Abstract
Here we describe 5 cases of molar malocclusions in adult pine voles (Microtus pinetorum) used for behavioral endocrinology studies. This species belongs to the subfamily Microtinae, which possess aradicular hypsodont molars. The abnormal molars identified caused apparent difficulty in mastication, resulting in poor body condition necessitating euthanasia. Postmortem examination of the oral cavity revealed grossly elongated mandibular and maxillary molars with abnormal wear at occlusal surfaces. This colony health problem was addressed successfully by adding autoclaved hardwood sticks to each cage as an enrichment tool.
Rodents of the genus Microtus typically are used as animal models in behavioral, physiologic, and ecologic studies. The genus Microtus, family Cricetidae, subfamily Arvicolinae20 or Microtinae,10 comprises voles, including the meadow vole (M. pennsylvanicus) and prairie vole (M. ochragaster). Pine voles (M. pinetorum), also known as woodland voles, are found throughout eastern North America, from southern Canada through northern Mexico.25 They are monogamous and possess several associated behavioral traits, such as cooperative breeding, the formation of pair bonds, and biparental care.3,9,17,18,27,28,30 These features have ensured their continued value to researchers engaged in behavioral neuroscience and endocrinologic fields.
Dental anomalies are relatively common problems in laboratory rodents and lagomorphs. These abnormalities most often manifest as malocclusions due to the continuously growing teeth of these species.5 These continuously growing teeth typically are maintained to an appropriate length through normal wear from gnawing behavior but can become maloccluded and overgrown if normal wear is prevented. The molars of rodents of the genus Microtus, unlike those of rats and mice, grow continuously. Consequently, rodents of the genus Microtus may experience molar malocclusions as well as incisor malocclusions. Here we describe molar malocclusions in a laboratory-housed breeding colony of pine voles.
Case Report
The pine voles (M. pinetorum) described in this report were descendants of voles originally trapped in Henderson County, North Carolina. No new animals had been introduced into the breeding colony for more than 6 y prior to this investigation. This colony, which supported studies in behavioral endocrinology,9 averaged 200 to 300 animals at any time. The average lifespan of animals in this colony was 2 to 3 y for those maintained for breeding purposes. Published25,27 and colony data indicate that the average adult weight is 20 to 25 g. Voles were housed according to standard operating procedures established at the Biological Resources Facility, (College of Agriculture and Life Sciences, North Carolina State University) in accordance with regulatory standards promulgated by the Animal Welfare Regulations and all applicable federal guidelines and policies. All animal use activity was approved by the Institutional Animal Care and Use Committee of North Carolina State University.
Feed (Mouse Diet 5015, Nestle, St Louis, MO) and water were provided ad libitum. Animals were maintained on a 14:10-h light:dark cycle in a room with a temperature set point of 21 °C and relative humidity of 30% to 70%. Colony animals were socially housed with siblings on corncob bedding in polypropylene cages (18 × 29 × 12.5 cm). Colony health was monitored quarterly by evaluation of serologic responses to a standard battery of mouse and rat pathogens by means of a dirty-bedding sentinel program. Serum samples from outbred female CD1 mice were evaluated quarterly by the Research Animal Diagnostic Laboratory (University of Missouri–Columbia) for serologic antibody responses to mouse hepatitis virus, mouse parvovirus, mouse minute virus, Sendai virus, Theiler mouse encephalitis virus, murine rotavirus, and Mycoplasma pulmonis. Outbred female Sprague–Dawley rats similarly were evaluated quarterly (Research Animal Diagnostic Laboratory) for serologic antibody responses to Sendai virus, Mycoplasma pulmonis, rat coronavirus, and rat parvovirus. Sentinels were also evaluated quarterly for ectoparasites and endoparasites. A review of sentinel results for the last 5 y did not reveal seroconversion or positive results to any of the above pathogens. A single PCR evaluation of feces for Helicobacter species by the Research Animal Diagnostic Laboratory revealed that 2 of 4 samples were positive for Helicobacter rodentium.
