Abstract
TNFα and TRAIL, two members of the tumor necrosis factor family, share many common signalling pathways to induce apoptosis. Although many cancer cells are sensitive to these proapoptotic agents, some develop resistance. Recently we have demonstrated that up-regulation of c-Fos/AP-1 is necessary, but insufficient for cancer cells to undergo TRAIL-induced apoptosis. Here we present a prostate cancer model with differential sensitivity to TNFα and TRAIL. We show that inhibition of NF-κB or activation of AP-1 can only partially sensitize resistant prostate cancer cells to proapoptotic effects of TNFα or TRAIL. Inhibition of NF-κB by silencing TRAF2, by silencing RIP or by ectopic expression of IκB partially sensitized resistant prostate cancer. Similarly, activation of c-Fos/AP-1 only partially sensitized resistant cancer cells to proapoptotic effects of TNFα or TRAIL. However, concomitant repression of NF-κB and activation of c-Fos/AP-1 significantly enhanced the proapoptotic effects of TNFα and TRAIL in resistant prostate cancer cells. Therefore, multiple molecular pathways may need to be modified, in order to overcome cancers that are resistant to proapoptotic therapies.
Keywords: Apoptosis, TRAIL, TNF-α, Mechanisms of resistance
INTRODUCTION
TNF (tumor necrosis factor) family members regulate a variety of biological processes such as cell development, differentiation, tumorigenesis, cell proliferation, cell survival and/or apoptosis 1. Among TNF members, Tumor Necrosis Factor α (TNFα) and Tumor Necrosis Factor-related Apoptosis Ligand (TRAIL) are two cytokines that possess strong anti-tumor activity. Both TNFα and TRAIL are capable of inducing cell death in cancer cells. However, TNFα is associated with significant cytotoxicity, which limits its clinical utility 1. In contrast, TRAIL promotes apoptosis in cancer cells with limited damage to normal cells, therefore, it is associated with minimal cytotoxicity – making TRAIL an ideal anti-cancer agent from the TNF family members 2, 3. Although many cancers are sensitive to TNFα and TRAIL-induced apoptosis, some develop resistance.
Both TNFα and TRAIL induce apoptosis through activating specific receptors. TNFα activates TNF receptor 1 (TNFR1) 4 and TNF receptor 2 (TNFR2) 5, while TRAIL activates DR4 (TRAIL-R1), DR5 (TRAIL-R2) and three other decoy receptors 6. Although TNFα- and TRAIL-induced apoptosis share many common intracellular pathways, there are some distinguishing differences between the two. TRAIL interacts with specific death domain receptors, DR4 and DR5, to rapidly induce intracellular cytoplasmic formation of the DISC (Death Inducing Signaling Complex) 3, 7, 8. DISC formation may involve the recruitment of caspase-8, FADD, TRADD, TRAFs, and RIP to the death domain of the activated receptor in order to induce the extrinsic apoptosis pathway 9.
In contrast to DISC formation by TRAIL-induced apoptosis, DISC formation induced by TNFα involves two sequential signaling complexes 10. Complex I consists of TNFR1, TRAF2, RIP and the adaptor TRADD, and it may rapidly activate NF-κB, thereafter increasing the expression of the anti-apoptotic molecule, c-FLIP, a homologous and competitive inhibitor of caspases 8/10 10. In a second step, caspase 8/10 and FADD can be recruited into the released complex I of TRAF2, RIP, TRADD and death domain, and assemble complex II. Complex II transduces signals of cell death when complex I fails to activate NF-κB.
NF-κB is a transcription factor that regulates death domain mediated apoptosis 11. NF-κB subunits, RelA/p65, cRel, RelB, NF-κB1/p50 and NF-κB2/p52, can form homodimeric or heterodimeric complexes. NF-κB is sequestered in the cytoplasm by its specific inhibitor IκB. IκB can be phosphorylated by IκB kinase (IKK) and quickly degraded via proteasome-mediated pathway, resulting in the rapid nuclear translocation of NF-κB and activation of NF-κB. Therefore, proteasome inhibitors such as MG132 can inhibit NF-κB activity by suppressing the degradation of IκB 12. NF-κB and its important modulators, TRAF2 and RIP, in TNFα-induced apoptosis have been well characterized 13. However, the effects of NF-κB on TRAIL signaling remain controversial -- while some reports suggest that NF-κB activation protects cells from TRAIL-induced apoptosis 14–16, others suggest that NF-κB may promote apoptosis 17. These discrepancies indicate that the role of NF-κB in TRAIL-induced apoptosis is unclear, and other important pathways may need to be considered when evaluating the true NF-κB function in regulating TRAIL-induced apoptosis.
Previously, we have found that TRAIL-induced apoptosis can be regulated by c-Fos 18, 19, a member of the AP-1 transcriptional factors 20. We have found that c-Fos, has a novel proapoptotic function in TRAIL-induced apoptosis in addition to its well-known oncogenic function. We have demonstrated that up-regulation of c-Fos/AP-1 is necessary, but insufficient for cancer cells to undergo TRAIL-induced apoptosis 19.
In the present study we identified a prostate cancer cell model with differential sensitivity to TNFα- and TRAIL-induced apoptosis. We demonstrate that in order for prostate cancer cells to be sensitive to TNFα or TRAIL, cancer cells reduce NF-κB activity and/or increase AP-1 activity. In resistant cancer cells, inhibition of NF-κB alone or activation of AP-1 alone can only partially sensitize cancer cells to TNFα or TRAIL. However, concomitant inhibition of NF-κB and activation of AP-1 significantly sensitizes prostate cancer cells to TRAIL- or TNFα-induced apoptosis. Therefore, multiple molecular pathways may need to be modified, in order to overcome cancers that are resistant to proapoptotic therapies.
MATERIALS AND METHODS
Materials
Recombinant human TRAIL/TNFSF10 and TNF-α/TNFSF1A were obtained from R&D System Inc. (Minneapolis, MN). Antibodies to RIP and c-Fos, Horseradish peroxidase-conjugated secondary antibodies (goat-anti-mouse, goat-anti-rabbit, goat-anti-rat antibodies) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies to TRAF2 and GAPDH were obtained from Abcam (Cambridge, MA). Antibodies to IκB and cleaved PARP were obtained from Cell signaling Technology, Inc. (Danvers, MA).
Cell Culture Material, Cell viability, Western Blot assays and Apoptosis
PC3 and LNCaP were obtained from the American Type Culture Collection (ATCC) (Manassas, VA). PC3-TR is a TRAIL resistant subline of PC3 cells that was derived in our laboratory 21. Cells were grown in RPMI 1640 medium supplemented with 2 mmol/L L-glutamine, 10% FBS and 5% penicillin at 37°C with 5% CO2. Cell viability was determined by MTT method (Roche Diagnostics, Indianapolis, IN) and Western Blot assays were carried out as previously described 21.
Flow cytometry was used to assess the sub-G1 DNA population of cells undergoing apoptosis. Cells were harvested, washed twice with cold PBS, and fixed with cold 70% ethanol for at least 1 hour at 4°C. Cells were washed twice with DNA extraction buffer (192 mM Na2HPO4, 4 mM citric acid, pH 7.8). Cells were incubated with propidium iodide (PI; 50 µg/ml, Molecular Probes, Eugene, OR) and RNase A at room temperature for 30 minutes before analyzed by flow cytometry. All results were from at least triplicate experiments.
Transfection of Plasmids and siRNA and Luciferase Assay
pNF-κB luciferase (25 ng/well) and pAP-1 luciferase reporter plasmids (25 ng/well) were purchased from Stratagene (La Jolla, CA). Renilla Luciferase Reporter was purchased from Promega (Madison, WI). TRAF2-specific siRNA and RIP-specific siRNA were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Negative control siRNA was purchased from Qiagen (Valencia, CA). pCMV-IκB-M plasmid was purchased from BD Biosciences (Franklin Lakes, NJ). Full length human c-Fos cDNA was provided by Dr. L Shemshedini, University of Toledo, OH 22. The plasmids and siRNA were transfected as previously described 18, 19, 21.
