Addictions are a diverse set of common, complex diseases that are to some extent tied together by shared genetic and environmental etiological factors. They are frequently chronic, with a relapsing/remitting course. Genetic studies and other analyses clarifying the origins of addiction help destigmatize addiction, leading to more prompt treatment. Knowledge of genetic factors in etiology and treatment response may enable the individualization of prevention and treatment, as well as the identification of new therapeutic targets.
Addictions are chronic relapsing psychiatric disorders characterized by the compulsive and dyscontrolled use of a drug or activity, with maladaptive and destructive outcomes. Although use of addictive agents is volitional, addiction leads to loss of volitional control. The use and abuse of legal and illegal psychoactive substances is a worldwide public health priority with repercussions extending from the level of the individual to the family, community, and society. The World Health Organization, in its 2004 Global Status Report on Alcohol (http://www.who.int/substance_abuse/publications/global_status_report_2004_overview.pdf), estimated that 2 billion people consume alcoholic beverages and 76.3 million of these have an alcohol use disorder. There are 1.3 billion tobacco users worldwide (http://www.who.int/substance_abuse/facts/global_burden/en/index.html). The United Nations estimated that in the late 1990s some 185 million people worldwide—4.3% of people aged 15 years and above—were consuming illicit drugs (http://www.unodc.org/unodc/en/data-and-analysis/WDR.html).
Three phenomena characterize addiction: craving (preoccupation/anticipation), binge/intoxication, and withdrawal/negative affect.1 Impulsivity and positive reinforcement often dominate the first stages, driving the motivation for drug seeking, and compulsivity and negative reinforcement dominate the terminal stages of the addiction cycle. Addictive drugs induce adaptive changes in gene expression in brain reward regions, including the striatum,2 representing a mechanism for tolerance and habit formation with craving and negative affect that persist long after consumption ceases. These neuroadaptive changes are key elements in relapse. Once an individual becomes addicted, the clinical options are untargeted and only partially effective. To what extent are the processes involved in the initiation and maintenance of drug use influenced by inherited variation, such that new therapies and preventive strategies could be developed and better targeted to individuals?
Genes and environment
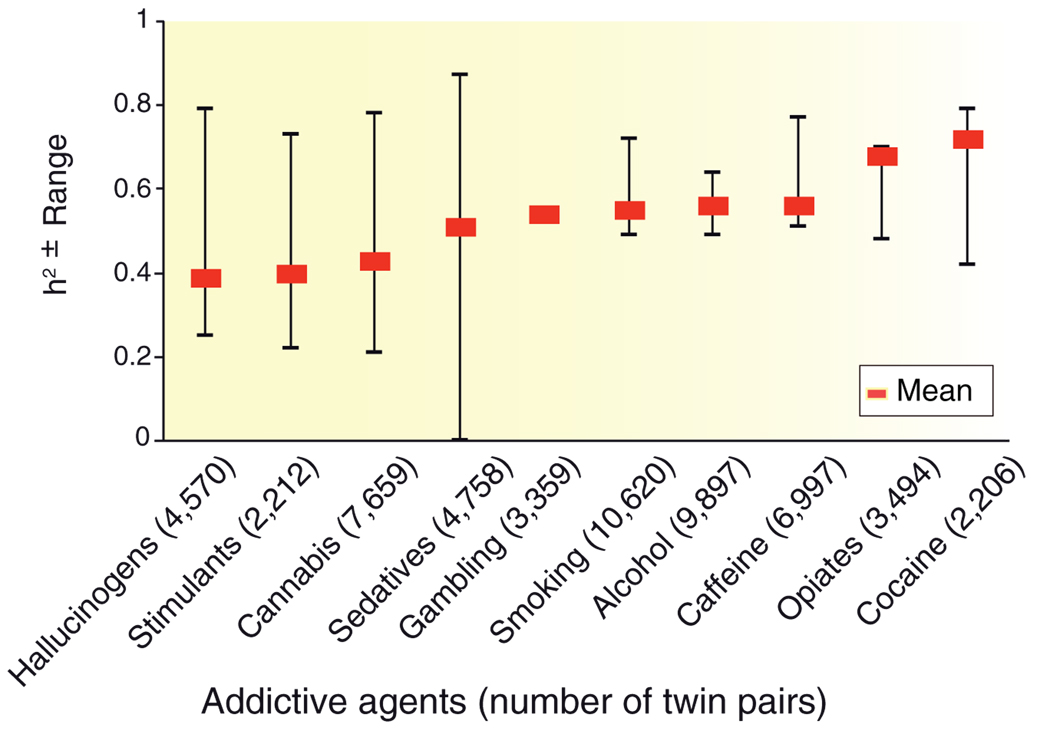
Both genetic and environmental variables contribute to the initiation of use of addictive agents and to the transition from use to addiction. Addictions are moderately to highly heritable. Family, adoption, and twin studies reveal that an individual’s risk tends to be proportional to the degree of genetic relationship to an addicted relative. Heritabilities of addictive disorders range from 0.39 for hallucinogens to 0.72 for cocaine3 (Figure 1). An important view of the shifting balance in importance of genetic and environmental influences has been obtained from the developmental perspective. The Virginia Twin Study revealed that in early adolescence the initiation and use of nicotine, alcohol, and cannabis are more strongly determined by familial and social factors, but these gradually decline in importance during the progression to young and middle adulthood, when the effects of genetic factors become maximal, declining somewhat with aging.4
Figure 1.
