Abstract
Mite infestation of mice remains a persistent problem for many institutions, leading to numerous health problems and creating unknown and unwanted variables for research. In this study, mice with mite infestation demonstrated significantly higher levels of inflammatory cytokines, both at draining lymph nodes (axillary) and systemically, as compared with mice without mites. In addition, histologic evaluation revealed significant inflammation in mite-infested mice. Inflammatory changes were still present in the skin of mice at 6 to 8 wk after treatment, despite absence of detectable infestation at that time. Because these significant and lasting local and systemic changes have the potential to alter research findings, eradication of mites infestations should be an important goal for all institutions.
Abbreviation: KC, keratinocyte-derived chemokine; MIP, macrophage inflammatory protein
Laboratory mice can harbor several species of acarids (fur mites), including Myobia musculi, Radfordia affinis, Myocoptes musculinus, and Psorergates simplex.11,14,29,40,45 Fur mites are an excluded pathogen in most research facilities, particularly within barrier suites, and in order to control or avoid mite infestations, many facilities, including those with ongoing infestations, will not accept infested animals from outside sources. Such policies can prevent or halt collaborative research between investigators in different institutions because mite infestation is a sporadic or endemic problem in many facilities that house mice under conventional conditions, despite attempts at eradication.12,22,25,43,62,69
Mite infestations cause several health problems in mice, including ulcerative dermatitis, amyloidosis, and other immune system alterations.2,12,22,27,29-31,37,44,45,61 For example, mite infestations are associated with increased serum concentrations of IgE and IgG in mice.30,44,48 Alterations in immune responses could alter research data and thereby perhaps alter the associated conclusions.36,65,66,70 Mice with mite infections often develop dermatitis, which can lead to bacterial infection and additional changes in immune status.15,30,31,45,61 Because any pathogenic infection can cause variability and alter basal measures of immune function, clinical chemistry, and behavior in mice, maintaining laboratory rodents in a disease-free state is crucial to their use for the collection of valid research data.51
The eradication of external parasites is a difficult process. Many reports have been published that attempt mite eradication using various drug treatments,5-7,17,18,23,24,35,39,41-43,46,47,49,50,57,59,67 with each method having distinct advantages and disadvantages. Some, but not all, of these treatment regimens have been compared directly.10 The mite life cycle complicates treatment, because eggs and larvae can be less susceptible to drugs than are adult parasites.2,19,20,55 In addition, mite eggs can contaminate the environment, providing a source for re-infection of treated animals.20,63,64 Some drugs (for example, ivermectin) have been associated with toxicity and death in mice, especially among specific transgenic lines.8,12,28,53,55,69 Other drugs may require frequent or repeated treatment of the mice. Furthermore, the drugs themselves may have properties that alter physiology or immune function in animals.2,13,60 The development of new veterinary drugs for treatment of parasites has increased the available therapies for rodent acariasis. Compounds such as fipronil and selamectin provide good efficacy against external parasites with limited side effects in mammals.9,22,68
Our facility housed a large colony of mice that occupied several rooms and were infested with Myocoptes musculinus and Myobia musculi. Although the majority of mite-infested mice had mild or no dermatitis, some infected mice had severe dermatitis. The goal of this study was to evaluate the local and systemic immune response in mice infested with mites. To our knowledge, this study is the first to comprehensively compare cytokine levels and histologic findings in mite-infested, treated, and mite-negative mice. We hypothesized that the immune response would be altered in mite-infested mice as demonstrated by significantly elevated cytokine levels in the draining lymph nodes or spleen as compared with mice that had never been infested with mites. In addition, we hypothesized that significant pathologic changes in the epidermis, dermis, and subcutaneous tissues would be present in response to mite infestation.
Materials and Methods
Animals.
Our institution housed several mouse colonies with endemic mite infestation. Given the availability of mice, we selected 3 lines for evaluation: an Ames dwarf strain, a growth hormone transgenic strain (Pepck-bGH-1) and a growth hormone receptor knock-out strain (GHRKO).4 Ames dwarfs are spontaneous mutants; GHRKOs were produced by targeted gene disruption, and transgenics were produced by pronuclear injection. All strains have heterogeneous genetic backgrounds. The background of Ames dwarfs is not related to any common inbred strain. The background of the GHRKO strain is derived from 129 Ola, BALB/c, C57BL/6 and C3H. The Pepck-bGH-1 transgenic mice have a mixed background derived from C57BL/6 and C3H strains.3
Four groups of mice were evaluated: mite-positive without treatment; mite-positive with treatment; mite-negative with treatment; and mite-negative without treatment. Each group consisted of 7 to 9 mice of each strain. The group comprising treated mite-positive mice was free of mites at the time of euthanasia (6 to 8 wk after last treatment). All groups were composed of both male and female animals, except for the mite-positive treated group, which contained only female mice. All mice in all 4 groups were 6 to 10 mo old at time of euthanasia and had lived in this colony for the duration of their lives. Two animals in the group of untreated mite-positive mice had ulcerative dermatitis at the time of euthanasia. Mice used for this study were not enrolled on another study at the time of testing and euthanasia.
All procedures were approved by the Southern Illinois University School of Medicine Laboratory Animal Care and Use Committee.
Facilities.
Mice were housed in an AAALAC-accredited facility with a 12:12-h light:dark cycle, ambient temperature of 20.6 to 25.6 ºC (69 to 78 °F), and humidity within generally accepted limits. The mice were housed under conventional husbandry practices in shoebox-style open-top cages on wood chip bedding (Beta Chip, Northeastern Products, Warrensburg, NY). All cage changes were performed on a cart in the room. Mouse colonies with mites were housed in separate rooms from mice without mites. Husbandry practices, such as a strict room-entry order, were in place to contain mites within the known-positive rooms. All mice were fed standard rodent chow (LabDiet 5001, PMI Nutrition International, St Louis, MO) and tap water ad libitum. Animal room air was exchanged at least 12 times hourly. Mice were euthanized by CO2 inhalation.
