Abstract
AIM: To examine the technical feasibility and clinical outcomes of the endoscopic insertion of a self-expandable metal stent (SEMS) for the palliation of a malignant anastomotic stricture caused by recurrent gastric cancer.
METHODS: The medical records of patients, who had obstructive symptoms caused by a malignant anastomotic stricture after gastric surgery and underwent endoscopic insertion of a SEMS from January 2001 to December 2007 at Kangnam St Mary’s Hospital, were reviewed retrospectively.
RESULTS: Twenty patients (15 male, mean age 63 years) were included. The operations were a total gastrectomy with esophagojejunostomy (n = 12), subtotal gastrectomy with Billroth-I reconstruction (n = 2) and subtotal gastrectomy with Billroth-II reconstruction (n = 8). The technical and clinical success rates were 100% and 70%, respectively. A small bowel or colon stricture was the reason for a lack of improvement in symptoms in 4 patients. Two of these patients showed improvement in symptoms after another stent was placed. Stent reobstruction caused by tumor ingrowth or overgrowth occurred in 3 patients (15%) within 1 mo after stenting. Stent migration occurred with a covered stent in 3 patients who underwent a subtotal gastrectomy with Billroth-II reconstruction. Two cases of partial stent migration were easily treated with a second stent or stent repositioning. The median stent patency was 56 d (range, 5-439 d). The median survival was 83 d (range, 12-439 d).
CONCLUSION: Endoscopic insertion of a SEMS provides safe and effective palliation of a recurrent anastomotic stricture caused by gastric cancer. A meticulous evaluation of the presence of other strictures before inserting the stent is essential for symptom improvement.
Keywords: Stents, Surgical anastomosis, Stricture, Endoscopic gastrointestinal surgery, Stomach neoplasms
INTRODUCTION
Local recurrence causing dysphagia occurs in approximately 20% of stomach cancer patients treated with a gastrectomy[1]. These patients are usually poor surgical candidates because of advanced malignancy, poor performance status or malnutrition. Palliative surgery carries a high risk of mortality and morbidity.
A self-expandable metal stent (SEMS) is currently the main palliative nonsurgical treatment for malignant gastric outlet obstructions[2]. Metal stents are also used to treat malignant anastomotic obstructions after esophagojejunostomy, gastrojejunostomy and gastroduodenostomy. However, there are only a few reports on the clinical outcome of SEMS for the palliation of a recurrent anastomotic obstruction after gastric surgery[3–11]. In particular, there is one study on endoscopic insertion of a SEMS in a recurrent anastomotic stricture[11] instead of a fluoroscopically-guided method. The clinical outcomes and complications might differ according to the surgical technique because of the different anastomotic angle or different anatomical alterations during surgery.
This study evaluated the technical feasibility and clinical effectiveness of endoscopic SEMS placement in the palliation of patients with a recurrent anastomotic obstruction after gastric surgery.
MATERIALS AND METHODS
Patients
Twenty consecutive patients (M:F = 15:5, mean age 63.1 ± 10.3 years), who had a documented postoperative anastomotic stricture caused by recurrent gastric cancer and had undergone endoscopic SEMS insertion from January 2001 to December 2007, were enrolled in this study. All patients had a symptomatic obstruction characterized by nausea, vomiting, reduced oral intake and weight loss. The recurrent gastric cancer, which was the underlying cause of the obstruction, was confirmed by pathological diagnosis in all patients. None of the patients were surgical candidates based on the presence of advanced, metastatic disease or medical comorbidity.
The exclusion criteria were patients who were mildly symptomatic or patients in whom an adult endoscope could be passed through the malignant anastomotic stricture or patients showing evidence of peritonitis. An abdominal computed tomography (CT) scan or contrast media radiographic study to document multiple strictures was not performed routinely.
The surgical technique was a total gastrectomy with esophagojejunostomy in 10 patients, subtotal gastrectomy with Billroth-I reconstruction in 2 patients and subtotal gastrectomy with Billroth-II reconstruction in 8 patients. The type of reconstruction after total gastrectomy was loop esophagojejunostomy except for 2 patients with a Roux-en-Y esophagojejunostomy. Gastrojejunostomy without jejunojejunostomy was used for Billroth-II reconstruction after subtotal gastrectomy. Strictures occurring in the efferent loop were included in this study. One patient had a stricture in both afferent and efferent loops. Therefore 2 stents were inserted in both sites. Patients with recurrent cancer only in the afferent loop were excluded. Table 1 lists the patients’ characteristics.
