Abstract
Nursery rearing is the single most important risk factor in the development of severe forms of abnormal behavior, such as self-biting, in rhesus macaques. This practice is common in research laboratories and typically involves continuous pair housing of infants without maternal contact. We examined the effects of variation in peer socialization on the behavioral development of rhesus infants by exposing 32 newborn infants to 4 different socialization routines: continuously paired; intermittently paired; continuously paired rotationally (partners rotated within the group once a week); and intermittently paired rotationally. Analyses revealed that infants paired intermittently exhibited ‘floating limb’ and self-biting behavior at significantly higher frequencies than those reared by using any other strategy. Results also suggested that continuous pairing was most effective in reducing the development of abnormal behaviors (that is, self-bite and floating limb), whereas intermittent pairing significantly reduced partner clinging and geckering. A principal component analysis revealed that floating limb behavior and self-biting are strongly associated. Self-biting began as early as 32 d of age, and a negative binomial regression on data of floating limb and self-biting revealed that early development of floating limb behavior predicts self-biting behavior later in development. Despite the significant effects of rearing strategies on the frequency of abnormal behaviors, we note that animals in all 4 treatment groups developed these traits to some degree. We suspect that the solitary incubator environment may be a trigger for the development of abnormal behaviors.
Abbreviation: CP, continuously paired; IP, intermittently paired; CRP, continuous rotationally paired; IRP, intermittent rotationally paired
Due to its high anatomic, physiologic, and genetic similarity to humans and its relative ease of maintenance and reproductive success in captivity, the rhesus macaque is the most commonly used nonhuman primate model in biomedical research.27 Despite their perceived robustness in captivity, laboratory-housed rhesus monkeys are known to exhibit a variety of abnormal behaviors.3,11,12,21-23,28 These behaviors may indicate current or past experiences of compromised welfare25 and thus present a serious issue in nonhuman primate care.
A survey of animals housed at a national regional primate center revealed that 88% of animals exhibited at least 1 type of abnormal behavior.22 Surveys of populations of individually housed macaques found that about 25% of these animals engaged in self-biting behavior.22 In 5% to 11% of cases, noninjurious self-biting may develop into self-injurious behavior resulting in physical harm to the animal,2,4,22 and about 14% of all single-housed macaques require veterinary care for self-inflicted wounding at some point in their lives.28
A typical characteristic of abnormal behaviors is that they are persistent and resistant to treatment once they have become established.21 Methods of intervention at our institution have included environmental enrichment aimed at promoting species-typical or incompatible behaviors; relocation to reduce social stress or sexual frustration; and pharmacologic approaches targeting possible underlying physiological imbalances.22,24,28,34 All of these methods have been largely unsuccessful at treating self-injurious behavior.
Because of the difficulties we face in the treatment of abnormal behaviors, recent efforts have been aimed at identifying risk factors for the development of behavioral pathologies in order to develop and implement preventive measures and thereby reduce the number of animals who develop abnormal behaviors.2,4,22,23 Some risk factors that appear to predispose animals to developing abnormal behaviors include single housing, age at first single housing, amount of time spent in single housing, number of relocations, and number of blood draws.4,22,24,29,30,34 However, disruption of the early rearing experience is by far the most important risk factor identified to date.4,22,24,29,30,34 Data analyzed retrospectively on colony records from our institution indicate that indoor-housed adult macaques that were nursery-reared without their mothers are 11 times more likely to exhibit self-abuse behavior than are adult animals that were reared indoors with their mothers.11
The effects of raising infant monkeys without their mothers were first examined in a seminal work on the development of affectional responses. This research16 showed that infant rhesus monkeys prefer a non-nourishing, cloth-covered inanimate surrogate over a nourishing wire model. This finding established the importance of contact comfort over the satisfaction of a primary drive, hunger, in the formation of early attachment. Surrogates served as a secure base for exploration in novel environments and their removal caused severe distress to the infants.
In the process of observing his subjects' responses to the different surrogates, the authors noted that the infants exhibited a suite of unusual behaviors, which they termed ‘isolation syndrome.’15 This group of behaviors mostly comprised self-directed behaviors such as self-sucking, self-clasping while rocking rhythmically, and self-biting. The authors attributed the emergence of these behaviors to the lack of social stimulation their subjects had experienced during early development. When introduced to age mates that had been reared with their mothers, the study subjects showed severe emotional distress and withdrew from any social interaction.39 However, exposure of the monkeys reared in isolation to younger mother-reared infants whose behavior more closely matched the subject's developmental stage resulted in a remarkable recovery. These ‘monkey therapists’ engaged isolates in play and other affiliative social interactions. In addition, abnormal behaviors also decreased to much lower frequencies.
Partly in response to these findings, the Guide for the Care and Use of Laboratory Animals17 states that “appropriate social interactions among conspecifics are essential for normal development” because “a social companion might buffer the effects of a stressful situation,13 reduce behavioral abnormality,33 increase opportunities for exercise,43 and expand species-typical behavior and cognitive stimulation.” Furthermore, US Department of Agriculture regulations40 recognize the importance of the developmental phase in maintaining colony health by stating that “certain nonhuman primates must be provided special attention regarding the enhancement of their environment.…Nonhuman primates requiring special attention are the following: 1) Infants and young juveniles; 2) Those who show signs of being in psychological distress through behavior or appearance.…” Infants that have been removed from their mothers and are being raised in the nursery satisfy both of these criteria, warranting increased research in the area of behavioral development in captive primates.