Mite testing.
Mites were detected in several rooms during routine health monitoring of mouse colonies. All mice in these rooms belonged to the same investigator. Additional testing of many mice in each room confirmed a mixed mite infestation of both Myobia musculi and Myocoptes musculinus. Mites and mite eggs were easily visible on gross and microscopic evaluation of pelts and ‘tape tests.’ All animals evaluated for this study were tested individually for the presence of mites by using tape tests. A piece of clear cellophane tape was applied to the back of each mouse to collect hair and, if present, mites. The tape was transferred to a microscope slide and evaluated for the presence of either adult mites or mite eggs on the hair shaft at 10× and 40× magnification.
Treatment for mite infection.
In an attempt to eradicate mites from the facility, all mice in the contaminated rooms were treated with topical selamectin (Revolution, Pfizer Animal Health, New York, NY), at a concentration of 120 mg/mL (14.2% selamectin in active ingredients) diluted 1:100 in isopropyl alcohol. Each mouse was dosed with 50 µL 3 times at 2-wk intervals.9,22,32,68 Because this was a breeding colony, we encountered many dams with litters. Pups were not treated until they were weaned and at least once thereafter. Pups born during the period of treatment were treated once or twice, depending on when during our course of treatment they were weaned. The mite-positive group of animals on this study was euthanized before treatment. A second mite-positive group of animals was euthanized 6 to 8 wk after last treatment was administered. One group of mite-negative animals was treated as described, and another group of mite negative animals was not treated.
Tissue collection.
Spleen, axillary lymph nodes (draining lymph nodes), and skin from the back and dorsal neck were harvested from each animal after euthanasia. Skin was fixed in 10% neutral buffered formalin for histologic evaluation. Spleen and lymph node samples were snap-frozen in liquid nitrogen and stored at −80 °C until processed for cytokine analysis.
Histology.
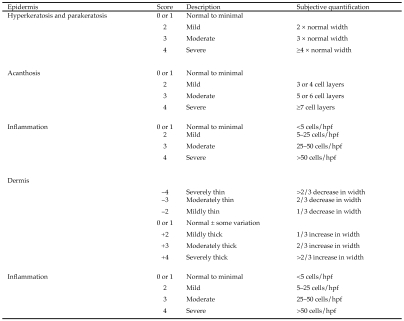
Histologic changes in skin and cytokine concentrations in spleen and lymph nodes were assessed in 7 mice from the untreated mite-positive group. Tissues from an additional 2 animals were submitted for histology only, bringing the group size to 9. In addition, histology and cytokine concentrations were assessed in 7 mice that underwent euthanasia at 6 to 8 wk after treatment (mite-positive with treatment); these mice were determined to be mite-negative at the time of euthanasia based on careful inspection and tape tests. Formalin-fixed skin samples were processed and embedded in paraffin, and 5-to 7-μm serial sections were stained with hematoxylin and eosin. A scoring system16 modified by 1 of the authors (SB-K) was used for histologic evaluation of the skin (Figure 1). During assignment of numerical scores, 6 to 10 areas were examined per section (that is, glass slide). If the inflammatory reaction was consistent, 6 areas were evaluated. If the inflammatory reaction was variable, 10 areas were scored and then averaged for the final numerical value. The noted histopathologic features and anatomic levels were evaluated to provide a basis for evaluating the type and degree of damage to the skin. The epidermis was evaluated for presence or absence of acanthosis (thickening of the stratum spinosum, diffuse epidermal hyperplasia), parakeratosis (retention of the keratinocyte nuclei in the stratum corneum), hyperkeratosis (thickening of the stratum corneum), and inflammation (intraepithelial inflammatory cell infiltration).54 Also noted were features such as erosion and ulceration (complete loss of epidermal cells). The superficial dermis and deep dermis, evaluated separately, were assessed for thickness and inflammation. In addition, features in the dermis such as fibrosis, necrosis, follicular keratosis, and the presence or absence of adnexa (hair follicles and sebaceous glands) were noted. The subcutaneous tissue was evaluated for inflammation.
Figure 1.
Scoring system for histologic evaluation of the skin. Superficial and deep dermis were evaluated separately. hpf, high-power (60×) field.
Expression of cytokines and chemokines in lymph node and spleen.
Preparation of tissue homogenates.
Lymph node and spleen were snap-frozen and stored at −80 °C until tissue homogenates were prepared. At the time of analysis, tissues was thawed in approximately 10 volumes (w/v) of ice-cold cell lysis buffer containing protease inhibitor cocktail (Cell Lysis Kit, 171-304012, BioRad, Hercules, CA) plus 2 μL 500 mM PMSF in dimethyl sulphoxide (Sigma, St Louis, MO). Samples were homogenized by using a 5-mL glass homogenizer and then sonicated on ice with 3 rapid pulses (approximately 1 s each) at setting 5 (model 100 Ultrasonic Dismembrator, Fisher Scientific, Pittsburgh, PA). Samples were centrifuged at 4500 × g for 15 min at 4 °C, and supernatants were collected and stored at −80 °C until analysis. Protein concentration of homogenates was measured by using the bicinchoninic acid protein assay (Pierce, Rockford, IL).
Cytokine multiplex assays.
