Synopsis
This review deals with development of myocardial architecture, crucial for contractile performance of the heart, and its conduction system, essential for generation and coordinated spread of electrical activity. Topics discussed include molecular determination of cardiac phenotype (contractile and conducting), remodeling of ventricular wall structure and its blood supply, and relation of trabecular compaction to noncompaction cardiomyopathy. Illustrated are the structure and function of tubular heart, time course of trabecular compaction and development of multilayered spiral system of the compact layer.
Keywords: ventricular myocardium, myocardial architecture, trabeculae, non-compaction
This review is focused on development of two distinct components of the myocardium: so-called working myocardium, which encompasses the bulk of the cardiac mass, and specialized network of pacemaking and conduction myocardium, known as the cardiac conduction system. Of course, the heart contains other critical tissues, including serous and fibrous covering (epicardium), fibrous tissue derivatives (valves and cardiac skeleton), blood supply (coronary arteries and cardiac veins), and an endocardial lining. Each of these tissues is important for proper heart function, and the developmental story of each is the subject of much ongoing research; for those interested in an overview of these dynamic sub-fields, for introduction to these topics, the reader is referred to a recent dedicated handbook 26.
Before we can consider the working myocardium and cardiac conduction system populations in detail, we have to treat briefly the events that lead to the formation of at least tubular heart from the precardiac mesoderm, and summarize current knowledge of genes involved in myocardial differentiation.
Early stages of heart formation
The heart takes its origin from paired cardiac mesodermal primordia that fuse in the midline to create a primitive tubular heart 76. Soon after initiation of heart beat, the cardiac tube undergoes a process of looping, which leads to creation of the first grossly visible asymmetry in the embryo. The looped heart then enters a period of chamber formation, with five compartments identifiable by morphological as well as molecular criteria. Following the blood flow, the first segment is the sinus venosus. The sinus venosus functions as a blood reservoir and pacemaker of the heart,, which correlates with its more robust expression of genes necessary for spontaneous action potential generation 37. Morphologically and histologically it is characterized by thin walls, small cell size, and scarce intracellular myofibrils. Next come the yet unseparated atrial chambers, with faster impulse propagation, absence of cardiac jelly, and no trabeculae (although they develop the pectinate muscles later on). The third segment is the atrioventricular channel. Atrioventricular channel myocardium is noted for a strongly circular myofiber alignment, as well as a lining of cardiac jelly; it also exhibits a slow conduction velocity. This gives it the role of delay generator for conduction between the atrial and ventricular myocardium (similar to the function of the atrioventricular node in the mature heart). The cardiac jelly is molded into atrioventricular cushions, which participate later on in chamber septation and formation of the atrioventricular valves. Next come the ventricles, which are distinguished by development of extensive trabecular network on the luminal side, fast impulse propagation, and most rapid differentiation of myocytes with respect to their contractility, channels, and energy metabolism. Most of our further discussion will focus on development of the ventricular myocardium, since the ventricles are the main pumping chambers of the heart, and cardiac failure is essentially failure of the ventricle(s). The last myocardial segment in the tubular heart is the conotruncus, or the outflow tract. Similar to already mentioned atrioventricular canal, its myocardium also has the characteristic of the earlier primitive tube 38, i.e. slow conduction 9,57 and prevailing circular myocyte alignment. Derived from the secondary heart field, it is a transitory structure that undergoes extensive remodeling to give rise to important structures in the mature heart. The outflow cushions take part in division of the cardiac outlet into aortic and pulmonary component, and their distal parts form the semilunar valves. Its myocardium mostly disappears through apoptosis 54, with the exception of the portion forming the pulmonary infundibulum.
Molecular determination of cardiac lineage (Table 1)
Table 1.
Genes involved in differentiation of myocardial lineage. Based on 26.
| Transcription factors (targets) |
| GATA |
| Nkx |
| Myocardin |
| MEF |
| Tbx |
| SRF |
| Stimulation |
| Activin |
| TGFbeta |
| Wnt (non-canonical) |
| BMP |
| FGF |
| Shh |
| Inhibition |
| Wnt (canonical) |
| Notch |
| Noggin |
The cardiomyocytes differentiate from horseshoe-shaped mesodermal primordia. In vitro studies have shown that there are more of these cells capable of forming cardiac myocytes than what actually occurs in vivo 10, pointing to a combination of positive and negative regulators of cardiomyogenic differentiation. A list of currently identified genes participating in commitment to, as well as restriction of this program, derived from a recent reference 26, is provided for overview (Table 1).
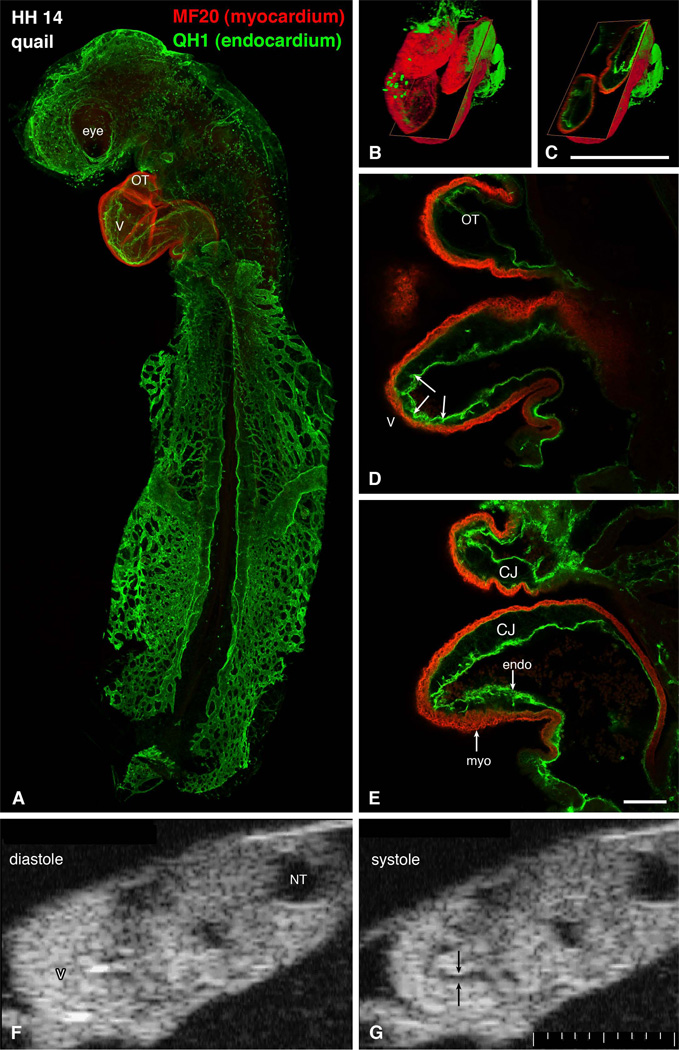
Structure and function of the tubular heart (Fig. 1, Movie 1)
Figure 1.
