Abstract
In cerebral folate deficiency syndrome, the presence of autoantibodies against the folate receptor (FR) explains decreased folate transport to the central nervous system and the clinical response to folinic acid. Autoantibody crossreactivity with milk FR from different species prompted us to test the effect of a milk-free diet. Intervention with a milk-free diet in 12 children (nine males, three females; mean age 6y [SD 4y 11mo], range l–19y), decreased autoantibody titer significantly from 2.08pmol of FR blocked per ml of serum (SD 2.1; range 0.24–8.35) to 0.35pmol (SD 0.49; range 0–1.32; p=0.012) over 3 to 13 months, whereas FR autoantibody titer increased significantly to 6.53 (SD 6.08; range 0.54–14.07; p=0.013) in nine children who were reexposed to milk for 6 to 14 weeks. In 12 children on a normal diet (eight males, four females; mean age 5y 5mo [SD 4y 1mo], range 1y 6mo–16y 4mo), the antibody titer increased significantly from 0.84pmol of FR blocked per ml (SD 0.39; range 0.24–1.44) to 3.04pmol (SD 1.42; range 0.84–6.01; p=0.001) over 10 to 24 months. Decreasing the autoantibody titer with a milk-free diet in conjunction with folinic acid therapy may be advocated for these patients.
Cerebral folate deficiency (CFD) syndrome is a neurological condition characterized by low levels of N5-methyltetrahy-drofolate (5MTHF) in the cerebrospinal fluid (CSF) and normal folate levels in plasma and red blood cells. Infantileonset CFD syndrome develops 4 to 6 months after birth and is defined as a clinical entity fulfilling three or more of the seven major criteria with initial manifestations of agitation and insomnia followed by deceleration of head growth, psychomotor retardation, hypotonia and ataxia, spasticity, dyskinesias, and epilepsy.1,2 In several of these children, autistic features are also present.2 A low level of 5MTHF in CSF can result from decreased transport across the blood–CSF barrier.3,4 Early diagnosis and treatment with pharmacological doses of reduced folates (5-formyltetrahydrofolate or 5-methyltetrahydrofolate) is necessary to prevent or reverse the neurological manifestations.
Autoantibodies against the folate receptor (FR) were first described in mothers with a neural-tube-defect pregnancy and provided an explanation for folate deficiency in the developing embryo resulting from autoantibodies blocking folate uptake via the FR.5 The finding of FR autoantibodies in children with CFD syndrome and the low level of 5MTHF in CSF suggested a similar mechanism by which binding of the autoantibodies to the FR on the choroid plexus would block folate transport into the CSF.6 In the CFD syndrome, the clinical manifestations typically occur after the switch to bovine milk. One likely mechanism for autoantibody production could be that exposure to soluble FR from milk elicits an immune response. Because of the structural homology with human FR, the autoantibodies cross-react with the FR on the epithelial cells of the choroid plexus, block folate transport, and ultimately produce the CFD syndrome.6,7 To test this hypothesis, patients with CFD with autoantibodies against FR and being treated with folinic acid to normalize their level of 5MTHF in CSF were asked to participate in the study. Twelve randomly chosen patients, after consent, were placed on a milk-free diet and a second group of 12 patients was placed on a regular milk-containing diet, and were evaluated for the effect of oral ingestion of milk FR on the titer of the autoantibodies and clinical response to therapy
Method
CLINICAL PRESENTATION AND DIAGNOSTIC CRITERIA OF THE CFD SYNDROME
All patients were referred to the first author (VR) at the Pediatric Neurology Clinic for evaluation. Those in whom a diagnosis of CFD was confirmed were asked to participate in the study. The 24 patients in this study fulfilled three or more of the seven major clinical criteria characterizing the CFD syndrome (Table I and Table II). Patients fulfilling the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria of autism were referred to the Department of Psychiatry for formal testing. Ten of these children presented with low-functioning autism on formal testing with the Autism Diagnostic Observation Schedules (ADOS) in conjunction with the Autism Diagnostic Interview (ADI).2 Patient 5 fulfilled two of these criteria (psychomotor retardation and severe ataxia) and patient 20 presented with irritability, psychomotor retardation, and autism. In all patients, serum and red blood cell folate levels, serum vitamin B12 levels, and serum homocysteine levels were normal in conjunction with consistent lowering of 5MTHF levels in CSF.
Table I.