Table 1.
Patients’ characteristics
| Age ± SD (yr) | 63.1 ± 10.3 |
| Male:Female | 15:5 |
| Prior surgery (n) | |
| Total gastrectomy with esophagojejunostomy (10) | Covered stent (6) |
| Uncovered stent (4) | |
| Subtotal gastrectomy with Billroth-I reconstruction (2) | Covered stent (1) |
| Uncovered stent (1) | |
| Subtotal gastrectomy with Billroth-II reconstruction (8) | Covered stent (6) |
| Uncovered stent (2) | |
| Chemotherapy after stent insertion (number of patients) | 10 |
| Follow-up loss (n, %) | 3 (15%) |
| 30-d mortality | 3/17 (18%) |
| Survival [median (range)] | 83 (12-439) d |
| Stent patency duration [median (range)] | 56 (5-439) d |
Methods
NITI-S® stents (Taewoong, Seoul, Korea, n = 10), Choo stent (M.I. Tech, Seoul, Korea, n = 10) were used. These stents are commonly used commercial pyloric stents. The degree, length and site of the stenosis were evaluated using an endoscopic procedure or barium meal prior to stent insertion. Thirteen covered stents and 7 uncovered stents were inserted. The covered stent was coated with polyurethane around the body and contained the proximal flare portion. The diameter of the body and flare portions were 18 and 26 mm, respectively. The length of these SEMS ranged from 8 to 22 cm. The outer diameter of the delivery system was 10F to 11F with an overall length of 180 cm. The stent delivery system was advanced over the guidewire. Under direct guidance of endoscopic and fluoroscopic vision, a guidewire was passed through the malignant stricture. The stent was then released and the position and location of the stent were assessed by both endoscopy and fluoroscopy. Compensatory hydrostatic dilatation of the stent was not required in any of the patients. The patients usually resumed a water or a liquid diet 24 h after stent placement. The patients started a soft or solid diet after the follow up X-ray showed full extension. There was one patient whose stent was not sufficiently expanded. He could not restart a soft diet.
After inserting the stent, a combination of 5-fluorouracil, cisplatin, and epirubicin or paclitaxel-based or docetaxel-based chemotherapy was administered when the oral intake improved and the Eastern Cooperative Oncology Group performance status was ≤ 2 (graded as follows: 0 = normal activity, 1 = symptoms but ambulatory, 2 = in bed less than 50% of time, 3 = in bed more than 50% of time, and 4 = totally bedridden). Palliative chemotherapy after stent insertion was performed in 10 patients (50%).
Definitions
The outcome of the stent was evaluated using the following parameters: (1) technical success and clinical success; (2) complications; (3) stent patency.
Technical success was defined as the successful insertion of a stent in the proper position and the confirmation of patency using a combination of endoscopy and fluoroscopy with oral contrast opacification.
Clinical success was defined as an improvement in the obstructive symptoms and oral intake 1 to 3 d after placing the stent. The degree of oral intake was assessed using the Gastric Outlet Obstruction Scoring System as follows: 0 = no oral intake; 1 = exclusively liquid diet; 2 = exclusively soft solids diet; 3 = full diet possible. The improvement in oral intake was evaluated as the best degree at least 3 d after stent insertion. A primary stent dysfunction was defined as a failure to resume an oral intake after stent insertion.
The stent patency time was defined as the duration between the initial stent placement and the recurrence of obstructive symptoms caused by a stent occlusion. It was considered to be equal to the survival time if there were no obstruction symptoms or stent occlusion.
Follow-up
The patients were followed up to determine their clinical outcomes until they died or the stent malfunctioned, such as by migration or occlusion by tumor ingrowth or overgrowth. The data were obtained from the hospital records, radiology or endoscopic records, the patients themselves during a clinical visit and their relatives by a telephone survey. The status of oral food intake was monitored at 1 mo intervals on an outpatient basis. A follow-up barium study or endoscopy was performed only if obstructive symptoms recurred in order to evaluate stent occlusion or migration.
Statistical analysis
The values for the patients’ characteristics are expressed as the median (range). The categorical data were examined using Fisher’s exact test. The degree of oral intake before and after stent insertion was compared by a Wilcoxon signed rank test. The overall survival and stent patency were estimated by Kaplan-Meier life table analysis. A P-value < 0.05 was considered significant. All analyses were carried out using SPSS version 10.0 (SPSS Inc, USA).
