Abstract
Secondary bacterial pneumonia is a common cause of death during influenza epidemics. We hypothesized that virus-specific factors could contribute to differences in annual excess mortality. A set of recombinant viruses with neuraminidases from representative influenza virus strains from the last 50 years was created and characterized. Their specific neuraminidase activities correlated with their ability to support secondary bacterial infections. Recombinant viruses with the 1957 and 1997 neuraminidases had the highest activities, while a virus with the 1968 neuraminidase had the lowest activity. The high activity of the 1957 neuraminidase, when compared to other neuraminidases, more strongly supported bacterial adherence in vitro and secondary pneumococcal pneumonia in a mouse model. These data lend support to our hypothesis that the neuraminidase of influenza viruses contributes to secondary bacterial infections and subsequent excess mortality.
Keywords: influenza virus, Streptococcus pneumoniae, pneumococcus, pneumonia, mouse model, neuraminidase, pandemic, synergism
Introduction
Influenza A virus causes epidemics annually and pandemics several times a century. Two subtypes of influenza A virus, H3N2 and H1N1, are currently circulating in the human population. H3N2 epidemics are associated with higher mortality in human populations than H1N1 or influenza B virus epidemics [1,2]. Pandemics are defined by acquisition of a new hemagglutinin (HA) and by a high attack rate and global spread. They are generally considered to result in high mortality. Understanding what factors are associated with increased mortality will help us prepare for the next influenza pandemic.
It has been suggested that antigenic novelty accounts for increased mortality during pandemics, but a comparison of mortality estimates between different pandemic years and between inter-pandemic years indicates that other factors are also involved. The capability of influenza strains to predispose to bacterial superinfection may be one of the factors that determine epidemic mortality. Secondary bacterial pneumonia is an important cause of influenza-associated death during both pandemic and inter-pandemic influenza. Studies during the influenza pandemics of 1957 and 1968 revealed a bacterial etiology in about 70% of patients with fatal or life-threatening pneumonia [3,4]. In inter-pandemic years, 44% to 57% of patients hospitalized with influenza have bacterial pneumonia [5−8], and, although it varies from year to year depending on the viral strain circulating, on average it is estimated that 25% of all deaths associated with influenza are due to secondary bacterial pneumonia [9].
Streptococcus pneumoniae is the leading cause of community-acquired pneumonia [10] and is a major pathogen causing pneumonia and other bacterial complications of influenza. We have recently developed a mouse model of synergism between influenza and S. pneumoniae [11,12]. Using this model, we established that viral neuraminidase (NA) is an important factor in viral-bacterial synergism [13,14]. Influenza viral NA activity is needed to release newly synthesized virus by cleaving sialic acid both from oligosaccharides of viral HA and NA, and from host cell glycoconjugates. This action of NA also promotes adherence and invasion of pneumococcus, as cleavage of sialic acid from the surface of host cells exposes cryptic receptors for pneumococcus [15−18]. Bacteria which can successfully invade the lower respiratory tract typically express NA for this purpose. The finding of generally higher NA activities in modern H3N2 than in H1N1 viruses [19] is consistent with the hypothesis that high NA activity leads to higher mortality from secondary bacterial pneumonia. We sought to define viral specific NA activity from several viruses within a subtype and correlate this with the biology of secondary bacterial pneumonia.
Materials and methods
Generation of recombinant viruses
Recombinant viruses were produced using an established eight-plasmid reverse-genetics system in co-culture of 293T and MDCK cells [20] from parental viruses from the influenza virus repository at St. Jude Children's Research Hospital. Gene segments of A/Puerto Rico/8/34 (H1N1; PR8) [21], the HA gene segment of A/Hong Kong/1/68 (H3N2; HK68), and the HA and NA segments of A/Fujian/411/02 (H3N2; Fuj02) had been cloned into the pHW2000 plasmid previously. NA gene segments from human H2N2 viruses A/Singapore/1/57 (Sing57) and A/England/12/62 (Eng62), human H3N2 viruses HK68, A/Memphis/102/72 (Mem72), A/Leningrad/516/86 (Len86) and A/Sydney/5/97 (Syd97), and chicken H9N2 virus A/Chicken/Hong Kong/WF2/99 (WF2) were amplified by PCR and cloned into pHW2000. HA and NA genes were sequenced and their identities were confirmed by comparison to the parental strain sequences. Virus stocks were grown in embryonated chicken eggs, centrifuged on a 25/75% sucrose cushion and then pelleted and resuspended in PBS.