Luciferase Assay
Cells were seeded into 24-well plates. When the cells were 80% confluent, both AP-1 luciferase reporter (25 ng/well) and Ranilla reporter (5 ng/well) from Stratagene (La Jolla, CA) or NF-κB reporter (25 ng/well) and Ranilla reporter from Stratagene (La Jolla, CA) were co-transfected into cells. Here, Ranilla served as an internal control for transfection efficiency. After 24 hours of transfection, cells were treated with TRAIL (100 ng/ml) or TNFα (100 ng/ml) for 4 hours, and then both attached and floating cells were collected, prepared and further detected by using Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Samples were stored at −20°C until detection. All results represent average of at least three independent experiments ± SD.
Cell Extracts and Electrophoretic Mobility Shift Assay (EMSA)
Frozen cell pellets were resuspended in 4 volume of lysis buffer: 20 mM HEPES (pH 7.9), 0.2 mM EDTA, 0.2 mM EGTA, 10% glycerol, 10 mM Na molybdate, 2 mM Na pyrophosphate, 2 mM Na orthovanadate, 0.5 mM spermidine, 0.15 mM spermine, 50 µM TPCK, 25 µM TLCK, 1 µg/mL each of aprotinin, pepstatin A, and leupeptin, 0.5 mM benzamidine, 1 mM DTT, and 0.5 mM PMSF. KCl was added to 400 mM final, and the extracts were incubated at 4°C for 30 min and centrifuged at 10,000 g for 5 min. The supernatant contained the whole cell extracts. The reactions were made using 3 µl of whole cell extract and 0.1–0.5 ng of 32P-labeled double-stranded specific oligonucleotides (5,000–25,000 cpm) and run on 5–7% polyacrylamide gels containing 0.5x Tris glycine EDTA. Gels were dried with Bio-Rad gel dryer (Hercules, CA) and imaged using Kodak BioMax MR Film (Fisher Scientific, Atlanta, GA). General AP-1 gel shift oligonucleotide was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The reaction containing 90% non-labeled and 10% 32P-labeled oligonucleotides prober was used as control.
RESULTS
Prostate cancer cell lines have differential sensitivity to TRAIL- and TNFα-induced apoptosis
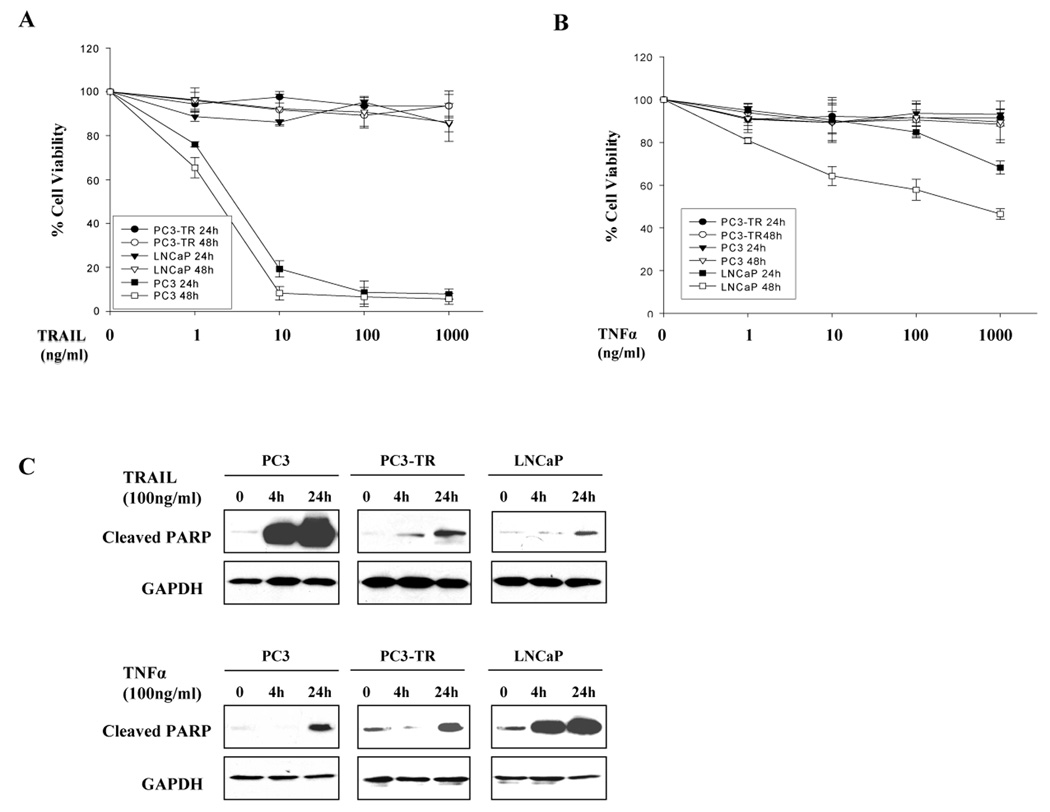
To evaluate the sensitivity of prostate cancer cells to proapoptotic agents, LNCaP, PC3, and PC3-TR cells, a subline of PC3 cells which is resistant to TRAIL treatment 21, were treated with TRAIL or TNFα in time-dependent and dose-dependent experiments. We found that PC3 cells were very sensitive to TRAIL, whereas, LNCaP and PC3-TR cells were resistant to TRAIL-induced apoptosis even with long exposures of 24, 48, and 72 hours (Fig. 1A and data not shown). In contrast, LNCaP cells were sensitive to TNFα in a dose- and time-dependent manner while PC3 and PC3-TR cells were resistant at 24, 48, and 72 hour exposures (Fig. 1B and data not shown). The results indicate that PC3 cells are sensitive to TRAIL- but resistant to TNFα-induced apoptosis. In contrast, LNCaP cells are sensitive to TNFα-induced apoptosis but resistant to TRAIL, while PC3-TR cells are resistant to both TRAIL- and TNFα-induced apoptosis. As a marker of apoptosis, cleaved PARP products was used to detect differential response of these cells to TRAIL or TNFα-induced apoptosis. Cleaved PARP was dramatically increased in TRAIL-sensitive PC3 cells or TNF-α sensitive LNCaP cells after treatment for 4 and 24 hours. However, cleaved PARP was only slightly increased in TRAIL-resistant or TNFα-resistant cells (Fig. 1C). These findings provide an in vitro prostate cancer model with differential sensitivity to TRAIL and TNFα, and enable us to investigate common and differential proapoptotic pathways for TRAIL- and TNFα-induced apoptosis.
Figure 1.
Prostate cancer cell lines PC3, PC3-TR and LNCaP have differential sensitivity to TRAIL and TNFα. PC3, PC3-TR and LNCaP cells were treated with TRAIL (A) or TNFα(B) with increasing concentrations and different time-course. Cell viability was measured by the MTT method at 24 and 48 hours after treatment. Error bars (SD) represent results of at least three independent experiments. (C) PARP cleavage product protein levels in PC3, PC3-TR and LNCaP cells after treatment with TRAIL (100 ng/ml) or TNFα (100 ng/ml) for 4 and 24 hours.