RESULTS
Technical and clinical success
Endoscopic stent placement was technically successful in all patients. Clinical success was achieved in 14 out of the 20 cases (70%). The reasons for the lack of improvement in obstructive symptoms were small bowel or colon stricture (n = 4), ileus induced by peritoneal dissemination (n = 1) and primary stent dysfunction caused by stent expansion failure (n = 1). The symptoms in 2 of the 4 patients with single small bowel or colon stricture improved after placing a second stent. Table 2 summarizes the improvement in the dietary status.
Table 2.
Improvement in the oral intake status compared to before stent insertion (n = 20)
| Oral intake status (by GOOSS) |
Number of cases |
|
| Pre-stenting | Post-stenting | |
| No oral intake (0) | 15 | 3 |
| Liquids only (1) | 3 | 6 |
| Soft solids (2) | 2 | 8 |
| Low-residual or full diet (3) | 0 | 3 |
| Mean scoreb | 0.35 ± 0.61 | 1.55 ± 0.94 |
P < 0.01 by Wilcoxon signed rank test; GOOSS: Gastric Outlet Obstruction Scoring System.
Complications
There was no procedure-related mortality. In one patient who underwent a distal gastrectomy with Billroth-I reconstruction, the uncovered stent did not expand fully and was compressed by the tumor mass until 5 d after stent placement. The symptoms were not improved. However, he refused further treatment and was lost to follow-up 7 d after stent placement.
Recurrent symptoms of an obstruction were observed in 3 patients (15%) as a result of tumor overgrowth (n = 2) or tumor ingrowth (n = 1) within 1 mo after stenting. The reobstruction rate (1/13 vs 2/7, P = 0.55) of a covered stent and uncovered stent, and stent patency duration [56 d (range, 7-439) for the covered stent vs 37 d (range, 15-141) for the uncovered stent, P = 0.7] were similar. Tumor overgrowth occurred in patients who underwent a total gastrectomy with esophagojejunostomy. Tumor ingrowth occurred in a patient who underwent a subtotal gastrectomy with Billroth-II reconstruction, in whom an uncovered stent was inserted. Two patients were treated successfully with an overlapping second covered stent. Stent migration occurred in 3 patients (15%) who underwent a subtotal gastrectomy with Billroth-II reconstruction, in whom covered stents was inserted. Complete stent migration occurred at 64 d in one patient who received palliative chemotherapy. The migrated stent was not detected until the endoscopy or radiologic study revealed no stent remaining at the previous stricture site. Therefore, the stent was believed to have migrated downward and pass out of the anus without the patient’s awareness. She was asymptomatic even though there was stent migration. The reobstructive symptoms appeared 319 d after stent migration. She was treated with the placement of 3 stents at the efferent loop, the afferent loop and distal colon stricture.
Partial stent migration to the more distal side of the efferent loop occurred in a patient 2 d after stent placement. The patient was treated by overlapping a second stent into the first stent. The proximal half of one stent slipped upward to the body of the stomach in one patient, which was repositioned by grasping with the forceps. The symptoms improved. Table 3 gives a summary of the complications.
Table 3.
Complications associated with stent placement
| Patient | Complication | Type of operation | Type of stent | Days after stenting | Treatment |
| 1 | Expansion failure | Billroth-I subtotal gastrectomy | Uncovered | 5 | Refusal of treatment |
| 2 | Tumor overgrowth | Total gastrectomy | Covered | 7 | Second stent |
| 3 | Tumor overgrowth | Total gastrectomy | Uncovered | 28 | TPN |
| 4 | Tumor ingrowth | Billroth-II subtotal gastrectomy | Uncovered | 15 | Second stent |
| 5 | Stent migration (complete) | Billroth-II subtotal gastrectomy | Covered | 64 | Not needed |
| 6 | Stent migration (partial) | Billroth-II subtotal gastrectomy | Covered | 20 | Reposition |
| 7 | Stent migration (partial) | Billroth-II subtotal gastrectomy | Covered | 2 | Second stent |
TPN: Total parenteral nutrition.
