Abstract
Purpose
Calcitriol potentiates cisplatin-mediated activity in a variety of tumor models. We examine here, the effect of calcitriol and cisplatin pre-clinically and clinically in canine spontaneous tumors through in vitro studies on tumor cells and through a phase I study of calcitriol and cisplatin to identify the maximum-tolerated dosage (MTD) of this combination in dogs with cancer and to characterize the pharmacokinetic disposition of calcitriol in dogs.
Methods
Canine tumor cells were investigated for calcitriol/cisplatin interactions on proliferation using an MTT assay in a median-dose effect analysis; data were used to derive a combination index (CI). Cisplatin was given at a fixed dosage of 60 mg/m2. Calcitriol was given i.v. and the dosage was escalated in cohorts of three dogs until the MTD was defined. Serum calcitriol concentrations were quantified by radioimmunoassay.
Results
In vitro, CIs<1.0 were obtained for all combinations of calcitriol/cisplatin examined. The MTD was 3.75 μg/kg calcitriol in combination with cisplatin, and hypercalcemia was the dose-limiting toxicosis. The relationship between calcitriol dosage and either Cmax or AUC was linear. Calcitriol dosages >1.5 μg/kg achieved Cmax ≥ 9.8 ng/mL and dosages >1.0 μg/kg achieved AUC ≥ 45 h ng/mL.
Conclusions
Calcitriol and cisplatin have synergistic antiproliferative effects on multiple canine tumor cells and high-dosages of i.v. calcitriol in combination with cisplatin can be safely administered to dogs. Cmax and AUC at the MTD 3.75 μg/kg calcitriol exceed concentrations associated with antitumor activity in a murine model, indicating this combination might have significant clinical utility in dogs.
Keywords: Vitamin D; 1,25-Dihydroxyvitamin D3; Platinum; Canine; Cancer; Pharmacokinetics
Introduction
Cisplatin (cis-diamminedichloroplatinum[II]) is one among the most active antitumor agents used in human and veterinary chemotherapy. In people, cisplatin is curative in testicular cancer and significantly prolongs survival in combination regimens for ovarian cancer. Cisplatin has therapeutic benefit in human head and neck, bladder, and lung cancer [28]. In veterinary oncology, cisplatin is widely used as adjunctive treatment for osteosarcoma [59] and there is demonstrated activity against numerous solid tumors including thyroid carcinoma [19], transitional cell carcinoma of the urinary bladder [41], and other high-grade sarcomas and carcinomas [30].
Cisplatin cytotoxicity results, in part, from the formation of intrastrand bifunctional N-7 adducts at adenine and guanine of DNA [28]. A number of signaling events occur after treatment of cultured cells with cisplatin. For example, ataxia telangiectasia-mutated kinase (ATM), which is involved in cell-cycle checkpoint activation, is activated by cisplatin. This kinase, in turn, phosphorylates and activates several downstream effectors that regulate cell cycle, DNA repair, cell survival, and apoptosis. These include p53, cAbl, and members of the mitogen-activated protein kinase (MAPK) pathway (extracellular signal-regulated kinase [ERK], c-Jun amino-terminal kinase [JNK], p38 kinase) [22, 28, 44]. Several reports have demonstrated an important role for stress signaling molecules such as mitogen-activating protein kinase kinase kinase-1 (MEKK-1) in regulating cisplatin sensitivity. In cisplatin-treated cultured cell lines, MEKK-1 becomes phosphorylated and then subsequently cleaved by caspase-3 [58]. Cleavage leads to the loss of intact MEKK-1; aberrant expression of unregulated MEKK-1 activity in the cytoplasm leads to the generation of proapoptotic signals via the c-Jun N-terminal kinase kinase (SEK1)-JNK-Jun and MKK3/MKK6-p38 MAPK stress pathways [13, 21, 50].
Calcitriol (1,25-dihydroxyvitamin D3; 1α25-dihydroxycholecalciferol), the principal biologically active form of vitamin D, exerts potent antineoplastic activity in vitro and in vivo in a broad range of tumor model systems [57]. Calcitriol induces G1/G0 cell cycle arrest, which might be mediated by increased expression of the cyclin-dependent kinase inhibitors p21waf/kip1 and p27kip1 [8, 24, 36], decreased cyclin-dependent kinase 2 (CDK2) activity, and hyperphorphorylation of the retinoblastoma protein [31]. In some cells, calcitriol induces apoptosis by down-regulating anti-apoptotic genes like Bcl-2 [15, 26]. Calcitriol decreases expression of epidermal growth factor receptors [56], induces transforming growth factors β1 and/or β2 [23], reduces insulin-like growth factor-1 signaling [6], and alters levels of hepatocyte growth factor [9]. Inhibition of tumor invasion through decreased matrix metalloproteinase 2 and 9 activity [52], antiproliferative effects on tumor-derived endothelial cells [12], and inhibition of the prostaglandin pathway have also been reported [32].
In vitro and in vivo, calcitriol potentiates the antitumor activity of numerous cytotoxic drugs [57]. Studies by our laboratory group show that calcitriol was synergistic with cisplatin in a murine squamous cell carcinoma (SCCVII/ SF) model system [25, 35]. The increased cytotoxicity resulted from cisplatin-enhancement of calcitriol-induced apoptotic signaling through upregulated MEKK-1 [25]. Evidence also suggests calcitriol might enhance cisplatin-mediated cytotoxicity through inhibitory effects on DNA repair [57]. The antiproliferative effect of calcitriol is dose-dependent; high doses of calcitriol are required to elicit antitumor activity and these doses might be associated with dose-limiting hypercalcemia [42].
To determine the maximum-tolerated dosage (MTD) of i.v. calcitriol that could safely be combined with cisplatin, we conducted this phase I trial in dogs with naturally occurring malignancies. Further objectives of the study reported here were to characterize the pharmacokinetic disposition of calcitriol in dogs and determine if calcitriol enhanced cisplatin-mediated cytotoxicity in canine tumor cell lines.
Materials and methods
In vitro studies
Chemicals and reagents
Calcitriol (1,25 dihydroxycholecalciferol; Hoffmann-LaRoche, Nutley, NJ, USA) was reconstituted in 100% EtOH and stored, protected from light, under nitrogen at −70°C. All handling of calcitriol was performed with indirect lighting. Calcitriol was diluted in tissue culture medium just before use. Cisplatin (Platinol-AQ; Bristol Laboratories, Princeton, NJ, USA, USA) was obtained as a 1 mg/mL solution and diluted in sterile saline or tissue culture medium immediately before use.
Canine tumor cells
Canine breast cancer cell line (CMT25) was provided by Allison Church Bird, Auburn University College of Veterinary Medicine, Auburn, AL 36849, USA. Canine osteosarcoma cell line (OS2.4) was obtained from Dr. Katrina Mealey, Washington Statue University College of Veterinary Medicine, Pullman, WA 99164, USA. Canine mast cell tumor cell line (C2) was provided by Dr. Cheryl London, The Ohio State University College of Veterinary Medicine, Columbus, OH 43210, USA. All cell lines were isolated from spontaneous dog tumors; isolation and characterization of the cell lines were previously described [14, 39, 60]. Cells were plated in RPMI 1640 supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT, USA) and 1% penicillin/streptomycin sulfate and incubated at 37°C in a humidified atmosphere containing 5% CO2. Cells were allowed to attach overnight and then treated and analyzed as described below. EtOH, used as a solvent control, never exceeded a final concentration of 0.0004%.
