28). Further, available instrumentation (noise loggers) and literature (equipment noise reference data) already make use of A weighting and are developed for the range of human hearing.
Before the expansion of our facility, we set forth to establish criteria for disruptive noise levels. We first characterized ambient daily noise within the vivarium and then compared this ambient noise with that of construction activities likely to cause disruption. The data from these studies allowed us to establish guidelines that set limits for construction-generated noise. In addition, we designed a composite noise barrier to help attenuate noise from outside construction activities. Because noise levels were expected to exceed our established limits even with the use of noise barriers, we designed a study to evaluate the effects of construction-generated noise on mouse reproduction and neonatal growth. Our hypothesis was that high levels of construction noise would decrease the number of live births and retard neonatal growth.
Materials and Methods
Baseline noise levels.
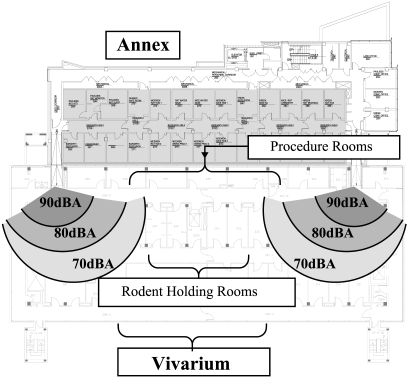
To establish baseline noise levels within the facility, we first examined areas of the vivarium where noise likely would be most deleterious to rodent colonies. Noise-sensitive areas were determined to be Levels 2 through 5 of our building. On these levels, labs and procedure rooms were directly adjacent to the east wall of the vivarium, where the future annex would be constructed. Animal-holding rooms located toward the core of the building just west of the labs and procedure rooms adjacent to planned breakthrough sites of the exterior wall were determined to be in a region where noise levels likely would exceed established noise limits (Figures 1 and 2).
Figure 2.
Noise transmission from construction into the vivarium. Conceptual illustration predicting the radius of noise attenuation to vivarium rodent-holding rooms during construction activities occurring at breakthrough points on each floor.
Short-term (20 min) and long-term (4 d) noise measurements (Nor140 noise monitor, Norsonic, Lierskogen, Norway) were conducted in 3 procedure rooms and 4 mouse-holding rooms, respectively. For the long-term noise measurements, noise monitors were used to log statistical hourly noise levels for 1 wk. Noise monitors were located in a central position in each room, typically on top of rodent housing racks. Short-term measurement data were acquired by digitally recording and analyzing ambient noise samples for about 20 min. All noise data were evaluated in terms of the common acoustical metric, Leq, which refers to the energy-equivalent sound level. Statistical distribution descriptors, L1, L10, and L90, were also used. The numerical subscript represents the measurement duration in minutes. Noise was reported by using an A-weighted sound scale (dBA). Room heating, cooling, and ventilation systems were operating in all spaces. Additional noise monitors were stationed on the exterior of the building at the construction sites to establish preconstruction outdoor noise levels over several days. In addition, the exterior shell was evaluated to determine the composite sound transmission loss provided by the concrete walls and the glass windows of the vivarium. Noise generated outside of the building was compared with that recorded inside the building to calculate noise attenuation across the concrete walls and glass windows of the vivarium.
To help establish potential effects of construction, noise monitors were placed in holding and procedure rooms during scheduled demolition of the concrete floor and slab foundation associated with an autoclave located on Level 1 of the vivarium. Short- and long-term noise monitoring measured noise transmission from Level 1 to Levels 3 through 5 during electric and pneumatic jackhammer use.
Finally noise generated by construction equipment, similar to what will be used during our building breakthroughs, was monitored and recorded. The test noise consisted of operating a hammer drill on the exterior wall and measuring noise levels at nearby locations within the building. The frequencies and intensity of the noise generated, along with the construction materials found in our facility, were used to predict sound attenuation horizontally at the site of our breakthroughs (Figure 2).
Mice.