Institutions that rear infant rhesus macaques in a nursery setting have used a variety of social rearing paradigms providing peer contact to developing infants. Current nursery-rearing practices are very different from those in the cited early studies15 in that infants receive much more exposure to humans and similarly aged conspecifics. Nonetheless, nursery-reared infants differ from their mother-reared counterparts in a number of measurable parameters, including physiology, immunology, and behavior.37
A number of rearing conditions that differ in the amount of social contact infant nonhuman primates experience during the first year of development have been studied. The first is the mother–peer rearing strategy, which was designed to resemble the species-typical social patterns infants experience under natural conditions. This strategy typically entails mother-rearing in an outdoor group setting in which infants are exposed to complex social interactions with other infants, juveniles, and adult males and females.
The behavior and physiology of infant macaques raised under the conditions of mother–peer rearing often serve as the ‘standard of normalcy’ with which other rearing conditions are compared. Several other strategies have been used that involve peer-rearing; 3 of these—continuous, intermittent, and rotational peer rearing—are reviewed here. All 3 approaches generally entail an early solitary period in an incubator, which can range from birth until about 24 to 37 d of age, followed by transfer into wire caging in 1 of 3 social contexts. During continuous pair rearing, infants are housed in pairs with the same age-matched partner throughout development. Intermittent peer housing allows for peer contact for limited periods during the day; the rest of the time, infants are housed singly. With the rotational peer-rearing condition infants are continuously peer-housed with a rotation of social partners every 2 to 7 d.22
The greatest differences in behavior exist between nursery- and mother-reared infants. Independent of peer-rearing condition, infants reared without maternal contact show an increased propensity to develop self-sucking, self-clinging, partner clinging, decreased exploration in novel environments, and insufficient social behaviors.9,10,14,31,35
Different species of nonhuman primates vary in their response to different nursery rearing conditions.22 However, studies conducted for over 30 y have shown that in general, raising infants in pairs continuously with the same partner causes behavioral deficiencies.10 Compared with those raised by using other rearing methods, these infants showed more fear, social withdrawal, aggression, and less nonsocial exploration in this10 and other14,35 studies examining this rearing condition. These differences were thought to be due to the infants' excessive and persistent partner clinging, which precluded independent exploration. Although clinging was initiated by both partners equally often, infants were physically unable to break the clasp of their partners. In addition, infants who received partner clinging often responded to their partner's behavior with aggression. In addition, continuously paired female infants engaged in more clinging than did male infants raised in this rearing environment and engaged in the least overall nonsocial exploration. When exposed to a more complex housing situation, continuously paired infants were “uniformly subordinate and appeared depressed.”35 However, typical disturbance behaviors indicative of fear were markedly decreased to absent in the novel environment of an open-field test of animals raised in continuous pairs.10
Intermittent peer-rearing environments produced very different effects. Although still inferior to mother-rearing, the intermittent peer-rearing condition decreased partner clinging, fear and withdrawal behaviors, and aggression while increasing nonsocial exploration and social play when compared with a continuous-pairing regime.10,35 However, infants raised in the intermittent peer-rearing environment showed high levels of self-directed behaviors such as rocking, huddling, self-clasping, and motor stereotypies.35
Research investigating the effects of a rotating-peer strategy showed inconsistent results. This approach was aimed at providing continuous social exposure to decrease disturbance behaviors while changing the composition of the social group to decrease partner clinging. One study9 found that although always housed with another monkey, the infants experiencing changing social composition due to rotation of partners exhibited little partner clinging. However, other research31 on the rotating-peer strategy revealed that infants experiencing change in social composition by rotating partners showed no decrease in clinging behavior and even less social exploration than did continuous peer-reared infants. At 6 mo of age, infants raised with rotating partners displayed behavioral repertoires that resembled those of continuous peer-reared subjects.
Institutions that rear infants in a nursery setting have used various social rearing paradigms providing peer contact to developing infants.9,31,35 Despite these efforts, nursery-reared infants differ from their mother-reared counterparts in a number of measurable parameters, including physiology, immunology, and behavior.36
The current study focused on exploring the effects of 4 different nursery-rearing strategies on several parameters of infant development and wellbeing. As presented earlier, several rearing paradigms have been tested, but none have yielded satisfactory results in terms of raising monkeys that develop independence and normal social skills without also acquiring abnormal behaviors. Despite evidence for the negative effects of continuous pair housing, the standard nursery rearing protocol at some primate research centers still calls for this procedure.
Though several other National Primate Research Centers have switched to a surrogate peer-rearing strategy that affords infants with only 2 h or less of group socialization daily, evidence is mounting that although this strategy decreases clinging and produces more normal social behaviors, surrogate peer-rearing may be increasing the incidence of self-directed behaviors and self-injury.23 This effect is thought to be due to early periods of single housing, an established risk factor for the development of self-biting and self-injurious behavior.36 The surrogate–peer rearing paradigm currently used at other primate centers assumes that a 2-h socialization period is the maximal amount of time before infants become attached and begin viewing their partners as their primary attachment figure. However, we are unaware of research testing longer daily socialization periods, which may prevent the ill effects of prolonged single housing. We sought to address this deficiency by testing our intermittent-pairing design.