MTT assay and dose-effect analysis
Canine tumor cells (CMT25, 1.5 × 104 cells/well; OS2.4, 0.15 × 104 cells/well; C2, 0.7 × 104 cells/well) were seeded into 96-well tissue culture plates. Cells were pretreated for 24 h by adding medium alone or medium containing calcitriol directly to the wells. Cells were either incubated without further treatment or treated with medium containing cisplatin for an additional 72 h. The final volume in each well was 0.2 ml. To quantitate cell viability, the cells were incubated with 20 μL of 0.5% MTT for 90 min at 37°C. The medium was removed, and the cells were solubilized for 20 min in 10% SDS/10 mM HCl. Absorbance was read with an ELISA plate reader (Bio-Tek Instruments, Winooski, VT, USA) at a wavelength of 490 nm. Median doses for each drug were determined from the dose response data using CalcuSyn software (Biosoft, Ferguson, MO, USA). Drug interactions were quantitated using the equation CI = (D)1/(Dx)1 + (D)2/(Dx)2. (D)1 and (D)2 are the doses of drugs 1 and 2 that, when given in combination, inhibit cell growth by a specified percentage. (Dx)1 and (Dx)2 are the doses of drugs 1 and 2 that, when given individually, inhibit cell growth by the same percentage. CI values of <1, 1, and >1 indicate synergism, additivity, and antagonism between the drugs, respectively.
In vivo study
Animals
This open-label phase I study used client-owned dogs and was performed at the Cornell University Hospital for Animals at Cornell University College of Veterinary Medicine. Dogs weighing >10 kg were considered eligible to receive calcitriol combined with cisplatin when they had a resected, recurrent, or metastatic spontaneously occurring neoplasm that had been confirmed histologically; an expected survival of at least 4 weeks; not received chemotherapy, immunotherapy, or radiotherapy for at least 4 weeks; and adequate bone marrow (absolute neutrophil count ≥3,000/μL, platelets ≥100,000/μL), renal (serum creatinine concentration ≤1.3 mg/dL), hepatic [serum bilirubin concentration ≤0.3 mg/dL; serum alanine transaminase (ALT) and aspartate transaminase (AST) activities ≤29× upper limit of reference range], and cardiac function. Corrected serum calcium was used to assess (and grade) hypercalcemia [corrected calcium = (serum calcium − serum albumin) + 3.5]. Dogs with corrected serum calcium ≥ 12.0 mg/dL (reference range, 9.3–11.6 mg/dL) were excluded from the study. Written informed consent was obtained from all clients. The study protocol and consent form were approved by the Institutional Animal Care and Use Committee at Cornell University.
Study design and treatment plan
The dosage of cisplatin (Sicor Pharmaceuticals Inc., Irvine, CA, USA) was fixed at 60 mg/m2 body surface area. Body surface area was calculated by use of the equation: (10.1×[body weight (g)0.67])/104. The dosage of i.v. calcitriol (Sicor Pharmaceuticals Inc.) was escalated in cohorts of three dogs. The first dose level was 0.1 μg/kg. Subsequent dose escalation levels were 0.25, 0.5, 1.0, 1.5, 2.25, 3.75, and 5.5 μg/kg. Intrapatient dose escalation was permitted; each dog could receive a maximum of two treatments. Each dosage was initially administered to three dogs, provided that none had dose-limiting toxicity (DLT). DLT was defined as: (1) corrected serum calcium ≥12.0 mg/dL persistent for ≥7 days; (2) corrected serum calcium ≥12.0 mg/dL with adverse clinical signs of hypercalcemia (e.g. vomiting, anorexia, diarrhea); (3) any corrected calcium ≥14.0 mg/dL; (4) increase in serum creatinine to >1.3 mg/dL; (5) any treatment interruption related to the protocol that persisted for ≥2 weeks. When one of three dogs in a group had DLT, three additional dogs were administered calcitriol at the same dosage. When no DLT was observed in the additional three dogs, the dosage was escalated. When two or more dogs in a group had DLT, at least three additional dogs were administered the preceding treatment dosage. The nontolerable dose level was defined as the dosage at which ≥2 of three or ≥2 of six dogs had DLT. The maximum-tolerated dose (MTD) was defined as the highest dosage below the nontolerable dose level that resulted in ≤1 of six dogs with DLT.
A routine protocol for administering cisplatin using antiemetics and fluid diuresis was delivered through an indwelling catheter inserted in a cephalic vein. Specifically, dogs received 0.9% NaCl solution (18.3 mL/kg/h, i.v., for 4 h) followed by a bolus of dolasetron (Aventis Pharmaceuticals Inc., Kansas City, MO, USA; 0.6 mg/kg, i.v.). The calculated dose of cisplatin (60 mg/m2 body surface area) was then infused during a 20-min period. A dose of butorphanol (Fort Dodge Animal Health, Fort Dodge, IA, USA; 0.4 mg/kg, i.m.) was given immediately after completion of the cisplatin administration. Diuresis with 0.9% NaCl solution was continued for another 2 h.
The prescribed dose of calcitriol was diluted in 0.9% NaCl solution, protected from light, and delivered i.v. over 60 min simultaneously with initiation of the pre-cisplatin fluid diuresis. Injectable calcitriol contains polysorbate 20 and dogs are known to experience hypersensitivity reactions to this vehicle [37]. Therefore, 24 h before treatment with calcitriol, dogs were premedicated with prednisone (Lloyd Inc., Shenandoah, IA, USA; 1 mg/kg, p.o.) and then 30 min before treatment dogs were premedicated with dexamethasone sodium phosphate (American Reagent Laboratories Inc., Shirley, NY, USA; 2 mg/kg, i.v.), cimetidine (Hospira Inc., Lake Forest, IL, USA; 4 mg/kg, i.v.), and diphenhydramine (Baxter Healthcare Corp, Deerfield, IL, USA; 4 mg/kg, i.m.) as described by Poirier et al. [48]. Following treatment with calcitriol/cisplatin, dogs received a prophylactic antiemetic (metoclopramide, Pliva, East Hanover, NJ, USA; 0.5 mg/kg, p.o., q8 h for 7 days).
Clinical evaluation and follow-up
Baseline evaluation included a complete medical history, physical examination, CBC with differential and platelet count, serum biochemical analysis, and urinalysis. Gross tumors were not required, but when present, tumors were either directly measured with calipers or imaged and measured by use of radiography, ultrasonography, or computed tomography. Medical history, physical examination, CBC, serum biochemical analysis, and urinalysis were repeated on days 7, 14, and 21 after calcitriol/cisplatin administration. Evaluation of toxic effects of calcitriol/cisplatin was monitored by evaluation of the medical histories obtained from dog-owners and results of laboratory data. Toxic effects were graded in accordance with the Veterinary Co-operative Oncology Group Common Terminology Criteria for Adverse Events 1.0 [1]. As previously mentioned, corrected serum calcium was used to grade hypercalcemia.