Clinical characteristics and laboratory findings of patients with cerebral folate deficiency syndrome treated with folinic acid and on a milk-free diet
| Characteristic | Patient no. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Sex | M | M | M | M | F | F | M | M | F | M | M | M |
| Age at diagnosis, y:mo | 1:0 | 2:5 | 2:6 | 3:6 | 4:1 | 4:6 | 4:8 | 5:2 | 6:5 | 7:0 | 11:11 | 19:0 |
| Irritability, marked unrest, sleep disturbances |
+/−/− | − | +/−/− | +/+/− | − | +/−/− | − | +/−/− | +/−/− | +/+/− | +/−/− | +/−/− |
| Head growth deceleration after 4mo of age |
− | +/−/− | +/−/− | − | − | +/−/− | − | +/−/− | − | +/−/− | − | − |
| Psychomotor retardation | +/+/+ | +/−/− | +/−/− | +/+/+ | +/−/− | +/+/+ | +/−/− | +/+/+ | +/+/+ | +/+/+ | +/+/+ | +/+/+ |
| Early infantile autism | +/−/− | − | − | − | − | +/−/− | − | +/±/± | +/±/± | +/±/− | +/±/± | − |
| Cerebellar ataxia | ||||||||||||
| Unsteady gait, difficulty in walking | −/+/− | −/−/+ | −/−/+ | −/−/+ | −/−/+ | −/+/− | −/+/− | −/−/+ | −/+/− | +/−/− | −/−/+ | −/−/− |
| Ataxia with frequent falls | −/−/− | −/+/− | −/+/− | −/−/− | −/+/− | −/−/− | +/−/− | −/+/− | +/−/− | − | −/+/− | −/−/− |
| Severe ataxia | +/−/− | +/−/− | +/−/− | +/+/− | +/−/− | +/−/− | − | +/−/− | − | − | +/−/− | +/+/+ |
| Pyramidal tract signs in legs | − | +/−/− | +/−/− | +/−/− | − | − | − | +/−/− | − | − | − | +/−/− |
| Dyskinesias, choreoathetosis, and ballismus |
− | − | − | − | − | − | − | − | +/−/− | − | − | +/−/− |
| Epilepsy | − | − | − | − | − | − | +/−/− | − | +/+/− | − | − | +/+/− |
| Laboratory findings | ||||||||||||
| Serum folate (nmol/l) | 34.8 | 32.4 | 26 | 19 | 23.5 | 32.1 | 15.3 | 13.9 | 34.4 | 11 | 22.3 | 12 |
| Initial 5MTHF level in CSF (nmol/l) | 45 | 33.4 | 33.6 | 24 | 6.4 | 50.5 | 22 | 4 | 30 | 41 | 5.9 | 7 |
| CSF/serum folate ratio | 1.3 | 1.0 | 1.3 | 1.3 | 0.3 | 1.6 | 1.4 | 0.3 | 0.9 | 3.7 | 0.3 | 0.6 |
| Blocking FR autoantibody titer | ||||||||||||
| (pmol FR blocked/ml serum) | ||||||||||||
| Initial (prior to milk-free diet) | 1.11 | 1.95 | 1.89 | 0.24 | 1.87 | 2.2 | 8.35 | 0.65 | 1.52 | 0.69 | 2.94 | 1.6 |
| After milk-free diet | 0 | 0.59 | 1.21 | 0 | 0 | 0 | 0.68 | 0 | 0 | 0 | 0.45 | 1.32 |
| Autoantibody type (s) | IgG | IgG | IgG | IgG | IgG | IgG | IgG | IgG, IgM | IgG | IgG | IgG | IgG |
| Autoantibody IgG isotype (s) | 4 | 1,4 | 4 | 4 | 1,4 | 1,3 | 4 | 4 | 1,3 | 1,4 | 1,4 | 1,4 |
Serum/CSF ratio in patient 10 was normal at the time of diagnosis when FR autoantibodies were very low at 0.19pmol of FR blocked per ml of serum. Before the milk-free diet the FR autoantibodies had risen to 0.69pmol/ml serum. 5MTHF levels in CSF vary with age: age 0 to 1 year, 64 to 182nmol/l; age 2 to 4 years, 63 to 111nmol/l; age above 5 years, 41 to 117nmol/l. The normal range of serum folate in this age group is 8.3 to 45nmol/l; the normal CSF/serum folate ratio in this age group is more than 2.5.The symbols +, −, and ± indicate respectively the presence, absence, or partial recovery from clinical signs and symptoms. The order of each symbol (1/2/3) indicates the status at the time of admission, after folinic acid treatment, and after being placed on a mik-free diet. 5MTHF, N5-methyltetrahydrofolate; CSF, cerebrospinal fluid; FR, folate receptor.
Table II.
Clinical characteristics and laboratory findings in patients with cerebral folate deficiency syndrome treated with folinic acid and on a milk-containing normal diet
| Characteristic | Patient no. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| Sex | M | M | F | F | F | M | M | M | M | M | F | M |
| Age at diagnosis, y:mo | 1:6 | 1:6 | 1:11 | 2:3 | 3:6 | 5:1 | 5:3 | 5:5 | 6:3 | 6:6 | 8:10 | 16:4 |
| Irritability, marked unrest, sleep disturbances |
+/− | +/− | +/+ | +/+ | +/− | +/+ | +/− | +/+ | +/− | − | − | − |
| Head growth deceleration after 4mo of age |
+/− | +/− | +/− | +/− | − | +/+ | − | − | − | +/− | +/− | +/− |
| Psychomotor retardation | +/− | +/+ | +/+ | +/+ | +/+ | +/+ | +/− | +/+ | +/− | +/− | +/+ | +/− |
| Early infantile autism | − | − | +/+ | +/+ | − | − | − | +/+ | − | − | +/+ | − |
| Cerebellar ataxia | ||||||||||||
| Unsteady gait, difficulty in walking | − | − | − | −/+ | − | − | +/− | − | +/− | − | − | − |
| Ataxia with frequent falls | +/− | +/− | − | +/− | − | − | − | − | − | +/− | +/− | +/− |
| Severe ataxia | − | − | +/+ | − | +/− | +/+ | − | − | − | − | − | − |
| Pyramidal tract signs in legs | +/− | − | +/− | +/− | − | +/− | − | − | − | − | − | +/− |
| Dyskinesias, choreoathetosis, and ballismus) |
− | − | +/+ | − | − | +/+ | − | − | − | − | − | − |
| Epilepsy | +/− | − | − | +/− | +/− | +/− | − | − | +/− | − | +/− | − |
| Laboratory findings | ||||||||||||
| Serum folate (nmol/l) | nd | 43.2 | >45 | 44 | >45 | 32.9 | >45 | nd | >47 | 14.2 | 17.8 | 45.3 |
| Initial 5MTHF level in CSF (nmol/l) | 30.3 | 43 | 62 | <1 | 46.3 | 19 | 21.8 | 32.8 | 38.8 | 14.2 | 26.7 | 32.4 |
| CSF/serum folate ratio | 1.0 | <1.4 | <0.1 | 1.0 | 0.6 | <0.5 | <0.8 | 1.0 | 1.5 | 0.7 | ||
| Blocking FR autoantibody titer | ||||||||||||