Survival
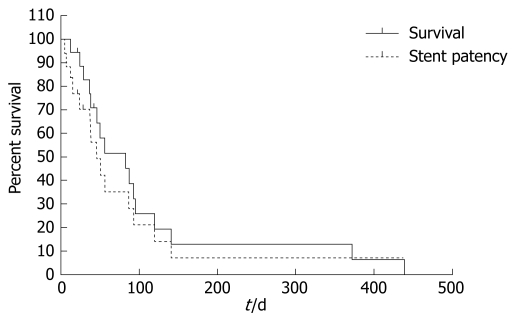
Three patients were lost during the follow-up period and the remaining 17 patients died. The median survival period was 83 d (range, 12-439 d) and the median stent patency was 56 d (range, 5-439 d, Figure 1). There were no differences in median survival or stent patency between the patients who received palliative chemotherapy and those who did not (P = 0.66).
Figure 1.
Cumulative survival and stent patency of 20 patients obtained using the Kaplan-Meier method.
DISCUSSION
A SEMS is a simple, safe and effective palliation treatment for patients with a malignant obstruction of the gastrointestinal tract[12,13]. A SEMS has clinical advantages, compared with surgical gastrojejunostomy, such as rapid resumption of oral intake, shorter hospital stay and rapid improvement in the quality of life in malignant gastric obstruction[14,15].
Patients with an anastomotic stricture caused by recurrent gastric cancer are likely to be severely debilitated. These patients generally have a relatively short life expectancy. Bypass or resective operations are usually impossible because of the extensive tumor invasion and metastasis[5]. Therefore, a less invasive procedure is preferred. This study evaluated the clinical effectiveness and the technical feasibility of SEMS insertion in the palliation of patients with a recurrent anastomotic stricture after various gastric surgical procedures.
The surgical techniques used were total gastrectomy with esophagojejunostomy (n = 10), subtotal gastrectomy with Billroth-I reconstruction (n = 2), subtotal gastrectomy with Billroth-II reconstruction (n = 8). All procedures were performed using endoscopic guidance. The technical success rate was 100%, which is comparable to those with a primary malignant gastric outlet obstruction (83%-100%)[15]. There is one report on endoscopically-guided stent insertion in a recurrent anastomotic stricture[11]. The advantages of endoscopically-guided stent insertion are the ease of accessing the stricture site and the avoidance of looping the delivery system through the dilated gastric lumen because endoscopy offers sufficient stiffness, so that the delivery system can easily pass through the dilated gastric lumen. There was no erroneous stent placement in the incorrect loop. The efferent loop was differentiated by identifying the ampulla of Vater in the afferent loop by endoscopy, which was confirmed by fluoroscopy during stent insertion. Before stenting, knowledge of the anatomy is important because it can be altered by the surgical procedures or recurrent tumor mass occluding the efferent loop[7].
The dietary intake improved in 14 out of the 20 patients (70%) after stent placement, which is comparable to the clinical success rate of SEMS insertion in a malignant gastric outlet obstruction (75%-85%)[13]. The improvement in symptoms after SEMS insertion in the anastomotic stricture caused by recurrent gastric cancer was reported to be 80%-90%[3–8]. The average score of the dietary status improved from 0.35 ± 0.61 to 1.55 ± 0.94 (P < 0.01). Five patients whose symptoms did not improve had another single stricture at the small intestine or colon, or ileus by peritoneal dissemination. The dietary state in 2 of them improved after inserting an additional stent. This suggests that a precise study of the distal bowel loop using a CT scan or barium study before stent insertion is essential in order to exclude a concealed obstruction. A single stent may not be helpful if there are multiple strictures. Moreover, the insertion of 2 stents at one time may be necessary if the patients have another single stricture.
Stent reobstruction caused by tumor ingrowth or overgrowth occurred in 3 patients (15%) within 1 mo after stent placement. A recent study reported that early restenosis within 1 mo tended to occur more frequently in postoperative anastomosis than a gastric outlet obstruction caused by primary cancer (4/6 vs 2/6, P < 0.01)[16]. The covered stents had the merit of less frequent reobstruction by tumor ingrowth[17]. However, in this study, the reobstruction rate and stent patency duration of covered stents and uncovered stents were similar. The incidence of stent reobstruction in recurrent anastomotic stricture after gastric surgery was reported to be 0%-17%[3–8]. Most studies used covered stents. In 2 studies using uncovered stents, Lee et al[6] reported that one out of 4 patients had tumor ingrowths, and Song et al[7] reported a 50% stent reocclusion rate within 2 wk of stent placement. A recent retrospective study suggested that a double coaxial stent had a longer patency and lower migration rate than an uncovered stent in postoperative anastomotic obstructions[11]. A prospective, randomized, comparative study to determine which stent is favorable in this situation will be needed.
