Abstract
Background
Transient elastography is a novel, noninvasive method for staging liver fibrosis. We compared elastography with histologic methods among hepatitis C virus (HCV)–infected and human immunodeficiency virus (HIV)–HCV-coinfected participants in an urban, predominantly black study population.
Methods
Participants recruited from the AIDS Linked to the Intravenous Experience and the Johns Hopkins HIV Clinical Cohort studies underwent elastography to determine liver stiffness measurements. Liver biopsy specimens were staged F0–F4 in accordance with the Metavir score. Diagnostic accuracy and determination of liver stiffness cutoff values, compared with histologic methods, were determined by receiver operating characteristic analysis. Logistic regression methods identified parameters associated with discordant classification status.
Results
Of 192 participants, 139 (72%) were coinfected with HIV and HCV, 121 (63%) had insignificant fibrosis, and 48 (25%) had cirrhosis. Overall, the area-under-the-curve receiver operating characteristic was 0.87 for detection of both significant fibrosis (95% confidence interval, 0.82–0.92) and cirrhosis (95% confidence interval, 0.81–0.93). With use of cutoff values of ≥9.3 kPa for fibrosis and ≥12.3 kPa for cirrhosis, 79%–83% of participants were correctly classified by liver stiffness measurement (compared with histologic methods); accuracy appeared to be higher among HIV-uninfected participants than among HIV-infected participants. Most discordance occurred when liver stiffness measurements indicated liver disease and histologic examination did not (in 16% of participants); the patients with these discordant results were more likely to have attributes that increased the odds of significant fibrosis, such as elevated serum fibrosis markers or HIV-related immunosuppression, compared with persons in whom low fibrosis was predicted by both examination of a biopsy specimen and elastography.
Conclusions
For most HCV-infected persons, fibrosis stage predicted by elastography is similar to that predicted by examination of a biopsy specimen. Elastography-based measurement of liver stiffness holds promise to expand liver disease screening and monitoring, particularly among injection drug users.
Coinfection with HIV is associated with more-rapid progression of hepatitis C virus (HCV) infection, leading to increased incidence of fibrosis, cirrhosis, and end-stage liver disease [1, 2]. Liver disease is increasing as a cause of morbidity and mortality among HIV-infected persons, predominantly among those with HIV-HCV coinfection [3]. Management of HCV-related liver disease relies on staging of fibrosis to ascertain the urgency for treatment and for hepatocellular carcinoma screening. Liver biopsy is the gold standard for fibrosis staging. However, the procedure is not as safe, accurate, or accessible as many standard medical screening tests [4, 5]. The application of liver biopsy is especially limited for injection drug users (IDUs) [6], who comprise more than two-thirds of Western HCV-infected persons. The low prevalence of liver disease staging likely contributes to the poor uptake of HCV treatment among IDUs [7, 8]. In response, significant research efforts have been directed toward identification of noninvasive methods for diagnosing fibrosis and cirrhosis.
Transient elastography uses ultrasound readings to measure the velocity of an elastic shear wave transmitted through the liver [9]. This measure of liver elasticity or stiffness is related to the degree of fibrosis, providing a quick, painless, and noninvasive assessment of fibrosis severity. Although elastography has been increasingly used in Europe [10, 11], it is not available outside research settings in the United States. Therefore, there are limited data based on the use of liver stiffness measurements to define fibrosis in North American populations, including IDUs, persons of African descent, and HIV-HCV–coinfected persons. The primary objective of this study was to evaluate the accuracy of elastography as a noninvasive method for diagnosis of fibrosis and cirrhosis in HCV-monoinfected and HIV-HCV–coinfected persons.
PATIENTS AND METHODS
Study participants
Participants were recruited from 2 ongoing cohorts in Baltimore, Maryland. The AIDS Linked to the Intravenous Experience (ALIVE) study comprises HIV-infected and HIV-uninfected IDUs who received semi-annual follow-up visits that involve systematic collection of behavioral and medical history data and biological specimens, as described in detail elsewhere [12]. Clinical outcomes are confirmed through standardized medical record review. HCV-infected participants, irrespective of HIV status, were invited to participate, including individuals previously enrolled in ALIVE biopsy studies [13, 14].