Resistance of prostate cancer cells to TRAIL or TNFα correlate with increased NF-κB and decreased AP-1 activities
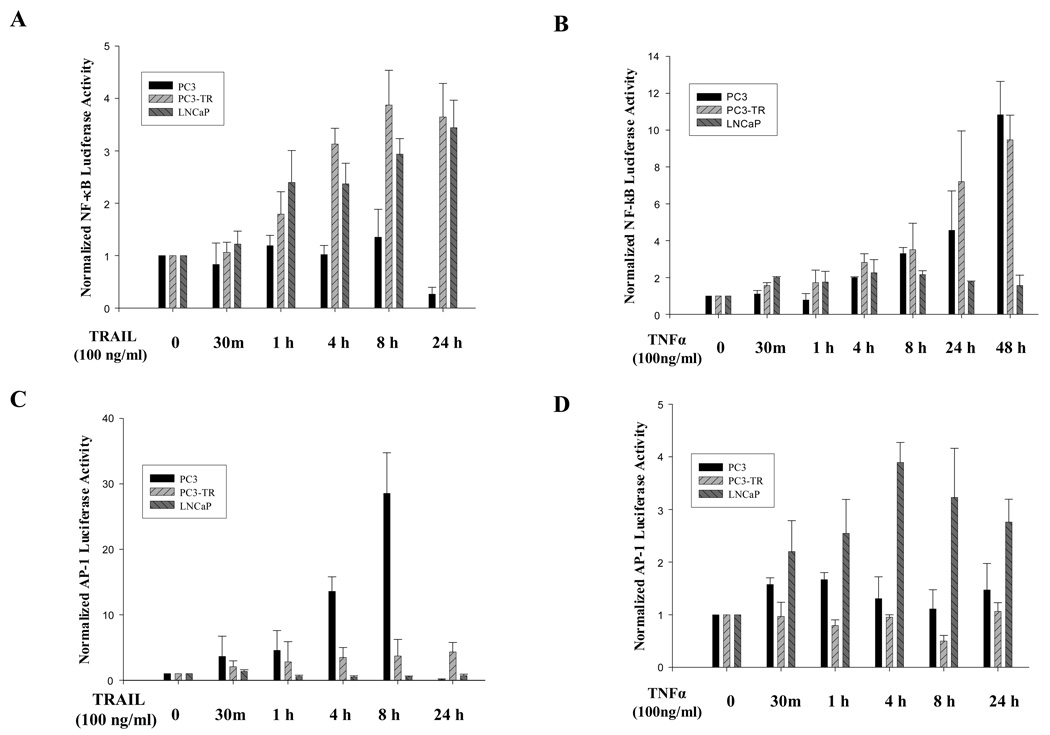
Since NF-κB is a key regulator of TNFα-induced apoptosis 23, and we have recently demonstrated that c-Fos/AP-1 activity is necessary, but insufficient for cancer cells to undergo TRAIL-induced apoptosis 18, 19, we wished to evaluate the role of both NF-κB and AP-1 activities in mediating TRAIL-induced and TNFα-induced apoptosis in prostate cancer cells. We found that NF-κB activity increased at one hour after TRAIL treatment in PC3-TR and LNCaP cells, which are both resistant to TRAIL-induced apoptosis. At 24hr after TRAIL treatment, the NF-κB activity was nearly 4 fold higher compared to the basal levels in the TRAIL-resistant cells. In contrast, in the TRAIL-sensitive PC3 cells NF-κB activity maintained at the same level and then decreased to 30% of basal level after 24 hours of TRAIL-treatment because most cells died at 24 hour treatment (Fig. 2A).
Figure 2.
Alterations in NF-κB and AP-1 activities correlate with sensitivity to TRAIL and TNFα in prostate cancer cells. NF-κB (A & B) and AP-1 (C & D) luciferase activities in PC3, PC3-TR and LNCaP cells in response to TRAIL (100 ng/ml) (A & C) and TNFα (100 ng/ml) (B & D). Error bars (SD) represent results of at least three independent experiments.
In a similar fashion, NF-κB activity in TNFα-resistant cell lines, PC3 and PC3-TR, increased as soon as 30 minutes after TNFα treatment and reached 10 folds higher than basal level at 48 hours after TNFα treatment. In contrast, NF-κB activity in LNCaP cells, which are sensitive to TNFα-induced apoptosis and have very low basal NF-κB activity 24, 25, was maintained at the same level even at 48 hours after treatment (Fig. 2B).
Given our prior findings that the AP-1 family members play a critical role in regulating TRAIL-induced apoptosis in prostate cancer cells 18, 19, we wished to evaluate the role of AP-1 family members in regulating both TRAIL-induced and TNFα-induced apoptosis. Luciferase reporter assay for AP-1 activity demonstrated that the AP-1 activity was dramatically increased after treatment with TRAIL in TRAIL-sensitive PC3 cells, but not in TRAIL-resistant PC3-TR and LNCaP cells, even though the baseline AP-1 activity was very low in LNCaP cells (Supplementary Data S1). However, at 24 hours after the treatment, the AP-1 activity was barely detectable in PC3 cells, mostly because majority of the PC3 cancer cells were dead at this time point (Fig. 2C).
AP-1 activity also increased in the TNF-α sensitive LNCaP cells after treatment with TNFα, but to a lesser degree than the TRAIL-sensitive prostate cancer cells after TRAIL treatment (Figs. 2C and 2D). Treatment with TNFα had no significant effect on AP-1 luciferase activity in the TNFα-resistant cells (PC3 and PC3-TR).
Table 1 summarizes our initial findings, which demonstrates that either down-regulation of NF-κB and/or upregulation of AP-1 activities are important components for cancer cells to be sensitive to TNFα-induced or TRAIL-induced apoptosis. Therefore, we postulate that decreased NF-κB activity and/or increased AP-1 activity may be important for sensitization of prostate cancer cells to TRAIL and TNFα treatments.
Table 1. Summary of changes of AP-1 and NF-κB activities and sensitivity to TRAIL and TNFα.
NF-κB and AP-1 activities in prostate cancer cells after either TRAIL or TNFα treatments.
| PC3 | PC3-TR | LNCaP | ||||
|---|---|---|---|---|---|---|
| TRAIL | TNFα | TRAIL | TNFα | TRAIL | TNFα | |
| NF-κB | ↓ | ↑ | ↑ | ↑ | ↑ | NC* |
| AP-1 | ↑ | NC | NC | NC | NC | ↑ |
| Sensitivity | S | R | R | R | R | S |
↑: Increased activity; ↓: decreased activity ; NC: no change; S: sensitive; R: resistant
Basal level is very low.
Silencing TRAF2 or RIP suppresses NF-κB activation and sensitize PC3 and PC3-TR cells to TNFα or TRAIL treatments
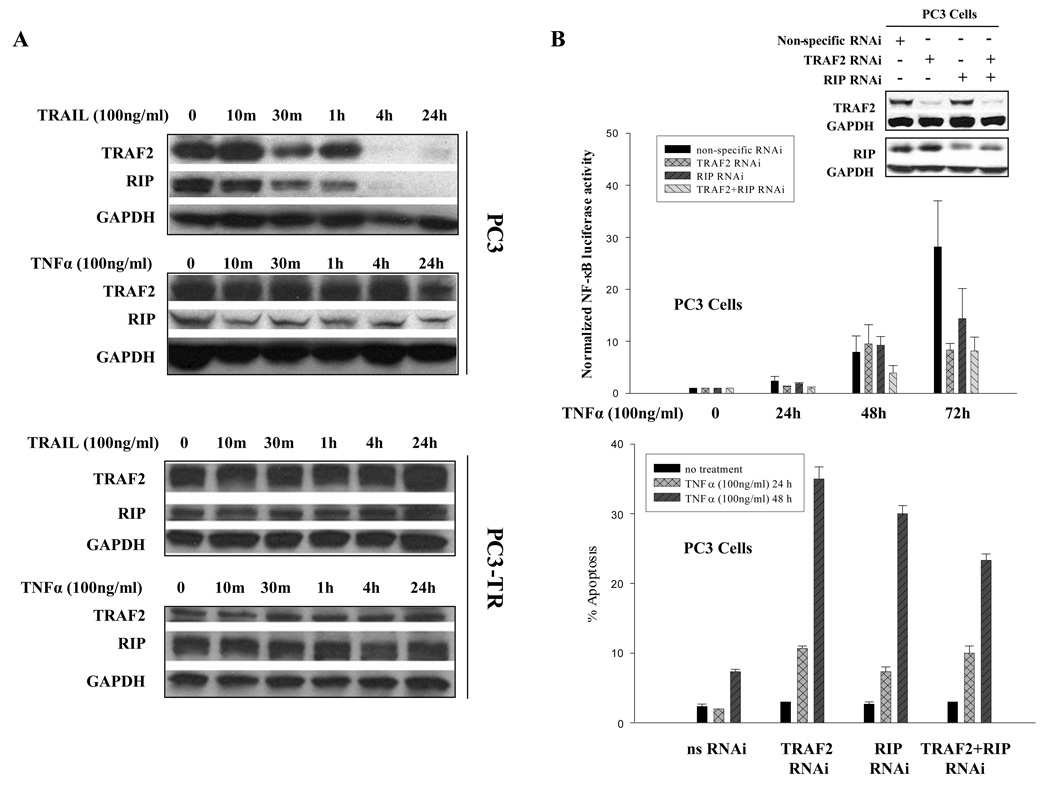
TRAF2 and RIP, two critical components of DISC 26, play a critical role in activation of NF-κB. Therefore, we evaluated the protein levels for TRAF2 and RIP in PC3 (sensitive to TRAIL but resistant to TNFα) and PC3-TR (resistant to both TRAIL and TNFα) cells. The PC3-TR cells are a subline of PC3 cells that were generated in our lab 21, and the two cell lines serve as a good model to investigate the molecular differences and similarities between TRAIL and TNFα sensitivity in cancer cells. We found that in PC3 cells, which are sensitive to TRAIL-induced apoptosis, the protein levels of both TRAF2 and RIP decreased and became undetectable at 4 hours after treatment with TRAIL. In contrast, the protein levels of TRAF2 and RIP were maintained in PC3 cells after treatment with TNFα, even after 24 hours of treatment (Fig. 3A). In PC3-TR cells, which are resistant to both TRAIL and TNFα, protein levels for TRAF2 and RIP were maintained after either TRAIL or TNFα treatments (Fig. 3A). We also observed modest reduction of TRAF2 and RIP in LNCaP cells (sensitive to TNFα and resistant to TRAIL) after TNFα treatment (data not shown). These results suggest that reduction of TRAF2 and RIP levels, two important NF-κB modulators, correlate with whether prostate cancer cells will undergo apoptosis after treatment with TRAIL and TNFα.