The obvious solution to preventing the occurrence of abnormal behaviors due to nursery rearing would be to raise all infants with their mothers. This paradigm should be a goal for primate centers around the world and has been for some time. However, colony managers are forced to balance animal welfare concerns with the need of investigators for animals that are free of pathogens that may interfere with research goals. The most reliable way to produce SPF animals has been to prevent transmission of viruses and other pathogens between mother and offspring by removing infants from their mothers at birth and raising infants in a clean SPF nursery environment. This procedure is practiced by many primate centers with SPF breeding protocols, although many facilities now have adult SPF dams that are allowed to raise their own infants, thus decreasing the need for nursery rearing. However, nursery rearing is sometimes necessary for other research, such as developmental and infectious studies.36 In addition, all primate centers must be able to raise infants that are orphaned or ill and cannot be fostered by another female monkey. Therefore, effective nursery rearing methods that produce healthy and behaviorally normal animals will always be necessary.
The goal of the current research was to test new rearing paradigms and to incorporate in novel ways the knowledge we have gained over many years, to produce behaviorally and physiologically healthy monkeys and improve the quality of a common biomedical research model. Findings from the previous work reviewed earlier led us to hypothesize that the quantity and quality of an infant's social experience will affect the frequency of partner clinging, aggression, fear, and self-directed behaviors. Specifically, we predicted that an intermittent-pairing regime would reduce the incidence of partner clinging, fear responses, and aggression. We also expected an increase in the frequency of self-directed behaviors. However, we predicted that this problem might be minimized through prolonged (8 h daily) socialization and therefore shortening the amount of time infants are housed singly. In addition, we predicted that a rotating-peer strategy would discourage clinging behavior and therefore decrease aggression and increase social play. Overall, we expected to see the most favorable results (low incidence of clinging, fear, aggression, and self-directed behaviors) in infants raised with a combination of treatments that includes intermittent pairing as well a rotation of social partners. We used behavioral observations in the home cage from the age of 28 d to approximately 10 to 11 mo to assess the effects of 4 socialization strategies on behavioral development.
Materials and Methods
The study was conducted at the California National Primate Research Center from May 2006 to May 2007. All research conducted and presented complied with protocols approved by the Institutional Animal Care and Use Committee at the University of California at Davis and adhered to the requirements of the Animal Welfare Act and US Department of Agriculture regulations.
We included 32 (14 female and 18 male) rhesus macaques (Macaca mulatta) who had been placed in the nursery as part of the yearly SPF breeding program at our institution. Infants had been removed from their mothers at birth and transferred to the nursery where they were housed in incubators measuring approximately 31 × 31 × 36 cm until 28 d of age. During this time they were provided with supplemental heat from a heating pad, soft cotton diapers, and a soft stuffed animal as a surrogate. Infants were weaned off their diapers and surrogates at 120 d of age, or earlier if they were destroying them, to prevent possible trauma to digits or intestinal blockage due to ingestion of pieces of fabric. All animals had free access to infant formula (Enfamil With Iron Liquid Formula, Mead Johnson, Evansville, IN) supplied in a pet nursing bottle attached to the side of the incubator wall. Subjects were encouraged to self-feed with initial support by nursery staff directing them to the bottle and propping them up on their surrogates to suckle for the first week of life. After this period, handling was restricted to daily weighing and cage cleaning. Infants were weaned off their pet bottles at around 56 d of age at which time they were provided with three hanging bottles (Lixit, Napa, CA) containing infant formula, citrus-flavored beverage (Tang, Kraft Foods, Northfield, IL), and water. Nursery rooms were kept at a timed cycle of lights on at 0600 and off at 2200, with lights on again during overnight feedings at 0100 and 0400. At 28 d of age, subjects began adaptation to quad cages by being transferred to nursery wire cages (Carter2Systems, Beaverton, OR) for 2 h daily. During this time infants were allowed visual access to their future pair mate through a clear plastic divider. Time in the quad cage was increased until infants were permanently transferred to quad cages at the age of 30 d. At this time the divider was removed and infants were paired with another animal of approximately the same age by using 1 of 4 pairing strategies.
Eight (4 pairs of 2) infants were assigned to the continuous pairing (CP) group (treatment). Two of these pairs were single-sex (male–male) and 2 were mixed-sex. This rearing condition functioned as the control condition and mirrored the standard nursery protocol at our institution. Infants were paired continuously with the same similar-age pair mate throughout their stay in the nursery. These infants were transferred with their pair mates to weanling rooms at 7 mo of age and remained together until they left the nursery permanently at 10 to 11 mo of age. A second set of 8 infants was assigned to the intermittent pairing (IP) group. Three of these pairs were single-sex (female–female) and 1 was mixed-sex. In this condition, subjects were paired for 8 h daily, from 0700 to 1500, and were separated by an opaque divider from 1500 until 0700. Eight additional infants were part of the continuous rotational pairing (CRP) group; 1 group of 4 animals consisted of 2 male and 2 female macaque whereas the second consisted of 4 male macaques. These subjects were continuously paired with another infant; in addition, partners were rotated once weekly within their group of 4 infants such that they were exposed to 3 different social partners throughout their stay in the nursery. Finally, 8 infants were assigned to the intermittent rotational pairing (IRP) group; 1 group of 4 infants consisted of 3 male and 1 female macaque whereas the other contained 4 female macaques. Infants in the IRP group were paired for 8 h daily as in the previously described IP group. In addition, partners were rotated within a group of 4 infants once weekly.