Structure of a tubular heart. A-E: whole mount immunostaining for myocardial marker myosin heavy chain (red) and endothelial QH1 marker (green) shows beautifully the shape of the heart (S-loop). Individual confocal sections (D, E) show the diminished amount of cardiac jelly in the ventricular apex, resulting in close contact between these two layers. Endocardial ruffles (arrowheads) show the site of prospective trabecular formation. Three-dimensional reconstructions (B, C) show the whole heart and an oblique section illustrating the variable extent of the cardiac jelly. Scale bars 500 µm (B, C) and 100 µm (D, E). Echocardiographic views (F, G) show the heart loop in two different phases of cardiac cycle. Note the echo-free acellular layer of cardiac jelly (arrows) along the inner curvature during systole. CJ, cardiac jelly, endo, endocardium, myo, myocardium, NT, neural tube, OT, outflow tract, V, ventricle. Scale bar 100 µm smallest division.
Although already diversified functionally 25, there is minimal variation in structure along the early cardiac tube. In section, the tube is composed of a one or two cells thick myocardial mantle, an acellular cardiac jelly, and the endocardium. The first morphological sign of regional myocardial diversification can be perceived in more pronounced circumferential arrangement of actin and fibronectin 61 in the inner layer. Regional differences in myofibrillar patterns become more obvious at the looping stage of the rat heart 46,74. Whether this regional asymmetry is responsible for the process of looping or its direction needs yet to be established.
The tubular heart (Fig. 1) differs in its mode of contraction from the mature one, or even pre-septation trabeculated heart, which is similar to the adult in e.g. observing the Frank-Starling law 8,19. Ventricular contraction in the tube heart is fundamentally asymmetric, a factor not often considered in biomechanical models of the tube heart 12,63. The fact that the opposite endocardial surfaces contract to fully occlude the cardiac lumen in a contractile wave from the atrioventricular canal to the muscularized outflow tract has implications for determination of cardiac function at these stages. There is no residual non-ejected systolic volume under normal conditions until the ventricular cardiac jelly regresses and cardiac ventricular outgrowth (‘ballooning”) begins 38. The ejection phase of contraction in the early tube heart therefore has little in common with ventricular systole in the septating and septated heart with their apex-to-base electromechanical activity. Because there is complete occlusion of the lumen at each point along the early tube he art (Movie 1), there is no back flow, and perhaps no dependence on valves as the whole tube acts as an impedance pump 12,32. The relevance of measurements of global systolic performance based on calculations of fractional shortening, ejection fraction and end-systolic volume measurements for measurement of cardiac function during these stages of development is not clear.
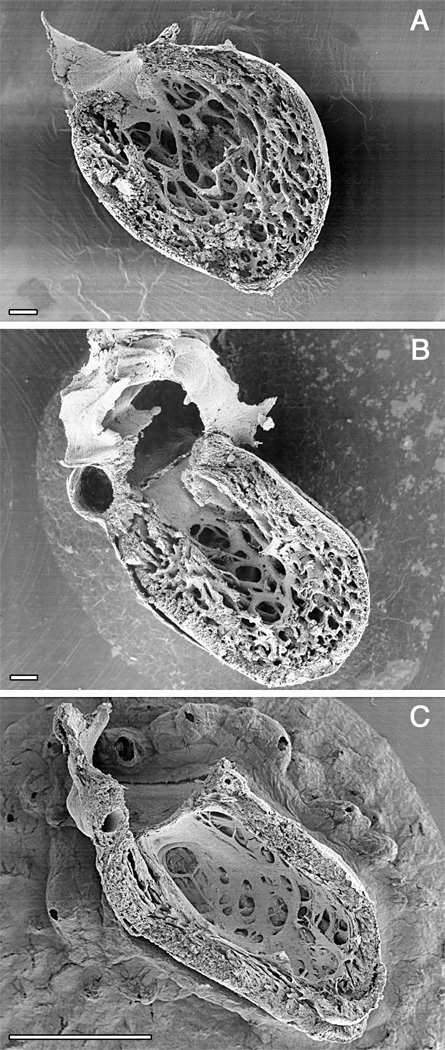
Development of myocardial trabeculation (Fig. 2, Movie 2)
Figure 2.
Time course of ventricular compaction in the human left ventricle. Note increasing proportion and thickness of the outer compact layer. A. Numerous fine trabeculae are present at 6 weeks. B. The trabeculae start to compact at their basal portion, contributing to added thickness of the compact layer at 12 weeks when ventricular septation is completed. C. The compact layer forms the bulk of the myocardial mass after completion of compaction in the early fetal period. Scale bars 100 microns (A, B), 1 mm (C). A. and C. were originally published in Sedmera et al. 56.