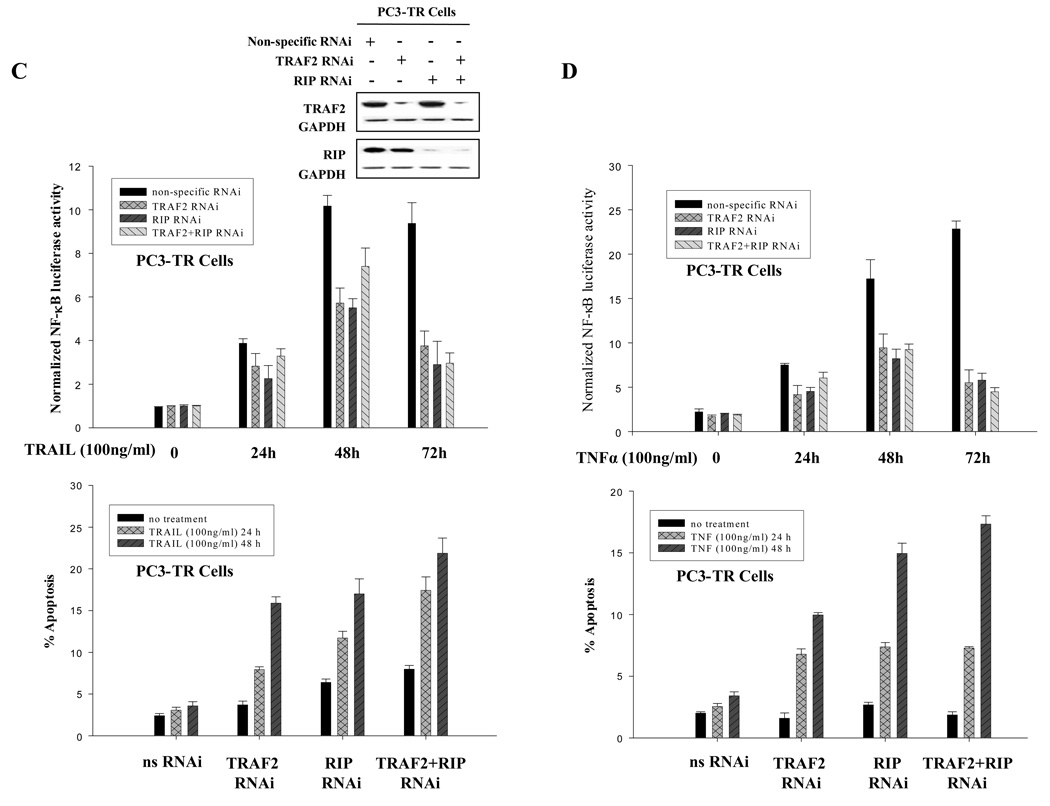
Figure 3.
TRAF2 and RIP as modulators of NF-κB and regulators of TRAIL- and TNFα-induced apoptosis. (A) Reduction of TRAF2 and RIP protein levels correlated with sensitivity to TRAIL- and TNFα-induced apoptosis. (B) Silencing TRAF2, RIP or both reduced NF-κB activity (top panel) and partially sensitized PC3 cells to TNFα (bottom panel). Western blots were performed with TRAF2- and RIP-specific antibodies to detect the efficiency of RNAi. Non-specific siRNA was used as negative control. GAPDH was used as loading control. (C) Silencing TRAF2, RIP or both reduced NF-κB activity (top panel) and partially sensitized PC3-TR cells to TRAIL (bottom panel). Western blots were performed to detect the efficiency of RNAi. (D) Silencing TRAF2, RIP or both reduced NF-κB activity (top panel) and partially sensitized PC3-TR cells to TNFα (bottom panel). Apoptosis is measured by flow cytometric analysis of the sub-G1 population. Error bars (SD) are results of at least three independent experiments.
In order to examine the effects of TRAF2 and RIP on NF-κB’s activity and the effect they may exert on prostate cancer cells, we silenced the expression of TRAF2 and RIP in PC3 and PC3-TR cells. As shown previously, NF-κB activity was induced in PC3 cells after treatment with TNFα, while silencing TRAF2 and/or RIP effectively suppressed NF-κB activation by 2–4 folds (Fig. 3B, top panel). Similarly in PC3-TR cells, the activation of NF-κB in response to TRAIL and TNFα treatment was suppressed by silencing TRAF2 or RIP expression (Fig. 3 C and D, top panel). Therefore, TRAF2 and RIP function as NF-κB activators in prostate cancer cells in response to TRAIL or TNFα treatments.
Next, we wished to determine whether suppression of NF-κB by silencing TRAF2 and/or RIP can affect the prostate cancer cells’ response to exposure to apoptotic agents. We found that both PC3 and PC3-TR cells were more prone to undergo apoptosis when treated with either TNFα or TRAIL (Figs. 3B, 3C and 3D, bottom panels). Silencing both TRAF2 and RIP did not have an additive effect in reducing the NF-κB activity or enhancing apoptosis, suggesting that TRAF2 and RIP function in series and have equivalent effects on NF-κB activity and sensitization of prostate cancer cells to undergo apoptosis.