HIV-HCV–coinfected participants of the Johns Hopkins University HIV Clinical Cohort (JHHCC) who had undergone liver biopsy were recruited to undergo elastography [15]. Information on clinical and laboratory parameters was obtained from the JHHCC database. As described in detail elsewhere [16], laboratory, radiological, and clinical data were periodically transferred from hospital administrative databases. Patient demographic and behavioral characteristics and clinical parameters were abstracted from charts by trained personnel at enrollment and at 6-month intervals. Additional behavioral and medical data were collected through computerized and interviewer-administered questionnaires at 6–12-month intervals.
From October 2005 through January 2007, we prospectively measured liver stiffness by elastography in 192 participants. Individuals were selected if they had a liver stiffness measurement obtained within 12 months after undergoing liver biopsy (157 persons; median time from biopsy to liver stiffness measurement, 1.7 months); cirrhosis established by prior examination of a biopsy specimen, with no intervening HCV treatment (30 persons); or clinical evidence of cirrhosis (5 persons) [17]. Johns Hopkins Institutional Review Boards approved this research; all participants provided written informed consent.
Liver stiffness measurement
Liver stiffness was determined by transient elastography with use of a Fibroscan machine (EchoSens) [9, 11]. In brief, an ultrasound transducer probe is mounted on the axis of a vibrator; vibrations of mild amplitude and low frequency induce an elastic shear wave that propagates through underlying liver tissue. Pulse-echo ultrasound acquisitions are used to follow propagation of the shear wave and measure its velocity. Results are instantaneously received as a single, quantitative parameter of liver stiffness measurement, reported in kilopascals. All elastography examinations were performed by certified operators (who were trained by the manufacturer) with use of a single device in the research clinic; the methods are described elsewhere [11]. Examinations with 8 validated measurements and a ≥60% success rate (the number of validated measurements divided by the total number of measurements) were considered to be reliable. During training, examinations were performed sequentially by 2 operators for 47 patients; the median interobserver difference was 0.0 kPa (interquartile range [IQR], −1.45 to 1.25 kPa).
Liver histology
Liver biopsies were performed and specimens were processed using identical protocols for all participants. Biopsies were performed by an experienced interventional radiologist under ultrasound guidance with use of a Monopty core biopsy device and a protocol designed to obtain 15-mm of tissue before fixation. Serial paraffin-embedded sections were cut and stained with hematoxylin and eosin, prussian blue, Mallory’s trichrome, and periodic acid-Schiff. Batched slides were read by an experienced hepatopathologist who was blinded to clinical data, including HIV status. The adequacy of the final tissue sample was judged by the pathologist. The median length of fixed and mounted biopsy specimens was 12 mm, and the median number of portal tracts was 11. The hepatopathologist graded the degree of inflammation with use of the Iskak scoring system and staged fibrosis according to the Metavir scoring system [18, 19]. Hepatic steatosis was classified on a 5-point scale [20]. Hepatic iron was graded as none, mild, and moderate to severe.
Laboratory methods
Standard laboratory assays were used for HCV antibody testing, HIV antibody testing, HIV load measurement, CD4 cell count measurement, and liver enzyme testing, as described elsewhere [12, 17, 20, 21]. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were examined as continuous variables and as 2.5 times the upper limit of the normal reference range (the upper limit of normal for AST level was 37 U/L and for ALT level was 40 U/L). The AST-to-platelet ratio index was calculated as described elsewhere [22].