Heritability (weighted mean and range) of 10 addictive disorders: dependence on or abuse of hallucinogens, stimulants, cannabis, sedatives, opiates, and cocaine; alcohol dependence; smoking persistence; caffeine consumption or heavy use; and pathological gambling. Weighted heritability means were computed using data from large national surveys of adult twins. Reprinted from ref. 3.
The moderate to high heritabilities of addictive disorders are paradoxical, because addictions initially depend on the availability of the addictive agent and the individual’s choice to use it. However, it should be taken into account that heritability studies are carried out in populations and age cohorts that tend to share likelihood of exposure and that also tend to be similar in experience of other environmental factors that influence risk. The availability of addictive agents is determined by culture, social policy, religion, economic status, and narco-trafficking, and it changes across time and space. Thus, the twin studies on addiction do not reveal the full reaction range of genotype but indicate that under particular (and highly relevant) societal scenarios, genotype plays a substantial role in vulnerability. Like other complex diseases, such as obesity, diabetes, cancer, coronary heart disease, and AIDS, the addictions are strongly influenced by genetic background and also profoundly influenced by lifestyle and individual choices. Although addictions show no clear pattern of mendelian inheritance and their complexity is poorly understood, on the basis of their moderate to high heritability it is evident that they are strongly influenced by inherited functional variations, and identification of these alleles is key to understanding the puzzle of causality.
Genetic approaches
Gene identification is accomplished both by genome-wide methods and by candidate gene studies, both of which can access intermediate phenotypes. Genome-wide analysis, including whole-genome linkage, whole-genome association, and mRNA expression analyses, allows the hypothesis-free mapping of disease-causing loci within the genome. Whole-genome linkage studies are used in family-based samples to test polymorphisms and meiotic linkage to a disease for chromosome regions that are shared more often among phenotypically concordant relatives than among ones who are phenotypically discordant. This approach is powerful in detecting effects of uncommon and rare alleles present in probands and their families. Whole-genome scans, by contrast, have greater power to detect effects of relatively common alleles (minor allele frequency >0.05) and allow for a more refined localization of signals to smaller chromosome regions. Whole-genome analysis can be conducted through the use of intermediate phenotypes with an increase of power. Intermediate phenotypes are the result of deconstruction of complex phenotypes to mechanism-related manifestations of genes and environment. Electrophysiological, psychological, neurochemical, and neuroimaging phenotypes, but also heritable, measurable biochemical, endocrinological, neuroanatomical, cognitive, and neuropsychological parameters, called endophenotypes, may predict diathesis to psychiatric disease. Stress resiliency and externalizing behaviors, characterized by disinhibition, aggression, and impulsivity, are both intermediate phenotypes thought to underlie the comorbidity between addictions and other psychiatric diseases, leading to the potential to track shared genetic factors.
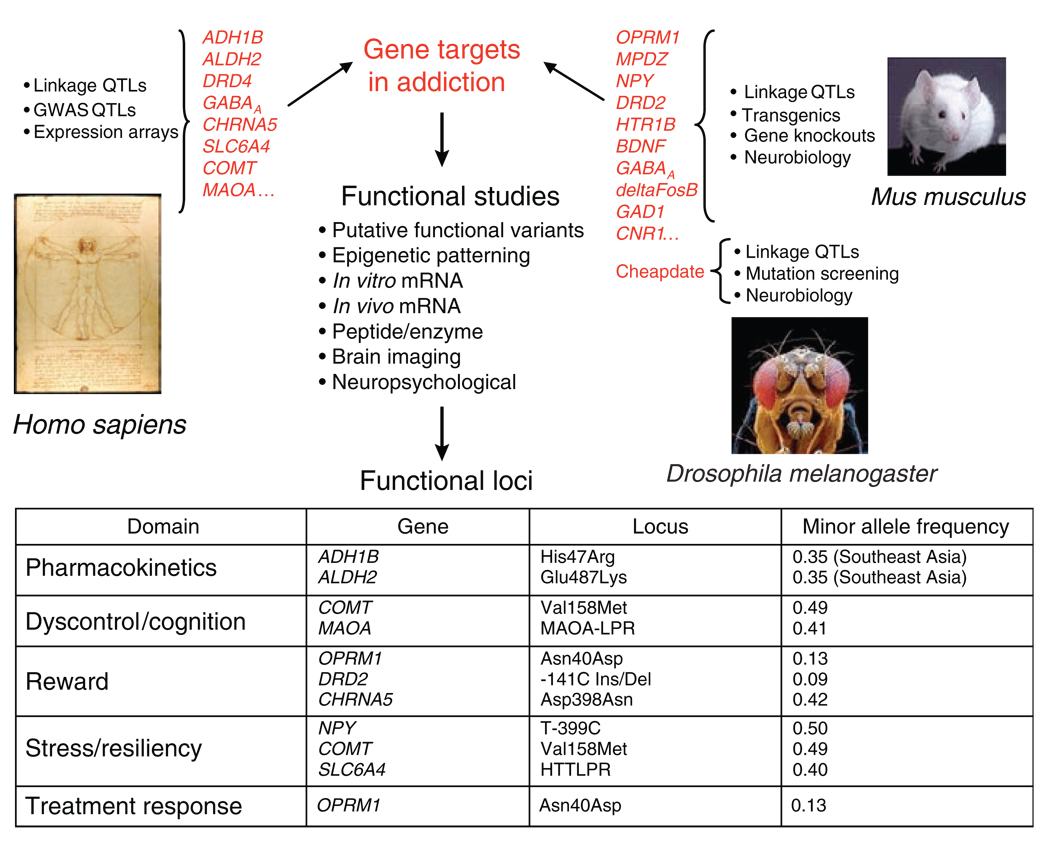
Intermediate phenotypes bridge the gap between target identification and candidate gene analysis, enabling the discovery of functional alleles, which may alter different aspects of drug response and which are noncomprehensively listed in Figure 2. Functional alleles can function in altered substance–specific vulnerabilities, such as variation in drug metabolism or drug receptors. They may also have a role in shared vulnerability or resiliency by altering factors that are not agent-specific, for example, variation in reward or stress resiliency. Gene discovery can reveal underlying mechanisms by which chronic drug exposure promotes stable changes in gene expression, brain structure and function, and, ultimately, behavior.
Figure 2.