IκB partially sensitizes resistant cancer cells to TRAIL and TNFα-induced apoptosis
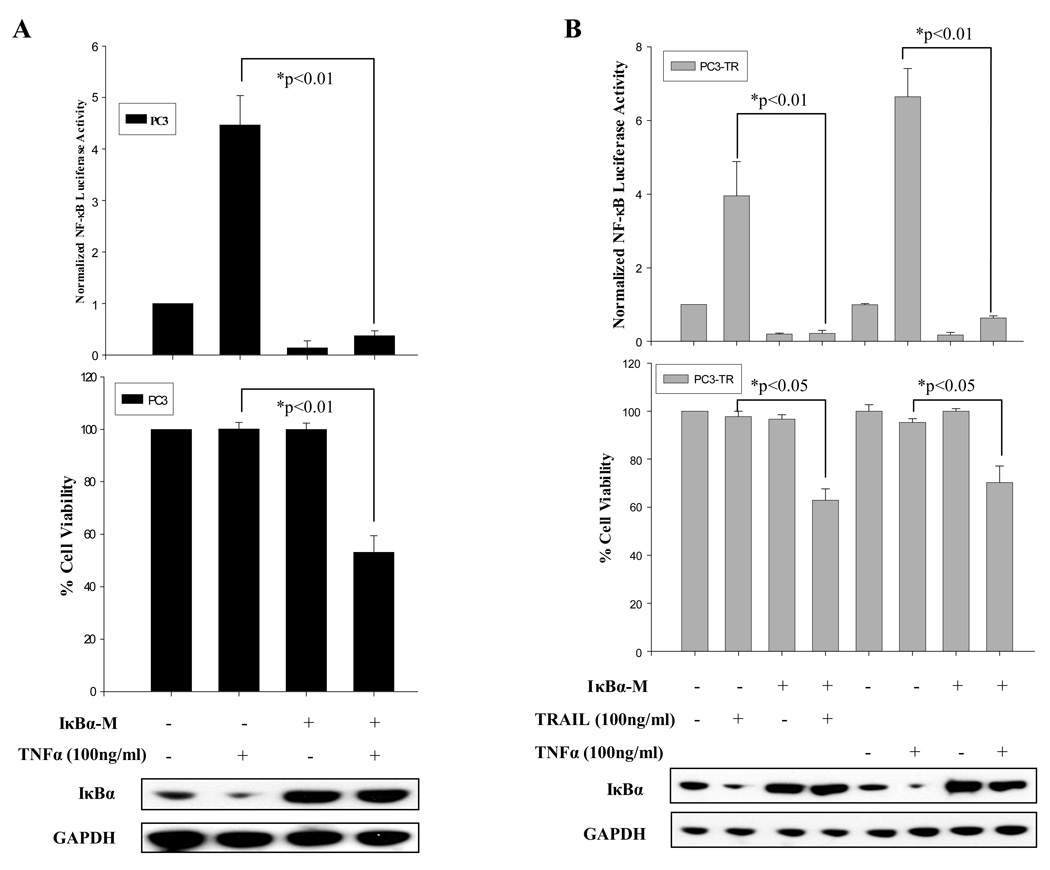
Since modulation of TRAF2 and RIP can affect NF-κB’s activation, and affect prostate cancer cells’ response to TNFα- and TRAIL-induced apoptosis (Fig. 3), here we wished to determine whether direct inhibition of NF-κB can modulate prostate cancer cells’ sensitivity to TNFα and TRAIL. To inhibit NF-κB, we ectopically expressed, IκB, an inhibitory subunit of NF-κB 11. pCMV-IκBα-M expresses IκBα with a point mutation which abrogates IκBα’s ability to be phosphorylated and subsequently degraded 27. After ectopic expression of pCMV-IκB (Fig. 4A and B – Western Blots), NF-κB’s activity was reduced in PC3 and PC3-TR cells and could not be activated when the cells were treated with TNFα or TRAIL (Fig. 4 A & B – upper panels). Subsequently, PC3 cells were treated with TNFα, and PC3-TR cells were treated with TRAIL or TNFα. The cells which expressed pCMV-IκBα-M were partially sensitized to TNFα (PC3 and PC3-TR cells) and TRAIL (PC3-TR cells) by demonstrating a lower percentage of viable cells (Fig. 4 A and B – lower panels). Therefore, although NF-κB is a key modulator of apoptosis in cancer cells 23, inhibition of NF-κB activity only partially sensitizes prostate cancer cells toward proapoptotic agents. Next, we wished to investigate other key regulators of TNFα-induced and TRAIL-induced apoptosis which may work in parallel with NF-κB’s activities.
Figure 4.
Inhibition of NF-κB activity by IκBα-M sensitizes prostate cancer cells to TRAIL- and TNFα-induced apoptosis. (A) NF-κB luciferase activity (top panel), cell viability (middle panel) of PC3 cells with ectopic expression of IκBα-M or control vector (Western blot -- bottom panel) with or without TNFα treatment for 24 hours. (B) NF-κB luciferase activity (top panel), cell viability (middle panel) of PC3-TR cells with ectopic expression of IκBα-M or control vector (Western blot -- bottom panel) with or without TRAIL or TNFα treatment for 24 hours. Transfection of empty vector was used as control (−). Cell viability was measured by MTT assay. Error bars (SD) are results of at least three independent experiments. “*” Refers to statistically significant differences between indicated groups.
Activation of AP-1 sensitizes resistant prostate cancer cells to TRAIL- and TNFα-induced apoptosis
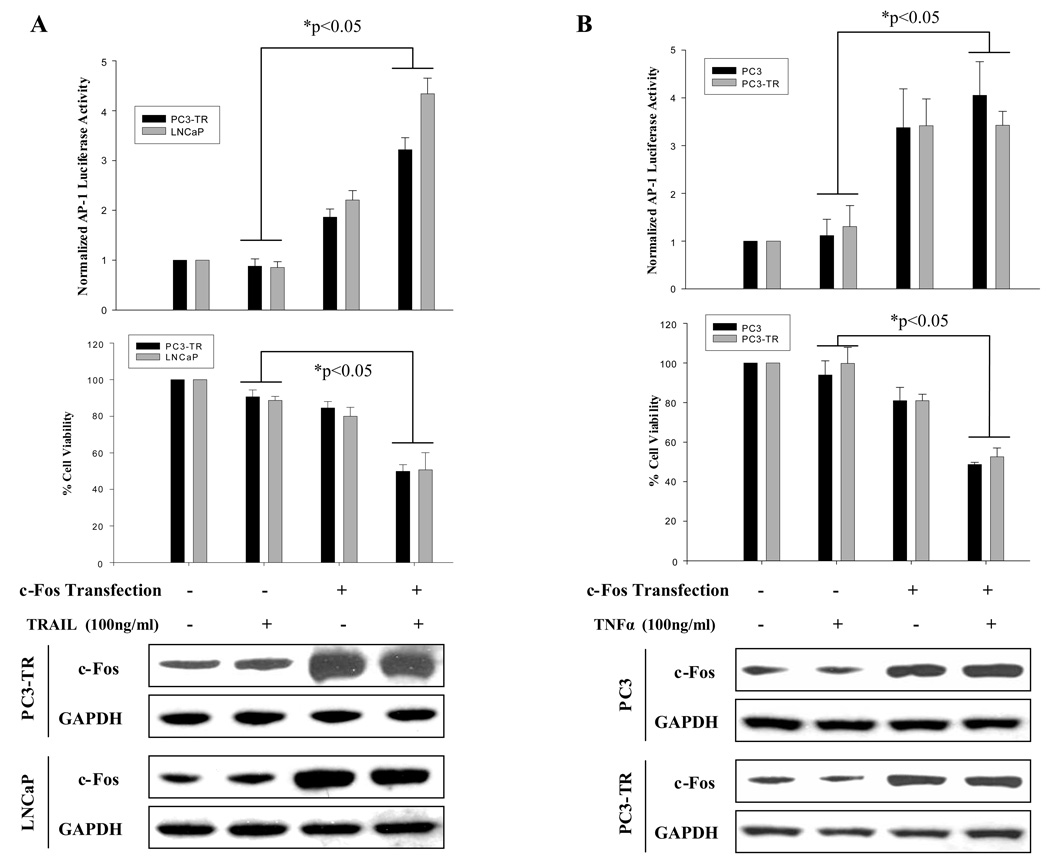
In addition to NF-κB’s role in regulating apoptosis in cancer cells, we have found that c-Fos/AP-1 represses the anti-apoptotic gene, c-FLIP(L), and primes prostate cancer cells to undergo TRAIL-induced apoptosis 19. We have demonstrated that activation of c-Fos/AP-1 is necessary but insufficient for TRAIL-induced apoptosis. In addition, we have demonstrated that AP-1 is activated in sensitive prostate cancer cells after treatment with either TRAIL or TNFα (Fig. 2). Here we wished to determine whether activation of c-Fos/AP-1 could affect TRAIL- and/or TNFα-induced apoptosis. We found that ectopic expression of wild type c-Fos led to increased expression of c-Fos and AP-1 activity and converted TRAIL-resistant prostate cancer cells (PC3-TR and LNCaP) and TNFα-resistant prostate cancer cells (PC3 and PC3-TR) to a more sensitive phenotype, respectively (Fig. 5 A and B).
Figure 5.