Pharmacokinetic analysis
An indwelling catheter was inserted in the jugular vein of each dog, and blood samples (3 ml) were collected into nonheparinized tubes before, immediately after (0 min), and at 0.5, 1, 1.5, 2, 3, 4, 5, and 24 h after the calcitriol infusion. Samples were protected from light, centrifuged for 10 min at 2,000×g, and serum was harvested in 1–2 ml aliquots at −70°C until analyzed. Serum calcitriol levels were determined using the 1,25-dihydroxyvitamin D3-[I125] radioimmunoassay kit from DiaSorin Co (Stillwater, MN, USA). The analytic characteristics of this assay have previously been described [53]. Noncompartmental analysis of pharmacokinetic data was done using WinNonlin® Version 5.1, Pharmasight (Mountain View, CA, USA). The pharmacokinetic parameters estimated were: peak levels (Cmax), area under the concentration–time curve from time 0–24 h (AUC0–24 h), terminal half life (t1/2), volume of distribution (Vz) and total body clearance (Cltb). The relationship between pharmacokinetic variables Cmax, AUC0–24 h, and Vz and calcitriol dosage were evaluated by use of linear regression analysis, and the correlation coefficient was defined. Associations between t1/2 and Cltb, and calcitriol dosage were analyzed by nonlinear regression using SAS software (Cary, NC, USA).
Results
In vitro studies
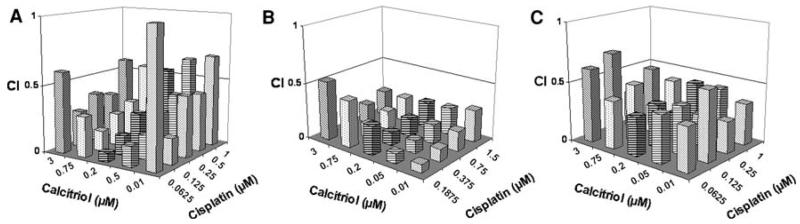
To explore the interaction between calcitriol and cisplatin in canine tumor cell lines, cells (CMT25, breast cancer; OS2.4, osteosarcoma, and C2, mast cell tumor), were treated with varying concentrations of either calcitriol or cisplatin alone, or they were pretreated with calcitriol followed by cisplatin; antiproliferative effects were measured using the MTT assay. As shown in Table 1, greater inhibition of cell growth was observed for the combination than for either single agent across all the canine cell lines. Utilizing a fixed ratio of calcitriol/cisplatin dose–effect data was determined and the combination index (CI) calculated (Fig. 1a-c). It was determined that the concentrations of calcitriol and cisplatin required for 50% growth inhibition were 3–8-fold lower, respectively, when the drugs were used in combination than when used individually. For most of the dose combinations tested, a CI value of <1 was obtained, indicating that the interaction between calcitriol and cisplatin is synergistic. Median dose–effect analysis also revealed apparent antagonism between the drugs when low concentrations of calcitriol were used in combination with higher cisplatin or calcitriol concentrations (data not shown).
Table 1.
Calcitriol and cisplatin induces growth inhibition (%) in canine tumor cell lines
| Cell line |
Calcitriol (0.2 μM) |
Cisplatin (0.25 μM) |
Calcitriol (0.2 μM) + Cisplatin (0.25 μM) |
|---|---|---|---|
| C2 | 0.1 | 8 | 69 |
| OS2.4 | 29 | 1 | 49 |
| CMT25 | 24 | 5 | 33 |
C2 mastocytoma, OS2.4 osteosarcoma, CMT25 breast cancer
Fig. 1.
Assessment of the interaction between calcitriol and cisplatin in canine tumor lines (a) breast CMT25, (b) osteosarcoma OS2.4, and (c) mastocytoma C2. Cells were plated into 96 well plates. After 24 h, cells were then either untreated or pre-treated for 24 h with various doses of calcitriol as indicated. Cells will then be either left with no further treatment or treated with cisplatin. After 72 h incubation, plates were harvested by staining with MTT and the dose–effect data obtained for each drug alone and in combination and these values will be used to calculate the CI as described in the “Materials and method”
In vivo study
Animals
Between February 2005 and October 2006, 22 dogs were entered into the phase I trial and received calcitriol at 8 dose levels. Patient characteristics are summarized in Table 2. Five of 22 (23%) were mixed-breed dogs and 17 (77%) were purebred including 3 Golden Retrievers, 3 Labrador Retrievers, 2 Rottweilers, 2 Australian Shepherds; 1 each were Boxer, Borzoi, Doberman Pinscher, German Shepherd, Gordon Setter, Greyhound, and Vizla. The majority (11 of 22, 50%) of dogs had osteosarcoma; tumors originated from the appendicular skeleton in 10 dogs and 1 had extraskeletal osteosarcoma of soft parts. Three (14%) dogs had chondrosarcoma, 2 (9%) squamous cell carcinoma, 2 melanoma, 2 soft tissue sarcoma, and 1 (4%) each had thyroid carcinoma and undifferentiated carcinoma. Fifteen dogs had previously undergone only surgery, 2 had received docetaxel chemotherapy, 1 had surgery and radiotherapy, 1 had surgery followed by radiotherapy and chemotherapy (doxorubicin and ifosfamide), and 3 dogs had no prior therapy.
Table 2.
Characteristics of 22 dogs with naturally occurring tumors treated with i.v. calcitriol combined with cisplatin
| Characteristics | No. of patients |
|---|---|
| Age (years) | |
| Median | 7 |
| Range | 3–14 |
| Weight (kg) | |
| Median | 33 |
| Range | 18–52 |
| Gender (male/female) | 13/9 |
| Breeds (purebred/mix) | 17/5 |
| Primary tumor | |
| Sarcoma | 16 |
| Carcinoma | 4 |
| Melanoma | 2 |
| Tumor volume | |
| Microscopic | 14 |
| Gross | 8 |
Determination of MTD and DLT
All 22 dogs were used for assessment of toxic effects after treatment with calcitriol/cisplatin. Dogs without DLT were able to receive the subsequent calcitriol dose level for a maximum of two treatments with calcitriol/cisplatin. Eight dose levels (32 treatments) were evaluated; 10 dogs received 2 dose levels and the remaining 12 dogs received 1 dose level. Hypercalcemia was the principal DLT (Table 3). At dose level 3 (0.5 μg/kg) 1 dog had dose-limiting renal toxicity. This dog had a serum creatinine concentration of 2.8 mg/dL (reference range 0.5–1.3 mg/dL) detected 7 days after treatment. Increased serum creatinine concentration was persistent when re-evaluated on days 14 and 21. Dose level 3 was expanded to a total of six dogs and there were no further episodes of DLT. At dose level 7 (3.75 μg/kg), one dog had hypercalcemia (serum calcium 14.5 mg/dL) with adverse gastrointestinal signs including grade 4 vomiting and abdominal pain (3 days after treatment). Dose level 7 was expanded to a total of seven dogs and there were no further episodes of DLT. At dose level 8 (5.5 μg/kg), two of four dogs had DLT consisting of hypercalcemia (serum calcium 13.7 mg/dL) and grade 4 vomiting in one dog (day 1 after treatment) and hypercalcemia (serum calcium 18.0 mg/dL) and grade 3 vomiting in the other dog (day 2 after treatment). This met the nontolerable dose level criterion of ≥2 of six affected dogs at a dosage. Thus, we concluded that i.v. calcitriol at a dosage of 3.75 μg/kg in combination with cisplatin at 60 mg/m2 was determined to be the MTD for future phase II trials in dogs.
Table 3.