The development of myocardial architecture of the ventricular wall passes through several distinct steps. At first, in the tubular heart, the myocardium has an epithelial character with just two layers of cells. The second step is the formation of sheet-like myocardial protrusions into the lumen, so-called trabeculations (Fig. 2). The next step is compaction of the basal portions of these trabeculations, which correlates with the establishment of the coronary vascular system derived from the epicardium. The final stage is the development of multilayered spiral system.
Only after looping is it possible to discern changes in the luminal appearance of the different components of the tube (Fig. 1), with trabeculations first becoming evident along the inner myocardial layers near the maximum or greater curvature of the looped primitive ventricle 2,5. This happens in the chick embryo at stage 16/17, in mouse at 10 days of gestation, rat at 11 days, and in humans at the end of the first month of gestation (Carnegie stage 12, the embryo is approximately 4 mm long 31). The pattern of early trabecular ridges in the ventricular apex runs dorsoventrally (circumferential to the heart tube) and appears similar in different species 56. At this early stage, the tube itself still contracts in a fashion most consistent with an impedance pump 4. Maximal stresses and the most highly differentiated cells are concentrated in the myocardial layers adjacent to the lumen, where they may serve to stimulate early trabecular formation 65. The spacing of such trabecular sheets may depend upon material properties 34,35 of the ventricular wall that result in buckling to relieve tension in response to compression along its longitudinal axis, similar to a long thick carpet, pushed from both ends. The early trabeculations effectively increase surface to volume ratio, enabling the myocardial mass to increase prior to establishment of a coronary circulation, and may also serve to compartmentalize blood flow as separate ventricular streams prior to septation 69.
After buckling, the distribution of stresses is no longer uniform, which favors growth of the trabeculations towards the center of the ventricle (Fig. 2). The trabeculae become transformed into fenestrated trabecular sheets with no free ends – this is conceptually and topologically different from villi in the intestine 59. Patterns of trabeculations specific for the morphologically left, as opposed to the right, ventricles become apparent at the beginning of ventricular septation 72,73.
The exact patterns of trabeculations in the adult heart show appreciable variability between individuals, and, similar to fingerprints, they seem to be unique for each individual heart. The arrangement of the moderator band, which contains the right bundle branch, and the anatomy of the medial papillary muscle complex of the mitral valve are more uniform, but by no means constant 49.
Atrial myoachitecture
Compared to the ventricle, the atrial myoarchitecture has received much less attention, except as a substrate for propagation of atrial arrhythmias 1,17. The pectinate muscles appear in the future atrial appendages after the beginning of the atrial septation. A preferential conduction pathway appears during development within the roof of the atria, transmitting the impulse rapidly from the right-sided sinoatrial node to the left atrium. The morphological substrate of this pathway - the bundle of Bachman – is apparent from embryonic day 6 in the chick onward, and is a prominent ridge of pectinate muscles continuous with the terminal crest 60. Similar activation patterns are apparent in the embryonic rat and mouse (D.S., unpublished observations). Further acceleration of impulse propagation (relative to interposed atrial myocardium) is noted along the ridges formed by the forming pectinate muscles, which branch from the terminal crest toward the atrioventricular sulcus. The pectinate muscles, hence, seem to serve a dual role – to strengthen the rather thin atrial wall akin to the struts of an umbrella, and as a morphological substrate of the preferential conduction pathways, which appear to exist to assure synchronous atrial activation and contraction rather than rapid impulse conduction between the sinoatrial and atrioventricular nodes.
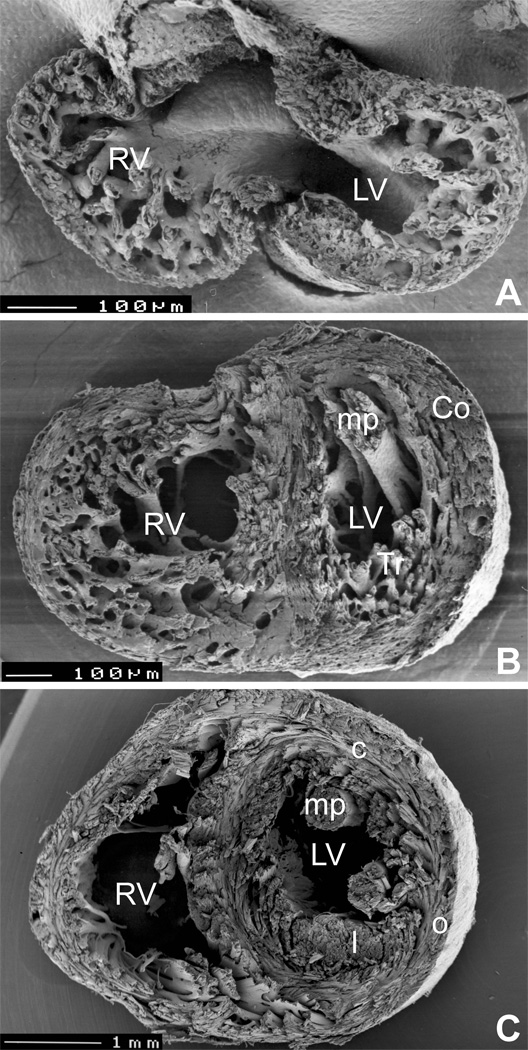
Myocardial (non)compaction (Fig. 2, Fig 3)
Figure 3.
Development of spiral architecture in the compact layer. A, pre-compaction stage (E13.5 embryonic mouse heart in transverse section) showing thin, avascular compact layer with circumferential arrangement of myocytes. B, post-compaction, vascularized compact layer at E15.5 shows spiral arrangement in both compact myocardium (Co) and trabeculae (Tr). C, in the adult mouse heart, the typical three-layered structure with inner predominantly longitudinal (l), middle circular (c) and outer oblique (o) orientation can be seen in the left ventricle (although recent quantitative studies show that the change in orientation occurs in continuum 29). LV, left ventricle, RV, right ventricle, mp, papillary muscles. Ventricular midportion slabs, viewed from the apex towards the outflow. The top two specimens were prepared by late Dr. Si Minh Pham at University of Lausanne.