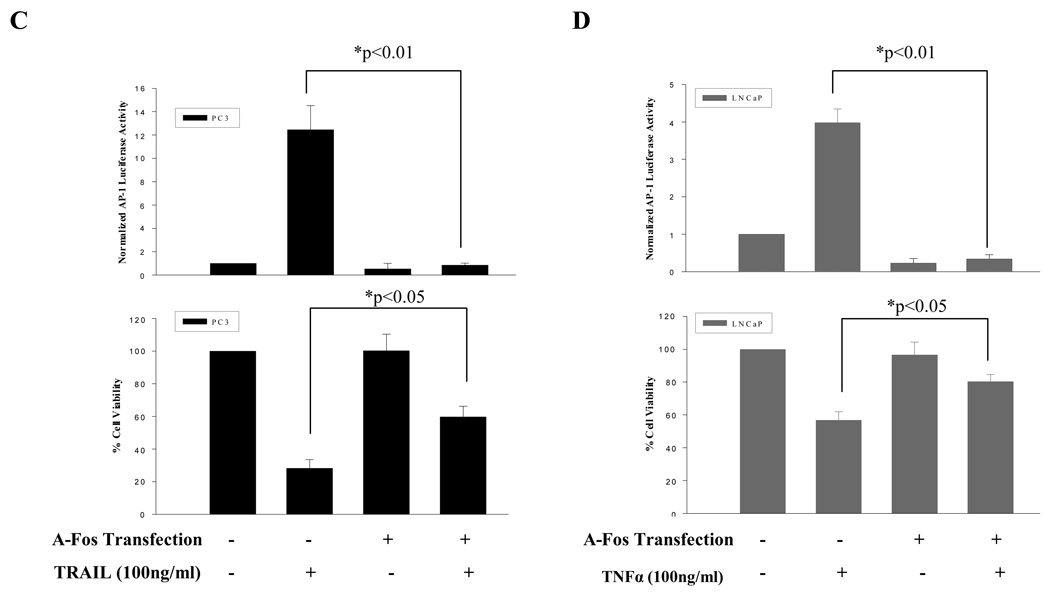
Increased AP-1 activity partially sensitizes resistant prostate cancer cells to TRAIL or TNFα, while inhibition of AP-1 activity partially reduced sensitivity to TRAIL and TNFα in prostate cancer cells. (A) Ectopic expression of c-Fos (bottom panel), increased AP-1 activity (top panel ) and partially sensitized TRAIL resistant PC3-TR and LNCaP cells to TRAIL (middle panel). (B) Ectopic expression of c-Fos (bottom panel) increased AP-1 activity (top panel) and partially sensitized TNFα resistant PC3 and PC3-TR cells to TNFα (middle panel). (C) Ectopic expression of A-Fos (an AP-1/c-Fos dominant negative) inhibited AP-1 activity (top panel) and partially reduced TRAIL-induced cell death in PC3 cells (bottom panel). (D) Ectopic expression of A-Fos inhibited AP-1 activity (top panel) and partially reduced TNFα-induced cell death in LNCaP cells (bottom panel). “−” Indicates transfection of empty vector was used as control. Error bars (SD) are results of at least three independent experiments. “*” Refers to statistically significant differences between indicated groups.
To test whether inhibition of c-Fos/AP-1 activity can alter the phenotype of TRAIL-sensitive (PC3) and TNFα-sensitive (LNCaP) prostate cancer cells, we inhibited the AP-1 activity by a dominant negative form of c-Fos/AP-1 (i.e. A-Fos) 28. We found that ectopic expression of A-Fos, reduced AP-1 luciferase activity in PC3 and LNCaP cells after TRAIL and TNFα treatments, respectively (Fig. 5 C and D, top panels). Reductions in AP-1 activity were associated with changing the phenotype of TRAIL-sensitive (PC3) and TNFα-sensitive (LNCaP) cells to a more resistant phenotype (Fig. 5 C and D, bottom panel).
This data demonstrates that AP-1 activity has a direct role in mediating TRAIL and TNFα apoptotic mediated responses. Therefore, manipulating AP-1 activity can alter sensitivity of prostate cancer cells to proapoptotic agents like TRAIL and TNFα.
Concomitant reduction of NF-κB and increase of AP-1 activities sensitize a significant portion of prostate cancer cells to TRAIL- or TNFα-induced apoptosis
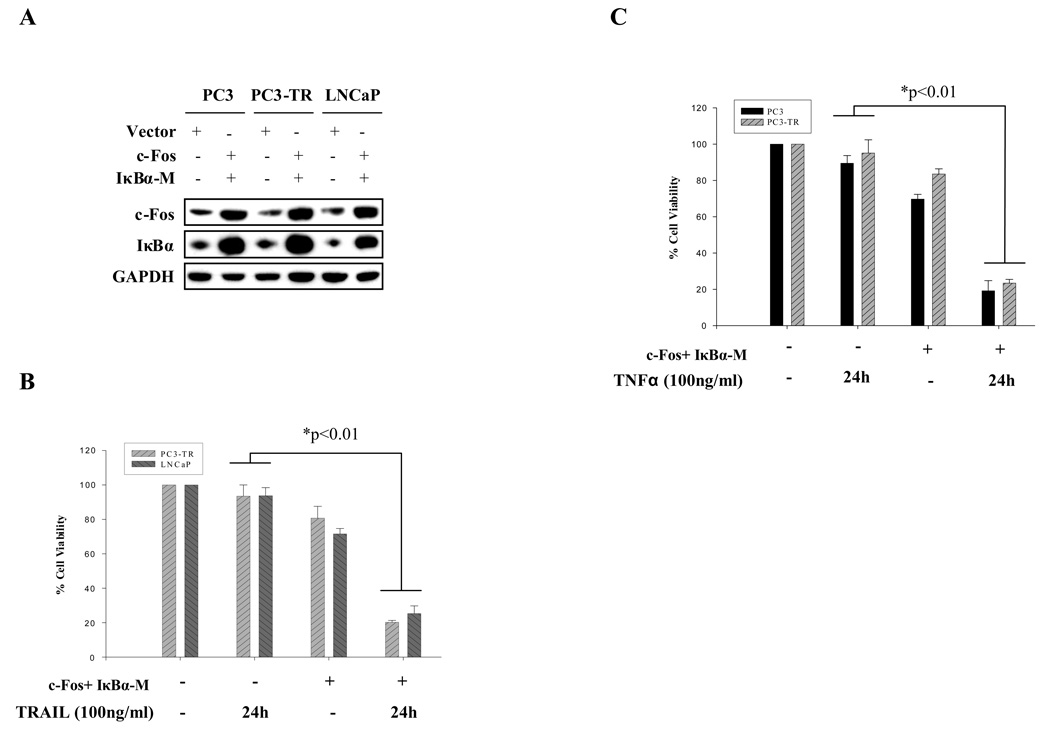
Our results showed that both NF-κB and AP-1 activity play essential roles in modulating sensitivity of prostate cancer cells to TRAIL- and TNFα-induced apoptosis. Suppressing NF-κB or increasing AP-1 activities alone partially sensitizes the cells to TRAIL- and TNFα-induced apoptosis. We postulate that combination of decreased NF-κB and increased AP-1 activities could potentiate the proapoptotic effects of TRAIL and TNFα. Therefore, IκBα-M and c-Fos were simultaneously introduced into prostate cancer cells in order to repress NF-κB and activate AP-1, respectively, in TRAIL-resistant (PC3-TR and LNCaP) or TNFα-resistant (PC3 and PC3-TR) prostate cancer cells. Concomitant expression of IκzBα-M and c-Fos (Fig. 6A) led to a much higher percentage of apoptosis (TRAIL treatment in PC3-TR and LNCaP cells; TNFα treatment in PC3 and PC3-TR cells) (Fig. 6B & C) than manipulating either gene family alone (Fig. 4 and Fig 5). These results suggest that simultaneous activation of AP-1 and inhibition of NF-κB can significantly enhance the efficacy of proapoptotic agents like TRAIL and TNFα.
Figure 6.