Pneumococci
A type 3 strain of S. pneumoniae that had been transformed with the lux operon (Kevin Francis and Jun Yu, Xenogen Corp.) was used in mouse studies. R6T, an unencapsulated laboratory strain, was used in adherence assays.
Characterization of recombinant viruses in cell culture, embryonated eggs, and in mice
Allantoic fluid and concentrated virus stocks were titrated in MDCK cells and in embryonated chicken eggs by standard methods to obtain the TCID50 and EID50. Groups of 4 mice were infected with serial dilutions of the allantoic fluid stocks to obtain an MLD50 by the method of Reed and Muench [22]. For lung viral titers, mouse lung homogenates were titrated in MDCK cells as described [12].
NA activity
Total NA activity of concentrated virus diluted in calcium saline buffer to a final substrate concentration of 10 μM was measured by fluorescence of 4-methylumbelliferone cleaved from 2’-(4-methylumbelliferyl)-N-acetylneuraminic acid (Mu-NANA; Sigma) as described [23]. Relative linkage specificity of NA was determined using N-acetylneuraminic acid bound to lactose through either α(2−3) or α(2−6) linkage as a substrate. Fetuin was used as a substrate to measure NA activity against a large molecule with both α(2−3) and α(2−6) sialic acid linkages. The amount of sialic acid released from NANA-lactose (substrate concentration of 0.1mM) or fetuin (substrate concentration 6.1 mM) was measured with the thiobarbituric acid assay as described [24]. All reactions were performed for 30 min at 37 °C. To relate NA activity to the predominant viral proteins, virus concentrates were run on 10% Tris-HCl gels. After staining with Sypro orange (Amersham Pharmacia), HA and nucleoprotein (NP) bands were quantified using a laser-excited gel scanner.
Immunoelectron microscopy
Immuno-EM was done as described previously [25], using a mixture of monoclonal antibodies against 7 different N2 NAs representing the range of NAs studied. Anti-NA monoclonal antibodies 152/6 (A/Japan/1/57), 25/4 (A/Tokyo/3/67), 5/2 (A/Aichi/2/68), 1/1 (A/Udorn/307/72), and E12−8 (A/Memphis/12/85) were provided by Dr. Robert Webster (St. Jude Children's Research Hospital) [26,27] and Mem4 and Mem5 (A/Memphis/31/98) were provided by Dr. Gillian Air (University of Oklahoma Health Sciences Center) [28]. Negative staining was done with 2% phosphotungstic acid, and positive staining with ethanolic uranyl acetate. Gold particles representing the amount of NA per virion were counted from positively stained samples.
Adherence assay
Adherence assays were performed using standard methods as described previously [13] using pneumococcal strain R6T following a 30 minute incubation with virus at 37 °C. Controls were treated identically, without the addition of virus. For inhibition of viral NA, the oseltamivir prodrug Ro 64−0796 (Roche Products Ltd.) was added to the virus suspension at a concentration of 10 μM, 30 min before incubation with monolayers (concentration of the active metabolite oseltamivir carboxylate was not determined).
Mice and infection model
Experimental procedures were done while the mice (8−10 week old female BALB/c, Jackson Laboratory) were under general anesthesia with inhaled isoflurane 2.5%. Infectious agents were administered intranasally in a volume of 100 μl of PBS. For synergism between influenza and pneumococcus, mice were infected first with influenza virus and then 7 days later with pneumococcus and monitored at least daily for illness and mortality. Groups of 9−10 mice were infected with 0.01 MLD50 of influenza and 100 cfu of pneumococcus and were imaged daily. Doses of virus were calculated in relation to the MLD50 rather than using a fixed number of infectious particles so that weight loss, viral lung titers, and damage to the respiratory tract prior to bacterial challenge were even between groups. Mice found to be moribund were euthanized and considered to have died that day. All animal experiments were approved by the St. Jude Children's Research Hospital Animal Care and Use Committee and were performed under biosafety level 2 conditions.
Imaging of live mice
Anesthetized mice were imaged for 20 seconds by an IVIS CCD camera (Xenogen Corp.).Total photon emission from selected and defined areas within the images of each mouse was quantified by the LivingImage software package version 2.20 (Xenogen Corp.), as described elsewhere [29], and was expressed as relative light units. Pneumonia was defined as detection of over 20,000 relative light units per minute from the thorax.
Statistical analysis
Comparison of survival rates in the groups of mice was done by Mantel-Cox χ2 test on Kaplan-Meier survival data, and comparisons in bacterial adherence were made by one-way analysis of variance followed by Dunn's test. P<0.05 was considered statistically significant.