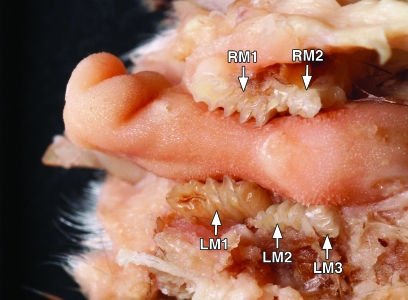
Five adult voles (age, 9 to 18 mo; 2 males and 3 females; mean weight, 16.9 g) were reported to the attending veterinarian for poor body condition and apparent weight loss. On examination, the animals' condition was determined to warrant euthanasia. Animals were euthanized by carbon dioxide inhalation and underwent gross necropsy. Postmortem examination of the oral cavity revealed grossly elongated molars in all 5 animals. To prepare them for detailed examination, 2 skulls were soaked in isopropanol for 24 h and then in 2% bleach for 24 h before tissues were removed gently by using dry gauze. Examination of these skulls revealed multiple mandibular and maxillary molars with abnormal wear of occlusal surfaces and growth of those surfaces in multiple directions (Figures 1 through 4). These molars often penetrated into the neighboring buccal mucosa. In addition, 1 vole had purulent discharge from a maxillary abscess caused by an extended mandibular molar (image unavailable). In another case, the right caudal mandibular molar penetrated caudally toward the tongue (Figure 4). In the remaining vole, inward growth of the mandibular molars led to entrapment of the tongue (Figure 5). Diagnoses of molar malocclusion were based on these findings from gross necropsy and comparison with the literature.10 Of the 5 animals in the colony identified at necropsy to have molar malocclusions, 4 had mandibular molar defects, 3 had maxillary defects, and 1 (Figure 5) demonstrated tongue entrapment by mandibular molars. Necropsy revealed no other abnormalities.
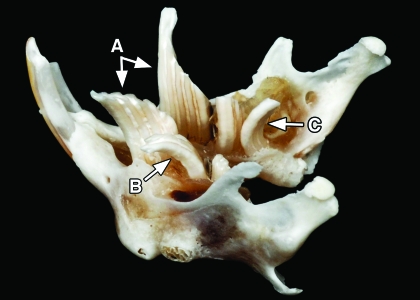
Figure 1.
Photograph of a skeletal mandible from a pine vole. Note (A) the grossly elongated left and right M1 molars, (B) an elongated, laterally-projecting left M2 molar, and (C) a caudally projecting elongated right M3 molar.
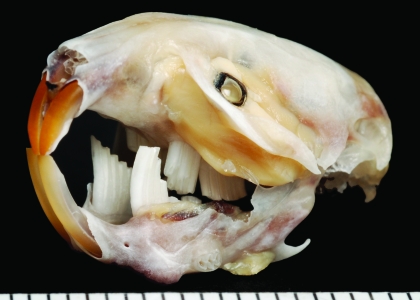
Figure 2.
Photograph of a vole skull with molar malocclusion.
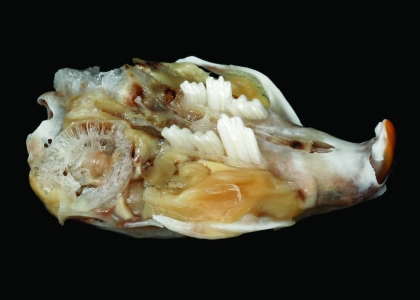
Figure 3.
Photograph of the maxillary region of the skull from Figure 2. In this oblique view, note the elongated maxillary molars.
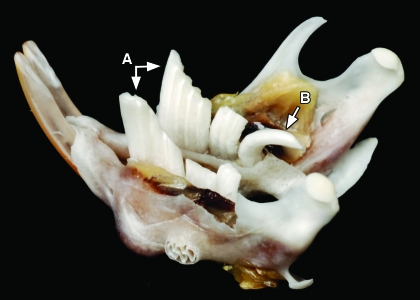
Figure 4.
Photograph of the mandible from Figure 2, showing (A) the grossly elongated left and right M1 mandibular molars and (B) a caudally projecting elongated right M3 mandibular molar.
Figure 5.
Formalin-fixed tissue specimen of the mandible of a pine vole, showing entrapment of the tongue due to inward growth patterns of the mandibular molars. The right M3 molar is missing from this specimen.