Statistical analysis
Using liver stiffness measurement as a log-transformed continuous variable, we performed nonparametric analysis of variance methods to compare liver stiffness measurement with ordinate fibrosis stage and with demographic, behavioral, clinical, laboratory, and histological factors. The diagnostic value of liver stiffness measurement relative to histological fibrosis staging (Metavir score, F0–F4) was determined on the basis of assessment of sensitivity, specificity, and predictive values and was determined by varying the threshold values and constructing receiver operating characteristic (ROC) curves. The primary comparisons were made (1) to distinguish significant fibrosis (Metavir score, F2, F3, or F4) from no or mild fibrosis (Metavir score, F0 or F1) and (2) to identify cirrhosis (Metavir score, F4). For these comparisons, examination of a biopsy specimen was considered to be the gold standard. We present area-under-the-ROC curves (AUC-ROC) as a global measure of liver stiffness measurement accuracy. Optimal liver stiffness measurement cutoff values for classification of the dichotomous histological outcomes were determined by maximizing the combination of sensitivity and specificity and the proportion of samples that were correctly classified. Similar diagnostic accuracy resulted from use of Ishak fibrosis scoring (data not shown).
Using participants for whom liver stiffness measurement and histologic findings concurred for absence of fibrosis (or cirrhosis) as the referent group, we performed univariate and multivariate logistic regression to identify parameters associated with discordance when liver stiffness measurement suggested disease but histologic findings did not. In addition to the aforementioned variables, we analyzed performance characteristics related to elastography (e.g., the ratio of the IQR to median liver stiffness measurement) or liver biopsy (e.g., biopsy specimen length and number of portal tracts).
For assessment of accuracy and cutoff value determinations, HIV-stratified analyses were performed. For discordant analyses, HIV status was included in models as a predictor variable. Analyses were performed using SAS, version 9.1 (SAS Institute), and Stata, version 9 (Stata).
RESULTS
Characteristics of study participants
A total of 192 persons were evaluated, including 89 (46%) from ALIVE and 104 (54%) from JHHCC (table 1). ALIVE participants were more likely to be male (73% vs. 58%; P < .05) or black (93% vs. 84%; P < .05) than were JHHCC participants. Overall, 72% of the participants were infected with HIV, including all JHHCC participants and 39% of ALIVE participants. The median CD4 cell count in HIV-infected participants was 369 cells/mm3; 69% had an HIV RNA level that was less than the limit of detection (i.e., 50 copies/mL). JHHCC participants had a higher median CD4 cell count than did ALIVE participants (433 vs. 248 cells/mm3; P < .01), but other clinical parameters were similar between participants from the 2 studies.
Table 1.
Characteristics of study participants who were assessed when elastography was performed.
| Characteristic | Participants (n = 192) |
|---|---|
| Age, years | 49 (45–53) |
| Male sex | 68 (35) |
| Black race | 171 (89) |
| ALIVE participant | 89 (46) |
| JHHCC participant | 103 (54) |
| BMI | 24.9 (22.7–27.8) |
| AST level, IU/L | 42 (31–75) |
| ALT level, IU/L | 41 (26–64) |
| Platelet count, × 1000 cells/µL | 205 (164–240) |
| APRI | 0.50 (0.32–0.96) |
| Albumin level, g/dL | 4.0 (3.8–4.3) |
| HIV infection | |
| All | 139 (72) |
| CD4 cell count, cells/mm3 | 369 (214–565) |
| HIV RNA level <50 copies/mLa | 64 (52) |
| HAART | |
| Ever | 104/139 (75) |
| Current | 82/139 (59) |
| Metavir fibrosis scoreb | |
| F0 | 46 (24) |
| F1 | 75 (39) |
| F2 | 15 (8) |
| F3 | 8 (4) |
| F4 | 48 (25) |
| Total MHAI score | |
| 0–3 | 66/150 (44) |
| 4–5 | 52/150 (35) |
| >5 | 32/150 (21) |
| Steatosis | |
| None | 89/150 (59) |
| <5% | 39/150 (26) |
| 5%–30% | 17/150 (11) |
| 31%–60% | 2/150 (1) |
| >60% | 3/150 (2) |
| Hepatic iron | |
| None | 98/150 (65) |
| Mild | 36/150 (24) |
| Moderate to severe | 16/150 (11) |
NOTE. Data are no. (%) of participants for categorical variables or median (interquartile range) for continuous variables. ALIVE, AIDS Linked to the Intravenous Experience; ALT, alanine aminotransferase; APRI, aspartate aminotransferase–to-platelet ratio; AST, aspartate aminotransferase; BMI, body mass index (calculated by the weight in kilograms divided by the square of the height in meters); JHHCC, Johns Hopkins University HIV Clinical Cohort; MHAI, modified hepatic activity index.