Results
Recombinant viruses differing only in neuraminidase
Influenza viruses with an N2 NA have been circulating in the human population since 1957. Representative NA genes were selected from 6 human pandemic and inter-pandemic H2N2 and H3N2 viruses that circulated between 1957 to 2004. Recombinant viruses that differed only in their NAs were rescued on the common 7 gene segment background (6 PR8 internal genes and the HK68 HA gene). For comparison with these human viruses, a virus with an NA from a chicken H9N2 influenza virus from 1999 was also rescued. All the recombinant viruses grew to high titers in embryonated eggs (Table 1). They caused morbidity and mortality in mice, and thus were suitable for further study of viral-bacterial synergism in a mouse model. The doses lethal to 50% of mice (MLD50) of the viruses with a human NA gene were similar, whereas the MLD50s of the virus with the chicken NA and that of the mouse adapted parental strain PR8 were 3 to 4 logs lower. A virus with the Fuj02 NA could not be rescued when paired with the HK68 HA and was therefore excluded from comparison to other isogenic viruses. However, it was rescued with its own HA (Fuj02 HA), and a comparison virus containing the HA from Fuj02 and the NA from Mem72 was also rescued. Because mutations may be generated during rescue of influenza viruses using the 8-plasmid system and during passage of influenza viruses in eggs, NA sequences of the recombinant viruses were compared with published sequences of the parental strains. Site directed mutagenesis was used to insure that the NA gene of all viruses matched the Genbank sequence of the target viruses.
Table 1.
Titers of the viruses and their virulence in mice.
| Virus | TCID50a (Log10/ml) | EID50 (Log10/ml) | MLD50b (Log10 EID50) |
|---|---|---|---|
| HK68/Sing57/PR8 | 7.75 | 8.75 | 7.0 |
| HK68/Eng62/PR8 | 8.0 | 8.25 | 6.25 |
| HK68/HK68/PR8 | 8.5 | 8.25 | 6.5 |
| HK68/Mem72/PR8 | 8.0 | 8.5 | 6.75 |
| HK68/Len86/PR8 | 7.5 | 8.75 | 6.5 |
| HK68/Syd97/PR8 | 7.75 | 7.5 | 6.25 |
| HK68/WF2/PR8 | 8.875 | 8.875 | 3.25 |
| A/PR/8/34 | 8.5 | 9.25 | 3.18 |
TCID50 and EID50 are the doses of virus infectious for 50% of wells containing MDCK cells and 50% of embryonated eggs, respectively.
The dose lethal to 50% of intranasally infected mice (MLD50) is expressed as the number of EID50s.
Neuraminidase activities in recombinant viruses
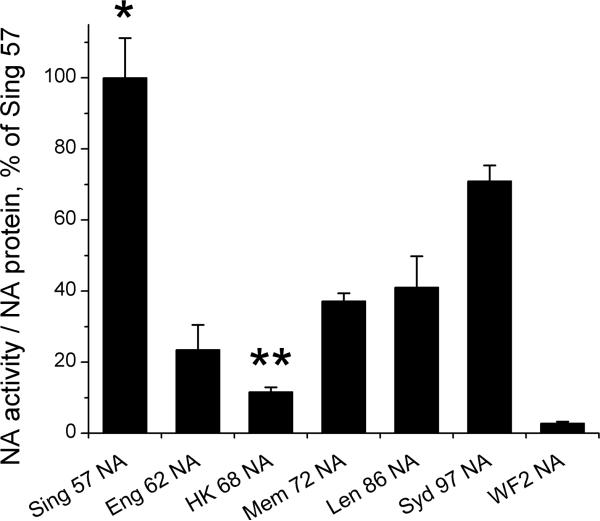
To characterize this set of viral tools, the NA activities in concentrated viruses were first measured using Mu-NANA as the substrate [23] and expressed as a function of the amount of NA protein (Fig. 1). NA activity was highest in the pandemic strain Sing57 NA. Activity decreased and was lowest in HK68 NA. From 1968 to 1997 a general increase in activity was seen such that Syd97 NA had the second highest activity. Although direct comparison to the isogenic set of viruses was not possible, the NAs of both the wild-type Fuj02 virus and the recombinant virus with the Fuj02 NA had NA specific activity at levels similar to that of the Syd97 NA (data not shown). WF2 NA from a recent chicken strain of the H9N2 subtype had a very low NA activity, only 2% of that of Sing57 NA. A similar pattern of NA activities was seen when values were expressed relative to the most abundant viral proteins HA and NP, or when fetuin was used as a substrate, showing a decline from 1957 to 1968, then a gradual increase from 1968 to 2002 (data not shown).
Figure 1.