Marked developmental modifications, significant in terms of both structure and function, occur in the compact myocardium. In early embryonic stages in birds and mammals 3, and also in hearts of lower vertebrates (summarized in 58), almost the entire thickness of the ventricular wall is made up of a trabecular layer, with little or no coronary vascular supply.
The initial thickening of the compact layer is based on cell proliferation 23. Subsequent thickening occurs due to compaction of trabeculations, this process coinciding with invasion by the developing coronary vasculature (discussed further in the following section) from the epicardium 52,70. Compaction is more pronounced in the left ventricle 56, and substantial further growth and compaction occur through the postnatal development 16 with increasing systemic pressure.
The process of trabecular compaction is fairly rapid (in chick, between ED7-10, in mouse, between ED13 and ED14, in human between 10 and 12 weeks 56). From ED16 in the mouse and the 4th month of gestation in human, the compact layer forms the bulk of the ventricular myocardium (Fig 2, Fig 3). Noncompaction of the myocardium presents serious functional consequences for the heart. Several mouse null mutants such as RXRalpha knockout present with a complete lack of compaction, which results in lethality around ED14 (reviewed in 56), stressing the importance of the compact myocardium for contractile performance in later fetal stages. In humans, ventricular noncompaction is usually localized. Although it can occur as an isolated entity, it can be associated with heart failure and sudden cardiac death 71. Even if only a minor proportion of ventricular wall is affected, ventricular contractile dynamics may be perturbed 22. This condition is nowadays diagnosed by ultrasound and is classified as a distinct cardiomyopathy, and several genetic etiologies are recognized 11,20,43,53. There is mounting evidence that the epicardium and the epicardially-derived cells play a crucial role in the development of the compact layer of the ventricular myocardial walls. Not only is the “thin compact myocardium syndrome” observed in several mouse models with perturbed epicardial development, such as in the VCAM-1 knockout 28, but also experimental studies in avian embryos in which epicardial development is perturbed also show inhibition of ventricular compaction 44. The exact molecular mechanism(s) responsible for the regulation of ventricular myocardial differentiation, growth, and trabecular compaction are currently poorly understood, although retinoid signaling from the epicardium to the myocardium appears to be crucial 6. More research into this area can bring more clues to the molecular etiology of this condition in humans.
The progressive thickening of the compact myocardium is accompanied by an improved level of its organization. Malpighi in 1672 had observed that “spiral muscle fibers were successively wrapped” around both ventricles of the chick from the 5th day of incubation onwards. This results in development of a three-layered spiral system of myocardial fibers 24,62. Development of the spiralling alignment of the fibers (Fig. 3) likely reflects the twisting pattern of contraction, and can be experimentally accelerated by increase workload 66.
Remodeling of blood supply
Once the size and metabolic activity of an animal exceed the limits of oxygen diffusion, a circulatory system needs to be established. This is true also for the heart, both phylogenetically and ontogenetically. The primitive pulsatile vessels or even sophisticated hearts of more complex invertebrates such as large marine crustaceans or mollusks are devoid of coronary vascularization.
If an increase in myocardial mass is necessary, the heart muscle is organized into a spongy network of trabeculae. This arrangement of myocardium is found in larger invertebrates (crabs, oysters), and some lower vertebrates, typically cold-blooded, relatively sedentary animals with low blood pressure, notably cetain fish 42,68 and amphibian species such as Xenopus 58 that have a ventricle devoid of coronary arteries.
The origin of the coronary vessels, including the capillaries, was for a long period controversial. Recent studies using retroviral targeting 33 and chick-quail chimeras 45 clearly showed the extracardiac origin of the angioblasts which form the primitive vascular bed which will become the coronary vasculature, and in that way supplanting older concepts of participation of the trabecular endocardium in the definitive coronary bed, or sprouting of coronary arteries from the aorta. Indeed, connection to the aorta is the final stage of development (reviewed in 67). The stimulus for the angioblast invasion is probably local tissue hypoxia 41,75, most pronounced in the outermost subepicardial layers. The mediators are fibroblast and vascular endothelial growth factors. Interestingly, the process of vascularization itself does not seem to be dependent on ventricular loading, as demonstrated by studies of Rongish et al. 50. They used the avascular rat embryonic heart transplanted into anterior eye chamber to study the relationship of the various components of the extracellular matrix to coronary vascularization, and found that angioblast invasion occurred even in the absence of any functional loading.
Unlike most organs in the vertebrate body, the heart has to function continuously from very early stages of its development to support the embryo’s increasing demands. Consequently, the coronary-less stages are recapitulated in the ontogenesis of the higher vertebrates, and the lacunar system of the trabeculated heart was recognized more than a century ago 36,51as a way to increase the surface to volume ratio in order to facilitate the exchange of nutrients and oxygen. The external, coronary blood supply becomes necessary when increasing circulatory demands cannot be met by the trabeculated heart, and increased thickness of the compact layer precludes its adequate nutrition and oxygenation by diffusion from the ventricular lumen.
Development of pacemaking and conduction system
The intrinsic rhythm of the adult heart is determined within the tissues of the cardiac pacemaker — the sinoatrial (SA) node. It is situated at the inflow portion of the heart, at the border of the superior caval vein and the right atrium. Following initiation of a cardiac action potential within the node, the activation wave is propagated through the atrial myocardium, eventually converging into the atrioventricular (AV) node. As its name suggests, the AV node is found at the junction of the atria and ventricles, and functions as a delay generator in the propagation of activation. Following the exit from the AV node, activation rapidly propagates along the His bundle and its branches, finally activating the ventricles via a ramified network of Purkinje fibers. Together, this rapid-conduction system of His-Purkinje tissues forms the last of the main elements of the cardiac conduction system.