| (pmol FR blocked/ml serum) | ||||||||||||
| Initial (at time of diagnosis) | 1.15 | 0.721 | 0.38 | 0.32 | 0.775 | 1.35 | 0.24 | 1.11 | 1.101 | 1.44 | 0.77 | 0.76 |
| Follow-up titer | 0.84 | 2.21 | 6.01 | 2.22 | 3.62 | 4.79 | 1.96 | 3.45 | 2.23 | 3.98 | 3.11 | 2.06 |
| Autoantibody type | IgG | IgG | IgG | IgG | IgG | IgG | IgG | IgG | IgG | IgG | IgG | IgG |
| Autoantibody IgG isotype(s) | 4 | 4 | 1,4 | 4 | 1,4 | 4 | 1,3 | 1,4 | 4 | 4 | 1,3 | 4 |
5MTHF level in CSF values vary with age: age 0 to 1 year, 64 to 182nmol/l; age 2 to 4 years, 63 to 111nmol/l; age above 5 years, 41 to 117nmol/l. The normal range of serum folate in this age group is 8.3 to 45nmol/l; the normal CSF/serum folate ratio in this age group is more than 2.5. Symbols: + indicates presence of clinical signs and symptoms; – indicates absence of clinical signs or symptoms. The position of each symbol (1/2) indicates respectively at the time of admission and after folinic acid therapy for 10 to 24 months. 5MTHF, N5-methyltetrahydrofolate; CSF, cerebrospinal fluid; FR, folate receptor, nd, not determined.
FOLINIC ACID TREATMENT, DIETARY INTERVENTION, AND FOLLOW-UP
Before introduction of the milk-free diet in 12 patients, all 24 patients were treated with folinic acid, which raised 5MTHF levels in CSF along with bringing about clinical improvement. Clinical improvement was assessed by evaluating all of the neurological signs and symptoms listed in Table I and Table II. While folinic acid supplementation was maintained, 12 of 24 patients were put on a milk-free diet (patients 1–12) and the remaining 12 patients (patients 13–24) remained on their normal diet containing milk. The parents were instructed by a dietician on maintaining the patient on a milk-free balanced diet replete with essential nutrients and protein-calorie requirements. Serum was assayed for autoantibodies before and after 3 to 13 months on a milk-free diet and after re-exposure to milk because of non-compliance with the milk-free diet. The second group of 12 patients (nos. 13–24) were maintained on a regular diet containing milk products. Serum was assayed for FR autoantibody at the time of diagnosis and after 10 to 24 months. All samples were assayed without identifiers. The study was approved by the institutional ethics committees, and all participants or their guardians provided written informed consent. The clinical evaluation and response to therapy was not blinded because these patients were on a long-term follow-up and were being treated with pharmacological doses of folinic acid. With the exception of a single patient who was diagnosed and treated at Massachusetts General Hospital, Boston, MA, USA, all children were diagnosed at the Pediatric Neurology Clinic, University Hospital Aachen, Germany. The first author (VR) was responsible for the recruitment of all participants and for clinical evaluation, treatment, and follow-up at regular intervals.
AUTOANTIBODY ANALYSES
Assay for folate-blocking antibodies
Testing for autoantibodies against FR was performed as described previously.5,6 Serum (300µl) was acidified with 900µl of 0.1M glycine/HCl pH2.6/0.5% Triton X-100/10mM EDTA and was added to a 12.5mg pellet of dextran-coated charcoal to remove free folate. After centrifugation, the supernatant was collected and the pH was raised to 7.4 with 180µl of 1M dibasic sodium phosphate. Aliquots of this sample, equivalent to 50 to 200µl of the original serum, were incubated overnight at 4°C with 0.34pmol of apo-FR purified from human placental membranes. Tritiated [3H]folic acid was added and the mixture was incubated for 20 minutes at room temperature. Free [3H]folic acid was adsorbed on dextran-coated charcoal, and receptor-bound radioactivity in the supernatant fraction was determined. Blocking autoantibodies prevented the binding of [3H]folic acid to FR; the titer of autoantibodies is expressed as picomoles of receptor blocked per milliliter of serum.
Identification of autoantibody type
The immunoglobulin type of the FR autoantibody was identified by an enzyme-linked immunosorbent assay (ELISA)8 whereby FR purified from bovine milk was immobilized in 96-well plates. Unreacted sites were blocked with normal rabbit serum, and processed serum samples (acidified, charcoal-treated, and neutralized) were incubated for 24 hours at 4°C with the FR in ELISA plates. The wells were washed with 0.1M sodium phosphate pH7.4 containing 0.1% Tween 20, and 100µl of biotin-conjugated goat antibodies against human IgG, IgA, IgM, or IgE (1:4000 dilution in normal goat serum) was added. After incubation for 1 hour at 25°C, the wells were washed with buffer; 40µl of an avidin–biotin–peroxidase complex (ABC reagent; Vector Labs) was added and incubated for 30 minutes at 25°C. The wells were then washed with buffer, and 100µl of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added. After 1 hour at 25°C, 100µl of 1M HCl was added and the color developed was quantified with an ELISA plate reader.