Dose-escalation for i.v. calcitriol administration to dogs with various tumors and resulting toxic effects
| Dosage (μg/kg) | No. of dogs treated |
No. that received previous dose level |
No. of dogs with DLT |
|---|---|---|---|
| 0.1 | 3 | - | 0 |
| 0.25 | 2 | 2 | 0 |
| 0.50 | 6 | 0 | 1 |
| 1.0 | 3 | 3 | 0 |
| 1.50 | 3 | 0 | 0 |
| 2.25 | 4 | 2 | 0 |
| 3.75 | 7 | 1 | 1a |
| 5.50 | 4 | 2 | 2b |
Intrapatient dose-escalation was permitted for a maximum of 2 treatments per dog
DLT dose-limiting toxicity
1 dog with DLT did not receive any previous dose levels
2 dogs with DLT did not receive any previous dose levels
Hematological toxicity
Only two dogs treated on this study developed ≥grade 3 (<500 cells/μL) neutropenia. At dose level 3 (0.5 μg/kg), one dog had grade 3 neutropenia (600 cells/μL). At dose level 6 (2.25 μg/kg), one dog had grade 4 neutropenia (400 cells/μL). Both episodes of neutropenia were detected on day 14 after treatment, were not associated with any clinical signs of sepsis, and were resolved when rechecked on day 21. No dogs developed ≥grade 2 (<100,000 cells/μL) thrombocytopenia and no dogs developed anemia.
Nonhematological toxicity
Hypersensitivity reactions
The most common nonhematological adverse events were hypersensitivity reactions presumably secondary to polysorbate contained within the calcitriol formulation. Hypersensitivity reactions occurred in 11 dogs during 13 (41%) of the 32 calcitriol infusions. All episodes were characterized by pruritis (eight intense pruritis, five mild pruritis). Dogs also had urticaria during six episodes and a rash during three episodes. Calcitriol infusion times were extended to 2 h in four dogs given 2.25, 3.75, 5.5, and 5.5 μg/kg, respectively. One dog (calcitriol dosage 3.75 μg/kg) required a second premedication and had the infusion time extended to 4 h. No dog had treatment discontinued because of a hypersensitivity reaction.
Gastrointestinal toxicities
Vomiting occurred after 12 treatments. In three dogs, vomiting occurred with dose-limiting hypercalcemia and required hospitalization for IV fluid and parenteral antiemetics. Diarrhea occurred after eight treatments and was self-limiting after all but three. Anorexia occurred after six treatments and commonly occurred concurrently with vomiting and/or diarrhea. Gastrointestinal toxicities are summarized in Table 4.
Table 4.
Gastrointestinal toxicoses in dogs after i.v. administration of calcitriol combined with cisplatin
| Calcitriol dosage (μg/kg) |
No. of dogs |
Vomitinga |
Anorexiaa |
Diarrheaa |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||
| 0.1 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.25 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.50 | 6 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 |
| 1.0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1.50 | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 2.25 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 3.75 | 7 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 5.50 | 4 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Grading criteria [1]: grade 1 (<3 episodes vomiting in 24 h; coaxing or dietary change required to maintain appetite; increase of >2 stools per day over baseline) grade 2 (3–5 episodes vomiting in 24 h or <3 episodes per day for>2 but <5 days; oral intake altered for <3 days; increase of 2–6 stools per day, parenteral fluids indicated for <24 h), grade 3 (>5 episodes vomiting in 24 h or vomiting >4 days, fluid therapy indicated; anorexia 3–5 days with weight loss; increase of >6 stools per day, incontinence, hospitalization for IV fluids), grade 4 (life-threatening vomiting or diarrhea leading to hemodynamic collapse; anorexia >5 days), grade 5 (death)
Pharmacokinetics
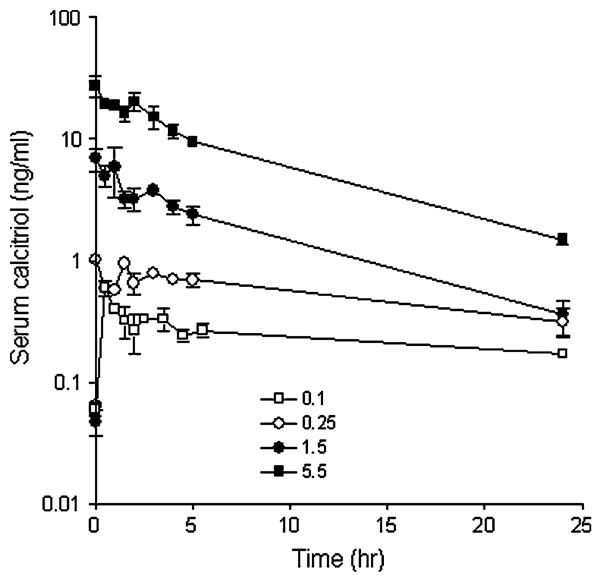
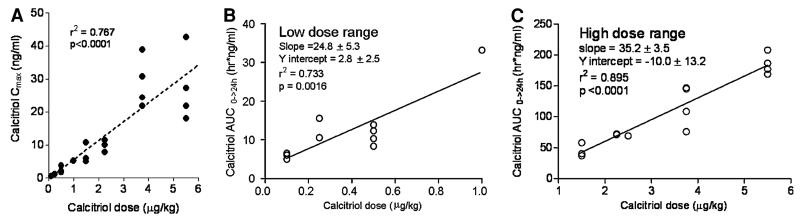
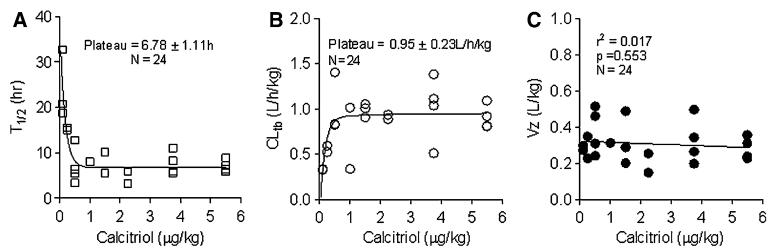
The median pretreatment serum calcitriol level was 60 pg/mL (range 5–95 pg/mL). Figure 2 shows concentration over time plots at two low calcitriol dosages (0.1 and 0.25 μg/kg), the intermediate calcitriol dosage (1.5 μg/kg) and at the highest calcitriol dosage administered (5.5 μg/kg). Peak serum levels were observed immediately after the calcitriol infusion and remained elevated 24 h post-treatment. A summary of the serum calcitriol pharmaco-kinetic variables at all calcitriol dose levels is shown in Table 5. The volume of distribution parameter indicates that the calcitriol distribution is primarily to the extracellular compartment. Figure 3 shows that the relationship between calcitriol dosage administered and either Cmax or AUC0–24 h. There is substantial variability in serum calcitriol Cmax at higher dosages (Fig. 3a). There is a linear relation between calcitriol dose and AUC, however, an expanded plots of AUC versus dose demonstrate the existence of two slopes—one for the low dose (<1 μg/kg) and another for high dose (>1 μg/kg). The slope for the higher dose (Fig. 3c) is steeper than the lower dose (Fig. 3b) implying a more rapid increase in AUC as the elimination half-life plateaus. Figure 4a, b show plots of t1/2 and Cltb versus dose, respectively. A fit to the equation t1/2 = a × (b + dose)/dose indicate that t1/2 was not proportional to dose (a = 6.78 ± 1.11 h and b = 0.33 ± 0.08/μg). The estimated a and b values for the fit of Cltb = a × dose/(b + dose) were 0.95 ± 0.23 L/h/kg and 0.13 ± 0.07/μg, respectively. If t1/2 and Cltb were proportional to dose, b = 0. The volume of distribution (Vz) was calcitriol dose-independent (Fig. 4c).