Concentrations of IL1α, IL1β, IL2, IL4, IL6, IL10, IL12(p40), IL12(p70), granulocyte colony-stimulating factor, keratinocyte-derived chemokine (KC), monocyte chemoattractant protein 1, macrophage inflammatory protein (MIP) 1α, MIP1β, TNFα, and IFNγ protein levels were measured in spleen and lymph node homogenates by using a mouse cytokine assay system (BioPlex, BioRad) on a flow-cytometry–based system (100IS System, Luminex) according to the manufacturers' instructions. The lower limits of detection for individual cytokines were 2 pg mL−1 [TNFα, IL6, IL10, IL12(p40), granulocyte colony-stimulating factor, and MIP1β], 3 pg mL−1 (IL2, IL4, and KC), 4 pg mL−1 [IL12(p70)], 6 pg mL−1 (IFNγ and TNFα), 7 pg mL−1 (IL1α), 14 pg mL−1 (monocyte chemoattractant protein 1), and 24 pg mL−1 (MIP1α). The technology of the measurement and readout system accommodated simultaneous determination of all 15 markers from a single 100-μL sample. All samples were assayed in duplicate, normalized to protein concentration of the tissue homogenate, and reported as picograms of cytokine per milligram of protein.
Statistical analysis.
Concentrations of individual cytokines in lymph node or spleen were analyzed separately by using 1-way ANOVA with Tukey posthoc comparisons. An α level of P ≤ 0.05 was considered to indicate a statistically significant difference among groups. Continuous histology data (dermal width, inflammation, hyperkeratosis, parakeratosis, and acanthosis) were analyzed by t test. Categorical histologic data (presence of ulcerations or erosions in the epidermis, fibrosis or necrosis of the superficial dermis, follicular keratosis, presence of hair follicles or sebaceous glands in the deep dermis) were analyzed by using the Fisher Exact Test. All results are presented as mean ± SEM for the indicated sample sizes. An α level of P ≤ 0.05 was considered to indicate a statistically significant effect for all histology data. Error bars shown in figures and tables indicate SEMs, and horizontal lines and asterisks represent significance levels.
Results
Histology.
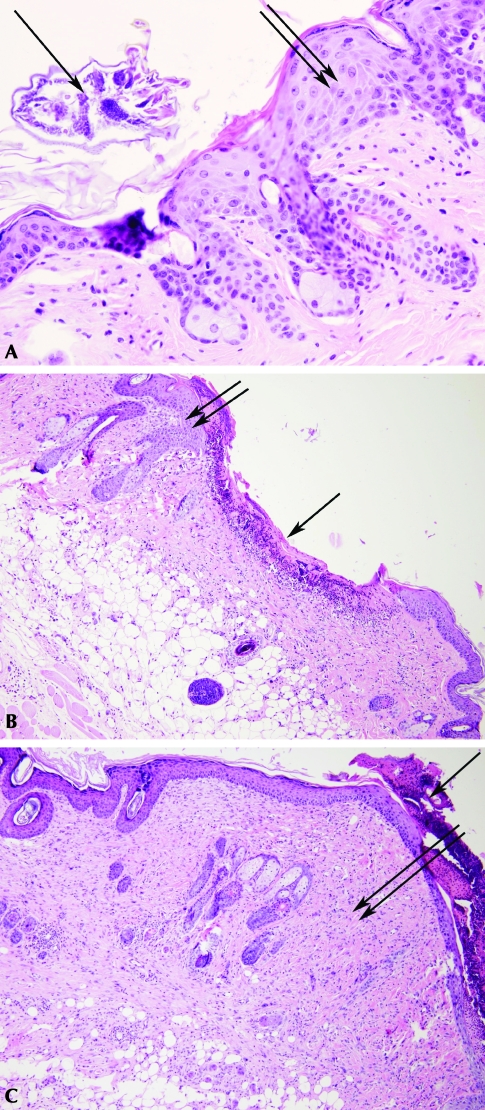
Seven mite-positive mice were evaluated prior to treatment. Histologic examination of skin samples confirmed the presence of mites in all mice that tested positive on the tape test (Figure 2 A). No mites were detected by either the tape test or histology of skin samples from mite-positive mice that were treated. Pathologic changes were evident in the skin samples all infested mice (Table 1). Mite-infested mice were significantly more likely (P ≤ 0.05) to have erosions and ulcerations in the epidermis (Figure 2 B, C) and follicular keratosis of the deep dermis (Figure 2 B). Ulcerations and erosions in the skin usually displayed histologic evidence of bacterial infection, but the presence of bacteria was not a specific criterion of histologic evaluation. Bacteria were noted in sections with ulceration or erosion (data not shown) but not in any other sections from these or other groups. In addition, areas of necrosis and fibrosis were present in the superficial or deep dermis of infected mice.
Figure 2.
(A) Cross-section of a luminal mite adjacent to the epidermis (single arrow). Mild parakeratosis and acanthosis of the epidermis (double arrows) is present. The superficial dermis is mildly infiltrated with mixed inflammatory cells. (B) Focal area of ulceration covered by a serocellular crust composed of proteinaceous debris, viable and nonviable inflammatory cells, red blood cells, fibrin, and keratin (single arrow). The underlying dermis is moderately infiltrated with mixed inflammatory cells. Note the moderate hyperkeratosis, parakeratosis, and acanthosis adjacent to the ulcerated region (double arrows). (C) Severe diffuse thickening of the superficial and deep dermis by large numbers of fibroblasts and increased deposition of collagen fibers (double arrows). The dermis is moderately infiltrated with mixed inflammatory cells and there is moderate loss of hair follicles and associated adnexa. Note the thick serocelluar crust overlying the parakeratotic epidermis (single arrow). Hematoxylin and eosin stain; magnification, ×400 (A), ×100 (B, C).
Table 1.