The main components of cardiac conduction system (CCS) show remarkable evolutionary conservation. The development of a mature CCS function during embryogenesis follows significant phases of cardiac morphogenesis 13. Initially, the impulse propagates in a slow and apparently isotropic fashion from the sinus venosus, the most caudal portion of the tubular heart, toward the cranially located primitive outflow tract 25. While the apparent speed of impulse propagation gradually increases (based on total activation time inferred from isochronal epicardial maps), the sequence of ventricular activation as the heart loops still follows the flow of blood. Eventually, the immature base-to-apex sequence of ventricular activation undergoes an apparent reversal, altering to a mature apex-to-base pattern 7,47,48, which is a hallmark of the His-Purkinje system function.
The cascade of events resulting in the differential gene expression that distinguishes CCS from the working myocardium is becoming unveiled 40,55. Molecular determinants of the SA node have been recently described 37, and consist of a combination of transcription factors Nkx2.5, Tbx3, and Pitx2c. Nkx2.5 in the atrial myocardium suppresses the expression of pacemaker channel gene Hcn4 and T-box transcription factor Tbx3, restricting their expression domain to the forming Nkx2.5-negative sinoatrial node and sinus horns and defining a gene expression boundary between the atrium and SA node. Tbx3 in turn suppresses the chamber differentiation program, providing an additional mechanism of reinforcing the nodal identity. Deficiency in Tbx3 results in an expansion of the atrial gene expression program into the nodal area and a partial loss of nodal gene expression, whereas its overexpression reciprocally suppresses the atrial program and induces a pacemaking phenotype 18. Pitx2c is a laterality determination gene that suppresses the program for SA node formation on the left side, resulting in the normal right-sided position of the node.
Determination of the AV node is linked to formation of the AV canal in the developing heart, or rather, differentiation of the atrial and ventricular chambers from the “primitive” myocardium of the cardiac tube 38. A combination of T-box transcription factors Tbx2 and Tbx5 signalling is involved 39, with Tbx5 being the determinant of the AV canal as well as the ventricular conduction system. Tbx2 is induced by BMP2 expression, and repressed by Hesr1 expressed in the atrial and Hesr2 in the ventricular myocardium 27. Nkx2.5 signalling is critical not only for the early stages of cardiogenesis 30 but is upregulated during the later stages of the ventricular conduction system formation, namely differentiation of the Purkinje fibers in the chick 15. In mammals, it is required for normal formation of the AV node and bundle branches 21.
In the chick, endothelin (ET) was identified as a secreted factor capable of turning on CCS markers in embryonic cardiomyocytes in vivo 14, and further studies suggest that it is also involved in turning on the Purkinje fiber-specific program in vivo 64. There is some evidence that it is also involved in CCS induction in mammals (reviewed in 55), but further research is needed to confirm this hypothesis.
Summary
Myocardium is derived from precardiac mesoderm, and its formation is subject of positive and negative regulation by a number of genes. After formation of tubular heart with mode of contraction distinct from subsequent stages, initial increase in ventricular mass during period of chamber formation is achieved by development of trabeculations, a hallmark of sponge-like hearts. Similar structures (pectinate muscles) develop later on in the atria. Trabecular compaction coincides with deployment of coronary circulation, and results in formation of ventricular chambers with significant, vascularized compact layer and clearly delineated lumen, which are capable of superior performance (pressure generation, ejection fraction) than the avascular, spongy ventricles. The contraction of the developing heart is orchestrated by its pacemaking and conduction system, which develops and changes in parallel with heart morphogenesis. While the molecular determination of the sinoatrial node and atrioventricular was recently unraveled, the pathways implicated in induction and patterning of ventricular His-Purkinje system are still subject of research.
Supplementary Material
Movie 1. Video recording (25 fps) of tubular heart action. Ventral view of a Stage 12 chick embryo in modified New culture. Best viewed as a loop.
Movie 2. Ultrasound recording of pre-septation trabeculated heart. Four-chamber view of a Stage 24 heart shows the reciprocating contraction of atria (center) and septating ventricle (right), as well as flowing blood in the branchial arch arteries (left) and paired dorsal aortae (far left, in cross-section). Recorded at 30 fps, smallest division on scale bar 100 µm.
Acknowledgments
This research was supported by NIH RR16434, MSMT VZ 206100-3, AS CR AVOZ50450515, and the Purkinje Fellowship of the Academy of Sciences of the Czech Republic. This work was conducted in part in a facility constructed with support from the National Institutes of Health, Grant Number C06 RR018823 from the Extramural Research Facilities Program of the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Permissions
Figure 2 is a reproduction of plate published originally in an editorial by Varnava (2001); however, I retained the copyright for this picture, as well as the original constituting images (CTA with Wiley grants me unrestricted right to use these images, with due citation, in my subsequent works).
Figure 1 and Figure 3 are original art work, as are the movies.