Subjects were assigned to treatment groups as pairs as they arrived in the nursery in the order of CP, IP, CRP, and IRP. This order was repeated until all treatment groups contained 4 pairs each. This process allowed us to match infants by age such that partners were of similar ages within pairs as well as rotational groups. Infants were paired permanently and transferred to weanling rooms with adult-size caging at 7 mo of age.
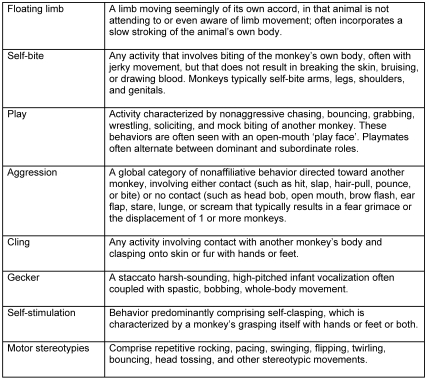
Subjects were monitored from their first day of pairing (at approximately 28 d of age) to the day of transfer from indoor housing to outdoor corn cribs (at approximately 10 to 11 mo of age). Infants were observed by using a 15-min 1-zero focal sample design1 with 15-s intervals (maximum score of 60 in a focal session) 3 times weekly until 3 mo of age, twice a week until 6 mo of age, and then once weekly until they left the indoor colony. Approximately 78 sessions (15 min each) were collected per animal across 42 wk. Two infants belonging to a pair were observed simultaneously by the same observer assessing 32 distinct behaviors. We restricted this report to the behaviors described in Figure 1.
Figure 1.
Ethogram of the behaviors reported in this study.
Infants were observed between 0700 and 1500 while they were paired, so that we could observe both partners at the same time. The order of pairs was randomized for each observation period. Therefore, infants were observed at different times each day depending on this predetermined order. All data collection was conducted by 2 trained observers (interobserver reliability, 84% agreement on 32 behaviors).
Data analysis
Data were structured such that averages for each behavioral variable were calculated and averaged by individual and week. We analyzed behavioral outcomes by using a multivariate generalized linear model (P < 0.10) with rearing condition and sex as fixed effects and individual as the covariate or repeated measure (SPSS, SPSS, Chicago, IL).
Estimated marginal means were used for multiple comparisons across rearing conditions (P < 0.05). Results are presented in mean frequencies, that is, the mean number of intervals over the duration of the study in which the behavior occurred. In addition, we used principal component analysis to analyze the relationships between behaviors (S-Plus 6.0.2, Insightful Corp, Seattle, WA). This process allowed us to view our data so that it is expressed in terms of the patterns between behavioral variables, where the patterns are the lines that most closely describe the relationships between the data.
Finally, negative binomial regression (Stata 9.0, StataCorp, College Station, TX) of floating limb and self-bite with individual as the grouped random effect was conducted to analyze data longitudinally by month in order to analyze whether one behavior was predictive of the other. Rates of floating limb were analyzed in relation to rates of self-biting behavior in the subsequent month.
Results
The multivariate generalized linear modeling revealed an overall main effect of rearing condition (F9,3843 = 5.548, P < 0.001), and sex (F9,1279 = 3.441, P < 0.001) as well as a sex × rearing condition interaction (F27,3843 = 1.496, P < 0.05).
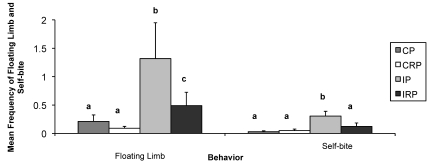
Mean frequencies of play (F3,1287 = 15.286, P < 0.001), floating limb (F3,1287 = 20.027, P < 0.001), and self-bite (F3,1287 = 7.585, P < 0.001) showed significant differences based on rearing condition (Figure 2). Specifically, IP monkeys played more than did CP (P < 0.001), CRP (P < 0.001), and IRP (P < 0.001) animals. In addition, CRP infants played more than did those in the CP (P < 0.01) and IRP (P < 0.01) groups. Monkeys in the IP group showed more floating limb than CP (P < 0.001), CRP (P < 0.001), and IRP animals (P < 0.001). In addition, IRP monkeys showed more floating limb than did CP (P < 0.01) and CRP (P < 0.01) monkeys. IP monkeys self-bit more than did those in the CP (P < 0.001), CRP (P < 0.001), and IRP (P < 0.01) groups.
Figure 2.
Effects of rearing condition on the frequency of floating limb and self-biting behavior. Mean frequency was calculated over the entire study period. Different letters indicate groups that were significantly different from one another. Bar, SEM; CP, continuous pairing; CRP, continuous rotational pairing; IP, intermittent pairing; IRP, intermittent rotational pairing.
Frequencies of social play (F1,1287 = 3.519, P < 0.10) and floating limb (F1,1287 = 4.611, P < 0.05) were greater in female than male monkeys. In the case of floating limb, this sex difference was not based on rearing group. Conversely frequency of aggression (F1,1287 = 9.979, P < 0.01) was greater in male than female monkeys, regardless of group.