Fig. 2.
Serum calcitriol concentration over time plots after i.v. administration of 0.1, 0.25, 1.5 and 5.5 μg/kg calcitriol. Slope of the elimination phase is different for the low and high calcitriol dosages
Table 5.
Pharmacokinetic variables for calcitriol in dogs after i.v. administration of a single dose
| Calcitriol dosage (μg/kg) | N | Cmax (ng/mL) | t1/2 (h) | AUC0->24 h (h ng/mL) | Vz (L/kg) | Cltb (L/h/kg) |
|---|---|---|---|---|---|---|
| 0.1 | 3 | 0.60 ± 0.02 | 24.1 ± 4.4 | 5.86 ± 0.46 | 0.29 ± 0.01 | 0.33 ± 0.00 |
| 0.25 | 2 | 1.13 ± 0.05 | 15.3 ± 0.2 | 13.1 ± 2.50 | 0.29 ± 0.06 | 0.56 ± 0.02 |
| 0.50 | 4 | 2.42 ± 0.47 | 7.1 ± 2.0 | 11.2 ± 1.20 | 0.38 ± 0.06 | 1.28 ± 0.29 |
| 1.0 | 1 | 5.20 | 8.1 | 33.2 | 0.24 | 1.01 |
| 1.50 | 3 | 7.26 ± 1.81 | 7.2 ± 1.6 | 45.1 ± 6.51 | 0.33 ± 0.09 | 0.99 ± 0.04 |
| 2.25 | 3 | 9.8 ± 1.1 | 4.1 ± 0.92 | 70.8 ± 0.91 | 0.16 ± 0.06 | 0.72 ± 0.19 |
| 3.75 | 4 | 29.0 ± 3.81 | 7.7 ± 1.3 | 119.3 ± 16.9 | 0.33 ± 0.06 | 1.01 ± 0.18 |
| 5.50 | 4 | 27.5 ± 5.43 | 7.1 ± 0.7 | 185.2 ± 8.43 | 0.28 ± 0.03 | 0.91 ± 0.07 |
Results represent the mean ± SEM
Fig. 3.
a Plot showing relationship between calcitriol dosage and serum calcitriol Cmax; b plot showing relationship between calcitriol dosage < 1 μg/kg and AUC achieved; c plot showing relationship between calcitriol dosage > 1 μg/kg and AUC achieved
Fig. 4.
Plot showing relationship between calcitriol dosage and serum calcitriol elimination t1/2 (a), total body calcitriol clearance (b) and volume of distribution (c)
In vivo antitumor activity
For inclusion in the study reported here, dogs did not need to have tumors of measurable volume, and assessment of tumor response to calcitriol/cisplatin was not the primary goal of the study. Nonetheless, eight dogs had measurable tumors and three had complete responses characterized by 100% reduction in tumor volume. Dogs that achieved complete response included one dog with pulmonary metastases of soft tissue sarcoma (calcitriol dosage 0.25 μg/kg), one dog with multifocal cutaneous squamous cell carcinoma (calcitriol dosage 1.0 μg/kg), and one dog with malignant melanoma of the maxillary gingiva and bone (2.25 μg/kg).
Discussion
On the basis of the findings reported here, a dosage of 3.75 μg of calcitriol per kg body weight was administered i.v. every 3 weeks with cisplatin appears to be appropriate for tumor-bearing dogs. This dosage of calcitriol is considerably higher than that used to treat other canine diseases. A daily oral calcitriol dosage of 0.0025 μg/kg is used to treat secondary renal hyperparathyroidism in end stage renal disease [7]. Calcitriol dosages of 0.01–0.02 μg/ kg per day are used to manage hypocalcemia in dogs with naturally occurring primary hypoparathyroidism or after extensive surgery for bilateral thyroid or parathyroid neo-plasia [18]. Preclinical data indicate that the antitumor activity of calcitriol is dose-dependent. In a murine squamous cell carcinoma model, doses of calcitriol that result in Cmax > 10.0 ng/mL and AUC > 40.0 h ng/mL are effective at suppressing tumor growth [42]. In the study reported herein, calcitriol dosages >1.5 μg/kg were associated with serum levels ≥9.8 ng/mL and calcitriol dosages >1.0 μg/ kg were associated with systemic exposure ≥45 h ng/mL. This data supports intermittent use of high-dosages of calcitriol as determined in our phase I study for future phase II and III studies in dogs with cancer.
As anticipated, the DLT was hypercalcemia. This was observed in two of four dogs given 5.5 μg calcitriol per kg. One dog also developed hypercalcemia at a lower dosage of calcitriol (3.75 μg/kg). Based on the study design, total serum calcium was measured beginning 7 days after treatment. All dogs with dose-limiting hypercalcemia had severe gastroenteritis that prompted laboratory evaluation before day 7. It is probable that the frequency of hypercalcemia was higher in other asymptomatic dogs in the study, but resolved within 1 week of treatment. Also, ionized calcium is the most biologically active fraction of serum calcium [20] but total serum calcium and adjusted total calcium concentration do not reliably predict ionized calcium status in dogs [51]. Future studies of high-dose calcitriol in dogs should monitor serum calcium, and possibly ionized calcium, 24–48 h after treatment. Glucorticoids not only enhance the antitumor effects of calcitriol [5, 61] but also might be useful to decrease calcitriol-induced hypercalcemia as has been shown in people [27].
Dogs with dose-limiting hypercalcemia had signs of gastroenteritis. Dogs studied after being given cholecal-ciferol-based rodenticide develop anorexia, hematemesis, and hematochezia due to hypercalcemia and soft-tissue mineralization in the lining of the stomach wall [49]. In a study of 41 dogs treated with single-agent cisplatin, 27 (66%) developed adverse gastrointestinal effects; the dosage of cisplatin was similar to that reported here [30]. Clinical signs of gastroenteritis observed in dogs in the present study might have been attributable to severe hypercalcemia, cisplatin, or a combination of these factors.
One dog on this trial developed dose-limiting renal toxicity. In the cases of severe hypercalcemia, kidney damage might occur secondary to renal vasoconstriction, tubular necrosis, and dystrophic mineralization of tubular epithelium [17]. In the dog in this study, however, elevated serum calcium was not detected when measured on days 7–21 after treatment. The adverse renal tubular effects of cisplatin are well characterized [55]. In a report of 18 dogs given 1–6 treatments of cisplatin, 4 (22%) experienced renal toxicity [11]. It is very likely that the renal damage in the dog in the current study occurred because of the cisplatin treatment.