This study was conducted at an AAALAC-accredited facility after the institutional animal care and use committee approved the research project. The study population comprised 120 female Tac:SW mice (age, 10 wk; Taconic, Germantown, NY). We chose Swiss Webster mice because they segregate the Cdh23 allele for hearing loss with no correlation between allele type and hearing function and, as a result, experience minimal age-related hearing loss.8,9,10,22,29 Mice were provided free access to irradiated feed (LabDiet 5053, Purina Mills International, St Louis, MO) and bottles containing chlorinated (2 ppm) water. Mice were maintained in a rodent housing room in which sentinel mice exposed to dirty bedding are comprehensively screened on a quarterly basis by using serology, bacteriology, and parasitology. Mice were housed 5 mice per cage in standard-sized ventilated microisolation caging (Thoren, Hazleton, PA) on irradiated corncob bedding (Bed-o'cobs and Pure-o'cel, The Andersons, Maumee, OH) supplemented with a synthetic absorbent material (ALPHA-dri, Shepherd Specialty Papers, Milford, NJ). Bedding was changed once weekly within a ventilated cage-change station by trained animal care staff. All mice were maintained on a 12:12-h light:dark cycle; animal room temperatures ranged between 20.0 and 22.2 °C (68 and 72 °F), with relative humidity ranging between 30% and 70%. Ambient continuous noise levels in the housing room ranged between 61 and 63 dBA. Maximal measured transient noise levels in the housing rooms ranged between 80 and 87 dBA. Room air changes were set for 10.5 changes hourly, with ventilated racks (external blower motors exhausting into the building heating–ventilation–air conditioning system) supplying approximately 50 air changes hourly to each cage. After arrival at our facility, mice were allowed to acclimate for 1 wk before embryo implantation.
Embryo implantation.
Personnel in Transgenic Laboratory Services (The Rockefeller University) performed embryo implantation of the Swiss Webster mice used in the gestational and neonatal growth experiments. B6CBAF1/J embryo donor mice were delivered to our facility at 4 wk of age; these mice were reported to be seronegative for Ectromelia virus, murine rotavirus, lymphocytic choriomeningitis virus, mouse hepatitis virus, mouse parvovirus, minute virus of mice, murine norovirus, pneumonia virus of mice, reovirus, Sendai virus, and Mycoplasma pulmonis and were also reported to be free of bacterial and parasitic infections. After a 1-wk acclimation period, donor mice were induced hormonally to superovulate and then mated with proven male mice (2:1 breeding ratio). Insemination was confirmed by the presence of a vaginal plug. Only 2-cell embryos were harvested and subsequently transferred to the recipient Swiss Webster mice. Prior to embryo transfer, Swiss Webster recipients were exposed to vasectomized male mice. Pseudopregnant Swiss Webster mice received tribromoethanol (5 mg/10 g body weight IP; Sigma-Aldrich, St Louis, MO), were prepared for aseptic surgery, and implanted with 15 embryos. After recovery from anesthesia, surrogate dams were returned to their home cages and were considered to be in the E2 stage of pregnancy the day after embryo transfer.
Gestational study.
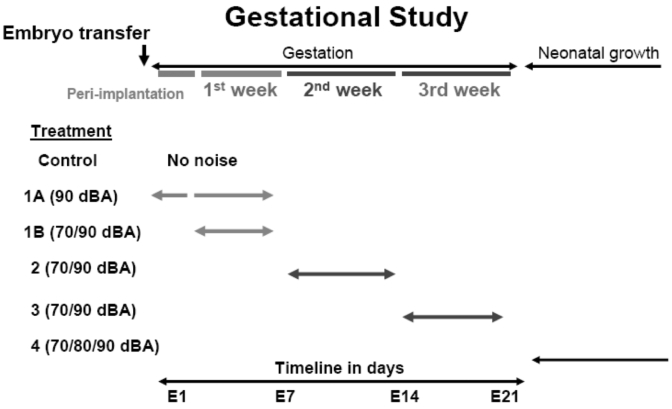
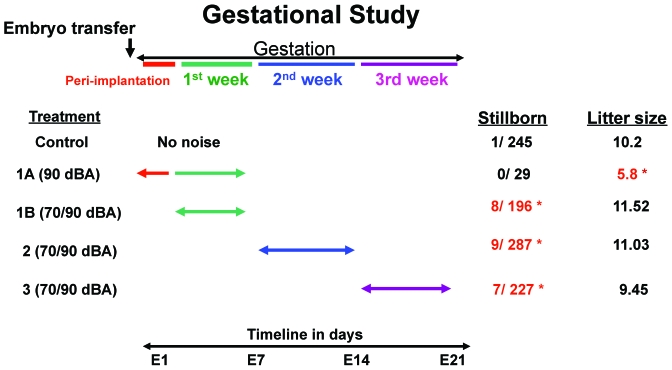
After embryo transfer, female Tac:SW mice were allocated randomly into a single control group of 24 mice and 4 experimental groups. The experimental groups were exposed for 1 h daily to a 6-min continuously looped audio sample of structure-borne noise from concrete saw cutting (provided by Wilson, Ihrig, and Associates, New York, NY) with dominant energy between 2 to 8 kHz. Daily noise exposure was administered between 1300 and1600 hrs. Experimental groups were as follows: group 1A (5 mice) was exposed to 90-dBA noise from the day after embryo transfer (E2) through E7; group 1B (9 mice at 90 dBA and 8 mice at 70 dBA) were exposed to noise during days E4 through E7; group 2 (13 mice at 90 dBA and 13 mice at 70 dBA) experienced noise on days E8 through E14; and group 3 (13 mice at 90 dBA and 12 mice at 70 dBA) were exposed to noise on days E15 until the end of the gestational period (E21; Figure 3).