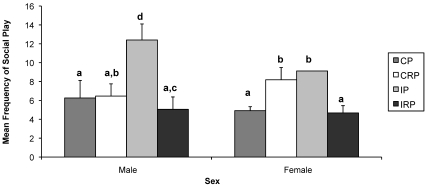
Overall differences in the mean frequency of social play (F3,1287 = 2.225, P < 0.10), cling (F3,1287 = 3.372, P < 0.01), and gecker (F3,1287 = 2.356, P < 0.10) among rearing conditions varied significantly based on sex. Specifically, CRP female monkeys played more than did CP female macaques (P < 0.05) and IP male macaques played more than CRP male macaques (P < 0.001). Both male and female IP monkeys played more than CP (P < 0.001 and P < 0.001, respectively) and IRP monkeys (P < 0.001 and P < 0.001, respectively). In addition, both male and female CRP monkeys played more than IRP monkeys (P < 0.10 and P < 0.05, respectively; Figure3).
Figure 3.
Effects of sex and rearing condition on the frequency of social play behavior. Mean frequency was calculated over the entire study period. Different letters indicate groups that were significantly different from one another. Bar, SEM; CP, continuous pairing; CRP, continuous rotational pairing; IP, intermittent pairing; IRP, intermittent rotational pairing.
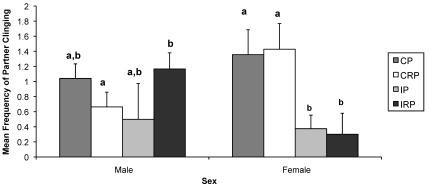
CP female monkeys clung significantly more than did IP (P < 0.05) and IRP (P < 0.05) female infants, and CRP female macaques clung significantly more than IP (P < 0.01) and IRP (P < 0.05) female macaques. Among male animals, those in the IRP group clung significantly (P = 0.054) more than did those in the CRP group (Figure 4).
Figure 4.
Effects of sex and rearing condition on the frequency of partner clinging. Mean frequency was calculated over the entire study period. Different letters indicate groups that were significantly different from one another. Bar, SEM; CP, continuous pairing; CRP, continuous rotational pairing; IP, intermittent pairing; IRP, intermittent rotational pairing.
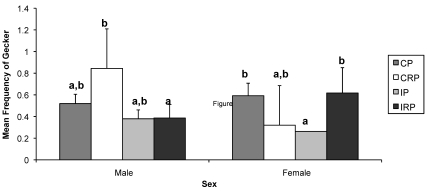
IP female monkeys geckered less than both CP (P = 0.053) and IRP (P < 0.05) female macaques. IRP male animals geckered less than did CRP male macaques (P < 0.01; Figure 5).
Figure 5.
Effects of sex and rearing condition on the frequency of gecker vocalizations. Mean frequency was calculated over the entire study period. Different letters indicate groups that were significantly different from one another. Bar, SEM; CP, continuous pairing; CRP, continuous rotational pairing; IP, intermittent pairing; IRP, intermittent rotational pairing.
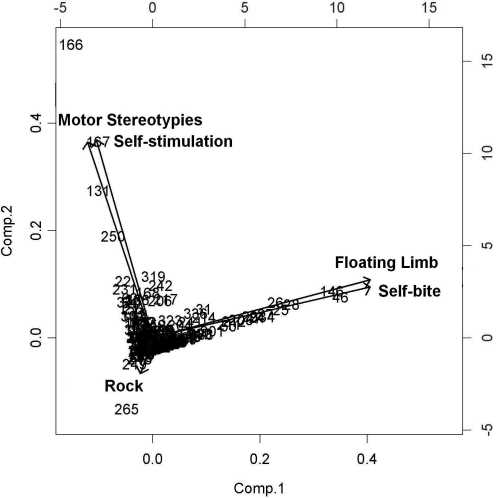
Principal component analysis showed that self-biting and floating limb are strongly associated; the same was true for self-stimulation (mostly self-clasping) and motor stereotypies. However, when separated out from other motor stereotypies, rocking did not load with either of the other behaviors (Figure 6).
Figure 6.
Principal component analysis showing that floating limb and self-biting are highly associated with each other, as are motor stereotypies and self-stimulation. Stereotypic rocking (rock) shows dissociation from other motor stereotypies. Comp, component.
Negative binomial regression of floating limb and self-biting revealed that animals that exhibited floating limb behavior earlier in development were significantly (β = 0.13, P < 0.0001) more likely to develop self-biting later in development. In addition, longitudinal data revealed that self-biting began as early as 32 d of age and floating limb as early as 28 d of age.
Discussion
Results of this study indicate important differences between rearing conditions for floating limb, self-bite, and play in nursery-reared rhesus macaques. Specifically, intermittent pairing was associated with a significant increase in the incidence of floating limb syndrome and self-biting. The same pattern was evident with the intermittent rotational strategy though to a lesser extent. These results indicate that floating limb and self-biting may be related to early single housing, as suggested previously,23 although rotation of peers may buffer this effect to some extent.