Multiple avenues for identification of gene targets in addictions (top), in human and in animal models, have been taken by multilevel studies (center), leading to the discovery of functional alleles. The genes and loci listed in the table are examples of mechanistic domains in which functional vulnerability alleles have been identified. GWAS, genome-wide association studies; mRNA, messenger RNA; QTLs, quantitative trait loci.
Identification of genes that are central to drug response and neuroadaptation is also being achieved through studies in vertebrate and invertebrate animal models of neuroanatomical circuits and cellular molecular networks that are crucial in addictions (Figure 2). Addiction-related behaviors have been altered by more than 100 mouse gene knockouts and transgenics, revealing the molecular complexity and multiplicity of pathways that may lead to addictions. Animal models can reveal associations between neurobiological phenotypes and addiction-related behaviors that are inaccessible in humans, and enable manipulations of both genes and environments. In mice, genes implicated in drug addiction have been discovered by quantitative trait loci studies, and online data sets (e.g., the Gene Network, http://webqtl.org) are available for the analysis of traits that are controlled by combinations of allelic variants and environmental factors. Enhanced ethanol sensitivity in Drosophila melanogaster is due to a mutation—Cheapdate, an allele of the memory mutant Amnesiac—that results in diminished activation of the cyclic adenosine monophosphate pathway. In nonhuman primates, it is possible to administer a defined early-life stress, namely, maternal deprivation. In rhesus macaques, such deprivation leads to behavioral dyscontrol, stress hyperresponsiveness, and increased alcohol consumption later in life. There is also a gene–environment interaction with a serotonin transporter promoter locus orthologous to a functional polymorphism in the human that also alters behavioral resiliency to stress.3
The understanding of the genome elements regulating gene expression, non-protein-coding transcripts, and protein-coding function of the genome is still very limited. However, deep resequencing, as in the 1000 Genomes Project, is providing a catalog of rare and common sequence variants that will be augmented and serve as a reference baseline for focused resequencing of individuals with addictive disorders. A new capability for the genome characterization of gene expression and chromatin variation made possible by high-throughput DNA sequencing is being implemented in the ENCODE (Encyclopedia of DNA Elements) study and via studies focused on drug-induced alterations in expression and chromatin structure. These studies promise to increase our knowledge of the genome’s role in neuroadaptation to drugs and the ways in which genetic variations and environmental exposures interact to lead to neuronal molecular changes integral to the vulnerability to addictions and to the processes of addiction and recovery.
Progress in the pharmacogenetics of addictions would reduce morbidity and mortality through better prevention and treatment. Progress is at this point limited and narrow in specificity. For example, the specific effect of the µ-opioid receptor polymorphism (OPRM1; Asn40Asp) Asp40 to predict favorable naltrexone treatment response in alcoholism was recently replicated,5 offering a narrowly defined clinical tool. In contrast, integrated pharmacogenetics of addictions would require an understanding of interacting effects in multiple biological mechanisms involved in tolerance, craving, anxiety, dysphoria, executive cognitive function, and reward. It is likely that this will be achieved by integrating this genetic information with information from other predictive and explanatory levels, enabling the clinical redefinition of the addictions on the more complete and informative basis of etiology.
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Phil. Trans. R. Soc. B. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman D, Oroszi G, Ducci F. The genetics of addiction: uncovering the genes. Nat. Rev. Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch. Gen. Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, et al. An evaluation of μ-opioid receptor (OPRM1) I as a predictor of naltrexone response in the treatment of alcohol dependence. Arch. Gen. Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]