Viral NA activities. NA activities of the viruses differing from each other only in NA were measured using Mu-NANA as the substrate. Activities are expressed per amount of viral protein. Mean ± SD from 3−5 independent measurements are shown as percentage of the activity in HK/Sing/PR8. An asterisk (*) indicates a significant difference in activity compared to all other viruses by ANOVA (p < 0.001). A double asterisk (**) indicates a significant difference compared to all other viruses containing a human NA (p < 0.05).
Changes in the amino acid sequences of the NAs of viruses isolated between 1957 and 1962 have been associated with a decrease in their NA activity, suggesting that these structural changes are responsible for the observed differences [30]. To determine whether these differences might reflect different preferences for α(2−3)- or α(2−6)-linked substrates, we performed additional experiments. Immunoelectron microscopy was used to quantify the amount of NA per virion (Fig. 2a-b). The differences in the amount of NA were minor (Fig. 2c) and did not correlate with differences in NA activity. Thus, it seems unlikely that changes in expression over time contributed significantly to changes in NA specific activity. In addition to demonstrating the relatively stable amount of NA protein per virion, immuno-EM studies confirmed the earlier finding of clustered distribution of NA on virions [25].
Figure 2.
Amount of NA per virion. NA protein was detected with immuno-EM using a mixture of monoclonal NA antibodies to bind gold particles to NA. (A) An example of a negatively stained immuno-EM sample where the viral structure is visualized more clearly than in positive staining, but the resolution of the gold particles (seen as black dots) is not as good. A clustered distribution of the NA can be seen. (B) The relative amount of NA per virion shown as the mean ± SD number of gold particles per virion. Gold particles were counted from 31 to 49 virions of each virus. (C) The amount of NA per virion was estimated by counting the gold particles from positively stained samples. Pictures of 4 representative viruses are shown. An asterisk (*) indicates a significant difference compared to viruses containing the Sing57 , Eng62, HK68, or Mem72 NAs by ANOVA (p < 0.001). A double asterisk (**) indicates a significant difference compared to viruses containing the Eng62 or Mem72 NAs (p < 0.05).
Next, the substrate preference of the recombinant viruses was examined. The ability of viral NA to liberate sialic acid from α(2−3)sialyllactose and α(2−6)sialyllactose as substrate was determined. All viruses had the majority of NA activity against α(2−3) linkage of sialic acid. As expected, Sing57 NA, as well as chicken WF2 NA had activity almost solely (98%) against substrate with an α(2−3) linkage, which is the primary linkage of sialic acid in the gastrointestinal tract of aquatic birds [31]. Consistent with previous findings [30,32], the ratio of α(2−6) activity to α(2−3) activity increased from 1957 to 1986. Even in NAs from 1986 or 1997, however, only about 11% of their activity was against the α(2−6) linkage. Thus, it seems unlikely that substrate preference is responsible for differences in N2 NA activities. It is noteworthy that passaging influenza viruses in eggs causes selective pressure in favor of α(2−3) NA activity, since only α(2−3)-linked sialic acid is found in chicken egg allantoic cells [33].
Adherence of pneumococci to human respiratory cells correlates with viral NA activity
We next tested the effect of the degree of NA specific activity on adherence of S. pneumoniae strain R6T. The recombinant viruses were diluted to the same amount of viral protein before incubation on A549 cell cultures. At a viral protein concentration of 1 μg/ml, all viruses clearly increased the adherence of R6T pneumococci to cells, but the HK68 NA with relatively low NA activity increased adherence only 2.1-fold, compared with a 3.8-fold increase after Sing57 NA and a 2.9-fold increase after Syd97 NA. At a lower viral protein concentration of 0.2 μg/ml, the virus with the most active NA, Sing57 NA, still increased adherence 3.6-fold, whereas at this concentration the other viruses caused only 1.3-fold to 1.7-fold increases in adherence (Fig. 3). The effect of all viruses on adherence of pneumococci was reversed when oseltamivir was added to the incubation mixture (only data against the higher concentration of virus are shown). Oseltamivir carboxylate is a specific inhibitor of influenza viral NA, with no effect on bacterial NAs [13]. The reversal by oseltamivir, together with a 30 minute incubation time that is too short for mechanisms involving viral replication, suggest that differences between viruses in their effects on the adherence of pneumococci are due to their intrinsic NA activities.
Figure 3.