References
- 1.Anderson RH, Ho SY. The architecture of the sinus node, the atrioventricular conduction axis, and the internodal atrial myocardium. J Cardiovasc Electrophysiol. 1998;9:1233. doi: 10.1111/j.1540-8167.1998.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Shachar G, Arcilla RA, Lucas RV, et al. Ventricular trabeculations in the chick embryo heart and their contribution to ventricular and muscular septal development. Circ Res. 1985;57:759. doi: 10.1161/01.res.57.5.759. [DOI] [PubMed] [Google Scholar]
- 3.Blausen BE, Johannes RS, Hutchins GM. Computer-based reconstructions of the cardiac ventricles of human embryos. Am J Cardiovasc Pathol. 1990;3:37. [PubMed] [Google Scholar]
- 4.Butcher JT, McQuinn TC, Sedmera D, et al. Transitions in early embryonic atrioventricular valvular function correspond with changes in cushion biomechanics that are predictable by tissue composition. Circ Res. 2007;100:1503. doi: 10.1161/CIRCRESAHA.107.148684. [DOI] [PubMed] [Google Scholar]
- 5.Challice CE, Viragh S. The embryonic development of the mammalian heart. In: Challice CE, Viragh S, editors. Ultrastructure of the mammalian heart. New York: Academic Press; 1973. p. 91. [Google Scholar]
- 6.Chen TH, Chang TC, Kang JO, et al. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev Biol. 2002;250:198. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- 7.Chuck ET, Freeman DM, Watanabe M, et al. Changing activation sequence in the embryonic chick heart. Implications for the development of the His-Purkinje system. Circ Res. 1997;81:470. doi: 10.1161/01.res.81.4.470. [DOI] [PubMed] [Google Scholar]
- 8.Clark EB, Hu N, Dummett JL, et al. Ventricular function and morphology in chick embryo from stages 18 to 29. Am J Physiol. 1986;250:H407. doi: 10.1152/ajpheart.1986.250.3.H407. [DOI] [PubMed] [Google Scholar]
- 9.de Jong F, Opthof T, Wilde AA, et al. Persisting zones of slow impulse conduction in developing chicken hearts. Circ Res. 1992;71:240. doi: 10.1161/01.res.71.2.240. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg LM, Eisenberg CA. An in vitro analysis of myocardial potential indicates that phenotypic plasticity is an innate property of early embryonic tissue. Stem Cells Dev. 2004;13:614. doi: 10.1089/scd.2004.13.614. [DOI] [PubMed] [Google Scholar]
- 11.Finsterer J, Stollberger C. Do cypher gene mutations cause left ventricular noncompaction with subclinical myopathy? J Am Coll Cardiol. 2004;44:1139. doi: 10.1016/j.jacc.2004.06.007. author reply 1139. [DOI] [PubMed] [Google Scholar]
- 12.Forouhar AS, Liebling M, Hickerson A, et al. The embryonic vertebrate heart tube is a dynamic suction pump. Science. 2006;312:751. doi: 10.1126/science.1123775. [DOI] [PubMed] [Google Scholar]
- 13.Gourdie RG, Harris BS, Bond J, et al. Development of the cardiac pacemaking and conduction system. Birth Defects Research. 2003;69C:46. doi: 10.1002/bdrc.10008. [DOI] [PubMed] [Google Scholar]
- 14.Gourdie RG, Wei Y, Kim D, et al. Endothelin-induced conversion of embryonic heart muscle cells into impulse-conducting Purkinje fibers. Proc Natl Acad Sci U S A. 1998;95:6815. doi: 10.1073/pnas.95.12.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris BS, Spruill L, Edmonson AM, et al. Differentiation of cardiac Purkinje fibers requires precise spatiotemporal regulation of Nkx2-5 expression. Dev Dyn. 2006;235:38. doi: 10.1002/dvdy.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirokawa K. A quantitative study on pre- and postnatal growth of human heart. Acta Pathol Jpn. 1972;22:613. [PubMed] [Google Scholar]
- 17.Ho SY, Anderson RH, Sanchez-Quintana D. Atrial structure and fibres: morphologic bases of atrial conduction. Cardiovasc Res. 2002;54:325. doi: 10.1016/s0008-6363(02)00226-2. [DOI] [PubMed] [Google Scholar]
- 18.Hoogaars WM, Engel A, Brons JF, et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007;21:1098. doi: 10.1101/gad.416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu N, Clark EB. Hemodynamics of the stage 12 to stage 29 chick embryo. Circ Res. 1989;65:1665. doi: 10.1161/01.res.65.6.1665. [DOI] [PubMed] [Google Scholar]
- 20.Ichida F, Tsubata S, Bowles KR, et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation. 2001;103:1256. doi: 10.1161/01.cir.103.9.1256. [DOI] [PubMed] [Google Scholar]
- 21.Jay PY, Harris BS, Maguire CT, et al. Nkx2-5 mutation causes anatomic hypoplasia of the cardiac conduction system. J Clin Invest. 2004;113:1130. doi: 10.1172/JCI19846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenni R, Oechslin E, Schneider J, et al. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeter JR, Jr, Cameron IL. Cell proliferation patterns during cytodifferentiation in embryonic chick tissues: liver, heart and erythrocytes. J Embryol Exp Morphol. 1971;25:405. [PubMed] [Google Scholar]
- 24.Jouk PS, Usson Y, Michalowicz G, et al. Three-dimensional cartography of the pattern of the myofibres in the second trimester fetal human heart. Anat Embryol (Berl) 2000;202:103. doi: 10.1007/s004290000103. [DOI] [PubMed] [Google Scholar]
- 25.Kamino K. Optical approaches to ontogeny of electrical activity and related functional organization during early heart development. Physiol Rev. 1991;71:53. doi: 10.1152/physrev.1991.71.1.53. [DOI] [PubMed] [Google Scholar]
- 26.Kirby ML. Cardiac Development. New York: Oxford University Press; 2007. [Google Scholar]