Discussion
The dental formula of voles, 2 × (I 1/1, C 0/0, P 0/0, M 3/3), is identical to that of rats and mice. Unlike those of rats and mice, the molars of voles of the genus Microtus grow continuously (that is, hypsodont). Hypsodont teeth frequently are described as being ‘high-crowned’12 or ‘long-crowned’.5 By definition, the term hypsodont refers to a tooth in which a section of the crown is subgingival (reserved crown): as the exposed crown wears down, additional submerged crown erupts from the gingiva.31 Aradicular hypsodont teeth, also known as ‘open-rooted’ or ‘continually growing’ teeth, continue to grow and produce new reserved crown throughout life.31 In contrast, in radicular hypsodont teeth, also known as ‘closed-rooted’ or ‘continually erupting’ teeth, new crown continues to erupt for most of the animal's life, but the root apices eventually do close and cease growth of new crown material.31 We will follow this terminology, but others have used slightly different terminology, in which teeth that grow continuously are termed ‘hypselodont,’ whereas the term ‘hypsodont’ refers to long-crowned teeth that erupt, but do not grow, continuously.12
The incisors of lagomorphs and rodents are aradicular hypsodont.5 Lagomorphs and hystricomorph (‘porcupine-like’) rodents such as guinea pigs and chinchillas also have aradicular hypsodont premolars and molars.5 Similarly, although they are myomorph (‘mouse-like’) rodents, which typically have nonhypsodont cheek teeth, Microtus rodents have aradicular hypsodont molars.
The molars of Microtus voles maintain persistent proliferative roots throughout life, and adult molar morphology resembles the embryonic tooth roots of nonhypsodont mammals.21 These continuously growing teeth require normal mastication on occlusal surfaces to maintain appropriate wear. Some voles that are not of the genus Microtus, such as of the group or clade Clethrionomys, lack aradicular hypsodont molars.23 That is, voles of the group Myodes (synonymous with Clethrionomys) have rooted molars, whereas the molars of Microtus voles are open-rooted.20
Dental malocclusions are a common clinical problem in laboratory rabbits and rodents. Traumatic malocclusion occurs when the opposing counterpart of a tooth has been lost or damaged, thus preventing normal wear and allowing overgrowth of the tooth.31 Causes of atraumatic malocclusions include genetic malpositioning and diet.31 Inadequate dietary roughage may prevent adequate tooth wear. Incisor malocclusions often result from improper angulation and occlusal wear patterns.11,14,22 Mandibular prognathism, maxillary brachygnathism, and abnormal or decreased molar wear also cause molar malocclusions and associated dental disease in rabbits.4,22 In addition, abnormal wear generally is considered the cause of molar malocclusions in guinea pigs.4 Clinical consequences of malocclusion include weight loss from reduced feed consumption due to difficult prehension or mastication or both.
Treatment of dental malocclusion in laboratory rabbits and rodents typically requires trimming of the affected teeth (sometimes under anesthesia) with appropriate equipment (often specialized dental units for larger rodent species) to achieve normal prehension and occlusion.16 Correction of premolar and molar malocclusions is more complicated than that of incisor malocclusion due to limited access to the cheek teeth, requiring anesthesia for adequate visualization to ‘float’ the teeth, as is done for horses.16 Because of their small body size and oral cavity, molar malocclusion in voles is even more difficult to identify than that in larger species. A small oral cavity complicates in vivo oral examination.
The hypsodont characteristics of Microtus molars have been recognized and studied by evolutionary biologists for many years.1,6,7,15,21,22,26 Unlike other rodents, the Microtus lineage developed hypsodont molars in the Pliocene and Pliestocene eras.15 In addition, comparison of rodent dental morphology has revealed marked differences in the molar cusp patterns of vole and mouse lineages, providing taxonomic information.13 In particular, the field vole (M. agrestis) has been used as a model for molar morphogenesis studies.24,32 However, reports of dental abnormalities in microtines are few.19,29 In 1 case, a bilaterally maloccluded maxillary molar in a Japanese field vole (M. montebellii) grew to penetrate the cranial cavity and resulted in brain deformity.29 In addition, several free-ranging long-tailed voles (M. longicaudus) with molar and incisor malocclusion have been reported.19 Although periodontitis and malocclusion have been diagnosed in M. montanus and M. ochrogaster,8 no dental abnormalities have previously been reported for M. pinetorum. Consequently, we believe this article is the first report of any dental abnormality in the pine vole.