Figure 3.
Experimental design. Duration and gestational timing of noise exposure. In the gestational study, noise was provided during the first, second, and third weeks of gestation (experimental groups 1B, 2, and 3). An additional treatment group received noise exposure from E2 (the peri-implantation period) through E7 (experimental group 1A). For the neonatal study, mice with litters were exposed to noise during the first week after parturition. (experimental group 4). Individual subgroups were exposed to either 70, 80, or 90 dBA.
An experimental housing cabinet with sound-attenuating properties was placed in a procedure room for use during the noise exposure study. Before experimentation, cages without mice were placed in various locations throughout the cabinet. A sound-level meter with attached microphone (Type II Sound Level Meter, model 824, Larson Davis, Provo, UT) then was placed within a cage to help to gauge sound levels and adjust speaker volume. A reference monitor (model MR5, Mackie, Woodinville, WA) was used to amplify the concrete saw sound sample and was adjusted until the noise sample could be run at both 70 and 90 dBA.
The control and experimental groups were housed in ventilated racks as described earlier. The control group was not exposed to the concrete saw noise sample and control animals were not transported to the experimental housing cabinet. For the experimental groups, the pregnant mice were left in their cages, with filter tops and water bottles removed. The cages then were placed in the cabinet for the 1-h exposure period. During the experimental phase, the sound meter was calibrated daily and was used to monitor sound output levels from the reference monitor. Depending on the subgroup, mice were exposed to either an Leq noise level of 70 ± 2 dBA (range, 68 to 72 dBA) or 90 ± 2 dBA noise daily at about the same time each afternoon. After 1 h, the pregnant mice were returned to their usual housing conditions.
Mice were housed individually during the last 48 h of gestation and were observed in the morning and afternoon for signs of parturition. The number of live pups born, weight of each pup at birth, and number of stillborn pups were recorded.
Neonatal growth study.
The control group from the gestational experiment was used for the neonatal growth study. Mice were monitored twice daily for signs of parturition. The number of live pups, number of stillborns, and the combined weight of all pups at birth were recorded. In addition, we tracked the individual weight gain of 3 neonates per litter from the day of birth through day 7 after parturition.
Dams with their litters remained in their home cages and were placed in the experimental cabinet for the daily 1-h exposure period. The sound-level meter (type II, model 824, Larson Davis) was calibrated daily and used to monitor sound output levels from the reference monitor (model MR5, Mackie). The control group of 9 mice and their litters were not exposed to the sample of concrete saw noise; each of the 3 experimental groups (5 dams each) was exposed for 1 h daily to an Leq noise level of 70 ± 2, 80 ± 2, or 90 ± 2 dBA noise at approximately the same time each afternoon for the first 7 d after parturition. After the experiment, dams and litters were returned to their usual housing conditions. Weights of individual pups were recorded once between 1400 and 1600 each day during the monitoring period from the day of birth through day 7 after parturition.