Concomitant inhibition of NF-κB and potentiation of AP-1 activities markedly changes TRAIL- or TNFα-resistance in prostate cancer cells. (A) Simultaneous ectopic expression of c-Fos and IκBα-M in prostate cancer PC3, PC3-TR and LNCaP cells. (B) TRAIL-induced cell death in PC3-TR and LNCaP cells before and after co-transfection of c-Fos and IκBα-M. (C) TNFα-induced cell death in PC3 and PC3-TR cells before and after co-transfection of c-Fos and IκBα-M. Error bars (SD) are results of at least three independent experiments. “*” Refers to statistically significant differences between indicated groups.
DISCUSSION
Although TRAIL and TNFα share many similar intracellular pathways, TNFα is associated with significant cytotoxicity, limiting TNFα’s clinical utility as a cancer therapeutic agent. Therefore, identifying similarities and differences between TRAIL-induced and TNFα-induced apoptosis can help differentiate between proapoptotic signals which may be responsible for the cytotoxicity that is associated with some proapoptotic agents. Here, we have identified a prostate cancer model where prostate cancer cells are differentially sensitive to TRAIL- and TNFα-induced apoptosis. We show that reduction of NF-κB activity or enhancement of AP-1 activity alone can only partially sensitize resistant prostate cancer cells to proapoptotic effects of TRAIL or TNFα. However, concomitant reduction of NF-κB and enhancement of AP-1 activities sensitize a high percentage of resistant prostate cancer cells to TRAIL- or TNFα-induced apoptosis.
NF-κB is a key transcription factor that suppresses TNFα-induced apoptosis. Although the precise basis for this signaling pathway continues to be explored, many studies have suggested that NF-κB mediated cell survival may be closely related to its downstream anti-apoptotic genes such as c-FLIP 29, Bcl-2 , IAPs, XIAP and Survivin 30. In our prostate cancer model, NF-κB activation serves as a survival signal which protects cells from apoptosis whether induced by TNFα or TRAIL. In the presence of TNFα and TRAIL, NF-κB activity increased in TRAIL-resistant (PC3-TR and LNCaP) and TNFα-resistant (PC3-TR and PC3) cancer cells (Fig. 2, A and B). On the other hand, NF-κB activity was decreased or maintained at low level in TRAIL-sensitive (PC3) and TNFα-sensitive (LNCaP) cells (Fig. 2, A and B).
We tested the expression of the well-documented NF-κB activating proteins TRAF2 and RIP in different prostate cancer cell lines after TRAIL and TNFα treatments. Although, some investigators have suggested that TRAF2 may have little role in TRAIL-induced NF-κB activation 31, our present prostate cancer model suggests that silencing TRAF2 leads to reduction of NF-κB activity and partially sensitizes prostate cancers to TRAIL-induced apoptosis (Fig. 3, C, D and E). This finding suggests that in addition to regulating TNFα-induced apoptosis, TRAF2 also plays an important role in TRAIL-induced apoptosis by regulating activation of NF-κB in prostate cancer cells. In addition to TRAF2, we found that RIP, another important regulator of DISC formation 26, regulates NF-κB and sensitivity of prostate cancer cells to TRAIL and TNFα (Fig. 3).
The relationship and interactions between TRAF2 and RIP for activation of NF-κB activation are controversial. Our results showed that simultaneous silencing of TRAF2 and RIP did not have any added benefit in reducing activity of NF-κB, or enhancing cell death, as compared to silencing of TRAF2 or RIP alone (Fig. 3, C, D and E). These results suggest that TRAF2 and RIP regulate activation of NF-κB in “series”, and not in a “parallel” cell signalling pathway – a finding that is consistent with previous reports that TRAF2-mediated ubiquitination leads to the activation of downstream kinases including RIP 32. However, NF-κB activation mediated by TRAF2 and RIP may be different between TRAIL-induce and TNFα-induced apoptotic pathways. After TNF binds to its receptor, RIP, TRADD and TRAF2 form complex I. Binding of TRAF2 to TNF DISC is TRADD dependent and complex I can activate NF-κB directly 33. In contrast, TRAIL-induced formation of DISC complex I requires FADD but not TRADD, while DISC complex II recruits RIP and TRADD 10, 33.
The precise mechanism of dual regulation of NF-κB and AP-1 is under investigation in our lab. We postulate that c-FLIP, a key anti-apoptosis molecule, may play an important role in mediating both NF-κB and AP-1 related pathways, in prostate cancer cells. We have shown in the past that c-FLIP(L) is an important regulator of TRAIL-induced apoptosis 21, 34, and its expression is negatively regulated by c-Fos/AP-1 19. Since c-FLIP’s expression can be induced by NF-κB 29, and in the present study, and our prior work we have shown that AP-1’s activity is required for cancer cells to be sensitive to TNFα and TRAIL, c-FLIP(L) may function as a common gene for cross-coupling NF-κB and AP-1 activities in prostate cancer cells undergoing apoptosis. Since there are three functional c-FLIP isoforms – c-FLIP(L) 35 , c-FLIP(s) 36 and FLIP(R) 37, each isoform may function differently in mediating the cross-talk between the NF-κB and AP-1 related pathways.
Although TRAIL- and TNFα-induced apoptosis may share some common pro-apoptotic pathways 38, Other important pathways such as Akt 39 and mitogen-activated protein kinases (MAPKs) 40 may differentially regulate TRAIL and TNFα signaling. We believe that regulation of TRAIL and TNFα signaling requires cross talks between multiple regulatory signaling networks, some of which include NF-κB and AP-1. For example, it has been suggested that AP-1 may directly regulate NF-κB by direct interactions with the NF-κB subunit, p65 41, 42. Other examples of potential cell signalling cross talks include Akt induction of NF-κB by phosphorylating I κB 43. Yet, another example is NF-κB’s ability to inhibit JNK and promote c-FLIP(L) in order to promote survival signals 44. In order to balance NF-κB’s survival signals, JNK has been shown to activate the E3 ubiquitin ligase, ITCH, thereby inhibiting c-FLIP(L) and potentiating proapoptotic signals 44.
In addition to the extrinsic pathway which is mediated by death receptors such as TNF and TRAIL receptors, the intrinsic pathway is regulated largely by the Bcl-2 family. Most epithelial cancer cells including prostate cancer are type II cells. The apoptosis process in these cells utilizes both intrinsic and extrinsic pathways. We have found that inhibition of caspase 8/10 (extrinsic pathway) and caspase 9 (intrinsic pathway) and caspase 3 can completely block TRAIL-induced apoptosis in PC3 cells (X. Zhang and A. Olumi, unpublished data). Many groups have shown the important role of Bcl-2 family members in mediating TRAIL or TNFα signaling pathways. However, the role of c-Fos/AP-1 in relation to Bcl-2 activity remains to be explored. Since c-Fos is translocated from the cytoplasm to the mitochondria after TRAIL treatment 45, it is possible that c-Fos/AP-1 activities may also play an important role in mediating the intrinsic apoptotic pathway and the Bcl-2 machinery.
Since normal development, growth and malignant progression of prostate cells are all dependent on androgens, one may postulate that androgens or the androgen-receptor may affect interactions in TRAIL- and TNFα-induced apoptosis. In fact, it’s been shown that the c-FLIP(L) promoter region contains multiple androgen-response element sequences 46. In our model system, we evaluated the hormone-dependent LNCaP cells to a series of hormone-independent LNCaP sub-lines 47. We did not find any difference of sensitivity to TRAIL-induced apoptosis, c-Fos or c-FLIP(L) expression between the hormone-dependent or the hormone-independent sub-lines (data not shown). Therefore, our preliminary studies does not suggest that androgen-dependence of prostate cancer cells may play a key role in TRAIL-induced apoptosis.
Conclusion
Our study demonstrates that concomitant reduction of NF-κB and enhancement of AP-1 activity potentiates the proapoptotic effects of TRAIL- and TNFα-induced apoptosis. Therefore, multiple molecular pathways, such as NF-κB and AP-1, may need to be modulated in order to overcome TRAIL or TNFα resistance for cancer therapies.