Standard health assessment of Microtus vole colonies should include observation for molar malocclusion and other dental abnormalities potentially signaled by weight loss or decreased food ingestion. Husbandry practices can be used to minimize clinical problems due to tooth overgrowth. The diet of free-ranging pine voles is sufficiently abrasive to grind the occlusal surfaces, helping to prevent tooth overgrowth.2 Pine voles have been known to eat the roots and bark of orchard trees to the extent that the resulting damage to the trees caused economic consequences.25 A review of husbandry practices for this pine vole colony resulted in changes designed to improve the enrichment program, including adding autoclaved hardwood sticks (length, 3 to 4 cm; diameter, 1 to 3 cm; apple, pecan, or maple, depending on availability) to each cage. Obvious preferences for 1 type of wood over another were not observed. Nonbreeding voles also were given additional pellets for increased roughage and improved molar occlusal wear. No health effects or obvious changes in body condition or breeding success due to the increased fiber intake from gnawing on hardwood sticks were noted.
After these husbandry changes, no cases of molar malocclusions occurred in this colony for at least 3 y, even after the colony was moved to a different institution (finding confirmed by the recipient institution's attending veterinarian). In conclusion, this report documents molar malocclusions with medical consequences in laboratory pine voles. This clinical colony problem ultimately was resolved by adding roughage in the form of hardwood sticks as enrichment to improve molar occlusal wear.
Acknowledgments
The authors gratefully acknowledge the contributions of Mr Chris Herron (Educational Resources, College of Veterinary Medicine, University of Georgia, Athens, GA) for expertise in photography. The authors also acknowledge Dr John Vandenbergh (College of Agriculture and Life Sciences, North Carolina State University, Raleigh, NC) for his expertise with this model and the staff of the Biological Resources Facility (College of Agriculture and Life Sciences, North Carolina State University) for the dedicated care they provide to research animals.
References
- 1.Abbazzi L, Masini F, Torre D. 1993. Evolutionary patterns in the first lower molar of the endemic murid microtia. Quat Int 19:63–70 [Google Scholar]
- 2.Boulton IC, Cooke JA, Johnson MS. 1997. Fluoride-induced lesions in the teeth of the short-tailed field vole (Microtus agrestis): a description of the dental pathology. J Morphol 232:155–167 [DOI] [PubMed] [Google Scholar]
- 3.Brant CL, Schwab TM, Vandenbergh JG, Schaefer RL, Solomon NG. 1998. Behavioural suppression of female pine voles after replacement of the breeding male. Anim Behav 55:615–627 [DOI] [PubMed] [Google Scholar]
- 4.Capello V. 2005. Dental diseases, Capello V, Gracis M, Lennox AM. Rabbit and rodent dentistry. Lake Worth (FL): Zoological Education Network [Google Scholar]
- 5.Capello V, Gracis M. 2005. Anatomy of the skull and teeth, Capello V, Gracis M, Lennox AM. Rabbit and rodent dentistry. Lake Worth (FL): Zoological Education Network [Google Scholar]
- 6.Chaline J, Graf J-D. 1988. Phylogeny of the Arvicolidae (Rodentia): biochemical and paleontological evidence. J Mammol. 69:22–33 [Google Scholar]
- 7.Chaline J, Laurin B, Brunet-Lecomte P, Viriot L. 1993. Morphological trends and rates of evolution in arvicolids (Arvicolidae, Rodentia): towards a punctuated equilibria–disequilibria model. Quat Int 19:27–39 [Google Scholar]
- 8.Donnelly TM, Quimby FW. 2002. Biology and diseases of other rodents,Fox JG, Anderson LC, Lowe FM, Quimby FW. Laboratory animal medicine, 2nd ed.San Diego (CA): Academic Press [Google Scholar]
- 9.Engell MD, Godwin J, Young LJ, Vandenbergh JG. 2006. Perinatal exposure to endocrine disrupting compounds alters behavior and brain in the female pine vole. Neurotoxicol Teratol 28:103–110 [DOI] [PubMed] [Google Scholar]
- 10.Gromov IM, Polyakov IY. 1992, Voles (Microtinae). New Delhi (India): Oxonian Press [Google Scholar]