Noise monitoring and regulation during building construction.
For continuous noise monitoring throughout the construction process, noise monitors (Nor140, Norsonic) were placed at 2 locations outside the building and in at least 1 room on each floor inside the vivarium. Monitors were linked to a website (maintained by Wilson, Ihrig, and Associates) that logged ongoing noise and accommodated retrospective access of suspected noise disturbances for any given date or time during the recording process. The website also was triggered to contact specific research personnel via text message and email notifications if the levels of noise exceeded our established noise limits
Statistical analysis.
Two-tailed t tests (Excel, Microsoft, Redmond, WA) were used to analyze numbers of stillborn pups, comparing ambient noise levels and noise treatment groups. Two-tailed t tests also were performed to compare the litter size of control groups with experimental groups exposed to noise during the peri-implantation period.
For neonatal growth studies, differences in weights among groups were compared in 2 ways. We first calculated the average weight of each litter for days 1 and 7, calculated the difference in average litter weight between days 1 and 7, and then compared these differences among groups by using ANOVA. We also calculated the individual change in weight between day 7 and day 1 for 3 pups in each litter. We examined the association of noise group on the weight change of individual pups between days 7 and 1 by using a linear regression model with general estimating equations to correct for correlations between pups within a litter (SAS version 9.1, SAS Institute, Cary, NC).
Results
Baseline noise evaluation.
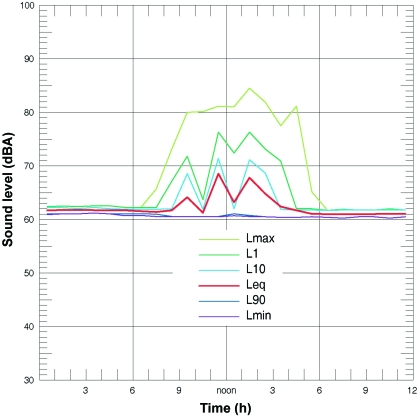
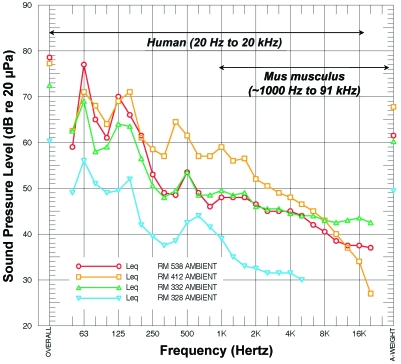
Ambient noise levels within rodent holding rooms averaged between 61 and 63 dBA Leq with staff-generated transient noise spikes of 80 to 87 dBA Lmax during working hours (Figure 4). The ambient noise levels on the order of 60 dBA resulted from the continuous operation of the ventilated microisolation racks. Building HVAC and rack ventilation noise was predominantly low-frequency (below 8 KHz) and therefore mostly below the hearing range of mice (Figure 5). Noise monitors were centrally located within rooms to allow for accurate comparison of ambient noise. Noise levels perceived by mice, however, may actually differ from that recorded outside their cages. Sound was attenuated by 2 dBA inside the cage when compared with noise levels immediately outside the cage.
Figure 4.
Ambient noise is increased due to human activity. The lines represent 24-h noise measurements taken from a rodent-holding room and are depicted as time-weighted, energy-equivalent noise levels (Leq). Statistical distribution descriptors were used, L1, L10, and L90, where the numerical subscript represents the measurement duration in minutes. Lmax and Lmin depict noise of the highest and the lowest intensities recorded during the measurement time period. Noise is increased during normal working hours, primarily because of human activity.
Figure 5.
Ambient noise in the vivarium. These representative 24-h spectral time-weighted averages (Leq) of measured noise levels were taken from 4 rodent-holding rooms. The figure illustrates the noise generated as it correlated to frequency and human and mouse hearing ranges (shown above graph lines).