Supplementary Material
Acknowledgments
Grant Support: Department of Defense Prostate Cancer Program (W81XWH-05-1-0080), NIH DK64062) and Howard Hughes Medical Institute/SPORE grant to the Biomedical Research Support Program at Harvard Medical School (53000234-0006) to AFO, and the National Natural Science Foundation of China (NSFC) (30572139) to XZ.
References
- 1.Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66:1403–1408. doi: 10.1016/s0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 2.O'Kane HF, Watson CJ, Johnston SR, Petak I, Watson RW, Williamson KE. Targeting death receptors in bladder, prostate and renal cancer. J Urol. 2006;175:432–438. doi: 10.1016/S0022-5347(05)00160-6. [DOI] [PubMed] [Google Scholar]
- 3.Bodmer JL, Holler N, Reynard S, Vinciguerra P, Schneider P, Juo P, Blenis J, Tschopp J. TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol. 2000;2:241–243. doi: 10.1038/35008667. [DOI] [PubMed] [Google Scholar]
- 4.Loetscher H, Pan YC, Lahm HW, Gentz R, Brockhaus M, Tabuchi H, Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990;61:351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- 5.Gray PW, Barrett K, Chantry D, Turner M, Feldmann M. Cloning of human tumor necrosis factor (TNF) receptor cDNA and expression of recombinant soluble TNF-binding protein. Proc Natl Acad Sci U S A. 1990;87:7380–7384. doi: 10.1073/pnas.87.19.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 7.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 8.Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, Krammer PH, Walczak H. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity. 2000;12:599–609. doi: 10.1016/s1074-7613(00)80211-3. [DOI] [PubMed] [Google Scholar]
- 9.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 10.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 11.Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 13.Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005;15:24–27. doi: 10.1038/sj.cr.7290259. [DOI] [PubMed] [Google Scholar]
- 14.Kim YS, Schwabe RF, Qian T, Lemasters JJ, Brenner DA. TRAIL-mediated apoptosis requires NF-kappaB inhibition and the mitochondrial permeability transition in human hepatoma cells. Hepatology. 2002;36:1498–1508. doi: 10.1053/jhep.2002.36942. [DOI] [PubMed] [Google Scholar]
- 15.Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene. 2003;22:3842–3852. doi: 10.1038/sj.onc.1206520. [DOI] [PubMed] [Google Scholar]
- 16.Guseva NV, Taghiyev AF, Sturm MT, Rokhlin OW, Cohen MB. Tumor necrosis factor-related apoptosis-inducing ligand-mediated activation of mitochondria-associated nuclear factor-kappaB in prostatic carcinoma cell lines. Mol Cancer Res. 2004;2:574–584. [PubMed] [Google Scholar]
- 17.Shetty S, Gladden JB, Henson ES, Hu X, Villanueva J, Haney N, Gibson SB. Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) up-regulates death receptor 5 (DR5) mediated by NFkappaB activation in epithelial derived cell lines. Apoptosis. 2002;7:413–420. doi: 10.1023/a:1020031023947. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Zhang X, Olumi AF. MG-132 Sensitizes TRAIL-Resistant Prostate Cancer Cells by Activating c-Fos/c-Jun Heterodimers and Repressing c-FLIP(L) Cancer Res. 2007;67:2247–2255. doi: 10.1158/0008-5472.CAN-06-3793. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Zhang L, Yang H, Huang X, Otu H, Libermann T, DeWolf WC, Khosravi-Far R, Olumi AF. c-Fos as a proapoptotic agent in TRAIL-induced apoptosis in prostate cancer cells. Cancer Res. 2007;67:9425–9434. doi: 10.1158/0008-5472.CAN-07-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Jin TG, Yang H, DeWolf WC, Khosravi-Far R, Olumi AF. Persistent c-FLIP(L) expression is necessary and sufficient to maintain resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in prostate cancer. Cancer Res. 2004;64:7086–7091. doi: 10.1158/0008-5472.CAN-04-1498. [DOI] [PubMed] [Google Scholar]
- 22.Tillman K, Oberfield JL, Shen XQ, Bubulya A, Shemshedini L. c-Fos dimerization with c-Jun represses c-Jun enhancement of androgen receptor transactivation. Endocrine. 1998;9:193–200. doi: 10.1385/ENDO:9:2:193. [DOI] [PubMed] [Google Scholar]
- 23.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 24.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 25.Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD. Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen. Oncogene. 1999;18:7389–7394. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 26.Hur GM, Lewis J, Yang Q, Lin Y, Nakano H, Nedospasov S, Liu ZG. The death domain kinase RIP has an essential role in DNA damage-induced NF-kappa B activation. Genes Dev. 2003;17:873–882. doi: 10.1101/gad.1062403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feig BW, Lu X, Hunt KK, Shan Q, Yu D, Pollock R, Chiao P. Inhibition of the transcription factor nuclear factor-kappa B by adenoviral-mediated expression of I kappa B alpha M results in tumor cell death. Surgery. 1999;126:399–405. [PubMed] [Google Scholar]
- 28.Gerdes MJ, Myakishev M, Frost NA, Rishi V, Moitra J, Acharya A, Levy MR, Park SW, Glick A, Yuspa SH, Vinson C. Activator protein-1 activity regulates epithelial tumor cell identity. Cancer Res. 2006;66:7578–7588. doi: 10.1158/0008-5472.CAN-06-1247. [DOI] [PubMed] [Google Scholar]
- 29.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y, Devin A, Cook A, Keane MM, Kelliher M, Lipkowitz S, Liu ZG. The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IkappaB kinase and c-Jun N-terminal kinase. Mol Cell Biol. 2000;20:6638–6645. doi: 10.1128/mcb.20.18.6638-6645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia ZP, Chen ZJ. TRAF2: a double-edged sword? Sci STKE. 2005;2005:pe7. doi: 10.1126/stke.2722005pe7. [DOI] [PubMed] [Google Scholar]
- 33.Jin Z, El-Deiry WS. Distinct signaling pathways in TRAIL- versus tumor necrosis factor-induced apoptosis. Mol Cell Biol. 2006;26:8136–8148. doi: 10.1128/MCB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Zhang L, Yang H, Huang X, Otu H, Libermann TA, DeWolf WC, Khosravi-Far R, Olumi AF. c-Fos as a proapoptotic agent in TRAIL-induced apoptosis in prostate cancer cells. Cancer Res. 2007;67:9425–9434. doi: 10.1158/0008-5472.CAN-07-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 36.Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- 37.Golks A, Brenner D, Fritsch C, Krammer PH, Lavrik IN. c-FLIPR, a new regulator of death receptor-induced apoptosis. J Biol Chem. 2005;280:14507–14513. doi: 10.1074/jbc.M414425200. [DOI] [PubMed] [Google Scholar]
- 38.Tamada K, Chen L. Renewed interest in cancer immunotherapy with the tumor necrosis factor superfamily molecules. Cancer Immunol Immunother. 2006;55:355–362. doi: 10.1007/s00262-005-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem. 2001;276:10767–10774. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- 40.Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene. 2003;22:4092–4101. doi: 10.1038/sj.onc.1206598. [DOI] [PubMed] [Google Scholar]
- 41.Li JJ, Cao Y, Young MR, Colburn NH. Induced expression of dominant-negative c-jun downregulates NFkappaB and AP-1 target genes and suppresses tumor phenotype in human keratinocytes. Mol Carcinog. 2000;29:159–169. doi: 10.1002/1098-2744(200011)29:3<159::aid-mc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 42.Stein B, Baldwin AS, Jr, Ballard DW, Greene WC, Angel P, Herrlich P. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. Embo J. 1993;12:3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 44.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Ameyar M, Wisniewska M, Weitzman JB. A role for AP-1 in apoptosis: the case for and against. Biochimie. 2003;85:747–752. doi: 10.1016/j.biochi.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, Wang YF, Wang Z. The androgen receptor directly targets the cellular Fas/FasL-associated death domain protein-like inhibitory protein gene to promote the androgen-independent growth of prostate cancer cells. Mol Endocrinol. 2005;19:1792–1802. doi: 10.1210/me.2004-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krueckl SL, Sikes RA, Edlund NM, Bell RH, Hurtado-Coll A, Fazli L, Gleave ME, Cox ME. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64:8620–8629. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.