The early onset of floating limb and self-biting behavior in our subjects may surprise those who consider young infants as too uncoordinated and behaviorally incomplete to exhibit such responses. At 1 mo of age, our subjects were alert and well-coordinated and we were careful not to include any potentially confounding behaviors during scoring, such as self-mouthing or self-sucking when scoring self-biting, and self-biting in young infants included the typical spastic or jerky movements associated with self-biting behavior in older aninals.
Our hypothesis that increasing the amount of social exposure to more than 2 h daily would minimize self-directed behaviors was not supported. Animals who receive early exposure to periods of single housing experience fewer opportunities for response-contingent stimulation than do those who are continuously paired and who receive more feedback from social partners about their behaviors. Therefore, intermittently paired animals can be viewed as having less control over their environment, beyond their interactions with simple manipulanda, than do those paired continuously, and this decreased control may reduce the monkeys' motivation to behaviorally interact with their surroundings. Previous research41,42 demonstrated this effect in human infants, who directed social behaviors towards a controllable stimulus but ignored the same stimulus when it provided no feedback.
Furthermore, the rotation of partners may simulate the periodic introduction of a novel stimulus to the infant's environment. This strategy may disrupt the development of stereotyped behavior patterns by stimulating animals to exhibit flexible behavioral responses during social interactions with less-familiar animals.
Alternatively, given the early onset of self-directed behaviors in our study animals, socialization strategies after the first month of life may not be the only factor affecting the development of these patterns. The early incubator environment may be an important contributor to the development of self-directed behaviors. Although early incubation may serve to protect the infant from dangers such as hypothermia and dehydration, it also subjects infants to a period of sensory deprivation. Handling time in the nursery at our institution typically is restricted to daily weighing and cleaning of the incubator and is dramatically different from the amount of handling and other sensory inputs received by mother–peer-reared infants. Elements of the early sensory stimulation lacking during the incubator period include kinesthetic stimulation normally provided by the mother's movement, tactile stimulation through maternal grooming and embracing, as well as vocal and visual stimulation received by hearing or observing the mother and other conspecifics or objects as the infant is carried about its environment. Therefore, early incubation may predispose infants to developing self-directed behaviors, which then are retained as coping strategies after incubation. As our data show, the occurrence of abnormal behaviors was not restricted to any particular treatment group. Monkeys in all groups exhibited floating limb and self-biting—the distinction between treatment groups was only apparent in the frequency with which animals showed the behaviors. Therefore, certain socialization strategies may function more as therapeutic approaches than preventive measures, affecting the quantity but not quality of behavioral responses. A large body of evidence concerning the effects of early stimulation on infant development supports this hypothesis.7,8,18,19,37,38 Therefore, increasing the amount of sensory stimulation received within the first 30 d of life may be an effective preventive measure for the development of behavioral pathologies.
Further analyses of the relationship between floating limb and self-biting revealed that these behaviors are highly associated and that floating limb behavior can be considered predictive of self-biting, as previously shown.5,6 This association may warrant increased monitoring and intervention when animals exhibit floating limb behavior. These findings are consistent with data from adult animals at our institution, showing that floating limb and self-bite are highly related in this age group also.34
In addition, treatment differences were evident for social play behavior. Again, IP animals showed the largest difference from all other groups, mostly due to increased play by female monkeys in this group and perhaps partially to the decrease in partner clinging in this group. Intermittently paired animals appear to use their limited social time by engaging in social play instead of clinging to their partners. One possible explanation for this finding is that CP animals direct their primary attachment behaviors to their peers whereas IP infants are more directed towards their own bodies or inanimate objects in their environment, such as stuffed toys and cloth diapers. Therefore, CP animals treat their social partners similar to surrogates, whereas IP infants consider them as playmates.
Geckers are a response to distress in wild rhesus populations.32 In the current study, gecker vocalizations were decreased significantly in IP animals. Because geckers most often occurred when infants were disturbed by their partners, this behavior may be indicative of a decrease in social stress with IP regimens. However, rather than reflecting distress, increased geckering simply may be a method for coping. If infants use geckering as a coping mechanism in stressful situations, they actually may be less stressed than infants who do not use such coping behaviors. A previous study on infant responses to maternal separation 20 supports this hypothesis: infants who vocalized more in response to maternal separation stress also showed lowered indices of psychologic arousal, such as plasma cortisol levels. In addition, differences in the frequency of gecker vocalizations depending on rearing condition may reflect differences in the infants' experience with reinforcement. Infants who gecker generally receive a response (whether desirable or not) from their partners. Any response to a behavior may serve as a reinforcer and encourage repetition. Therefore, CP infants presumably receive feedback to geckering more often than do IP animals, given the differences in socialization duration in these 2 groups. When IP animals are separated, geckering does not receive a response and therefore is not reinforced, thus supporting our findings of decreased vocalization in these subjects. This hypothesis is supported further by the authors of a previous study,32 who stated that infants in wild rhesus populations use gecker vocalizations to receive attention from their mothers.
Results from the PCA group suggest that rocking and other motor stereotypies may have different etiologies. In fact, previous research suggests that rocking may be a very early response to the deprivation of maternal movement in infancy,26 whereas other motor stereotypies develop later and may be a response to a different set of frustrated motivations, such as an increased need for unrestricted whole-body movement. Our data showed a similar pattern, with other motor stereotypies developing later than rocking.