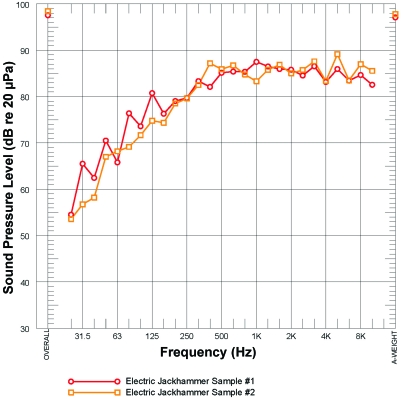
Our analysis of a slab demolition conducted within the vivarium showed noise levels of 10 kHz and higher as the result of jackhammer use (Figure 6). Despite the high sound levels near the slab at the lower floors, substantial noise reduction to upper floors during this activity demonstrated the potential for the building to attenuate sound as it moved through the building to the upper floors of the vivarium.
Figure 6.
Noise generated from construction activities. These noise measurements were taken during a jackhammer slab demolition on the first floor. High-decibel noise was present at high frequencies (greater than 8 kHz) well within the hearing range of mice.
Previous analysis of construction equipment used for the facility expansion demonstrated that noise levels during construction were 95 to 110 dBA and most construction equipment would have predominant energy at or below 10 kHz (data not shown). Construction equipment can generate noise at higher frequencies (above 10 kHz). However, much of the noise in the ultrasonic range (above 20 kHz) tends to be of substantially lower intensity (data not published).28 Noise data of concrete saw cutting (data not shown) indicated relatively low levels of noise in the ultrasonic frequency range compared with the dominant saw noise energy between 2 and 8 kHz.
The exterior concrete wall provided substantial attenuation of construction noise transmitted to the interior of the building. The windows facing the construction site, however, did not have as substantial sound attenuation properties. Noise barriers made from composite materials were designed to improve the attenuation of noise transmission by the windows. The barriers consisted of 2 layers of 5/8-in. thick exterior sheathing on metal studs with mineral wool insulation. Noise barriers were installed on the outside surface of all windows facing the construction site.
Exterior to interior sound transmission studies with and without the window barriers confirmed the high level of sound attenuation achieved. In addition, attenuation was much greater for high-frequency noise than low-frequency noise, suggesting that ultrasonic noise produced outside will have virtually no effect on the rodent colonies inside the building.
Effect of noise on gestation.
Only 1 of the 245 pups born to the control group of 24 mice was stillborn. In comparison, more pups were stillborn when mice were exposed to noise during the first (P = 0.016), second (P = 0.024), or third (P = 0.031) week of pregnancy (Figure 7). Although the effect varied among exposure groups, more pups were stillborn from dams exposed to noise of 70 or 90 dBA as compared with the control dams. In particular, the average litter size of the mice exposed to 90 dBA during the peri-implantation period (5.8 pups) was significantly (P = 0.005) smaller than that of controls (10.2 pups).
Figure 7.
Noise affects litter size and the number of stillborn pups. Noise during the first (excluding the peri-implantation period), second, and third weeks of gestation increased the incidence of stillborn pups (Experimental groups 1B, 2, and 3). Noise exposure during the peri-implantation period decreased litter size (Experimental group 1A). *, P < 0.05.
Effect of noise on neonatal growth.
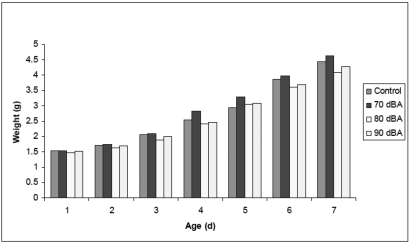
During the first 7 d after birth, the pups' weight increased over time as expected and varied depending on litter size. Growth rates of litters exposed to noise did not differ significantly when pooled weights [P = 0.93 (ANOVA)] or individual weights [P = 0.64 (linear regression model)] were compared with those of mice not exposed to noise (Figure 8).
Figure 8.
Growth rates of neonatal pups exposed to noise. Average growth weights of pups from similar-sized litters exposed to ambient noise (control, n = 80 pups; 70 dBA, n = 44 pups; 80 dBA, n = 60 pups; and 90 dBA, n = 52 pups). During the first 7 d after birth, the pups' weight increased over time as expected, and daily 1-h exposure to noise had no significant effect on growth rates.