The linear relationship between calcitriol dosage and either Cmax or AUC observed in this study is in agreement with our recent report showing similar linear relationships in people with cancer treated with high doses of i.v. calcitriol [16]. Hypersensitivity reactions occurred during 41% of the calcitriol infusions administered to the dogs in our study; the etiology of this toxicity is suspected to be a result of the solubilizing agent, polysorbate, in which parenteral calcitriol is formulated [37]. Oral administration of calcitriol would circumvent this adverse effect, but in people there is a lack of a dose-dependent increase in calcitriol serum concentrations when oral dosages above 0.5 μg/kg of commercially available calcitriol are given [3, 4, 43]. However, a new oral formulation of calcitriol designed specifically for cancer therapy (DN101, Novacea, Inc., San Francisco, CA, USA) has recently been shown to exhibit linear relationships between dose and either Cmax or AUC although substantial variability was observed in people treated with high doses [3]. In dogs, it is unknown if the MTD and/or pharmacokinetic characteristics of oral calcitriol (commercial caplets or DN101) will differ from i.v. calcitriol. Studies to evaluate commercially available calcitriol preparations in dogs are not reasonable since the largest caplet size is 0.5 μg, and administering 3.75 μg/kg to an average-sized dog would require approximately 250 caplets. Investigation of the pharmacokinetic disposition of DN101 in dogs is warranted.
The relationship between calcitriol dosage and elimination t1/2, which we observed in the study, suggests saturable clearance mechanisms at calcitriol dosages ≥1.0 μg/kg. Although the pharmacologic basis for this observation is unknown, the attainment of maximal induction of CYP24A1-mediated metabolic clearance at calcitriol dosages ≥1.0 μg/kg is a potential mechanism for the plateau of the calcitriol elimination t1/2. CYP24A1 is the major vitamin D3 catabolizing enzyme [2, 45, 46] and delayed calcitriol clearance in CYP24A1 knockout mice indicates the pivotal role of CYP24 in regulating vitamin D3 homeostasis [38, 54]. The lack of similar observations in people could be attributed to species differences in calcitriol dosages required to maximally induce CYP24A1 activity. Furthermore, calcitriol dosages ≥3.0 μg/kg have not been administered intravenously to people with cancer. Other contributing factors in need of further investigation include species differences in polymorphisms in vitamin D receptor and vitamin D3 metabolizing enzymes such as CYP24A1.
Our in vitro studies show calcitriol significantly enhanced cisplatin-mediated antitumor activity in multiple cell lines. Similar synergistic effects have been observed in murine squamous cell carcinoma [25, 35] and human breast carcinoma [10, 47], prostatic carcinoma [40], and leukemia [47] cell lines. Single-agent calcitriol has been shown to exert antiproliferative effects against various canine epithelial malignancies including squamous cell carcinoma [33], adenocarcinoma [34], and transitional cell carcinoma [29]. To date, this is the first study to examine the effects of calcitriol and calcitriol combined with cisplatin against nonepithelial tumor cells.
In summary, our in vitro data demonstrate that calcitriol and cisplatin have synergistic antiproliferative effects on multiple canine tumor cells. Our in vivo data demonstrate that a high-dosage of i.v. calcitriol can be safely combined with cisplatin in dogs and that the pharmacokinetic parameters achieved exceed those associated with antitumor activity in a mouse model. Although assessment of tumor response was not the primary goal of our in vivo study, antineoplastic activity was observed in three of eight dogs with measurable tumors treated by administration of calcitriol/cisplatin. Taken together, these results indicate that calcitriol/cisplatin-based combination therapies might have significant clinical activity in the treatment of a variety of solid tumors.
Acknowledgments
This work was supported by grants (NCI and DOD Grants CA67267, CA95045, and PCRP050202) and The Sprecher Institute for Comparative Cancer Research, Cornell University. The authors thank Dr. Lili Tian, Associate Professor of Biostatistics, University of Buffalo, for assistance with statistical analysis.
Footnotes
This article was presented in part as a poster presentation at the 13th Workshop on Vitamin D, Victoria, British Columbia, Canada, 8–12 April 2006.
Contributor Information
Kenneth M. Rassnick, Department of Clinical Sciences, Cornell University College of Veterinary Medicine, Box 31, Ithaca, NY 14853, USA
Josephia R. Muindi, Department of Pharmacology and Therapeutics, Roswell Park Cancer Institute, Buffalo, NY 14263, USA
Candace S. Johnson, Department of Pharmacology and Therapeutics, Roswell Park Cancer Institute, Buffalo, NY 14263, USA
Cheryl E. Balkman, Department of Clinical Sciences, Cornell University College of Veterinary Medicine, Box 31, Ithaca, NY 14853, USA
Nithya Ramnath, Department of Medicine, Roswell Park Cancer Institute, Buffalo, NY 14263, USA.
Wei-Dong Yu, Department of Pharmacology and Therapeutics, Roswell Park Cancer Institute, Buffalo, NY 14263, USA.
Kristie L. Engler, Department of Pharmacology and Therapeutics, Roswell Park Cancer Institute, Buffalo, NY 14263, USA
Rodney L. Page, Department of Clinical Sciences, Cornell University College of Veterinary Medicine, Box 31, Ithaca, NY 14853, USA
Donald L. Trump, Department of Medicine, Roswell Park Cancer Institute, Buffalo, NY 14263, USA
References
- 1.Veterinary co-operative oncology group common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Compar Oncol. 2004;2:195–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 2.Beckman MJ, Tadikonda P, Werner E, Prahl J, Yamada S, DeLuca HF. Human 25-hydroxyvitamin D3–24-hydroxylase, a multicatalytic enzyme. Biochemistry. 1996;35:8465–8472. doi: 10.1021/bi960658i. [DOI] [PubMed] [Google Scholar]
- 3.Beer TM, Javle MM, Ryan CW, Garzotto M, Lam GN, Wong A, Henner WD, Johnson CS, Trump DL. Phase I study of weekly DN-101, a new formulation of calcitriol, in patients with cancer. Cancer Chemother Pharmacol. 2007;59:581–587. doi: 10.1007/s00280-006-0299-1. [DOI] [PubMed] [Google Scholar]
- 4.Beer TM, Munar M, Henner WD. A phase I trial of pulse calcitriol in patients with refractory malignancies: pulse dosing permits substantial dose escalation. Cancer. 2001;91:2431–2439. [PubMed] [Google Scholar]