- 11.Jackson Laboratories [Internet] 2003. Malocclusion in the laboratory mouse. [Cited 28 Oct 2008] Available at http://jaxmice.jax.org/jaxnotes/archive/489h.html
- 12.Janis CM, Fortelius M. 1988. On the means whereby mammals achieve increased functional durability of their dentitions, with special reference to limiting factors. Biol Rev Camb Philos Soc 63:197–230 [DOI] [PubMed] [Google Scholar]
- 13.Jernvall J, Keränen SVE, Thesleff I. 2000. Evolutionary modification of development in mammalian teeth: quantifying gene expression patterns and topography. Proc Natl Acad Sci USA 97:14444–14448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Lee JY, Han TS, Han KB, Kang SS, Bae CS, Choi SH. 2005. A case of maloccluded incisor teeth in a beaver (Castor canadensis). J Vet Sci 6:173–175 [PubMed] [Google Scholar]
- 15.Koenigswald WV. 1993. Heterochronies in morphology and schmelzmuster of hypsodont molars in the muroidea (Rodentia). Quat Int 19:57–61 [Google Scholar]
- 16.Legendre LF. 2002. Malocclusions in guinea pigs, chinchillas, and rabbits. Can Vet J 43:385–390 [PMC free article] [PubMed] [Google Scholar]
- 17.Lepri JJ, Vandenbergh JG. 1986. Puberty in pine voles (Microtus pinetorum) and the influence of chemosignals on female reproduction. Biol Reprod 34:370–377 [DOI] [PubMed] [Google Scholar]
- 18.Lim MM, Tsivkovskaia NO, Bai Y, Young LJ, Ryabinin AE. 2006. Distribution of corticotropin-releasing factor and urocortin 1 in the vole brain. Brain Behav Evol 68:229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maser C, Hooven EF. 1970. Dental abnormalities in Microtus longicaudus. The Murrelet 51:11 [Google Scholar]
- 20.Musser GG, Carleton MD. 2005. Order Rodentia, superfamily Muroidea, Wilson DE, Reeder DM. Mammal species of the world: a taxonomic and geographic reference, vol 2. Baltimore (MD): The Johns Hopkins University Press [Google Scholar]
- 21.Oxberry BA. 1975. An anatomical, histochemical, and autoradiographic study of the ever-growing molar dentition of Microtus with comments on the role of structure in growth and eruption. J Morphol 147:337–354 [DOI] [PubMed] [Google Scholar]
- 22.Percy DH, Barthold SW. 2007. Pathology of laboratory rodents and rabbits, p 102–303 Ames (IA): Blackwell Publishing [Google Scholar]
- 23.Phillips CJ, Oxberry B. 1972. Comparative histology of molar dentitions of Microtus and Clerithromys, with comments on dental evolution in microtine rodents. J Mammal 53:1–21 [PubMed] [Google Scholar]
- 24.Setkova J, Lesot H, Malatova E, Witter K, Matulova P, Misek I. 2006. Proliferation and apoptosis in early molar morphogenesis—voles as models in odontogenesis. Int J Dev Biol 50:481–489 [DOI] [PubMed] [Google Scholar]
- 25.Smolen MJ. 1981. Microtus pinetorum. Mamm Species 147:1–7 [Google Scholar]
- 26.Smolen MJ, Keller BL. 1987. Microtus longicaudus. Mamm Species 271:1–7 [Google Scholar]
- 27.Solomon NG, Vandenberg JG. 1994. Management, breeding, and reproductive performance of pine voles. Lab Anim Sci 44:613–617 [PubMed] [Google Scholar]
- 28.Solomon NG, Vandenbergh JG, Wekesa KS, Barghusen L. 1996. Chemical cues are necessary but insufficient for reproductive activation of female pine vole (Microtus pinetorum). Biol Reprod 54:1038–1045 [DOI] [PubMed] [Google Scholar]
- 29.Sugita S, Uchiumi O, Fujiwara K, Niida S, Fukuta K. 1995. [Brain deformation caused by hyperplasia molar teeth (macrodonts) in the Japanese field vole (Microtus montebellli)] Article in Japanese. Exp Anim 43:769–772 [DOI] [PubMed] [Google Scholar]
- 30.Wekesa KS, Vandenbergh JG. 1996. Androgen exposure and reproductive behavior of an induced ovulator, the pine vole (Microtus pinetorum). Horm Behav 30:416–423 [DOI] [PubMed] [Google Scholar]
- 31.Wiggs RB, Lobprise HB. 1997. Oral anatomy and physiology, Wiggs RB, Lobprise HB. Veterinary dentistry principles and practice. Philadelphia (PA): Lippincott–Raven Publishers [Google Scholar]
- 32.Witter K, Lesot H, Peterka M, Vonesch J-L, Misek I, Peterkova R. 2005. Origin and developmental fate of vestigial tooth primordial in the upper diastema of the field vole (Microtus agrestis, Rodentia). Arch Oral Biol 50:401–409 [DOI] [PubMed] [Google Scholar]