Discussion
Our ambient noise study revealed that mice within our vivarium are exposed continuously to moderate (less than 65 dBA) levels of noise. Background noise levels found in housing rooms originated mainly from the building heating, ventilation, and cooling systems and the rack ventilation systems. This background noise is predominantly low-frequency and therefore mostly inaudible to the mice. In procedure rooms, most of the noise generated was due to human activity during normal working hours. This finding is consistent with a previous study,27 which established that most vivarium noise either originates from personnel within the facility or is the result of animals responding to personnel within the facility.
Noise limits for construction were established based on the ambient noise levels logged in the rodent housing rooms. Because mice housed within the vivarium were maintained in an environment that routinely exposed them to moderate levels of noise, we predicted that continuous noise below 65 dBA would not have a negative effect. We established that noise should not exceed 75 dBA for 1 h and set a maximum noise allowance of 85 dBA. The 85-dBA noise limit was based on preliminary studies evaluating the behavior of nursing dams: mice exposed to 90 dBA of noise stopped nursing pups during the period of noise exposure (data not shown). Ultrasonic noise measurement data for construction equipment at close range is an area for further study because building elements such as walls, floors, or other potential transmission paths act as a mechanical filter and attenuate higher frequencies more substantially than lower frequencies.
Because noise exposure from construction activities was predicted to exceed these limits, we secured composite noise barriers over windows to increase noise attenuation across glass. The composite barriers kept noise levels within established limits during most outdoor construction activities. With the exception of a few construction activities, such as breaking through walls to connect the new building to the old, the composite barriers provided adequate sound attenuation of exterior construction noise. For the breakthroughs, supplementary noise barriers were installed inside the building to minimize noise transmission to the nearest housing rooms.
In our study, we attempted to isolate noise exposure as an independent variable in both the gestational and neonatal growth studies. Other external factors, such as cage changes, transportation of cages to the sound chamber or other environmental factors, although unlikely, may also influence fetal viability. We used a simulated noise sample as the independent variable. This simulated noise from the amplifier may have caused the mice to experience some limited mechanosensation during noise transmission, which could have contributed to the overall observed effects on fetal viability.
Our gestational study revealed a statistically significant correlation between the number of stillborn pups and noise exposure, even at 70 dBA for 1 h daily, and noise exposure during the peri-implantation period decreased litter size. These reproductive effects could be related to a “fight-or-flight” response that noise may trigger in plasma catecholamines and the neuroendocrine system.4,7,11,12,17,18,21,26,27 As mice are a prey species, noise is one of the first sensory systems that allows them to respond to predators.26
Noise exposure has been linked to increased levels of plasma catecholamines (norepinephrine and epinephrine).11,17 In rats, norepinephrine infusion acutely reduces ovarian and uterine blood flow,11 and in guinea pigs, infusion of norepinephrine decreases placental blood flow by 24% to 46%, depending on the dose administered.17 Therefore, increased norepinephrine could cause decreases in blood flow that could adversely influence implantation and fetal health.
High levels of noise activate the neuroendocrine response system and increase corticosterone levels in rodents.27 Increased corticosterone levels induced by restraint during the peri-implantation period can lead to implantation failure in rodents.12 In addition, changes in maternal plasma cortisol levels impair fetal and placental growth in sheep.18 Thus, fetal health could be influenced by neuroendocrine-induced changes in placental blood flow, fetal hormone levels, or placental structure.
Corticosteroids have a direct effect on estrogen and progesterone levels.5,12 Estrogen and progesterone in turn differentially regulate the expression and secretion of inflammatory cytokines IL1α and IL6, which directly influence mouse blastocyst implantation.5,12,16,25 In rats, restraint increases IL1 expression in the brain and IL6 expression in the liver.12 IL1 is present in early-stage embryos and may have a role in embryo implantation.5,12,25 IL6 reduces the rate of blastocyst attachment and embryo outgrowth in culture.12,16 Increases in IL1α and IL6 expression as a result of noise-induced elevations in corticosterone levels may explain the reduced litter size in mice exposed to noise during the peri-implantation period.