Identification of the autoantibody isotype
For individuals with IgG autoantibodies, the IgG isotype or isotypes were also determined.9,10 ELISA plates prepared and reacted with serum extracts as described above were incubated with 100µl of biotin-conjugated mouse monoclonal antibodies against human IgG1, IgG2, IgG3, or IgG4 (dilution 1:1000 to 1:40 000) and the bound mouse antibody was identified by reacting with avidin–biotin–peroxidase.
Antigenic cross-reactivity of the blocking autoantibodies
The concentration of each purified FR from placental membranes, human milk, bovine milk, and goat milk was adjusted to bind about 10 000cpm of [3H]folic acid (0.34pmol of FR) and was incubated with processed serum that was diluted to adjust the titer of the autoantibody to block about 0.1pmol of human placental FR. After incubation overnight at 4°C, [3H]folic acid (16 000cpm; 0.55pmol) was added and incubated for 20 minutes at 25°C. Unbound [3H]folic acid was adsorbed on dextran-coated charcoal and the amount of FR-bound [3H]folic acid in the supernatant was determined. Any decrease in FR-bound [3H]folic acid represents the amount of [3H]folic acid blocked from binding to FR and provides a measure of antigenic cross-reactivity of the blocking autoantibodies.
STATISTICAL ANALYSIS
The t-test for two independent samples was used to compare serum folate and 5MTHF levels in CSF before and after folinic acid treatment with values for healthy age-matched controls.6 The Mann-Whitney U test (two-tailed p values using 95% confidence intervals) was also used to evaluate the two groups. In addition, the Kruskal–Wallis test and Dunn's Multiple Comparison test was used for more than two data sets to confirm significant differences observed with the paired t-test. The paired t-test was used for comparing the autoantibody titers in the various groups and for evaluating the cross-reactivity of the autoantibodies.
Results
The clinical characteristics and laboratory findings in 24 participants who were treated with folinic acid initially and thereafter switched to a milk-free diet (n=12) or were maintained on a regular diet (n=12) are shown in Table I and Table II. The 5MTHF level in CSF in all participants (mean 27.9nmol/l, [SD 16.1], range <1–62) was significantly lower than in 100 normal controls (mean 82nmol/l [SD 31]; range 44–181; p<0.001). However, all 24 patients had a normal or elevated serum folate level (mean 29.8nmol/l [SD 12.8]; range 11–47) compared with healthy controls (mean 29.0nmol/l [SD 13.6]; range 8.3–45). The serum of all 24 patients contained blocking autoantibodies against FR (mean 1.17 [SD 0.83pmol of FR blocked per ml of serum]; range 0.19–3.47). The presence of autoantibodies against the FR antigen was also confirmed by ELISA plate assay. All 24 participants had IgG autoantibodies and one child had both IgG and IgM autoantibodies in the serum. Twenty of the participants had the IgG4 isotype, and 12 of these also had the IgG1 isotype. Four of the 24 participants had a combination of IgG1 and IgG3 isotypes (Table I and Table II).
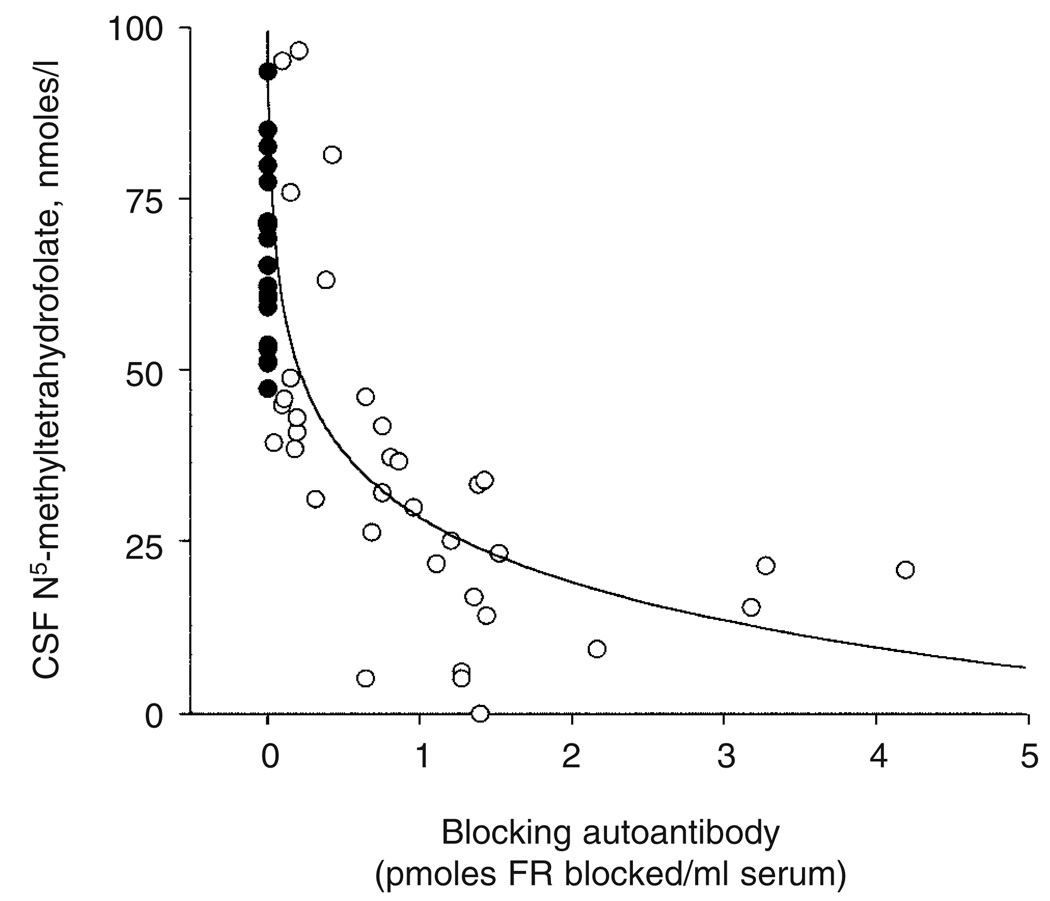