- 27.Kokubo H, Tomita-Miyagawa S, Hamada Y, et al. Hesr1 and Hesr2 regulate atrioventricular boundary formation in the developing heart through the repression of Tbx2. Development. 2007;134:747. doi: 10.1242/dev.02777. [DOI] [PubMed] [Google Scholar]
- 28.Kwee L, Baldwin HS, Shen HM, et al. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 29.Lunkenheimer PP, Redmann K, Kling N, et al. Three-dimensional architecture of the left ventricular myocardium. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:565. doi: 10.1002/ar.a.20326. [DOI] [PubMed] [Google Scholar]
- 30.Lyons I, Parsons LM, Hartley L, et al. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5. Genes Dev. 1995;9:1654. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 31.Mall FP. On the development of the human heart. Am J Anat. 1912;13:249. [Google Scholar]
- 32.McQuinn TC, Bratoeva M, Dealmeida A, et al. High-frequency ultrasonographic imaging of avian cardiovascular development. Dev Dyn. 2007;236:3503–3513. doi: 10.1002/dvdy.21357. [DOI] [PubMed] [Google Scholar]
- 33.Mikawa T, Borisov A, Brown AM, et al. Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus: I. Formation of the ventricular myocardium. Dev Dyn. 1992;193:11. doi: 10.1002/aja.1001930104. [DOI] [PubMed] [Google Scholar]
- 34.Miller CE, Wong CL. Trabeculated embryonic myocardium shows rapid stress relaxation and non-quasi-linear viscoelastic behavior. J Biomech. 2000;33:615. doi: 10.1016/s0021-9290(99)00212-2. [DOI] [PubMed] [Google Scholar]
- 35.Miller CE, Wong CL, Sedmera D. Pressure overload alters stress-strain properties of the developing chick heart. Am J Physiol Heart Circ Physiol. 2003;285:H1849. doi: 10.1152/ajpheart.00384.2002. [DOI] [PubMed] [Google Scholar]
- 36.Minot CS. On a hitherto unrecognised circulation without capillaries in the organs of Vertebrata. Proc Boston Soc Nat Hist. 1901;29:185. [PMC free article] [PubMed] [Google Scholar]
- 37.Mommersteeg MT, Hoogaars WM, Prall OW, et al. Molecular Pathway for the Localized Formation of the Sinoatrial Node. Circ Res. 2007;100:354. doi: 10.1161/01.RES.0000258019.74591.b3. [DOI] [PubMed] [Google Scholar]
- 38.Moorman AF, Christoffels VM. Cardiac chamber formation: development, genes, and evolution. Physiol Rev. 2003;83:1223. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- 39.Moskowitz IP, Pizard A, Patel VV, et al. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development. 2004;131:4107. doi: 10.1242/dev.01265. [DOI] [PubMed] [Google Scholar]
- 40.Myers DC, Fishman GI. Toward an understanding of the genetics of murine cardiac pacemaking and conduction system development. Anat Rec. 2004;280A:1018. doi: 10.1002/ar.a.20077. [DOI] [PubMed] [Google Scholar]
- 41.Nanka O, Valasek P, Dvorakova M, et al. Experimental hypoxia and embryonic angiogenesis. Dev Dyn. 2006;235:723. doi: 10.1002/dvdy.20689. [DOI] [PubMed] [Google Scholar]
- 42.Ostadal B, Schiebler TH. The terminal blood bed in the heart of fish. Z Anat Entwicklungsgesch. 1971;134:101. [PubMed] [Google Scholar]
- 43.Pauli RM, Scheib-Wixted S, Cripe L, et al. Ventricular noncompaction and distal chromosome 5q deletion. Am J Med Genet. 1999;85:419. [PubMed] [Google Scholar]
- 44.Perez-Pomares JM, Phelps A, Sedmerova M, et al. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs) Dev Biol. 2002;247:307. doi: 10.1006/dbio.2002.0706. [DOI] [PubMed] [Google Scholar]
- 45.Poelmann RE, Gittenberger-de Groot AC, Mentink MM, et al. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res. 1993;73:559. doi: 10.1161/01.res.73.3.559. [DOI] [PubMed] [Google Scholar]
- 46.Price RL, Chintanowonges C, Shiraishi I, et al. Local and regional variations in myofibrillar patterns in looping rat hearts. Anat Rec. 1996;245:83. doi: 10.1002/(SICI)1097-0185(199605)245:1<83::AID-AR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 47.Reckova M, Rosengarten C, deAlmeida A, et al. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res. 2003;93:77. doi: 10.1161/01.RES.0000079488.91342.B7. [DOI] [PubMed] [Google Scholar]
- 48.Rentschler S, Vaidya DM, Tamaddon H, et al. Visualization and functional characterization of the developing murine cardiac conduction system. Development. 2001;128:1785. doi: 10.1242/dev.128.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Restivo A, Smith A, Wilkinson JL, et al. The medial papillary muscle complex and its related septomarginal trabeculation. A normal anatomical study on human hearts. J Anat. 1989;163:231. [PMC free article] [PubMed] [Google Scholar]
- 50.Rongish BJ, Hinchman G, Doty MK, et al. Relationship of the extracellular matrix to coronary neovascularization during development. J Mol Cell Cardiol. 1996;28:2203. doi: 10.1006/jmcc.1996.0212. [DOI] [PubMed] [Google Scholar]
- 51.Rychter Z, Ostadal B. Fate of "sinusoidal" intertrabecular spaces of the cardiac wall after development of the coronary vascular bed in chick embryo. Folia Morphol. 1971;19:31. [PubMed] [Google Scholar]
- 52.Rychterova V. Principle of growth in thickness of the heart ventricular wall in the chick embryo. Folia Morphol (Praha) 1971;19:262. [PubMed] [Google Scholar]
- 53.Sasse-Klaassen S, Probst S, Gerull B, et al. Novel gene locus for autosomal dominant left ventricular noncompaction maps to chromosome 11p15. Circulation. 2004;109:2720. doi: 10.1161/01.CIR.0000131865.21260.56. [DOI] [PubMed] [Google Scholar]
- 54.Schaefer KS, Doughman YQ, Fisher SA, et al. Dynamic patterns of apoptosis in the developing chicken heart. Dev Dyn. 2004;229:489. doi: 10.1002/dvdy.10463. [DOI] [PubMed] [Google Scholar]