We hypothesized that daily noise exposure would disrupt nursing or alter the maternal behavior of dams, resulting in retarded pup growth rates. However, the data revealed that 1 h of noise exposure daily at 70, 80, or 90 dBA does not significantly alter pup growth rates. Perhaps 1 h of noise exposure does not appreciably reduce overall milk consumption over 24 h, even if the maternal behavior of dams is altered during that time, as occurred in our preliminary study. We speculate that prolonged noise exposure would decrease neonatal growth rates by altering maternal behavior enough to reduce milk ingestion by pups over 24 h, resulting in retardation of growth rates. Additional studies with longer noise exposure times need to be conducted to test this hypothesis.
In our vivarium we chose to mitigate the negative effects of noise on fetal viability by designing and placing composite noise barriers to effectively attenuate noise produced outside the building. In addition, a detailed construction schedule was developed characterizing predicted levels of noise during all phases of construction. Investigators, therefore, knew in advance when high levels of noise were going to occur so they could schedule noise-sensitive studies accordingly. Finally, noise monitors were placed at various locations on the construction site and within the existing vivarium to allow continual assessment of construction noise and to confirm that noise levels did not exceed established limits.
In conclusion, we determined that mice are exposed to moderate levels of ambient noise in the traditional housing environment of our vivarium. Taking measures to control noise during construction is important when trying to maintain mouse reproductive performance. Exposure to modest to high levels of noise, as expected during construction, significantly decreased the reproductive efficiency of mice by decreasing the number of pups born and increasing the number of stillborn pups. The observed decrease in fetal viability associated with noise exposure probably results from multiple systemic factors associated with these underlying sympathetic and neuroendocrine responses to noise. Additional studies to measure relevant stress hormones are necessary in order to better understand the physiologic mechanisms by which noise compromises fetal health.
Acknowledgments
We thank Roxana Cubias, Wei Tang, Lua Lu, and Jahnney Torres (The Rockefeller University Transgenic Core) for technical assistance with the embryo transfers. We also thank Elyn Riedel (Memorial Sloan Kettering Cancer Center) for help with statistical analysis.
References
- 1.Acoustical Society of America [Internet] Glossary of acoustical terms and definitions of terminology related to the science of acoustics. [Cited 2009 Jan 3]. Available from: http://www.webref.org/acoustics/acoustics.htm
- 2.Baldwin AL, Bell IR. 2007. Effect of noise on microvascular integrity in laboratory rats. J Am Assoc Lab Anim Sci 46:58–65 [PubMed] [Google Scholar]
- 3.Baldwin AL, Primeau RL, Johnson WE. 2006. Effect of noise on the morphology of the intestinal mucosa in laboratory rats. J Am Assoc Lab Anim Sci 45:74–82 [PubMed] [Google Scholar]
- 4.Baldwin AL, Schwartz GE, Hopp DH. 2007. Are investigators aware of enviromental noise in animal facilities and that this noise may affect experimental data? J Am Assoc Lab Anim Sci 46:45–51 [PubMed] [Google Scholar]
- 5.Basak S, Dubanchet S, Zourbas S, Chaouat G, Das C. 2002. Expression of pro-inflammatory cytokines in mouse blastocyst during implantation: modulation by steroid hormones. Am J Reprod Immunol 47:2–11 [DOI] [PubMed] [Google Scholar]
- 6.Brennan TJ, Seeley WW, Kilgard M, Schreiner CE, Tecott LH. 1997. Sound-induced seizures in serotonin 5HT2c receptor mutant mice. Nat Genet 16:387–390 [DOI] [PubMed] [Google Scholar]
- 7.Cook RO, Nawrot PS, Hamm CW. 1982. Effects of high-frequency noise on prenatal development and maternal plasma and uterine catecholamine levels in the CD1 mouse. Toxicol Appl Pharmacol 66:338–348 [DOI] [PubMed] [Google Scholar]