Effect of viruses on pneumococcal adherence to A549 cells. Cells were incubated for 30 min with 1 μg/ml or 0.2 μg/ml of influenza viruses, with or without the NA inhibitor oseltamivir, before the assay of adherence of R6T pneumococcus to A549 cells. Comparison of HK/Sing/PR8, which has high NA activity, with 3 representative viruses with lower NA activities is shown. Data are shown as mean ± SD of 3−6 consecutive, independent experiments. An asterisk (*) indicates a significant difference between different treatments with the same virus by ANOVA (P<0.05). A double asterisk (**) indicates a significant difference between HK/Sing/PR8 and the other viruses (0.2 μg/ml) without oseltamivir treatment by ANOVA (P<0.05).
Higher NA activity induces higher mortality from secondary pneumococcal pneumonia in mice
Influenza infection primes mice for subsequent lethal pneumonia from pneumococcus [11,12]. We infected mice with influenza viruses and 7 days later infected them with a strain of pneumococcus that had been transformed with the luciferase-expressing lux operon that permits bioluminescent imaging of pneumonia in live anesthetized mice [13]. We compared pairs of recombinant viruses that differed approximately two-fold in NA activity (Sing57 NA vs. Syd97 NA matched with the HK68 HA, and Fuj02 NA vs. Mem72 NA matched with the Fuj02 HA). The mean weight loss at the time of secondary infection was similar in paired groups of mice, 7.9% in Sing57 NA mice vs. 8.9% in Syd97 NA mice, and 0.1% in Fuj02 NA mice vs. 1.3% in Mem72 NA mice, showing that morbidity from the two different influenza virus infections was similar. However, a significant difference in survival from secondary challenge was observed, as 8/19 (42%) of mice infected with Sing57 NA vs. 15/19 (79%) infected with Syd97 NA (P<0.05) lived (Fig. 5a), and 3/9 (33%) infected with the Fuj02 NA vs. 8/9 (89%) infected with the Mem72 NA (P<0.05) lived (Fig. 4d). Daily imaging showed the development of pneumococcal pneumonia 2 to 3 days before death (Fig. 4a,c,e; two representative mice are pictured). Studies using several other recombinant viruses showed similar differences when viruses with high and low NA specific activity were compared (data not shown). These data establish that even small differences in NA activity are reflected in differences in the ability of influenza viruses to permit the development of secondary bacterial infections in a mouse model. Data for the Syd97 NA (Fig. 4a, b) and Fuj02 NA (Fig. 4c,d) are not directly comparable despite the similarities in measured NA activity as the HA differs between the two experiments and could independently contribute to secondary bacterial infections.
Figure 4.
Lethal pneumococcal pneumonia in mice after infection with pairs of recombinant influenza viruses differing ∼ 2-fold in NA specific activity. Mice were challenged intranasally with 100 cfu of D39 lux pneumococcus 7 days after intranasal influenza infection. (A) Detection of pneumococcal pneumonia with a live imaging system in mice infected with HK/Sing/PR8 or HK/Syd/PR8 (9 mice/group). Pneumonia was defined as over 20,000 relative light units from the thorax. (B) Survival curves of the mice infected with HK/Sing/PR8 or HK/Syd/PR8 (19 mice/group). (C) Detection of pneumococcal pneumonia in mice infected with Fuj/Mem/PR8 or Fuj/Fuj/PR8 (9 mice/group). (D) Survival curves of the mice infected with Fuj/Mem/PR8 or Fuj/Fuj/PR8 (9 mice/group). (E) Daily live imaging of two representative mice. The mouse infected with HK/Sing/PR8 died of pneumonia on day 6 after pneumococcal challenge. The mouse infected with HK/Syd/PR8 survived through the experiment without pneumonia. Scale for the relative light units is shown on the right. One mouse infected with HK/Syd/PR8 and one with Fuj/Fuj/PR8 recovered from pneumonia; all the other mice that developed pneumonia died. An asterisk (*) indicates a significant difference in development of pneumonia or survival between the groups (P<0.05 by the Mantel-Cox χ2 test on Kaplan-Meier data).
The experiment utilizing the Sing57 and Syd97 NAs was repeated and lung viral titers were measured 3 and 7 days after infection to make sure that the kinetics of viral infection in the two groups of mice were comparable. On day 3 post infection, groups of 4 mice infected with Sing57 NA had mean viral titers of 6.5 ± 0.4 log10 TCID50/ml as compared to 6.3 ± 0.3 log10 TCID50/ml in Syd97 NA infected mice. On day 7, the titers were 2.4 ± 0.7 log10 TCID50/ml and 2.5 ± 0.4 log10 TCID50/ml, with no detectable virus in the lungs of 1 mouse from each group of 4 mice.