Three cases of stent migration (15%, 3/20) were encountered in patients who underwent a subtotal gastrectomy with Billroth-II reconstruction and had a covered stent inserted. Complete stent migration occurred in one patient who received palliative chemotherapy after approximately 64 d. Because chemotherapy might stabilize or reduce the tumor burden, it could influence stent migration. Two cases of partial stent migration were easily treated by repositioning the stent and overlapping a second stent. The incidence of stent migration was reported to be 0%-16% in studies using a covered stent in an anastomotic stricture in various types of gastric cancer surgery[3–8]. The surgical technique can influence the rate of migration. The relatively acute angle between anastomosis and the efferent loop in gastrojejunostomy compared with the relatively obtuse angle in esophagojejunostomy or gastroduodenostomy, the radial force of the stent in the angulated loop, or the use of a covered stent may influence stent migration.
In this study, the 30-d mortality was 18%. The median survival was 83 d (range, 12-432 d). The median stent patency was 56 d (range, 5-439 d). Because the median survival in an anastomotic obstruction is comparable to that in a malignant gastric outlet obstruction, strategies to prolong stent patency and avoid the need for additional intervention are important in patients with recurrent cancer, particularly those with a relatively good performance status or who are expected to have a longer survival.
In summary, endoscopic insertion of a SEMS is a safe, technically feasible, and effective treatment for the palliation of anastomotic strictures caused by recurrent gastric cancer. A meticulous evaluation of the presence of another stricture before inserting the stent is essential for symptom improvement.
COMMENTS
Background
A self-expandable metal stent (SEMS) was used to treat malignant anastomotic obstruction during esophagojejunostomy, gastrojejunostomy and gastroduodenostomy. There are only a few reports on the clinical outcome of SEMS insertion for the palliation of a recurrent anastomotic obstruction after gastric surgery.
Research frontiers
The authors aimed to evaluate the technical feasibility and clinical effectiveness of an endoscopic SEMS placement in the palliation of patients with a recurrent anastomotic obstruction after gastric surgery.
Innovations and breakthroughs
This retrospective study has shown that the technical and clinical success of SEMS insertion for anastomotic strictures caused by recurrent gastric cancer were 100% and 70%, respectively. The main reasons for the clinical failure were small bowel or colon stricture in addition to anastomotic stricture. Stent migration (15%) was encountered in patients who underwent subtotal gastrectomy with Billroth-II reconstruction and had a covered stent inserted.
Applications
A meticulous evaluation of the presence of other strictures is essential before inserting the stent for anastomotic strictures from recurrent gastric cancer. The possibility of stent migration is a consideration in anastomotic strictures after gastrojejunostomy.
Peer review
This paper is a good retrospective report on the usage of a SEMS for palliation of malignant anastomotic stricture caused by recurrent gastric cancer, showing that it is a safe and effective palliation treatment.