Pathologic findings in mite-positive and treated mice.
| Mite-positive (n = 7) |
Mite-negative (treated; n = 9) |
|||
| Pathology | no. affected | % | no. affected | % |
| Mites | 7 | 100 | 0 | 0 |
| Erosions or ulcerations of epidermis | 5 | 71.4a | 0 | 0a |
| Fibrosis of superficial dermis | 3 | 43 | 0 | 0 |
| Necrosis of superficial dermis | 2 | 29 | 0 | 0 |
| Follicular keratosis of deep dermis | 5 | 71.4a | 0 | 0a |
| Presence of hair follicles in deep dermis | 1 | 14 | 2 | 22% |
| Presence of sebaceous glands in deep dermis | 1 | 14 | 2 | 22% |
Lesions are more prevalent in mite-positive animals. Lesions in the deep dermis are residual damage from previous mite infestation injury.
Significant (P < 0.01) difference between groups.
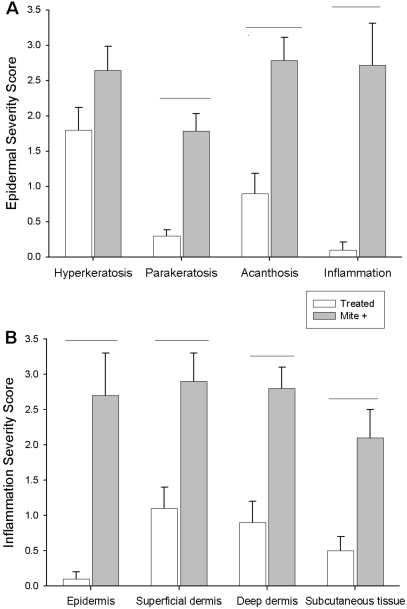
Mite-positive mice that had been treated were evaluated 6 to 8 wk after the last treatment. None of the treated mice showed epidermal inflammation, but inflammation was present in the superficial dermis (3 of 9 mice) and deep dermis (2 of 9 mice). The severity of the inflammation differed significantly (P < 0.05) between treated and untreated mice in all regions examined (Figure 3 B). The inflammatory infiltrate consisted of either mononuclear cells or a mixed mononuclear cell–neutrophil population. Of the 7 mite-positive mice evaluated, 6 had inflammation of the epidermis (3 with severe inflammation), and 7 had inflammation of the superficial and deep dermis (4 with severe inflammation). Parakeratosis, acanthosis, and inflammation were significantly more severe (P < 0.05) in mite infested mice as compared with treated mice. Hyperkeratosis of the epidermis was also more severe in (P < 0.05) mite-infested mice (Figure 2 A).
Figure 3.
Histologic assessment of epidermis, dermis, and subcutaneous tissues. (A) Severity scores for histologic markers of inflammation in the epidermis. (B) Severity scores for inflammation in tissues indicated. Horizontal lines denote significant differences (P < 0.01).
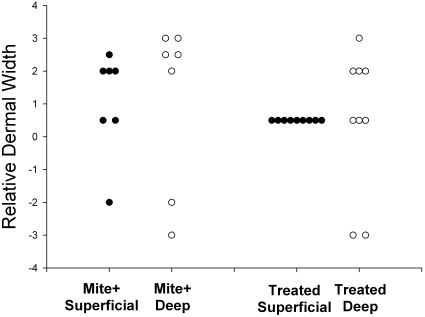
All 7 of the mite-positive untreated mice had marked variation in dermal thickness, ranging from mildly thin to moderately thick. Among treated mice, the superficial dermis was within the normal range of values, but the deep dermis showed persistent damage even at 6 to 8 wk after treatment (Figure 4).
Figure 4.
Relative superficial and deep dermal width in mite-positive and treated animals. Scores of 0 or 1 represent normal width. Increased dermal width indicative of inflammation is found in both superficial and deep dermis of mite-infested animals. Increased dermal width is also present in the deep dermis of treated animals, indicating that the dermal pathology from mite infestation is long-lasting.
Cytokines.
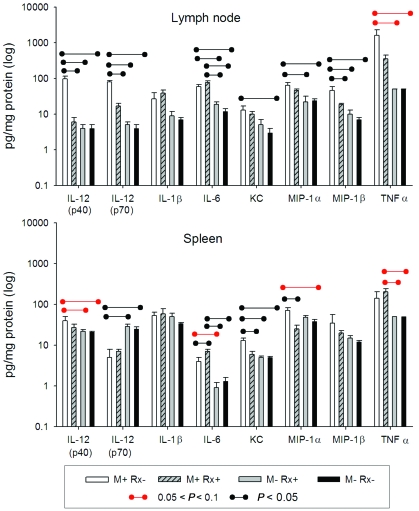
Analyses of a panel of cytokines in homogenates of axillary lymph nodes revealed that concentrations of IL12(p40), IL12(p70), and MIP1β were significantly higher in the mite-positive (untreated) mice as compared with all other groups of mite-negative mice, both treated and untreated (P < 0.05). Concentrations of IL6, KC, and MIP1α were significantly (P < 0.05) higher in the mite-positive (untreated) mice as compared with at least 1 other mite-negative group (Figure 4). In addition, concentrations of TNFα tended (P < 0.1) to be higher in the mite-positive mice as compared with mite-negative mice. Other cytokines measured showed no significant elevations or differences (data not shown).
Splenic concentrations of IL6, KC, and MIP1α were significantly (P < 0.05) higher in untreated, mite-infested mice than in mite-negative treated and mite-naïve mice (Figure 5). In addition, concentrations of IL12(p40) and TNFα showed trends (P < 0.1) toward higher values in the mite-positive mice as compared with mite-negative mice. IL12(p70) was increased approximately 10-fold in the lymph nodes of infested mice, yet was not significantly elevated in the spleens (Figure 5).
Figure 5.
Cytokine and chemokine values in axillary lymph node (top) and spleen (bottom). Values below limits of detection or lacking significant difference are not shown.