Data on HIV RNA level were available for 124 participants.
F4 category includes 5 participants with clinically defined cirrhosis.
Liver histologic findings
The majority of participants (121 [63%]) had no or minimal fibrosis (table 1). Of 71 (37%) participants with significant fibrosis (Metavir score, F2, F3, or F4), 48 (25% of all patients) had cirrhosis (Metavir score, F4). The prevalence of significant hepatic inflammation, as determined by a total modified histologic activity index >5, was 21%. Of 150 participants who underwent steatosis grading, 59% had no detectable fatty change, consistent with prior observations [20]. Significant steatosis, defined as a fatty change of >30%, was uncommon (in 3% of participants).
Liver stiffness measurement
Elastography was extremely well accepted and tolerated. A total of 198 patients were offered the procedure, and all complied; valid results were obtained for 192 (97%) of these patients. The median liver stiffness measurement was 8.85 kPa (range, 3–75 kPa; IQR, 6.13–14.0 kPa). In linear regression models, Metavir fibrosis score was very strongly associated with liver stiffness measurement (P < .001), as were laboratory (AST level, ALT level, platelet count, AST-to-platelet ratio index, and albumin level) and histologic (steatosis and inflammation score) markers of liver disease. In multivariate analysis (table 2), Metavir fibrosis score, male sex, AST-to-platelet ratio index >0.5, and any steatosis were significantly associated with higher liver stiffness measurement.
Table 2.
Association of demographic, clinical, elastographic, and histologic parameters with liver stiffness measurements.
| Variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Coefficient | P | Coefficient | P | |
| Age, per 5-year increment | 0.03 | .380 | … | |
| Male sex | 0.06 | .514 | 0.12 | .047 |
| Black race | −0.26 | .070 | … | |
| Study group | 0.008 | .934 | … | |
| BMI 128 | 0.17 | .128 | … | |
| AST level | 0.009 | <.001 | … | |
| AST level >2.5 × ULN | 0.52 | <.001 | … | |
| ALT level | 0.006 | <.001 | … | |
| ALT level >2.5 × ULN | 0.29 | .096 | … | |
| Platelet count | −0.004 | <.001 | … | |
| APRI | 0.24 | <.001 | … | |
| APRI >0.5 | 0.64 | <.001 | 0.26 | <.001 |
| Albumin level | −0.44 | <.001 | … | |
| Metavir fibrosis score | 0.27 | <.001 | 0.13 | <.001 |
| Total MHAI score >5 | 0.28 | .005 | … | |
| Steatosis (any vs. none) | 0.34 | <.001 | 0.17 | .010 |
| Hepatic iron (any vs. none) | 0.07 | .451 | … | |
| HIV infection | 0.03 | .79 | … | |
| HIV-infected patients | ||||
| HIV RNA level, per log10 copies/mL | −0.03 | .622 | … | |
| CD4 cell count, per 100 cells/mm3 increase | −0.004 | .855 | … | |
NOTE. Linear regression models were performed with the outcome variable of log-transformed liver stiffness measurement. ALT, alanine aminotransferase; APRI, aspartate aminotransferase–to-platelet ratio; AST, aspartate aminotransferase; BMI, body mass index (calculated by the weight in kilograms divided by the square of the height in meters); MHAI, modified hepatic activity index; ULN, upper limit of normal.