- 55.Sedmera D. Development of cardiac conduction system in mammals. Journal of Applied Biomedicine. 2007;5:115. [Google Scholar]
- 56.Sedmera D, Pexieder T, Vuillemin M, et al. Developmental patterning of the myocardium. Anat Rec. 2000;258:319. doi: 10.1002/(SICI)1097-0185(20000401)258:4<319::AID-AR1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 57.Sedmera D, Reckova M, DeAlmeida A, et al. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat Rec. 2003;274A:773. doi: 10.1002/ar.a.10085. [DOI] [PubMed] [Google Scholar]
- 58.Sedmera D, Reckova M, DeAlmeida A, et al. Functional and morphological evidence for a ventricular conduction system in the zebrafish and Xenopus heart. Am J Physiol Heart Circ Physiol. 2003;284:H1152. doi: 10.1152/ajpheart.00870.2002. [DOI] [PubMed] [Google Scholar]
- 59.Sedmera D, Thomas PS. Trabeculation in the embryonic heart [letter] Bioessays. 1996;18:607. doi: 10.1002/bies.950180714. [DOI] [PubMed] [Google Scholar]
- 60.Sedmera D, Wessels A, Trusk TC, et al. Changes in activation sequence of embryonic chick atria correlate with developing myocardial architecture. Am J Physiol Heart Circ Physiol. 2006;291:H1646. doi: 10.1152/ajpheart.01007.2005. [DOI] [PubMed] [Google Scholar]
- 61.Shiraishi I, Takamatsu T, Fujita S. Three-dimensional observation with a confocal scanning laser microscope of fibronectin immunolabeling during cardiac looping in the chick embryo. Anat Embryol (Berl) 1995;191:183. doi: 10.1007/BF00187817. [DOI] [PubMed] [Google Scholar]
- 62.Streeeter DDJ. Gross Morphology and fiber geometry of the heart. In: Berne RMSN, Geiger SR, editors. Handbook of Physiology - Section 2: The Cardiovascular System. Bethesda: Am Physiol Soc; 1979. p. 61. [Google Scholar]
- 63.Taber LA. Biomechanics of cardiovascular development. Annu Rev Biomed Eng. 2001;3:1. doi: 10.1146/annurev.bioeng.3.1.1. [DOI] [PubMed] [Google Scholar]
- 64.Takebayashi-Suzuki K, Yanagisawa M, Gourdie RG, et al. In vivo induction of cardiac Purkinje fiber differentiation by coexpression of preproendothelin-1 and endothelin converting enzyme-1. Development. 2000;127:3523. doi: 10.1242/dev.127.16.3523. [DOI] [PubMed] [Google Scholar]
- 65.Thompsonq RP, Reckova M, DeAlmeida A, et al. The oldest, toughest cells in the heart. In: Chadwick DJ, Goode J, editors. Development of the cardiac conduction system. Vol 250. Chichester: Wiley; 2003. p. 157. [Google Scholar]
- 66.Tobita K, Garrison JB, Li JJ, et al. Three-dimensional myofiber architecture of the embryonic left ventricle during normal development and altered mechanical loads. Anat Rec A Discov Mol Cell Evol Biol. 2005;283:193. doi: 10.1002/ar.a.20133. [DOI] [PubMed] [Google Scholar]
- 67.Tomanek RJ. Formation of the coronary vasculature: a brief review. Cardiovasc Res. 1996;31(Spec No):E46. [PubMed] [Google Scholar]
- 68.Tota B, Cimini V, Salvatore G, et al. Comparative study of the arterial and lacunary systems of the ventricular myocardium of elasmobranch and teleost fishes. Am J Anat. 1983;167:15. doi: 10.1002/aja.1001670103. [DOI] [PubMed] [Google Scholar]
- 69.Van Mierop LHS, Kutsche LM. Comparative Anatomy and Embryology of the Ventricles and Arterial Pole of the Vertebrate heart. In: Nora JJTA, editor. Congenital Heart Disease: Causes and Processes. New York: Futura Publishing; 1984. p. 459. [Google Scholar]
- 70.Vrancken Peeters MP, Gittenberger-de Groot AC, Mentink MM, et al. The development of the coronary vessels and their differentiation into arteries and veins in the embryonic quail heart. Dev Dyn. 1997;208:338. doi: 10.1002/(SICI)1097-0177(199703)208:3<338::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 71.Waller BF, Smith ER, Blackbourne BD, et al. Congenital hypoplasia of portions of both right and left ventricular myocardial walls. Clinical and necropsy observations in two patients with parchment heart syndrome. Am J Cardiol. 1980;46:885. doi: 10.1016/0002-9149(80)90444-0. [DOI] [PubMed] [Google Scholar]
- 72.Wenink AC. Quantitative morphology of the embryonic heart: an approach to development of the atrioventricular valves. Anat Rec. 1992;234:129. doi: 10.1002/ar.1092340114. [DOI] [PubMed] [Google Scholar]
- 73.Wenink AC, Gittenberger-de Groot AC. Left and right ventricular trabecular patterns. Consequence of ventricular septation and valve development. Br Heart J. 1982;48:462. doi: 10.1136/hrt.48.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wenink AC, Knaapen MW, Vrolijk BC, et al. Development of myocardial fiber organization in the rat heart. Anat Embryol (Berl) 1996;193:559. doi: 10.1007/BF00187927. [DOI] [PubMed] [Google Scholar]
- 75.Wikenheiser J, Doughman YQ, Fisher SA, et al. Differential levels of tissue hypoxia in the developing chicken heart. Dev Dyn. 2006;235:115. doi: 10.1002/dvdy.20499. [DOI] [PubMed] [Google Scholar]
- 76.Yutzey KE, Bader D. Diversification of cardiomyogenic cell lineages during early heart development. Circ Res. 1995;77:216. doi: 10.1161/01.res.77.2.216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1. Video recording (25 fps) of tubular heart action. Ventral view of a Stage 12 chick embryo in modified New culture. Best viewed as a loop.
Movie 2. Ultrasound recording of pre-septation trabeculated heart. Four-chamber view of a Stage 24 heart shows the reciprocating contraction of atria (center) and septating ventricle (right), as well as flowing blood in the branchial arch arteries (left) and paired dorsal aortae (far left, in cross-section). Recorded at 30 fps, smallest division on scale bar 100 µm.