Discussion
Mite infestation has been reported to cause pathologic changes in the skin, altered behavior,45 and eventual secondary amyloidosis.30,31,45 Our study expands this knowledge to include alterations in selected cytokines indicative of an ongoing immune response both locally and systemically in mite-infested mice. The data presented here provide quantitative documentation of the altered basal immune measures and variation across animals that can occur in mice infected with mites. Furthermore, although the local inflammatory response in the draining lymph node largely was resolved 6 to 8 wk after treatment, as demonstrated by the return of most cytokines to basal levels in treated mice, multifocal inflammatory infiltrates were detected in the skin of several mice even at this stage. In addition, moderate to severe thinning of the dermis (6 of 9 mice) and loss of hair follicles (7 of 9) or sebaceous glands (7 of 9) in some treated mice demonstrates that mite infestation caused marked and possibly permanent damage to the skin in these mice. Earlier treatment to eliminate mites may have prevented these changes.
Our study revealed moderate to severe inflammatory changes in the skin of all mite-infested mice, with pathology still evident 6 to 8 wk after mite eradication. In infested mice, the epidermis and dermis both showed severe inflammation, with frequent epidermal ulcerations and necrosis of the superficial dermis in infested mice. These lesions were often associated with secondary bacterial infections, as revealed on histopathology. In several cases, the clinical severity or extent of these lesions was sufficient to warrant euthanasia. The most severe and extensive lesions included marked hyperkeratosis and inflammation that extended into the deep dermis. Inflammation in the superficial or deep dermis often reflected the severity of the injury. As the severity of the injury increased, greater inflammation developed in the deep dermis as compared with the superficial dermis. Histologic assessment indicated that the residual effects of mite infestation, such as lost adnexa (hair follicles and sebaceous glands) and variation in dermal thickness, were still present in the dermis of treated mice at 6 to 8 wk after completion of treatment. Injured tissue does not regain lost adnexa.54 The superficial and deep dermis may recover normal thickness as inflammation resolves and environmental factors such as lysosomal enzymes and fibrogenic cytokines regain normal balance. However, 6 to 8 wk is too soon after treatment to reveal the permanency of pathologic change. Hyperkeratosis and parakeratosis (both involving the stratum corneum) reflect the response to irritation or injury to 2 layers of the epidermis, the stratum corneum and the stratum granulosum, in which keratohyalin granules accumulate and nuclei are lost. Injury to the skin often causes thickening of both the stratum spinosum (acanthosis) and the stratum granulosum. Depending on the degree and duration of injury, this thickening can be accompanied by hyperkeratosis (increased thickening of the corneum) and parakeratosis (retention of nuclei in the corneum), both of which can reflect decreased desquamation.54
Unless a function of body location, dermal thickness usually reflects the severity or chronicity of inflammation. The dermis thickens during both acute and chronic inflammation, but the changes differ qualitatively. Acute inflammation is characterized by edema, congestion and significant inflammatory infiltration, whereas chronic inflammation also includes infiltration and activation of fibroblasts and deposition of collagen fibers without complete resolution of inflammation (therefore inflammatory cells are still present).54 In our study population, 5 mice (3 positive for mites but untreated and 2 that were treated) showed a thin dermis. An abnormally thin dermis usually reflects endocrine dysfunction or autoimmune disease that targets fibroblasts or collagen but also can occur after trauma if nutritional status is compromised. During inflammation, a thin dermis can also reflect an imbalance in collagen degradation versus deposition, especially during a chronic inflammatory process that has not yet resolved or does not resolve.31,54 In this study, the thin dermis probably occurred secondary to chronic inflammation coupled with secondary infection and impaired systemic health.
The alterations in cytokine concentrations that we measured in infested mice are consistent with an ongoing, primarily local immune response. Cytokines are signaling proteins produced by many cell types. They are necessary for the normal development and functioning of the innate and adaptive immune responses.34,58 We selected the evaluated cytokines as representative of a complete panel of mouse proinflammatory cytokines; in addition, the cytokines targeted in this study are mediators and regulators of innate immunity. The primary cell sources of these cytokines are macrophages and natural killer calls. Cytokines are released in response to challenges from infectious agents.33 For example, IL12 is responsible for initiating the cascade of events that results in the eradication of intracellular microbes and KC and MIP1α and β aid in neutrophil recruitment.1,56 Other reports also have shown increased levels of cytokines in mite-infested animals,48 yet few describe the changes at either the local or systemic level, as our study demonstrates. Levels of 7 inflammatory cytokines were significantly elevated in the axillary lymph nodes of mite-infested mice as compared with mite-naïve mice. Systemic immune responses, as reflected by alterations in splenic cytokine concentrations, were less robust than those in the axillary lymph nodes but were still altered, as demonstrated by the high concentrations of 5 inflammatory cytokines measured in the spleens of mite-infested mice. The increase in splenic IL12(p70) was unexpected and may be an artifact related to analyzing the samples in separate batches.
The alterations in inflammatory cytokines that develop in response to mite infestation can create variables in research and alter experimental outcomes. Animals used as models in fields such as basic immunology and infection studies, graft rejection, or autoimmune studies21,38 should be free of fur mites to reduce unwanted immune variables, and proinflammatory cytokines are associated with processes as diverse as tumor metastisis26,52 to diabetes.26In such studies, altered values of proinflammatory cytokines would be related in part to the mite infestation, rather than being solely related to experimental manipulation.