Diagnostic accuracy of liver stiffness measurements, compared with that of histologic examination
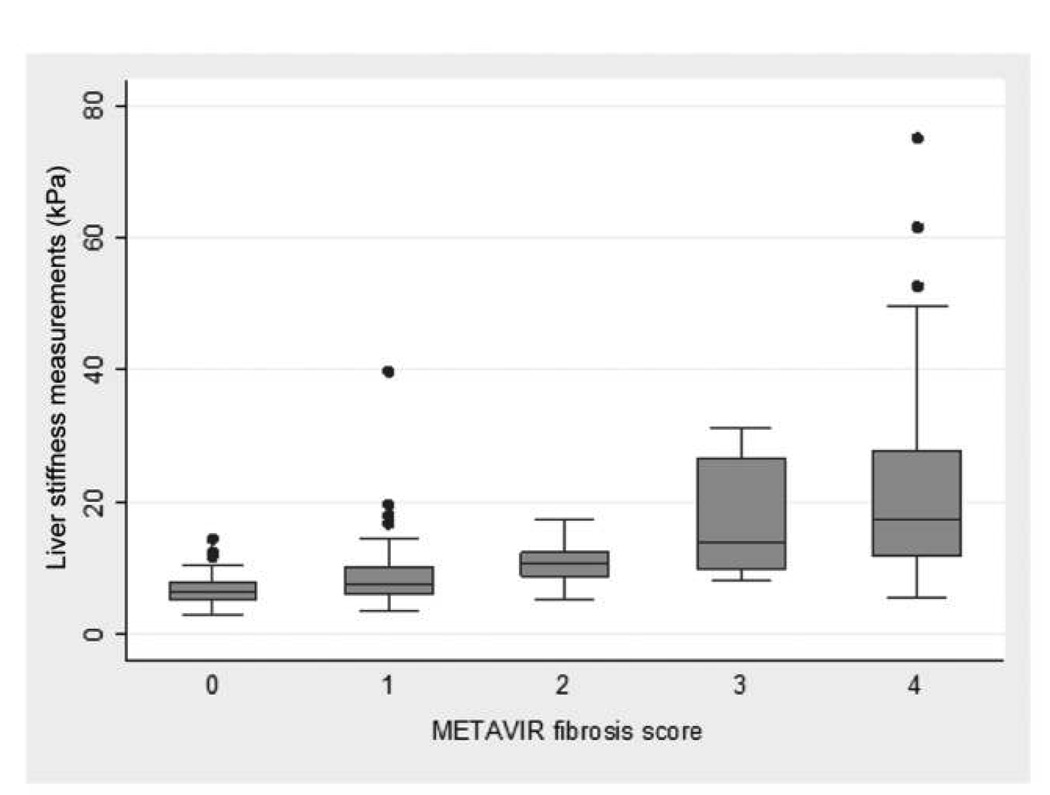
The distributions of liver stiffness measurement values by Metavir score are presented in figure 1. The median liver stiffness measurement in participants histologically categorized as having no or mild fibrosis was 6.8 kPa (IQR, 5.7–9.2 kPa), compared with 14.4 kPa (IQR, 10.4–22.4 kPa) in those with significant fibrosis. Participants with cirrhosis had a median liver stiffness measurement of 17.45 kPa (IQR, 11.9–27.9 kPa); the 5 participants with clinically defined cirrhosis had a median liver stiffness measurement of 42.2 kPa (IQR, 21.3–48.0 kPa).
Figure 1.
Box-plots of liver stiffness measurements, by Metavir fibrosis score (F0–F4).
Overall, the diagnostic accuracy of liver stiffness measurement for significant fibrosis by AUC-ROC analysis was 0.87 (95% CI, 0.82–0.92), which was nearly identical to that for cirrhosis (0.87; 95% CI, 0.81–0.93). For both fibrosis and cirrhosis, diagnostic performance was higher among HIV-uninfected participants (0.94 [95% CI, 0.89–1.00] and 0.92 [95% CI, 0.85–0.99], respectively) than among HIV-infected participants (0.84 [95% CI, 0.77–0.91] and 0.85 [95% CI, 0.77–0.93], respectively). For significant fibrosis, a liver stiffness measurement diagnostic cutoff value of 9.3 kPa correctly classified 79% of participants, with 86% sensitivity and 75% specificity (table 3). For cirrhosis, a liver stiffness measurement cutoff value of 12.3 kPa correctly classified 83% of participants, with 75% sensitivity and 86% specificity. In stratified analysis using these cutoff values, the proportion of persons correctly classified as having fibrosis was higher among HIV-uninfected participants than among HIV-infected participants (87% vs. 76%); no differences were seen by HIV status for cirrhosis (83% for both).