Discussion
A previous study using a panel of reassortant duck viruses containing human N2 NAs suggested that influenza virus NA specific activity decreased markedly between 1957 and 1968 [30]. We have confirmed and extended this observation using a set of human viruses generated by reverse genetics that contain N2 NAs representative of viruses circulating from 1957 to 2005. NA activity decreased from 1957 to 1968, and increased again from 1968 to 1997. This trend in NA activities correlates with the observed historic influenza mortality from H3N2 viruses which was highest in 1957, decreased over the following decade, but increased again in the 1990s (Table 2). Low NA activity in the 1968 virus is consistent with lower mortality from this pandemic compared to the 1957 pandemic or H3N2 epidemics during the 1990s. It has been suggested that conservation of the N2 NA in the 1968 virus resulted in relatively low pandemic mortality, but this does not explain why later viruses with no antigenic shift in HA or NA caused higher mortality. The 1997 N2 NA had the second highest activity, and this virus caused the highest epidemic mortality since the 1957 pandemic [2,34,35].
Table 2.
Comparison of NA activity with excess mortality from influenza.
| Season | Circulating virus | NA activity (%)a | Deathsb | Excess mortality rate/100,000 | Ref. |
|---|---|---|---|---|---|
| 1957−1958 | A/Singapore/1/57 | 100 | 66,000 | 39 | [35] |
| 1962−1963 | A/England/12/62 | 24 | 46,000 | 25 | [35] |
| 1968−1969 | A/Hong Kong/1/68 | 12 | 28,100 | 14 | [35] |
| 1972−1973 | A/Memphis/102/72 | 37 | 20,700 | 10 | [44] |
| 1987−1988 | A/Leningrad/86 | 41 | 30,800 | 13 | [2] |
| 1997−1998 | A/Sydney/1/97 | 71 | 72,400 | 27 | [2] |
NA activity was measured in recombinant viruses with a common background of HA and the internal genes.
Although the correlation we report here between NA activity, secondary bacterial pneumonia in our model, and historical excess mortality in humans is intriguing, other factors must be involved. A large proportion of excess deaths related to influenza are coded as cardiovascular disease, cerebrovascular disease, or diabetes [36] and most of these are presumably not related to bacterial super-infection. Virus specific factors contribute to excess morbidity and mortality and differences in these virulence factors account for differences from season to season. Our data suggest that NA activity is one of these factors, and that its impact can be seen in secondary bacterial pneumonia deaths. Other virulence factors such as antigenic novelty of the HA, modulation of interferon [37] or cytokine expression by NS1 [38], or interactions between several genes may be involved, and the NA may have effects on the host unrelated to sialic acid cleavage as suggested by its ability to activate TGF-beta [39].
Our previous studies indicated that pharmacological inhibition of the viral NA improves survival from secondary pneumococcal pneumonia following influenza [13,14]. However, these studies were done using a single virus and could not establish whether a dose effect based on the activities of different viruses might exist. In this study, we created a set of recombinant viruses that differ from each other only in NA activity. A hierarchy of support for bacterial adherence and secondary bacterial pneumonia could be seen when recombinant viruses were compared in cell culture and animal models of viral-bacterial interactions. The NA of highest activity (from the 1957 pandemic influenza strain) was capable of inducing more pneumococcal adherence to cultured respiratory epithelial cells and higher mortality from secondary pneumococcal pneumonia in mice than an NA with 2-fold less activity (Syd97). Similar weight loss and lung viral titers in the influenza phase of the infections indicated that differences in viral kinetics were not interfering in the comparisons. A virus with the NA of the recent Fuj02 strain, with NA specific activity comparable to that of the Syd97 NA, more effectively supported secondary bacterial pneumonia than a virus with another 2-fold decrease in relative activity (Mem72) when tested on the same background (paired with the Fuj02 HA). The Fujian virus caused high epidemic mortality during the most reason influenza season (2003−2004) and contributed to a number of well-publicized deaths from methicillin-resistant Staphylococcus aureus (MRSA) [40]. The activity of the 1918 NA has not been reported, and activities of N1 NAs from before 1957 have not been studied in a comprehensive fashion. Our data provide direct evidence that influenza virus NA activity is a predictor of mortality from secondary bacterial pneumonia.
These results, together with our previous data showing that oseltamivir treatment prevents secondary pneumococcal pneumonia in mice even when administered late in the viral infection [13], warrant clinical studies of prevention and treatment of bacterial complications of influenza with NA inhibitors. Effectiveness studies of these drugs in treatment of influenza have already shown decreases in complications, although the populations studied have most often not included those most vulnerable to secondary infections due to underlying illnesses or extremities of age [41−43]. Pandemic planning must take into account the possibility that many of the deaths during the next pandemic will be from bacterial complications of influenza, and an increased focus on the NA including stockpiling of NA inhibitors is therefore essential.