Folinic acid treatment was started at a dose of 0.5 to 1.0mg/kg per day divided into two equal doses and the amount was adjusted to between 0.4 and 2.5mg/kg per day on the basis of response to therapy and CSF folate concentration. This treatment was administered for a period of at least 7 months before dietary intervention. Folinic acid therapy had an overall positive effect on irritability and insomnia, arrested further head growth deceleration, decreased ataxia, spasticity, and seizure frequency, and had a variable effect on mental retardation* and dyskinesias. Among the 10 patients with low-IQ autism associated with neurological deficits (patients 1, 6, 8, 9, 10, 11, 15, 16, 20, and 23), a marked improvement was observed in two (patients 1 and 6) and a partial response in four (patients 8, 9, 10, and 11) with regard to improved attention, communication, and less stereotypies. Lumbar puncture during treatment with folinic acid showed a normalization of 5MTHF level in CSF (mean 78.9nmol/l, SD 27; range 52.2–158). The presence of autoantibodies against FR in CFD suggested that the decrease in 5MTHF level in CSF could be related to the antibody titer. This inverse correlation was confirmed when initial 5MTHF level in CSF was plotted against antibody titer: there was a lower 5MTHF level in CSF with a higher antibody titer in 55 individuals in whom both determinants were available (Fig. 1). These children were referred to the Pediatric Neurology Clinic at the University Hospital Aachen, and include the 24 patients described in Table I and Table II. Even though clinical improvement was evident with folinic acid therapy, the autoantibody titer did not decrease; a milk-free diet was therefore introduced on the assumption that milk, which contains about 5 to 8mg/l FR,11 might be a source of the antigen and that a breakdown of the immune barrier in the gastrointestinal tract could be the route of exposure to the antigen.
Figure 1.
Correlation between blocking folate receptor (FR) autoantibody titer and cerebrospinal fluid (CSF) folate level in patients with cerebral folate deficiency (CFD). Filled circles represent non-CFD patients with normal levels of folate in CSF who tested negative for the autoantibody
Nonparametric analyses with the Mann–Whitney U test showed no significant differences in the ages, initial 5MTHF level in CSF, serum:CSF folate ratios, or the FR-autoantibody titer between the two groups. The Kruskal–Wallis test and Dunn's Multiple Comparison test on both CSF 5MTHF and the FR autoantibody titer showed no significant difference between the two groups before one group was placed on the milk-free diet.
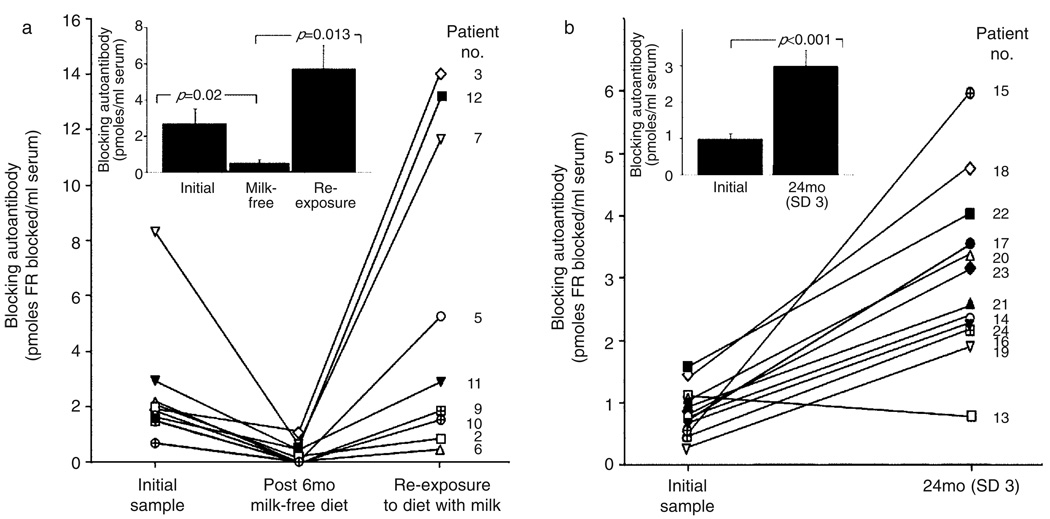
Before a milk-free diet, the mean baseline titer of FR autoantibodies for the 12 patients was 2.08pmol of FR blocked per ml of serum (SD 2.11; range 0.24–8.35) and decreased significantly to a mean of 0.35pmol of FR blocked per ml of serum (SD 0.49; range 0–1.32; p=0.012 by paired t-test) after 3 to 13 months on a milk-free diet. In 7 of the 12 patients, FR autoantibody titers decreased below detectable limits (less than 0.1pmol of FR blocked per ml), and in the remaining five patients, a significant decrease in the antibody titer was observed (Fig. 2a). The antibody titer monitored for a period between 10 and 24 months in the second group of 12 patients maintained on folinic acid and a regular diet containing milk products did not decrease (Fig. 2b). In these patients, a significant increase (p<0.001) in the autoantibody titer (mean 3.04pmol of FR blocked per ml, SD 1.42; range 0.84–6.01) from an initial mean value of 0.84 (SD 0.39; range 0.24–1.44) was observed.