Table 3.
Diagnostic accuracy of liver stiffness measurements (LSMs) for detection of significant liver fibrosis and cirrhosis, compared with liver histologic examination.
| Characteristic | Fibrosis | Cirrhosis |
|---|---|---|
| LSM cutoff value, kPa | ≥9.3 | ≥12.3 |
| AUC-ROC, % (95% CI) | 0.81 (0.75–0.86) | 0.81 (0.74–0.87) |
| Sensitivity, % (95% CI) | 85.9 (75.6–93.0) | 75.0 (60.4–86.4) |
| Specificity, % (95% CI) | 75.2 (66.5–82.6) | 86.1 (79.4–91.3) |
| PPV, % (95% CI) | 67.0 (56.4–76.5) | 64.3 (50.4–76.6) |
| NPV, % (95% CI) | 90.1 (82.5–95.1) | 91.2 (85.1–95.4) |
| Percentage of cases that were correctly classified | 79.2 | 83.3 |
NOTE. Performance characteristics were determined for dichotomous LSM categorizations, compared with dichotomous histological categorizations based on Metavir fibrosis score, with fibrosis classified by a score of F2, F3, or F4 and cirrhosis classified by a score of F4. AUC-ROC, area under the curve for receiver operating characteristics; NPV, negative predictive value; PPV, positive predictive value.
Correlates of discordant classification between liver stiffness measurement and histologic examination
For diagnosis of significant fibrosis, 40 persons had discordant liver stiffness measurement and liver histologic results. For 30 (15.6% of all participants), liver stiffness measurement estimated a higher degree of fibrosis than did histologic examination. Compared with the 91 participants for whom both tests predicted nonsignificant fibrosis, these 30 participants with discordant results—with histologic findings revealing a fibrosis score of F0 or F1 but with a liver stiffness measurement ≥9.3 kPa—more frequently had other indicators of higher disease stage, including increased AST level, ALT level, AST-to-platelet ratio index, and modified histologic activity index and lower platelet count (table 4). In multivariate analysis, an AST-to-platelet ratio index >0.50 and HIV infection (with a CD4 cell count <350 cells/mm3) were significantly associated with liver stiffness measurement ≥9.3 kPa, compared with concordant predictions of low fibrosis. No associations with discordance were detected in biopsy or elastography quality measures or with body mass index (table 4). Subsequent liver stiffness measurement assessments were available for 9 patients who had discordant results; 6 displayed consistently elevated liver stiffness measurements, and all 9 had AST or ALT level elevations during the follow-up period. Prior results from a liver biopsy examination (3–5 years before the study) were available for 11 of 30 patients who had discordant results: 4 had prior histological evidence of significant fibrosis, and 7 did not. Overall, these results suggest that, in some instances, fibrosis may have been underestimated by histologic findings.
Table 4.
Factors associated with discordant classification compared with those associated with concordant classification by elastography in participants without histological evidence of liver fibrosis.