With the availability of new parasitacides, effective treatment of acariasis has become more feasible in mice. Although older drugs were labor-intensive to use, produced unwanted side effects, or were costly or ineffective for eradication, newer drugs such as fipronil and selamectin have proven to be safe and effective for most strains of mice. The experience of 1 the authors (NAJ) supports other reports68 of the effectiveness of these newer drugs. Our study demonstrates that the immune response, as reflected by cytokine concentrations in lymph node and spleen, is significantly altered in untreated mite-infested mice as compared with treated and naive mice. Furthermore, mite-induced damage can persist for months after treatment. Treatment of uninfested mice with selamectin did not produce significant elevations in proinflammatory cytokines. A substantial body of evidence2,12,22,27,29-31,37,44,45,61 documents the detrimental effects of mite infestation on animal health and their suitability as research subjects. The availability of superior treatment options makes mite eradication a reasonable and important goal for all institutions.
References
- 1.Abbas AK, Lichtman AH. 2005. Cellular and molecular immunology. Philadelphia (PA): Elsevier Saunders [Google Scholar]
- 2.Baker DG. 1998. Natural pathogens of laboratory mice, rats, and rabbits and their effect on research. Clin Microbiol Rev 11:231–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartke A.2008. Personal communication.
- 4.Bartke A, Masternak MM, Al-Regaiey KA, Bonkowski MS. 2007. Effects of dietary restriction on the expression of insulin-signaling-related genes in long-lived mutant mice. Interdiscip Top Gerontol 35:69–82 [DOI] [PubMed] [Google Scholar]
- 5.Baumans V, Havenaar R, Van Herck H. 1988. The use of repeated treatment with Ivomec and Neguvon spray in the control of murine fur mites and oxyurid worms. Lab Anim 22:246–249 [DOI] [PubMed] [Google Scholar]
- 6.Baumans V, Havenaar R, Van Herck H, Rooymans TP. 1988. The effectiveness of Ivomec and Neguvon in the control of murine mites. Lab Anim 22:243–245 [DOI] [PubMed] [Google Scholar]
- 7.Bean-Knudsen DE, Wagner JE, Hall RD. 1986. Evaluation of the control of Myobia musculi infestations on laboratory mice with permethrin. Lab Anim Sci 36:268–270 [PubMed] [Google Scholar]
- 8.Blakley BR, Rousseaux CG. 1991. Effect of ivermectin on the immune response in mice. Am J Vet Res 52:593–595 [PubMed] [Google Scholar]
- 9.Bornstein DA, Scola J, Rath A, Warren HB. 2006. Multimodal approach to treatment for control of fur mites. J Am Assoc Lab Anim Sci 45:29–32 [PubMed] [Google Scholar]
- 10.Burdett EC, Heckmann RA, Ochoa R. 1997. Evaluation of five treatment regimens and five diagnostic methods for murine mites (Myocoptes musculinus and Myobia musculi). Contemp Top Lab Anim Sci 36:73–76 [PubMed] [Google Scholar]
- 11.Chu DK, Couto MA. 2005. Arthropod infestation in a colony of mice. Lab Anim (NY) 34:25–27 [DOI] [PubMed] [Google Scholar]
- 12.Cole JS, Sabol-Jones M, Karolewski B, Byford T. 2005. Ornithonyssus bacoti infestation and elimination from a mouse colony. Contemp Top Lab Anim Sci 44:27–30 [PubMed] [Google Scholar]
- 13.Conole J, Wilkinson MJ, McKellar QA. 2003. Some observations on the pharmacological properties of ivermectin during treatment of a mite infestation in mice. Contemp Top Lab Anim Sci 42:42–45 [PubMed] [Google Scholar]
- 14.Csiza CK, McMartin DN. 1976. Apparent acaridal dermatitis in a C57BL/6 Nya mouse colony. Lab Anim Sci 26:781–787 [PubMed] [Google Scholar]
- 15.Dawson DV, Whitmore SP, Bresnahan JF. 1986. Genetic control of susceptibility to mite-associated ulcerative dermatitis. Lab Anim Sci 36:262–267 [PubMed] [Google Scholar]
- 16.Fick JL, Novo RE, Kirchhof N. 2005. Comparison of gross and histologic tissue responses of skin incisions closed by use of absorbable subcuticular staples, cutaneous metal staples, and ployglactin 910 suture in pigs. Am J Vet Res 66:1975–1984 [DOI] [PubMed] [Google Scholar]
- 17.Fraser J, Joiner GN, Jardine JH. 1974. The use of pelleted dichlorvos in the control of murine acariasis. Lab Anim 8:271–274 [DOI] [PubMed] [Google Scholar]
- 18.French AW. 1987. Elimination of Ornithonyssus bacoti in a colony of aging mice. Lab Anim Sci 37:670–672 [PubMed] [Google Scholar]
- 19.Friedman S, Weisbroth SH. 1975. The parasitic ecology of the rodent mite Myobia musculi. II. Genetic factors. Lab Anim Sci 25:440–445 [PubMed] [Google Scholar]
- 20.Friedman S, Weisbroth SH. 1977. The parasitic ecology of the rodent mite, Myobia musculi. IV. Life cycle. Lab Anim Sci 27:34–37 [PubMed] [Google Scholar]
- 21.Goncalves Dasilva A, Yong VW. 2009. Matrix metalloproteinase-12 deficiency worsens relapsing-remitting experimental autoimmune encephalomyelitis in association with cytokine and chemokine dysregulation. Am J Pathol 174:898–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonenc B, Sarimehmetoglu HO, Ica A, Kozan E. 2006. Efficacy of selamectin against mites (Myobia musculi, Myocoptes musculinus, and Radfordia ensifera) and nematodes (Aspiculuris tetraptera and Syphacia obvelata) in mice. Lab Anim 40:210–213 [DOI] [PubMed] [Google Scholar]