Acknowledgments
We thank G. Air for providing us with Mem4 and Mem5 monoclonal antibodies, E. Hoffmann, D. R. Perez, L. Widjaja and R. G. Webster for PR8, HK68 HA, and Fuj02 HA and NA plasmids, and R. McKeon for technical support. We thank R.G. Webster and E.I. Tuomanen for critical reading of the manuscript.
This work was supported by NIH (grants AI-49178 and AI-54802), the St. Jude Cancer Center Support (CORE) grant CA-21765, and the American Lebanese Syrian Associated Charities (ALSAC). V.T.P. was supported by the Academy of Finland, the Leiras Research Foundation, and the Pediatric Research Foundation of Finland.
Footnotes
The authors do not have a commercial or other association that might pose a conflict of interest.
References
- 1.Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87:1944–50. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Hers JFP, Masurel N, Mulder J. Bacteriology and histopathology of the respiratory tract and lungs of fatal Asian influenza. Lancet. 1958;2:1164–5. doi: 10.1016/s0140-6736(58)92404-8. [DOI] [PubMed] [Google Scholar]
- 4.Lindsay MI, Jr., Herrmann EC, Jr., Morrow GW, Jr., Brown AL., Jr. Hong Kong influenza: clinical, microbiologic, and pathologic features in 127 cases. JAMA. 1970;214:1825–32. doi: 10.1001/jama.214.10.1825. [DOI] [PubMed] [Google Scholar]
- 5.Scadding JG. Lung changes in influenza. Quart J Med. 1937;6:425–65. [Google Scholar]
- 6.Stuart-Harris CH, Laird J, Tyrrell DA, Kelsall MH, Franks ZC. The relationship between influenza and pneumonia. J Hygiene. 1949;47:434–48. doi: 10.1017/s0022172400014789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyrrell DAJ. The pulmonary complications of influenza as seen in Sheffield in 1949. Quart J Med. 1952;21:291–306. [PubMed] [Google Scholar]
- 8.Nicholson KG. Human influenza. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. Blackwell Science, Ltd.; London: 1998. pp. 222–223. [Google Scholar]
- 9.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17(Suppl 1):S3–10. doi: 10.1016/s0264-410x(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 10.Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med. 1995;333:1618–24. doi: 10.1056/NEJM199512143332408. [DOI] [PubMed] [Google Scholar]
- 11.McCullers JA, Webster RG. A mouse model of dual infection with influenza virus and Streptococcus pneumoniae. In: Cox N, Hampson AW, editors. Osterhaus ADME. Elsevier Science B.V.; Amsterdam: 2001. pp. 601–607. [Google Scholar]
- 12.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186:341–50. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 13.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187:1000–9. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 14.McCullers JA. Effect of antiviral treatment on the outcome of secondary bacterial pneumonia after influenza. J Infect Dis. 2004;190:519–26. doi: 10.1086/421525. [DOI] [PubMed] [Google Scholar]
- 15.Tong HH, McIver MA, Fisher LM, DeMaria TF. Effect of lacto-N-neotetraose, asialoganglioside-GM1 and neuraminidase on adherence of otitis media-associated serotypes of Streptococcus pneumoniae to chinchilla tracheal epithelium. Microb Pathog. 1999;26:111–9. doi: 10.1006/mpat.1998.0257. [DOI] [PubMed] [Google Scholar]
- 16.LaMarco KL, Diven WF, Glew RH. Experimental alteration of chinchilla middle ear mucosae by bacterial neuraminidase. Ann Otol Rhinol Laryngol. 1986;95:304–8. doi: 10.1177/000348948609500319. [DOI] [PubMed] [Google Scholar]
- 17.Linder TE, Lim DJ, DeMaria TF. Changes in the structure of the cell surface carbohydrates of the chinchilla tubotympanum following Streptococcus pneumoniae-induced otitis media. Microb Pathog. 1992;13:293–303. doi: 10.1016/0882-4010(92)90039-q. [DOI] [PubMed] [Google Scholar]
- 18.Tong HH, Grants I, Liu X, DeMaria TF. Comparison of alteration of cell surface carbohydrates of the chinchilla tubotympanum and colonial opacity phenotype of Streptococcus pneumoniae during experimental pneumococcal otitis media with or without an antecedent influenza A virus infection. Infect Immun. 2002;70:4292–301. doi: 10.1128/IAI.70.8.4292-4301.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner R, Wolff T, Herwig A, Pleschka S, Klenk HD. Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: a study by reverse genetics. J Virol. 2000;74:6316–23. doi: 10.1128/jvi.74.14.6316-6323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A. 2000;97:6108–13. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20:3165–70. doi: 10.1016/s0264-410x(02)00268-2. [DOI] [PubMed] [Google Scholar]