| Variable | LSM |
OR (95% CI) |
||
|---|---|---|---|---|
| <9.3 kPa | ≥9.3 kPa | Univariate analysis | Multivariate analysis | |
| Demographic characteristic | ||||
| Age, per 5-year increment | 48 (44–52) | 49 (43–52) | 1.14 (0.84–1.54) | … |
| Male sex | 57 (62.6) | 22 (73.3) | 1.64 (0.66–4.10) | … |
| Black race | 83 (91.2) | 26 (86.7) | 0.63 (0.17–2.25) | … |
| JHHCC | 48 (52.8) | 16 (53.3) | 1.02 (0.45–2.34) | 0.23 (0.06–0.93) |
| Clinical parameters | ||||
| BMI 128 | 17 (19.8) | 8 (28.6) | 1.62 (0.61–4.31) | … |
| AST level, IU/L | 33 (27–41) | 58 (33–98) | 1.03 (1.01–1.05) | … |
| AST level >2.5 × ULN | 5 (5.9) | 7 (23.3) | 4.87 (1.41–16.8) | … |
| ALT level, IU/L | 33 (24–46) | 42 (30–90) | 1.02 (1.01–1.03) | … |
| ALT level >2.5 × ULN | 3 (3.5) | 6 (20.0) | 6.83 (1.59–29.4) | … |
| Platelet count, × 1000 cells/µL | 225 (192–271) | 175 (149–218) | 0.99 (0.98–0.99) | … |
| Platelet count <200,000 cells/µL | 26 (30.6) | 19 (63.3) | 3.92 (1.64–9.40) | … |
| APRI | 0.35 (0.27–0.48) | 0.87 (0.45–1.30) | 8.06 (2.87–22.1) | … |
| APRI >0.5 | 19 (22.6) | 20 (71.4) | 8.55 (3.25–22.5) | 8.81 (3.02–25.7) |
| Albumin level, g/dL | 4.2 (3.9–4.3) | 4.1 (3.8–4.4) | 0.62 (0.25–1.52) | … |
| HIV status | ||||
| HIV uninfected | 32 (37.2) | 6 (20.0) | 1.00 | 1.00 |
| HIV infected | ||||
| CD4 cell count ≥350 cells/mm3 | 29 (33.7) | 8 (26.7) | 1.47 (0.46–4.75) | 3.69 (0.66–20.46) |
| CD4 cell count <350 cells/mm3 | 25 (29.1) | 13 (48.2) | 2.77 (0.92–8.33) | 5.04 (1.15–22.1) |
| Biopsy factor | ||||
| Time from biopsy to LSM, months | 2.11 (0.79–5.76) | 1.17 (0.33–5.49) | 0.94 (0.82–1.08) | … |
| Biopsy specimen length | 12 (11–14) | 11 (10–14) | 0.90 (0.77–1.06) | … |
| No. of portal tracts | 10 (8–13) | 10 (8–12) | 0.90 (0.76–1.07) | … |
| Total MHAI score 15 | 11 (12.4) | 10 (35.7) | 3.89 (1.43–10.6) | … |
| Any steatosis | 28 (31.8) | 10 (35.7) | 1.19 (0.49–2.91) | … |
| Any hepatic iron | 28 (31.5) | 10 (34.5) | 1.15 (0.47–2.78) | … |
| Elastography factors | ||||
| Operator 1 | 32 (35.2) | 8 (26.7) | 1.00 | … |
| Operator 2 | 20 (22.0) | 6 (20.0) | 1.20 (0.36–3.97) | … |
| Operator 3 | 39 (42.9) | 16 (53.3) | 1.64 (0.62–4.20) | … |
| Period | ||||
| October 2005–March 2006 | 24 (26.4) | 7 (23.3) | 1.00 | … |
| April 2006–August 2006 | 47 (51.7) | 15 (50.0) | 1.09 (0.39–3.04) | … |
| September 2006–January 2007 | 20 (22.0) | 8 (26.7) | 1.37 (0.42–4.44) | … |
| Median LSM:IQR 10.3 | 5 (5.5) | 2 (6.7) | 1.23 (0.23–6.69) | … |
| HIV-infected patients only | ||||
| HIV RNA level, per log10 copies/mL | 2.30 (2.30–3.88) | 2.30 (2.30–4.28) | 0.95 (0.60–1.52) | … |
| CD4 cell count, per 100 cells/mm3 increase | 380 (211–565) | 316 (222–491) | 0.92 (0.73–1.16) | … |
| CD4 cell count <350 cells/mm3 | 25/54 (46.3) | 13/24 (61.9) | 1.89 (0.67–5.28) | … |
NOTE. Data are no. (%) of patients for categorical variables and median value (interquartile range [IQR]) for continuous variables. Numbers and percentages of patients are inclusive of patients with available data. Multivariate models were adjusted for all other variables, with the presented estimated risk. Participants without histological evidence of fibrosis were those with a fibrosis score of F0 or F1. Discordant was classified as a liver stiffness measurement (LSM) ≥9.3 kPa, and concordant was classified as an LSM <9.3 kPa). ALT, alanine aminotransferase; APRI, aspartate aminotransferase–to-platelet ratio; AST, aspartate aminotransferase; BMI, body mass index (calculated as the weight in kilograms divided by the square of the height in meters); JHHCC, Johns Hopkins University HIV Clinical Cohort; MHAI, modified hepatic activity index. ULN, upper limit of normal.