Disparate results between rearing studies likely are influenced highly by the use of different nursery-rearing protocols, especially differences in the amount of early handling received by infants. Because early cognitive testing and hand-feeding are not a routine part of the nursery rearing protocol at our institution, early stimulation through handling by human caretakers was minimal. This situation most certainly affected the development of our subjects in comparison to those raised at other primate centers, which use more handling-intensive regimens.
Results of the current and previous studies showed that the sex of the infants is an important variable in the course of behavioral development. Because our subjects were part of the established SPF breeding protocol, we had no control over the composition of pairs in our study—infants were paired to be as close in age to each other. Therefore, sex differences and the uncontrolled composition of pairs in our treatment groups may have influenced outcome in our study. That is, male–male pairs, for example, may show very different behavioral dynamics than those of mixed-sex pairs. Future studies should control for sex effects by controlling pair composition.
In summary, intermittent pairing during infancy of rhesus macaques appears to decrease the strength of social attachment between partners and is associated with an concerning increase in the abnormal behaviors of such as floating limb and self-biting. Further research on other surrogate–peer rearing methods and the effects of the early incubator environment on behavioral development is needed.
Acknowledgments
The research conducted and presented in this study complied with protocols approved by the Institutional Animal Care and Use Committee at UC Davis and adhered to the legal requirements of the US Department of Agriculture and the Animal Welfare Act.
We would like to thank the California National Primate Research Center for supporting this project with a pilot study grant funded by National Institutes of Health grant P51 RR000169. Special appreciation also goes to the primate center nursery staff, especially nursery supervisor Kelly Weaver. We also would like to thank Drs John Capitanio and Joy Mench for their comments on the master's thesis on which this paper was based and two anonymous reviewers for their comments on an earlier version of this publication. Finally we thank Dr Nicholas Lerche for his support throughout this project.
References
- 1.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49:227–267 [DOI] [PubMed] [Google Scholar]
- 2.Bayne K, Haines M, Dexter S, Woodman D, Evans C. 1995. Nonhuman primate wounding prevalence: a retrospective analysis. Lab Anim 24:40–44 [Google Scholar]
- 3.Bayne K, Mainzer H, Dexter S, Campbell G, Yamada F, Suomi SJ. 1991. The reduction of abnormal behaviors in individually housed rhesus monkeys (Macaca mulatta) with a foraging–grooming board. Am J Primatol 23:23–35 [DOI] [PubMed] [Google Scholar]
- 4.Bellanca RU, Crockett CM. 2002. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. Am J Primatol 58:57–69 [DOI] [PubMed] [Google Scholar]
- 5.Bentson K, Bellanca R, Crockett C. 2005. Rate of floating limb activity at WANPRC varies by sex, age, project assignment and, in Macaca nemestrina, by origin. Am J Primatol 66:37 [Google Scholar]
- 6.Bentson K, Montgomery H, Bellanca R, Lee G, Thom J, Crockett C. 2005. Floating limb activity is associated with self-biting in 4 monkey species (Macaca mulatta, M. fascicularis, M. nemestrina, and Papio cynocephalus). Am J Primatol 66:181 [Google Scholar]
- 7.Bernstein L. 1952. A note on Christie's experimental naivete and experiential naivete. Psychol Bull 49:38–40 [DOI] [PubMed] [Google Scholar]
- 8.Casler L. 1965. The effects of extra tactile stimulation on a group of institutionalized infants. Genet Psychol Monogr 71:137–175 [PubMed] [Google Scholar]
- 9.Chamove AS. 1973. Rearing infant rhesus together. Behaviour 47:48–66 [DOI] [PubMed] [Google Scholar]
- 10.Chamove AS, Rosenblum LA, Harlow HF. 1973. Monkeys (Macaca mulatta) raised only with peers. A pilot study. Anim Behav 21:316–325 [DOI] [PubMed] [Google Scholar]
- 11.Eaton GG, Kelley ST, Axthelm MK, Iliff-Sizemore SA, Shiigi SM. 1994. Psychological wellbeing in paired adult female rhesus (Macaca mulatta). Am J Primatol 33:89–99 [DOI] [PubMed] [Google Scholar]
- 12.Erwin J, Mitchell G, Maple T. 1973. Abnormal behavior in nonisolate-reared rhesus monkeys. Psychol Rep 33:515–523 [DOI] [PubMed] [Google Scholar]
- 13.Gust DA, Gordon TP, Brodie AR, McClure HM. 1994. Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiol Behav 55:681–684 [DOI] [PubMed] [Google Scholar]
- 14.Harlow HF. The maternal affectional system. Foss BM. Determinants of infant behavior. New York (NY): John Wiley and Sons [Google Scholar]