- 5.Bernardi RJ, Trump DL, Yu WD, McGuire TF, Hershberger PA, Johnson CS. Combination of 1alpha,25-dihydroxyvitamin D(3) with dexamethasone enhances cell cycle arrest and apoptosis: role of nuclear receptor cross-talk and Erk/Akt signaling. Clin Cancer Res. 2001;7:4164–4173. [PubMed] [Google Scholar]
- 6.Boyle BJ, Zhao XY, Cohen P, Feldman D. Insulin-like growth factor binding protein-3 mediates 1 alpha,25-dihydroxyvitamin d(3) growth inhibition in the LNCaP prostate cancer cell line through p21/WAF1. J Urol. 2001;165:1319–1324. [PubMed] [Google Scholar]
- 7.Brown SA, Finco DR. Reassessment of the use of calcitriol in chronic renal failure. In: Bonagura JD, editor. Kirk's current veterinary therapy XII small animal practice. W.B. Saunders Co; Philadelphia: 1995. pp. 963–966. [Google Scholar]
- 8.Campbell MJ, Koeffler HP. Toward therapeutic intervention of cancer by vitamin D compounds. J Natl Cancer Inst. 1997;89:182–185. doi: 10.1093/jnci/89.3.182. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay N, MacLeod RJ, Tfelt-Hansen J, Brown EM. 1alpha,25(OH)2-vitamin D3 inhibits HGF synthesis and secretion from MG-63 human osteosarcoma cells. Am J Physiol Endocrinol Metab. 2003;284:E219–E227. doi: 10.1152/ajpendo.00247.2002. [DOI] [PubMed] [Google Scholar]
- 10.Cho YL, Christensen C, Saunders DE, Lawrence WD, Deppe G, Malviya VK, Malone JM. Combined effects of 1,25-dihydroxyvitamin D3 and platinum drugs on the growth of MCF-7 cells. Cancer Res. 1991;51:2848–2853. [PubMed] [Google Scholar]
- 11.Chun R, Knapp DW, Widmer WR, Glickman NW, DeNicola DB, Bonney PL. Cisplatin treatment of transitional cell carcinoma of the urinary bladder in dogs: 18 cases (1983–1993) J Am Vet Med Assoc. 1996;209:1588–1591. [PubMed] [Google Scholar]
- 12.Chung I, Yu WD, Karpf AR, Flynn G, Bernardi RJ, Modzelewski RA, Johnson CS, Trump DL. Anti-proliferative effects of calcitriol on endothelial cells derived from two different micro-environments. J Steroid Biochem Mol Biol. 2007;103:768–770. doi: 10.1016/j.jsbmb.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 13.Deak JC, Cross JV, Lewis M, Qian Y, Parrott LA, Distelhorst CW, Templeton DJ. Fas-induced proteolytic activation and intracellular redistribution of the stress-signaling kinase MEKK1. Proc Natl Acad Sci USA. 1998;95:5595–5600. doi: 10.1073/pnas.95.10.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeVinney R, Gold WM. Establishment of two dog mastocytoma cell lines in continuous culture. Am J Respir Cell Mol Biol. 1990;3:413–420. doi: 10.1165/ajrcmb/3.5.413. [DOI] [PubMed] [Google Scholar]
- 15.Elstner E, Heber D, Koeffler HP. 20-Epi-vitamin D3 analogs. Potent modulators of proliferation and differentiation of breast cancer cell lines in vitro. Adv Exp Med Biol. 1996;399:53–70. [PubMed] [Google Scholar]
- 16.Fakih MG, Trump DL, Muindi JR, Black JD, Bernardi RJ, Creaven PJ, Schwartz J, Brattain MG, Hutson A, French R, Johnson CS. A phase I pharmacokinetic and pharmaco-dynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13:1216–1223. doi: 10.1158/1078-0432.CCR-06-1165. [DOI] [PubMed] [Google Scholar]
- 17.Feldman EC, Nelson RW. Hypercalcemia and hyperparathyroidism. In: Feldman EC, Nelson RW, editors. Canine and feline endocrinology and reproduction. Saunders; St. Louis: 2004. pp. 660–715. [Google Scholar]
- 18.Feldman EC, Nelson RW. Hypocalcemia and primary hypoparathyroidism. In: Feldman EC, Nelson RW, editors. Canine and feline endocrinology and reproduction. Saunders; St. Louis: 2004. pp. 716–742. [Google Scholar]
- 19.Fineman LS, Hamilton TA, de Gortari A, Bonney P. Cisplatin chemotherapy for treatment of thyroid carcinoma in dogs: 13 cases. J Am Anim Hosp Assoc. 1998;34:109–112. doi: 10.5326/15473317-34-2-109. [DOI] [PubMed] [Google Scholar]
- 20.Forman DT, Lorenzo L. Ionized calcium: its significance and clinical usefulness. Ann Clin Lab Sci. 1991;21:297–304. [PubMed] [Google Scholar]
- 21.Gebauer G, Mirakhur B, Nguyen Q, Shore SK, Simpkins H, Dhanasekaran N. Cisplatin-resistance involves the defective processing of MEKK1 in human ovarian adenocarcinoma 2008/C13 cells. Int J Oncol. 2000;16:321–325. doi: 10.3892/ijo.16.2.321. [DOI] [PubMed] [Google Scholar]
- 22.Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG, Jr, Levrero M, Wang JY. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 23.Haugen JD, Pittelkow MR, Zinsmeister AR, Kumar R. 1 Alpha,25-dihydroxyvitamin D3 inhibits normal human keratinocyte growth by increasing transforming growth factor beta 2 release. Biochem Biophys Res Commun. 1996;229:618–623. doi: 10.1006/bbrc.1996.1853. [DOI] [PubMed] [Google Scholar]
- 24.Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 25.Hershberger PA, McGuire TF, Yu WD, Zuhowski EG, Schellens JH, Egorin MJ, Trump DL, Johnson CS. Cisplatin potentiates 1,25-dihydroxyvitamin D3-induced apoptosis in association with increased mitogen-activated protein kinase kinase kinase 1 (MEKK-1) expression. Mol Cancer Ther. 2002;1:821–829. [PubMed] [Google Scholar]
- 26.James SY, Mackay AG, Colston KW. Effects of 1,25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:395–401. doi: 10.1016/0960-0760(96)00048-9. [DOI] [PubMed] [Google Scholar]
- 27.Johnson CS, Muindi JR, Hershberger PA, Trump DL. The antitumor efficacy of calcitriol: preclinical studies. Anticancer Res. 2006;26:2543–2549. [PubMed] [Google Scholar]
- 28.Johnson SW, O'Dwyer PJ. Cisplatin and its analogues. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer principles and practice of oncology. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 344–358. [Google Scholar]
- 29.Kaewsakhorn T, Kisseberth WC, Capen CC, Hayes KA, Calverley MJ, Inpanbutr N. Effects of calcitriol, seocalcitol, and medium-chain triglyceride on a canine transitional cell carcinoma cell line. Anticancer Res. 2005;25:2689–2696. [PubMed] [Google Scholar]
- 30.Knapp DW, Richardson RC, Bonney PL, Hahn K. Cisplatin therapy in 41 dogs with malignant tumors. J Vet Intern Med. 1988;2:41–46. doi: 10.1111/j.1939-1676.1988.tb01976.x. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi T, Hashimoto K, Yoshikawa K. Growth inhibition of human keratinocytes by 1,25-dihydroxyvitamin D3 is linked to dephosphorylation of retinoblastoma gene product. Biochem Biophys Res Commun. 1993;196:487–493. doi: 10.1006/bbrc.1993.2276. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan AV, Moreno J, Nonn L, Malloy P, Swami S, Peng L, Peehl DM, Feldman D. Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J Steroid Biochem Mol Biol. 2007;103:694–702. doi: 10.1016/j.jsbmb.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 33.Kunakornsawat S, Rosol TJ, Capen CC, Middleton RP, Hannah SS, Inpanbutr N. Effects of 1,25(OH)2D3, EB1089, and analog V on PTHrP production, PTHrP mRNA expression and cell growth in SCC 2/88. Anticancer Res. 2001;21:3355–3363. [PubMed] [Google Scholar]