- 8.Davis RR, Kozel P, Erway LC. 2003. Genetic influences in individual susceptibility to noise: a review. Noise Health 5:19–28 [PubMed] [Google Scholar]
- 9.Drayton M, Noben-Trauth K. 2006. Mapping quantitative loci for hearing loss in Black Swiss mice. Hear Res 212:128–139 [DOI] [PubMed] [Google Scholar]
- 10.Erway LC, Shiau YW, Davis RR, Krieg EF. 1996. Genetics of age-related hearing loss in mice. III. Susceptibility of inbred and F1 hybrid strains to noise-induced hearing loss. Hear Res 93:181–187 [DOI] [PubMed] [Google Scholar]
- 11.Gafvels M, Olofssen J, Norjavaara E, Selstam G. 1988. Hormonal influence on utero-ovarian blood flow distribution in the midluteal pseudopregnant rat. Acta Physiol Scand 132:329–334 [DOI] [PubMed] [Google Scholar]
- 12.Golub MS, Campbell MA, Kaufman FL, Iyer P, Li L, Donald JM, Morgan JE. 2004. Effects of restraint stress in gestation: implications for rodent developmental toxicology studies. Birth Defects Res B Dev Reprod Toxicol 71:26–36 [DOI] [PubMed] [Google Scholar]
- 13.Heffner HE, Heffner RS. 2007. Hearing ranges of laboratory animals. J Am Assoc Lab Anim Sci 46:20–22 [PubMed] [Google Scholar]
- 14.Hughes LF. 2007. The fundamentals of sound and its measurements. J Am Assoc Lab Anim Sci 46:14–19 [PubMed] [Google Scholar]
- 15.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [DOI] [PubMed] [Google Scholar]
- 16.Jacobs AL, Sehgal PB, Julian J, Carson DD. 1992. Secretion and hormonal regulation of interleukin-6 production by mouse uterine stromal and polarized epithelial cells cultured in vitro. Endocrinology 131:1037–1046 [DOI] [PubMed] [Google Scholar]
- 17.Jansson T. 1988. Responsiveness to norepinephrine of the vessels supplying the placenta of growth-retarded fetuses. Am J Obstet Gynecol 158:1233–1237 [DOI] [PubMed] [Google Scholar]
- 18.Jensen E, Wood CE, Keller-Wood M. 2004. Chronic alterations in ovine maternal corticosteroid levels influence uterine blood flow and placental and fetal growth. Am J Physiol Regul Integr Comp Physiol 288:R54–R61 [DOI] [PubMed] [Google Scholar]
- 19.Meyer RE, Aldrich TE, Easterly CE. 1989. Effects of noise and electromagnetic fields on reproductive outcomes. Environ Health Perspect 81:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naff KA, Riva CM, Craig SL, Gray KN. 2007. Noise produced by vacuuming exceeds the hearing thresholds of C57Bl/6 and CD1 mice. J Am Assoc Lab Anim Sci 46:52–57 [PubMed] [Google Scholar]
- 21.Nawrot PS, Cook RO, Staples RE. 1980. Embryotoxicity of various noise stimuli in the mouse. Teratology 22:279–289 [DOI] [PubMed] [Google Scholar]
- 22.Ohlemiller KK, Wright JS, Heidbreder AF. 2000. Vulnerability to noise-induced hearing loss in ‘middle-aged’ and young adult mice: a dose–response approach in CBA, C57BL, and BALB inbred strains. Hear Res 149:239–247 [DOI] [PubMed] [Google Scholar]
- 23.Portfors CV. 2007. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci 46:28–34 [PubMed] [Google Scholar]
- 24.Rabat A. 2007. Extra-auditory effects of noise in laboratory animals: the relationship between noise and sleep. J Am Assoc Lab Anim Sci 46:35–41 [PubMed] [Google Scholar]
- 25.Simon C, Frances A, Piquette GN, Danasouri I, Zurawski G, Dang W, Polan ML. 1994. Embryonic Implantation in mice is blocked by interleukin 1 receptor antagonist. Endocrinology 134:521–528 [DOI] [PubMed] [Google Scholar]
- 26.Turner JG, Bauer CA, Rybak LP. 2007. Noise in animal facilities: why it matters. J Am Assoc Lab Anim Sci 46:10–13 [PubMed] [Google Scholar]
- 27.Turner JG, Parrish JL, Hughes LF, Toth LA, Caspary DM. 2005. Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med 55:12–23 [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson I, and Associates 2008
- 29.Zheng QY, Johnson KR, Erway LC. 1999. Assessment of hearing in 80 inbred strains of mice by ABR threshold analysis. Hear Res 130:94–107 [DOI] [PMC free article] [PubMed] [Google Scholar]