- 15.Harlow HF, Mc Kinney WT. 1971. Nonhuman primates and psychoses. J Autism Dev Disord 1:368–375 [DOI] [PubMed] [Google Scholar]
- 16.Harlow HF, Zimmermann RR. 1958. The development of affectional responses in infant monkeys. Proc Am Philos Soc 102:501–509 [Google Scholar]
- 17.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 18.Joffe JM, Rawson RA, Mulick JA. 1973. Control of their environment reduces emotionality in rats. Science 180:1383–1384 [DOI] [PubMed] [Google Scholar]
- 19.Levine S, Otis LS. 1958. The effects of handling before and after weaning on the resistance of albino rats to later deprivation. Can J Psychol 12:103–108 [DOI] [PubMed] [Google Scholar]
- 20.Levine S, Wiener SG. 1988. Psychoendocrine aspects of mother–infant relationships in nonhuman primates. Psychoneuroendocrinology 13:143–154 [DOI] [PubMed] [Google Scholar]
- 21.Lutz C, Tiefenbacher S, Meyer J, Novak M. 2004. Extinction deficits in male rhesus macaques with a history of self-Injurious behavior. Am J Primatol 63:41–48 [DOI] [PubMed] [Google Scholar]
- 22.Lutz C, Well A, Novak M. 2003. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol 60:1–15 [DOI] [PubMed] [Google Scholar]
- 23.Lutz CK, Davis EB, Ruggiero AM, Suomi SJ. 2007. Early predictors of self-biting in socially housed rhesus macaques (Macaca mulatta). Am J Primatol 69:584–590 [DOI] [PubMed] [Google Scholar]
- 24.Lutz CK, Novak MA. 2005. Environmental enrichment for nonhuman primates: theory and application. ILAR J 46:178–191 [DOI] [PubMed] [Google Scholar]
- 25.Mason GJ, Latham NR. 2004. Can't stop, won't stop: is stereotypy a reliable animal welfare indicator? Anim Welf 13:S57–S69 [Google Scholar]
- 26.Mason WA, Berkson G. 1975. Effects of maternal mobility on the development of rocking and other behaviors in rhesus monkeys: a study with artificial mothers. Dev Psychobiol 8:197–211 [DOI] [PubMed] [Google Scholar]
- 27.Mitruka BM, Rawnsley HM, Vadehra DV. 1976. Animals for medical research: models for the study of human disease. New York (NY): Wiley [Google Scholar]
- 28.Novak MA. 2003. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am J Primatol 59:3–19 [DOI] [PubMed] [Google Scholar]
- 29.Novak MA, Petto AJ. 1991. Perspectives on psychological wellbeing in captive primates: through the looking glass. Novak MA, Petto AJ. Through the looking glass: issues of psychological wellbeing in captive nonhuman primates. Washington (DC): American Psychological Association [Google Scholar]
- 30.Novak MA, Suomi SJ. 1988. Psychological wellbeing of primates in captivity. Am Psychol 43:765–773 [DOI] [PubMed] [Google Scholar]
- 31.Novak MF, Sackett GP. 1997. Pair-rearing infant monkeys (Macaca nemestrina) using a“ rotating-peer” strategy. Am J Primatol 41:141–149 [DOI] [PubMed] [Google Scholar]
- 32.Patel ER, Owren MJ. 2007. Acoustics and behavioral contexts of ‘gecker’ vocalizations in young rhesus macaques (Macaca mulatta). J Acoust Soc Am 121:575–585 [DOI] [PubMed] [Google Scholar]
- 33.Reinhardt V, Houser D, Eisele S, Cowley D, Vertein R. 1988. Behavioral responses of unrelated rhesus monkey females paired for the purpose of environmental enrichment. Am J Primatol 14:135–140 [DOI] [PubMed] [Google Scholar]
- 34.Rommeck I, McCowan B, Anderson K, Heagerty A, Cameron A. 2009. Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. J Appl Anim Welf Sci 12:61–72 [DOI] [PubMed] [Google Scholar]
- 35.Ruppenthal GC, Walker CG, Sackett GP. 1991. Rearing infant monkeys (Macaca nemestrina) in pairs produces deficient social development compared with rearing in single cages. Am J Primatol 25:103–113 [DOI] [PubMed] [Google Scholar]
- 36.Sackett GP, Ruppenthal GC, Elias K. 2006. Nursery rearing of nonhuman primates in the 21st century. Chicago (IL): Springer [Google Scholar]
- 37.Solkoff N, Matuszak D. 1975. Tactile stimulation and behavioral development among low-birthweight infants. Child Psychiatry Hum Dev 6:33–37 [DOI] [PubMed] [Google Scholar]
- 38.Solkoff N, Yaffe S, Weintraub D, Blase B. 1969. Effects of handling on the subsequent development of premature infants. Dev Psychol 1:765–768 [Google Scholar]
- 39.Suomi SJ, Harlow HF, McKinney WT. 1972. Monkey psychiatrists. Am J Psychiatr 128:927–932 [DOI] [PubMed] [Google Scholar]
- 40.USDA 1991. Animal Welfare, Standards, Final Rule (Part 3, Subpart D: Specifications for the humane handling, care, treatment, and transportation of nonhuman primates). Fed Regist 56:6495–6505 [Google Scholar]
- 41.Watson JS. 1972. Smiling, cooing, and ‘the game.’ Merrill–Palmer Q 18:323–339 [Google Scholar]
- 42.Watson JS. 1985. Contingency perception in early social development. Field TM, Fox NA. Social perception in infants. Norwood (NJ): Ablex Publishing Corp [Google Scholar]
- 43.Whary M, Peper R, Borkowski G, Lawrence W, Ferguson F. 1993. The effects of group housing on the research use of the laboratory rabbit. Lab Anim 27:330–341 [DOI] [PubMed] [Google Scholar]