Figure 2.
(a) Effect of dietary intervention with a milk-free diet followed by re-exposure to milk on the antibody titer of nine patients with cerebral folate deficiency (CFD) with autoantibodies against the folate receptor (FR) protein. The remaining three children who continued on a milk-free diet and whose autoantibody titer remained low are not shown. Each individual is depicted by a symbol and identified by the patient number from Table I. (b) Blocking autoantibody titer in 12 CFD patients on a normal diet containing milk and milk-derived products. Each patient is depicted by a symbol and identified by the patient number from Table II. Inset shows the mean, SEM, and statistical significance (determined by the paired t-test) of the autoantibody titer for all participants in each group.
During the 3 to 13 months on a milk-free diet, signs of ataxia improved or disappeared completely in all except the oldest patient, who had been diagnosed at age 19 years (patient 12). One patient with severe ataxia (patient 4), who had remained non-ambulatory during treatment with folinic acid, started to walk. The diet also led to complete seizure control in patients 9 and 12. One autistic patient (patient 10), who had partly improved with folinic acid, showed a marked further improvement in communication skills with fewer stereotypies. All of the clinical improvement during this period may be considered to be due to a combination of folinic acid supplementation and a decrease in the autoantibody titer. Considering the nature of the disorder with the risk of regression and the long-term treatment needed, withholding folinic acid to test the effects of dietary intervention alone was not an acceptable option. However, reducing the autoantibody titer by eliminating the antigen might have had an additional beneficial effect.
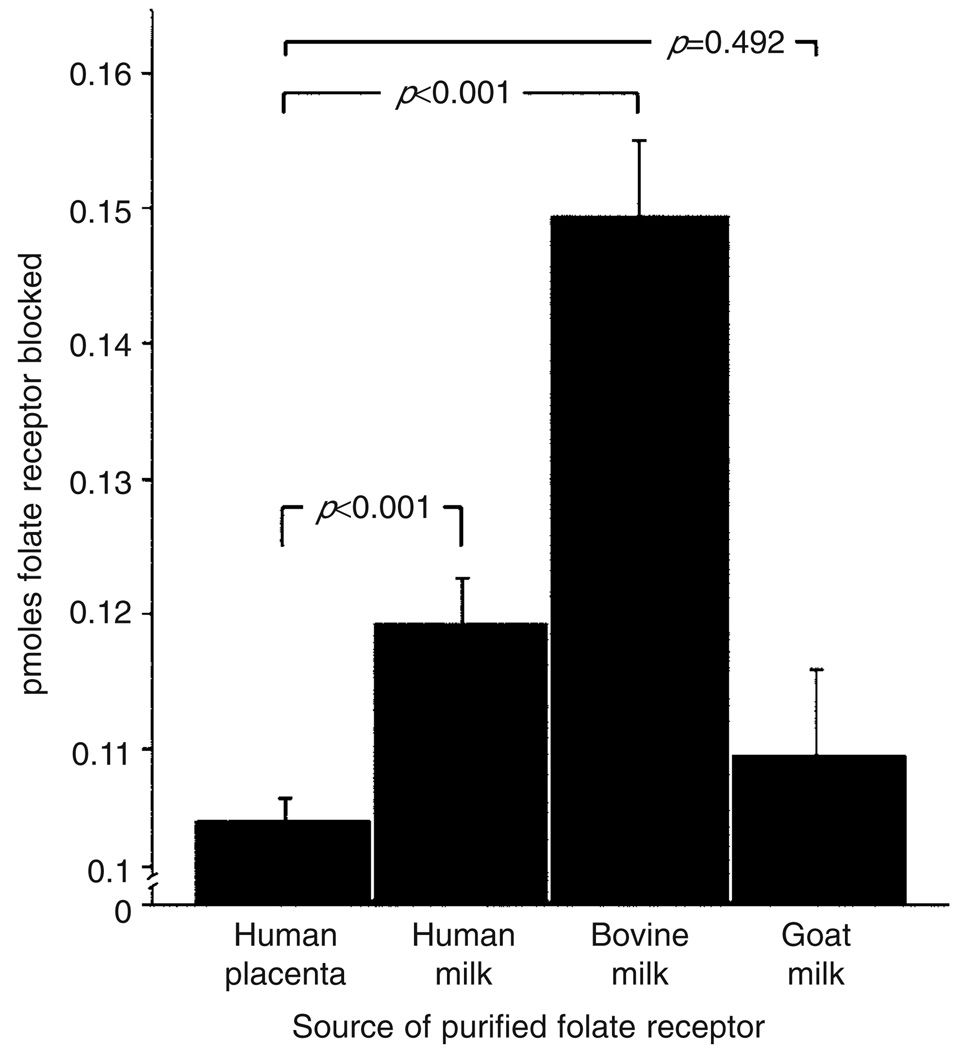
After 6 months of the milk-free diet, nine children (patients 2, 3, 5, 6, 7, 9, 10, 11, and 12) reverted to a regular diet that included milk. In these nine patients, the initial mean titer of the autoantibody was 2.56pmol of FR blocked per ml of serum (SD 2.25; range 0.69–8.35) and had decreased significantly to 0.47 (SD 0.53; range 0–1.32; p=0.023) on a milk-free diet. However, non-compliance over a period of 6 to 14 weeks resulted in a significant increase in the mean titer to 6.53 (SD 6.08; range 0.54–l4.07; p=0.013 by the paired t-test; Fig. 2a). This increase in titer was not accompanied by clinical deterioration because folinic acid supplementation was maintained throughout. Further characterization of the FR autoantibodies from these nine patients showed that the autoantibodies blocked the binding of folate to the FR purified from human placental membranes, human milk, bovine milk, and goat milk with highest cross-reactivity against FR from bovine milk (Fig. 3).