- 23.Green CJ, Needham JR. 1974. Control of mange mites in a large mouse colony. Lab Anim 8:245–251 [DOI] [PubMed] [Google Scholar]
- 24.Hill WA, Randolph MM, Boyd KL, Mandrell TD. 2005. Use of permethrin eradicated the tropical rat mite (Ornithonyssus bacoti) from a colony of mutagenized and transgenic mice. Contemp Top Lab Anim Sci 44:31–34 [PubMed] [Google Scholar]
- 25.Huerkamp MJ, Zitzow LA, Webb S, Pullium JK. 2005. Cross-fostering in combination with ivermectin therapy: a method to eradicate murine fur mites. Contemp Top Lab Anim Sci 44:12–16 [PubMed] [Google Scholar]
- 26.Hultcrantz M, Jacobson S, Hill NJ, Santamaria P, Flodström-Tullberg M. 2009. The target cell response to cytokines governs the autoreactive T cell repertoire in the pancreas of NOD mice. Diabetologia 52:299–305 [DOI] [PubMed] [Google Scholar]
- 27.Iijima OT, Takeda H, Komatsu Y, Matsumiya T, Takahashi H. 2000. Atopic dermatitis in NC/Jic mice associated with Myobia musculi infestation. Comp Med 50:225–228 [PubMed] [Google Scholar]
- 28.Jackson TA, Boivin GP, Hall JE, Luo W, Stedelin JR. 1997. Ivermectin toxicosis in mice of multiple transgenic lines. Contemp Top Lab Anim Sci 36:77 [Google Scholar]
- 29.Jacoby RO, Fox JG, Davisson M. 2002. Biology and diseases of mice, Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine. New York (NY): Academic Press [Google Scholar]
- 30.Jungmann P, Freitas A, Bandeira A, Nobrega A, Coutinho A, Marcos M-A, Minoprio P. 1996. Murine acariasis. II. Immunological dysfunction and evidence for chronic activation of Th2 lymphocytes. Scand J Immunol 43:604–612 [DOI] [PubMed] [Google Scholar]
- 31.Jungmann P, Guenet J-L, Cazenave P-A, Cazenave P-A, Coutinho A, Huerre M. 1996. Murine acariasis: I. Pathological and clinical evidence suggesting cutaneous allergy and wasting syndrome in BALB/c mouse. Res Immunol 147:27–38 [DOI] [PubMed] [Google Scholar]
- 32.Kalishman J. 2007. Selamectin treatment for mice. Personal communication
- 33.Kobayashi Y. 2006. Neutrophil infiltration and chemokines. Crit Rev Immunol 26:307–316 [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi Y. 2008. The role of chemokines in neutrophil biology. Front Biosci 13:2400–2407 [DOI] [PubMed] [Google Scholar]
- 35.Kondo S, Taylor A, Chun S. 1998. Elimination of an infestation of rat fur mites (Radfordia ensifera) from a colony of Long Evans rats, using the microdot technique for topical administration of 1% ivermectin. Contemp Top Lab Anim Sci 37:58–61 [PubMed] [Google Scholar]
- 36.Laltoo H, Kind LS. 1979. Reduction of contact sensitivity reactions to oxazolone mite-infested mice. Infect Immun 26:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laltoo H, Van Zoost T, Kind LS. 1979. IgE antibody response to mite antigens in mite-infested mice. Immunol Commun 8:1–9 [DOI] [PubMed] [Google Scholar]
- 38.Lee JD, Huh JE, Baek YH, Cho KC, Choi DY, Park DS. 2009. The efficacy and mechanism action of RvCSd, a new herbal agent, on immune suppression and cartilage protection in a mouse model of rheumatoid arthritis. J Pharmacol Sci 109:211–221 [DOI] [PubMed] [Google Scholar]
- 39.Levee EM, Klinger MM, Kaiser CC, Serrano LJ. 1994. A practical delivery method for oral administration of ivermectin to large colonies of rodents. Contemp Top Lab Anim Sci 33:68–70 [PubMed] [Google Scholar]
- 40.Levine JF, Lage AL. 1984. House mouse mites infesting laboratory rodents. Lab Anim Sci 34:393–394 [PubMed] [Google Scholar]
- 41.Mather TN, Lausen NCG. 1990. A new insecticide delivery method for control of fur mite infestations in laboratory mice. Lab Anim 9:25–29 [Google Scholar]
- 42.Mehlhorn H, Schmahl G, Mevissen I. 2005. Efficacy of a combination of imidacloprid and moxidectin against parasites of reptiles and rodents: case reports. Parasitol Res 97:S97–S101 [DOI] [PubMed] [Google Scholar]
- 43.Mook DM, Benjamin KA. 2008. Use of selamectin and moxidectin in the treatment of mouse fur mites. J Am Assoc Lab Anim Sci 47:20–24 [PMC free article] [PubMed] [Google Scholar]
- 44.Morita E, Kaneko S, Hiragun T, Shindo H, Tanaka T, Furukawa T, Nobukiyo A, Yamamoto S. 1999. Fur mites induce dermatitis associated with IgE hyperproduction in an inbred strain of mice, NC/Kuj. J Dermatol Sci 19:37–43 [DOI] [PubMed] [Google Scholar]
- 45.National Research Council 1991. Infectious diseases of mice and rats: a report of the Institute of Laboratory Animal Resources Committee on Infectious Diseases of Mice and Rats. Washington (DC): National Academy Press [Google Scholar]
- 46.Papini R, Marconcini A. 1991. Treatment with ivermectin in drinking water against Myobia musculi and Myocoptes musculinus mange in naturally infected laboratory mice. Angew Parasitol 32:11–13 [PubMed] [Google Scholar]