Peer reviewer: Wai-Man Wong, MD, Department of Medicine, University of Hong Kong, St Paul’s Hospital, 2 Eastern Hospital Road, Causeway Bay, Hong Kong, China
S- Editor Li LF L- Editor Cant MR E- Editor Lin YP
References
- 1.Iwanaga T, Koyama H, Furukawa H, Taniguchi H, Wada A, Tateishi R. Mechanisms of late recurrence after radical surgery for gastric carcinoma. Am J Surg. 1978;135:637–640. doi: 10.1016/0002-9610(78)90126-5. [DOI] [PubMed] [Google Scholar]
- 2.Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543–550. doi: 10.1055/s-2004-814434. [DOI] [PubMed] [Google Scholar]
- 3.Cheung HY, Chung SC. Covered metal stent for tumor obstruction of efferent loop recurrence after gastrectomy. Surg Endosc. 1997;11:936–938. doi: 10.1007/s004649900491. [DOI] [PubMed] [Google Scholar]
- 4.Jeong JY, Kim YJ, Han JK, Lee JM, Lee KH, Choi BI, Yang HK, Lee KU. Palliation of anastomotic obstructions in recurrent gastric carcinoma with the use of covered metallic stents: clinical results in 25 patients. Surgery. 2004;135:171–177. doi: 10.1016/s0039-6060(03)00346-5. [DOI] [PubMed] [Google Scholar]
- 5.Jeong JY, Han JK, Kim AY, Lee KH, Lee JY, Kang JW, Kim TJ, Shin SH, Choi BI. Fluoroscopically guided placement of a covered self-expandable metallic stent for malignant antroduodenal obstructions: preliminary results in 18 patients. AJR Am J Roentgenol. 2002;178:847–852. doi: 10.2214/ajr.178.4.1780847. [DOI] [PubMed] [Google Scholar]
- 6.Lee JM, Han YM, Kim CS, Lee SY, Lee ST, Yang DH. Fluoroscopic-guided covered metallic stent placement for gastric outlet obstruction and post-operative gastroenterostomy anastomotic stricture. Clin Radiol. 2001;56:560–567. doi: 10.1053/crad.2001.0700. [DOI] [PubMed] [Google Scholar]
- 7.Song HY, Kim TH, Choi EK, Kim JH, Kim KR, Shin JH, Lee SK, Kim TW, Yook JH, Kim BS. Metallic stent placement in patients with recurrent cancer after gastrojejunostomy. J Vasc Interv Radiol. 2007;18:1538–1546. doi: 10.1016/j.jvir.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 8.Yang ZQ, Song HY, Kim JH, Shin JH, Kim TW, Yook JH, Kim BS. Covered stent placement in patients with recurrent cancer after a Billroth I reconstruction. J Vasc Interv Radiol. 2007;18:1533–1537. doi: 10.1016/j.jvir.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Wayman J, Bliss R, Richardson DL, Griffin SM. Self-expanding metal stents in the palliation of small bowel stenosis secondary to recurrent gastric cancer. Gastrointest Endosc. 1998;47:286–290. doi: 10.1016/s0016-5107(98)70328-1. [DOI] [PubMed] [Google Scholar]
- 10.Patton JT, Carter R. Endoscopic stenting for recurrent malignant gastric outlet obstruction. Br J Surg. 1997;84:865–866. [PubMed] [Google Scholar]
- 11.Song GA, Kang DH, Kim TO, Heo J, Kim GH, Cho M, Heo JH, Kim JY, Lee JS, Jeoung YJ, et al. Endoscopic stenting in patients with recurrent malignant obstruction after gastric surgery: uncovered versus simultaneously deployed uncovered and covered (double) self-expandable metal stents. Gastrointest Endosc. 2007;65:782–787. doi: 10.1016/j.gie.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Baron TH. Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med. 2001;344:1681–1687. doi: 10.1056/NEJM200105313442206. [DOI] [PubMed] [Google Scholar]
- 13.Adler DG, Merwat SN. Endoscopic approaches for palliation of luminal gastrointestinal obstruction. Gastroenterol Clin North Am. 2006;35:65–82, viii. doi: 10.1016/j.gtc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Maetani I, Tada T, Ukita T, Inoue H, Sakai Y, Nagao J. Comparison of duodenal stent placement with surgical gastrojejunostomy for palliation in patients with duodenal obstructions caused by pancreaticobiliary malignancies. Endoscopy. 2004;36:73–78. doi: 10.1055/s-2004-814123. [DOI] [PubMed] [Google Scholar]
- 15.Jeurnink SM, van Eijck CH, Steyerberg EW, Kuipers EJ, Siersema PD. Stent versus gastrojejunostomy for the palliation of gastric outlet obstruction: a systematic review. BMC Gastroenterol. 2007;7:18. doi: 10.1186/1471-230X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim GH, Kang DH, Lee DH, Heo J, Song GA, Cho M, Yang US. Which types of stent, uncovered or covered, should be used in gastric outlet obstructions? Scand J Gastroenterol. 2004;39:1010–1014. doi: 10.1080/00365520410003146. [DOI] [PubMed] [Google Scholar]
- 17.Jung GS, Song HY, Kang SG, Huh JD, Park SJ, Koo JY, Cho YD. Malignant gastroduodenal obstructions: treatment by means of a covered expandable metallic stent-initial experience. Radiology. 2000;216:758–763. doi: 10.1148/radiology.216.3.r00au05758. [DOI] [PubMed] [Google Scholar]