Figure 3.
Cross-reactivity of folate receptor autoantibodies against folate receptor from different species (n=9; mean and SEM). The p values were determined by paired t-test.
Discussion
In CFD patients with autoantibodies against FR, oral folinic acid treatment leads to substantial clinical improvement, especially if the treatment is started early in the disorder. Avoidance of milk downregulates these autoantibodies, and re-exposure to milk is followed by an increase in the autoantibody titer. Milk contains substantial amounts of FR and seems to present the triggering antigen for the autoantibody response. This indicates that the gastrointestinal tract is a likely route of exposure to the antigen and that a compromised immune barrier in this system can be considered as the potential cause of the autoimmune response. The familial occurrence of CFD in some siblings, such as that observed in three brothers, suggests a genetic component to this disorder. An autoimmune response against FR may result from genetically dictated errors during early thymic negative selection of autoreactive T-cells (CD4+ T helper cells and regulatory T cells) that fail to undergo apoptosis or do not remain in a state of anergy toward the FR antigen.12,13 Further research in families could identify the specific genetic components involved.14 After ingestion of milk, immunogenic peptides could cross a damaged intestinal barrier and activate peripheral macrophages, B cells, and T cells. We were unable to obtain detailed histories of early infancy to identify food allergies or allergy to milk or other conditions that may have triggered an inflammatory response and compromised the integrity of the intestinal barriers. The onset of the disorder during the first 3 to 6 months of life after the switch to bovine milk would suggest a delayed or disrupted synergy between the adaptive and innate immune systems in the gut. Because of the time-dependent evolution of typical signs and symptoms and the slow progression of CFD syndrome, diagnosis of the neurological phenotype is established much later in the disease process.
The cross-reactivity of the blocking autoantibodies with antigen from different sources is consistent with the significant homology in the native structure of these proteins. FR is well conserved across species, with more than 90% amino acid sequence homology between human FRα and bovine FRα,15,16 and this could contribute to common antigenic epitopes and cross-reactivity of the autoantibodies. The antibody showed better reactivity with the FR from bovine milk than with the FR from human placenta, human milk, or goat milk, suggesting bovine FR as the likely primary antigen. Quantitatively, there was more cross-reactivity with FR purified from human milk than with FR from human placenta. This discordance may be due to the presence of both the a and β isoforms of FR in human placenta, whereas milk contains only the FRα isoform;16,17 this would support the conclusion that the autoantibodies react better with epitopes on FRα, the putative antigen for the initial immune response. Most of the FR autoantibodies belong to the IgG4 subclass, and the switch to this class of antibodies seems to be influenced by repeated exposure to the antigen over a prolonged period,18,19 which supports the conclusion that repeated exposure to milk FR in the digestive tract is the likely mechanism for autoantibody generation.
Even though the pathology of B12 deficiency in the nervous system has been established, the association of neurological disease with folate deficiency is ill-defined, especially during neural development in early infancy. The association of neural tube defects in the developing embryo with folate deficiency due to either dietary deficiency, folate antagonists,20 deletion of the gene encoding FRα,21 or an autoantibody to FR that blocks folate uptake5 provides compelling evidence in support of a requirement for folate in neural development during early embryogenesis. The CFD syndrome provides additional evidence for a requirement for folate in infancy and during childhood for normal function of the central nervous system.2 The association of the FR autoantibody with the CFD syndrome, the folate blocking property of this antibody, and the clinical response to folinic acid therapy all suggest that decreasing the antibody titer would be beneficial in this disorder. Because milk and milk-derived products are the primary source of nutrition during infancy, a formulation devoid of the FR antigen and supplemented with folate may be recommended for children at risk or showing clinical signs of the CFD syndrome that may present independently or in association with other related neurological conditions, such as the autism spectrum disorders and Rett syndrome.22,23
Acknowledgements
This work was supported by NIH grant HD051880 (to EVQ) and Swiss National Science Foundation grant 310000-107500 (to NB).
List of abbreviations
- 5MTHF
N5-methyltetrahydrofolate
- CFD
Cerebral folate deficiency
- ELISA
Enzyme-linked immunosorbent assay
- FR
Folate receptor
Footnotes
UK usage: learning disability.
Contributor Information
Vincent T Ramaekers, Department of Paediatric Neurology, Centre Hospitalier Universitaire, Liege, Belgium.