- 47.Pence BC, Demick DS, Richard BC, Buddingh F. 1991. The efficacy and safety of chlorpyrifos (Dursban) for control of Myobia musculi infestation in mice. Lab Anim Sci 41:139–142 [PubMed] [Google Scholar]
- 48.Pochanke V, Hatak S, Hengartner H, Zinkernagel RM, McCoy KD. 2006. Induction of IgE and allergic-type responses in fur mite-infested mice. Eur J Immunol 36:2434–2445 [DOI] [PubMed] [Google Scholar]
- 49.Pollicino P, Rossi L, Rambozzi L, Farca AM, Peano A. 2008. Oral administration of moxidectin for treatment of murine acariasis due to Radfordia affinis. Vet Parasitol 151:355–357 [DOI] [PubMed] [Google Scholar]
- 50.Pullium JK, Brooks WJ, Langley AD, Huerkamp MJ. 2005. A single dose of topical moxidectin as an effective treatment for murine acariasis due to Myocoptes musculinus. Contemp Top Lab Anim Sci 44:26–28 [PubMed] [Google Scholar]
- 51.Sebesteny A. 1991. Necessity of a more standardized microbial characterization of rodents for aging studies. Neurobiol Aging 12:663–668 [DOI] [PubMed] [Google Scholar]
- 52.Seeger H, Wallwiener D, Mueck AO. 2008. Effects of estradiol and progestogens on tumor-necrosis factor α-induced changes of biochemical markers for breast cancer growth and metastasis. Gynecol Endocrinol 24:576–579 [DOI] [PubMed] [Google Scholar]
- 53.Skopets B, Wilson RP, Griffith JW, Lang CW. 1996. Ivermectin toxicity in young mice. Lab Anim Sci 46:111–112 [PubMed] [Google Scholar]
- 54.Sundburg JP, King LE., Jr 2000. Skin and its appendages: normal anatomy and pathology of spontaneous, transgenic, and targeted mouse mutations, Ward JM, Mahler JF, Maronpot RR, Sundberg JP. Pathology of genetically engineered mice. Ames (IA): Iowa State University Press [Google Scholar]
- 55.Toth LA, Oberbeck C, Straign CM, Frazier S, Rehg JE. 2000. Toxicity evaluation of prophylactic treatments for mites and pinworms in mice. Contemp Top Lab Anim Sci 39:18–21 [PubMed] [Google Scholar]
- 56.Uchi H, Terao H, Koga T, Furue M. 2000. Cytokines and chemokines in the epidermis. J Dermatol Sci 24:S29–S38 [DOI] [PubMed] [Google Scholar]
- 57.Vachon P, Aubry L. 1996. [The use of ivermectin for the treatment of mites, Myobia musculi and Mycoptes musculinus, in a colony of transgenic mice.] Article in French. Can Vet J 37:231–232 [PMC free article] [PubMed] [Google Scholar]
- 58.Van der Meide PH, Schellekens H. 1996. Cytokines and the immune response. Biotherapy 8:243–249 [DOI] [PubMed] [Google Scholar]
- 59.Wagner JE. 1969. Control of mouse ectoparasites with resin vaporizer strips containing Vapona. Lab Anim Care 19:804–807 [PubMed] [Google Scholar]
- 60.Wagner JE, Johnson DR. 1970. Toxicity of dichlorvos for laboratory mice—LD50 and effect on serum cholinesterase. Lab Anim Care 20:45–47 [PubMed] [Google Scholar]
- 61.Watson DP. 1961. The effect of the mite Myocoptes musculinus (CL Koch 1840) on the skin of the white laboratory mouse and its control. Parasitology 51:373–378 [DOI] [PubMed] [Google Scholar]
- 62.Watson J. 2008. New building, old parasite: mesostigmatid mites,–an ever-present threat to barrier facilities. ILAR J 49:303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weisbroth SH, Friedman S, Powell M, Scher S. 1974. The parasitic ecology of the rodent mite Myobia musculi. I. Grooming factors. Lab Anim Sci 24:510–516 [PubMed] [Google Scholar]
- 64.Weisbroth SH, Friedman S, Scher S. 1976. The parasitic ecology of the rodent mite, Myobia musculi. III. Lesions in certain host strains. Lab Anim Sci 26:725–735 [PubMed] [Google Scholar]
- 65.Welter A, Mineo JR, de Oliveira Silva DA, Lourenco EV, Vieira Ferro EA, Roque-Barreira MC, Maria da Silva N. 2007. BALB/c mice resistant to Toxoplasma gondii infection proved to be highly susceptible when previously infected with Myocoptes musculinus fur mites. Int J Exp Pathol 88:325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welter A, Mineo JR, Silva DA, Lourenco EV, Ferro EA, Roque-Barreira MC, da Silva NM. 2006. An opposite role is exerted by the acarian Myocoptes musculinus in the outcome of Toxoplasma gondii infection according to the route of the protozoa inoculation. Microbes Infect 8:2618–2628 [DOI] [PubMed] [Google Scholar]
- 67.West WL, Schofield JC, Bennett BT. 1992. Efficacy of the ‘microdot’ technique for administering topical 1% ivermectin for the control of pinworms and fur mites in mice. Contemp Top Lab Anim Sci 31:7–10 [Google Scholar]
- 68.Winchester M, Farrell B, Hayes Y, Bellinger D. 2004. The use of fipronil (Frontline) and selamectin (Revolution) for the treatment and control of parasites in mice. Contemp Top Lab Anim Sci 43:65 [Google Scholar]
- 69.Wing SR, Courtney CH, Young MD. 1985. Effect of ivermectin on murine mites. J Am Vet Med Assoc 187:1191–1192 [PubMed] [Google Scholar]
- 70.Yamaguchi T, Maekawa T, Nishikawa Y, Nojima H, Kaneko M, Kawakita T, Miyamoto T, Kuraishi Y. 2001. Characterization of itch-associated responses of NC mice with mite-induced chronic dermatitis. J Dermatol Sci 25:20–28 [DOI] [PubMed] [Google Scholar]