- 34.Kunakornsawat S, Rosol TJ, Capen CC, Reddy GS, Binderup L, Inpanbutr N. Effects of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] and its analogues (EB1089 and analog V) on canine adenocarcinoma (CAC-8) in nude mice. Biol Pharm Bull. 2002;25:642–647. doi: 10.1248/bpb.25.642. [DOI] [PubMed] [Google Scholar]
- 35.Light BW, Yu WD, McElwain MC, Russell DM, Trump DL, Johnson CS. Potentiation of cisplatin antitumor activity using a vitamin D analogue in a murine squamous cell carcinoma model system. Cancer Res. 1997;57:3759–3764. [PubMed] [Google Scholar]
- 36.Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 37.Masini E, Planchenault J, Pezziardi F, Gautier P, Gagnol JP. Histamine-releasing properties of Polysorbate 80 in vitro and in vivo: correlation with its hypotensive action in the dog. Agents Actions. 1985;16:470–477. doi: 10.1007/BF01983649. [DOI] [PubMed] [Google Scholar]
- 38.Masuda S, Byford V, Arabian A, Sakai Y, Demay MB, Arnaud R, Jones G. Altered pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology. 2005;146:825–834. doi: 10.1210/en.2004-1116. [DOI] [PubMed] [Google Scholar]
- 39.Mealey KL, Barhoumi R, Rogers K, Kochevar DT. Doxorubicin induced expression of P-glycoprotein in a canine osteosarcoma cell line. Cancer Lett. 1998;126:187–192. doi: 10.1016/s0304-3835(98)00004-4. [DOI] [PubMed] [Google Scholar]
- 40.Moffatt KA, Johannes WU, Miller GJ. 1Alpha,25dihydroxyvitamin D3 and platinum drugs act synergistically to inhibit the growth of prostate cancer cell lines. Clin Cancer Res. 1999;5:695–703. [PubMed] [Google Scholar]
- 41.Moore AS, Cardona A, Shapiro W, Madewell BR. Cisplatin (cisdiamminedichloroplatinum) for treatment of transitional cell carcinoma of the urinary bladder or urethra. A retrospective study of 15 dogs. J Vet Intern Med. 1990;4:148–152. doi: 10.1111/j.1939-1676.1990.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 42.Muindi JR, Modzelewski RA, Peng Y, Trump DL, Johnson CS. Pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 in normal mice after systemic exposure to effective and safe antitumor doses. Oncology. 2004;66:62–66. doi: 10.1159/000076336. [DOI] [PubMed] [Google Scholar]
- 43.Muindi JR, Potter DM, Peng Y, Johnson CS, Trump DL. Pharmacokinetics of liquid calcitriol formulation in advanced solid tumor patients: comparison with caplet formulation. Cancer Chemother Pharmacol. 2005;56:492–496. doi: 10.1007/s00280-005-1015-2. [DOI] [PubMed] [Google Scholar]
- 44.Nehme A, Baskaran R, Nebel S, Fink D, Howell SB, Wang JY, Christen RD. Induction of JNK and c-Abl signalling by cisplatin and oxaliplatin in mismatch repair-proficient and -deficient cells. Br J Cancer. 1999;79:1104–1110. doi: 10.1038/sj.bjc.6690176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okuda K, Usui E, Ohyama Y. Recent progress in enzymology and molecular biology of enzymes involved in vitamin D metabolism. J Lipid Res. 1995;36:1641–1652. [PubMed] [Google Scholar]
- 46.Omdahl JL, Morris HA, May BK. Hydroxylase enzymes of the vitamin D pathway: expression, function, and regulation. Annu Rev Nutr. 2002;22:139–166. doi: 10.1146/annurev.nutr.22.120501.150216. [DOI] [PubMed] [Google Scholar]
- 47.Pelczynska M, Switalska M, Maciejewska M, Jaroszewicz I, Kutner A, Opolski A. Antiproliferative activity of vitamin D compounds in combination with cytostatics. Anticancer Res. 2006;26:2701–2705. [PubMed] [Google Scholar]
- 48.Poirier VJ, Hershey AE, Burgess KE, Phillips B, Turek MM, Forrest LJ, Beaver L, Vail DM. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J Vet Intern Med. 2004;18:219–222. doi: 10.1892/0891-6640(2004)18<219:eatopt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Rumbeiha WK, Kruger JM, Fitzgerald SF, Nachreiner RF, Kaneene JB, Braselton WE, Chiapuzio CL. Use of pamidronate to reverse vitamin D3-induced toxicosis in dogs. Am J Vet Res. 1999;60:1092–1097. [PubMed] [Google Scholar]
- 50.Sanchez-Perez I, Perona R. Lack of c-Jun activity increases survival to cisplatin. FEBS Lett. 1999;453:151–158. doi: 10.1016/s0014-5793(99)00690-0. [DOI] [PubMed] [Google Scholar]
- 51.Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by use of serum total calcium concentration in dogs. Am J Vet Res. 2005;66:1330–1336. doi: 10.2460/ajvr.2005.66.1330. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz GG, Wang MH, Zang M, Singh RK, Siegal GP. 1 alpha,25-Dihydroxyvitamin D (calcitriol) inhibits the invasiveness of human prostate cancer cells. Cancer Epidemiol Biomarkers Prev. 1997;6:727–732. [PubMed] [Google Scholar]
- 53.Smith DC, Johnson CS, Freeman CC, Muindi J, Wilson JW, Trump DL. A phase I trial of calcitriol (1,25-dihydroxycholecalciferol) in patients with advanced malignancy. Clin Cancer Res. 1999;5:1339–1345. [PubMed] [Google Scholar]
- 54.Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay MB, Glorieux FH. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
- 55.Taguchi T, Nazneen A, Abid MR, Razzaque MS. Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol. 2005;148:107–121. doi: 10.1159/000086055. [DOI] [PubMed] [Google Scholar]
- 56.Tang W, Ziboh VA, Isseroff RR, Martinez D. Novel regulatory actions of 1 alpha,25-dihydroxyvitamin D3 on the metabolism of polyphosphoinositides in murine epidermal keratinocytes. J Cell Physiol. 1987;132:131–136. doi: 10.1002/jcp.1041320118. [DOI] [PubMed] [Google Scholar]
- 57.Trump DL, Muindi J, Fakih M, Yu WD, Johnson CS. Vitamin D compounds: clinical development as cancer therapy and prevention agents. Anticancer Res. 2006;26:2551–2556. [PubMed] [Google Scholar]
- 58.Widmann C, Gerwins P, Johnson NL, Jarpe MB, Johnson GL. MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol Cell Biol. 1998;18:2416–2429. doi: 10.1128/mcb.18.4.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Withrow SJ, Liptak JM, Straw RC, Dernell WS, Jameson VJ, Powers BE, Johnson JL, Brekke JH, Douple EB. Biodegradable cisplatin polymer in limb-sparing surgery for canine osteosarcoma. Ann Surg Oncol. 2004;11:705–713. doi: 10.1245/ASO.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Wolfe LG, Smith BB, Toivio-Kinnucan MA, Sartin EA, Kwapien RP, Henderson RA, Barnes S. Biologic properties of cell lines derived from canine mammary carcinomas. J Natl Cancer Inst. 1986;77:783–792. doi: 10.1093/jnci/77.3.783. [DOI] [PubMed] [Google Scholar]
- 61.Yu WD, McElwain MC, Modzelewski RA, Russell DM, Smith DC, Trump DL, Johnson CS. Enhancement of 1,25-dihydroxyvitamin D3-mediated antitumor activity with dexamethasone. J Natl Cancer Inst. 1998;90:134–141. doi: 10.1093/jnci/90.2.134. [DOI] [PubMed] [Google Scholar]