- 22.Reed LJ, Muench H. A simple method for estimating fifty percent endpoints. Am J Hygiene. 1938;27:493–7. [Google Scholar]
- 23.Warner TG, O'Brien JS. Synthesis of 2'-(4-methylumbelliferyl)-alpha-D-N-acetylneuraminic acid and detection of skin fibroblast neuraminidase in normal humans and in sialidosis. Biochemistry. 1979;18:2783–7. doi: 10.1021/bi00580a014. [DOI] [PubMed] [Google Scholar]
- 24.Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234:1971–5. [PubMed] [Google Scholar]
- 25.Murti KG, Webster RG. Distribution of hemagglutinin and neuraminidase on influenza virions as revealed by immunoelectron microscopy. Virology. 1986;149:36–43. doi: 10.1016/0042-6822(86)90084-x. [DOI] [PubMed] [Google Scholar]
- 26.Webster RG, Brown LE, Laver WG. Antigenic and biological characterization of influenza virus neuraminidase (N2) with monoclonal antibodies. Virology. 1984;135:30–42. doi: 10.1016/0042-6822(84)90114-4. [DOI] [PubMed] [Google Scholar]
- 27.Air GM, Els MC, Brown LE, Laver WG, Webster RG. Location of antigenic sites on the three-dimensional structure of the influenza N2 virus neuraminidase. Virology. 1985;145:237–48. doi: 10.1016/0042-6822(85)90157-6. [DOI] [PubMed] [Google Scholar]
- 28.Gulati U, Hwang CC, Venkatramani L, et al. Antibody epitopes on the neuraminidase of a recent H3N2 influenza virus (A/Memphis/31/98). J Virol. 2002;76:12274–80. doi: 10.1128/JVI.76.23.12274-12280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francis KP, Yu J, Bellinger-Kawahara C, et al. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram- positive lux transposon. Infect Immun. 2001;69:3350–8. doi: 10.1128/IAI.69.5.3350-3358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobasa D, Wells K, Kawaoka Y. Amino acids responsible for the absolute sialidase activity of the influenza A virus neuraminidase: relationship to growth in the duck intestine. J Virol. 2001;75:11773–80. doi: 10.1128/JVI.75.23.11773-11780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito T, Couceiro JN, Kelm S, et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998;72:7367–73. doi: 10.1128/jvi.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baum LG, Paulson JC. The N2 neuraminidase of human influenza virus has acquired a substrate specificity complementary to the hemagglutinin receptor specificity. Virology. 1991;180:10–5. doi: 10.1016/0042-6822(91)90003-t. [DOI] [PubMed] [Google Scholar]
- 33.Ito T, Suzuki Y, Takada A, et al. Differences in sialic acid-galactose linkages in the chicken egg amnion and allantois influence human influenza virus receptor specificity and variant selection. J Virol. 1997;71:3357–62. doi: 10.1128/jvi.71.4.3357-3362.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998;178:53–60. doi: 10.1086/515616. [DOI] [PubMed] [Google Scholar]
- 35.Housworth WJ, Spoon MM. The age distribution of excess mortality during A2 Hong Kong influenza epidemics compared with earlier A2 outbreaks. Am J Epidemiol. 1971;94:348–50. doi: 10.1093/oxfordjournals.aje.a121329. [DOI] [PubMed] [Google Scholar]
- 36.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959−1999. Am J Epidemiol. 2004;160:492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Sastre A, Egorov A, Matassov D, et al. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–30. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 38.Guan Y, Poon LL, Cheung CY, et al. H5N1 influenza: a protean pandemic threat. Proc Natl Acad Sci U S A. 2004;101:8156–61. doi: 10.1073/pnas.0402443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz-Cherry S, Hinshaw VS. Influenza virus neuraminidase activates latent transforming growth factor beta. J Virol. 1996;70:8624–9. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control Update: influenza-associated deaths reported among children aged <18 years--United States, 2003−04 influenza season. MMWR Morb Mortal Wkly Rep. 2004;52:1254–5. [PubMed] [Google Scholar]
- 41.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–24. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 42.Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20:127–33. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 43.The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352:1877–81. [PubMed] [Google Scholar]
- 44.Lui KJ, Kendal AP. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health 1987. 77:712–6. doi: 10.2105/ajph.77.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]