Jeffrey M Sequeira, Departments of Medicine and Cell Biology, SUNY-Downstate Medical Center, Brooklyn, NY, USA.
Nenad Blau, Division of Chemistry and Biochemistry, University Children’s Hospital, Zurich, Switzerland.
Edward V Quadros, Departments of Medicine and Cell Biology, SUNY-Downstate Medical Center, Brooklyn, NY, USA.
References
- 1.Ramaekers VT, Hausler M, Opladen T, Heimann G, Blau N. Psychomotor retardation, spastic paraplegia, cerebellar ataxia and dyskinesia associated with low 5-methyltetrahydrofolate in cerebrospinal fluid: a novel neurometabolic condition responding to folinic acid substitution. Neuropediatrics. 2002;33:301–308. doi: 10.1055/s-2002-37082. [DOI] [PubMed] [Google Scholar]
- 2.Ramaekers VT, Blau N. Cerebral folate deficiency. Dev Med Child Neurol. 2004;46:843–851. doi: 10.1017/s0012162204001471. [DOI] [PubMed] [Google Scholar]
- 3.Holm J, Hansen SI, Hoier-Madsen M, Bostad L. High-affinity folate binding in human choroid plexus. Characterization of radioligand binding, immunoreactivity, molecular heterogeneity and hydrophobic domain of the binding protein. Biochem J. 1991;280:267–271. doi: 10.1042/bj2800267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector R, Johanson C. Micronutrient and urate transport in choroid plexus and kidney: implications for drug therapy. Pharm Res. 2006;23:2515–2524. doi: 10.1007/s11095-006-9091-5. [DOI] [PubMed] [Google Scholar]
- 5.Rothenberg SP, da Costa MP, Sequeira JM, et al. Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. N Engl J Med. 2004;350:134–142. doi: 10.1056/NEJMoa031145. [DOI] [PubMed] [Google Scholar]
- 6.Ramaekers VT, Rothenberg SP, Sequeira JM, et al. Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. N Engl J Med. 2005;352:1985–1991. doi: 10.1056/NEJMoa043160. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz RS. Autoimmune folate deficiency and the rise and fall of ‘horror autotoxicus’. N Engl J Med. 2005;352:1948–1950. doi: 10.1056/NEJMp058034. [DOI] [PubMed] [Google Scholar]
- 8.Jefferis R, Reimer CB, Skvaril F, et al. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10:223–252. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton RG, Reimer CB, Rodkey LS. Quality control of murine monoclonal antibodies using isoelectric focusing affinity immunoblot analysis. Hybridoma. 1987;6:205–217. doi: 10.1089/hyb.1987.6.205. [DOI] [PubMed] [Google Scholar]
- 10.Reimer CB, Phillips DJ, Aloisio CH, et al. Evaluation of thirty-one mouse monoclonal antibodies to human IgG epitopes. Hybridoma. 1984;3:263–275. doi: 10.1089/hyb.1984.3.263. [DOI] [PubMed] [Google Scholar]
- 11.Forssen KM, Jagerstad MI, Wigertz K, Witthoft CM. Folates and dairy products: a critical update. J Am Coll Nutr. 2000;19(Suppl 2):100S–110S. doi: 10.1080/07315724.2000.10718071. [DOI] [PubMed] [Google Scholar]
- 12.Bowcock AM, Lovett M. Zeroing in on tolerance. Nat Med. 2001;7:279–281. doi: 10.1038/85408. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi PS. T-cell signalling and autoimmunity: molecular mechanisms of disease. Nat Rev Immunol. 2002;2:427–438. doi: 10.1038/nri822. [DOI] [PubMed] [Google Scholar]
- 14.Pearce SH, Merriman TR. Genetic progress towards the molecular basis of autoimmunity. Trends Mol Med. 2006;12:90–98. doi: 10.1016/j.molmed.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antony AC. The biological chemistry of folate receptors. Blood. 1992;79:2807–2820. [PubMed] [Google Scholar]
- 17.Henderson GB. Folate-binding proteins. Annu Rev Nutr. 1990;10:319–335. doi: 10.1146/annurev.nu.10.070190.001535. [DOI] [PubMed] [Google Scholar]
- 18.Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–726. [PubMed] [Google Scholar]
- 19.Ottesen EA, Skvaril F, Tripathy SP, Poindexter RW, Hussain R. Prominence of IgG4 in the IgG antibody response to human filariasis. J Immunol. 1985;134:2707–2712. [PubMed] [Google Scholar]
- 20.Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343:1608–1614. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 21.Finnell RH, Gelineau-van Waes J, Bennett GD, et al. Genetic basis of susceptibility to environmentally induced neural tube defects. Ann NY Acad Sci. 2000;919:261–277. doi: 10.1111/j.1749-6632.2000.tb06886.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramaekers VT, Sequeira JM, Artuch R, et al. Folate receptor autoantibodies and spinal fluid 5-methyltetrahydrofolate deficiency in Rett syndrome. Neuropediatrics. 2007;38:179–183. doi: 10.1055/s-2007-991148. [DOI] [PubMed] [Google Scholar]
- 23.Ramaekers VT, Blau N, Sequeira JM, Nassogne MC, Quadros EV. Folate receptor autoimmunity and cerebral folate deficiency in low-functioning autism with neurological deficits. Neuropediatrics. doi: 10.1055/s-2008-1065354. (Forthcoming) [DOI] [PubMed] [Google Scholar]