Among 144 participants without histological evidence of cirrhosis (Metavir score, F0, F1, F2, or F3), 20 (13.9% of all participants) had a liver stiffness measurement ≥12.3 kPa. Compared with the 124 participants for whom both tests predicted the absence of cirrhosis, the 20 participants who had discordant results more frequently had indicators of higher disease stage, including older age, AST-to-platelet ratio index >0.50, and histological evidence of steatosis or hepatic iron (table 5). Furthermore, characteristics related to biopsy or elastography performance were not significantly associated with discordant classification.
Table 5.
Factors associated with discordant classification compared with those associated with concordant classification by elastography in participants without histological evidence of cirrhosis.
| Variable | LSM |
OR (95% CI) |
||
|---|---|---|---|---|
| <9.3 kPa | ≥9.3 kPa | Univariate analysis, | Multivariate analysis | |
| Demographic factor | ||||
| Age, per 5-year increment | 48 (44–51) | 51 (47–55) | 1.46 (1.00–2.14) | 2.69 (1.43–5.07) |
| Male sex | 79 (63.7) | 15 (75.0) | 1.71 (0.58–5.01) | … |
| Black race | 111 (89.5) | 19 (95.0) | 2.23 (0.27–18.0) | … |
| JHHCC | 67 (54.0) | 7 (35.0) | 0.46 (0.17–1.23) | … |
| Clinical parameters | ||||
| BMI 128 | 22 (19.0) | 9 (45.0) | 3.50 (1.29–9.46) | … |
| AST level, IU/L | 35 (28–51) | 60 (36–97) | 1.02 (1.01–1.03) | … |
| AST level >2.5 × ULN | 11 (9.3) | 5 (25.0) | 3.24 (0.99–10.6) | … |
| ALT level, IU/L | 35 (25–52) | 48 (36–79) | 1.02 (1.01–1.03) | … |
| ALT level >2.5 × ULN | 3 (3.5) | 6 (20.0) | 6.83 (1.59–29.4) | … |
| Platelet count, × 1000 cells/µL | 218 (181–267) | 178 (145–216) | 0.99 (0.98–1.00) | … |
| Platelet count <200,000 cells/µL | 44 (37.3) | 12 (60.0) | 2.52 (0.96–6.65) | … |
| APRI | 0.39 (0.28–0.59) | 0.87 (0.47–1.27) | 3.71 (1.72–7.98) | … |
| APRI >0.5 | 38 (33.0) | 14 (70.0) | 4.73 (1.68–13.3) | 11.4 (2.37–55.2) |
| Albumin level, g/dL | 4.1 (3.9–4.3) | 4.1 (3.9–4.4) | 0.60 (0.22–1.66) | … |
| HIV status | ||||
| HIV uninfected | 36 (30.3) | 7 (35.0) | 1.00 | … |
| HIV infected | ||||
| CD4 cell count ≥350 cells/mm3 | 41 (34.5) | 6 (30.0) | 0.86 (0.28–2.68) | … |
| CD4 cell count <350 cells/mm3 | 42 (35.3) | 7 (35.0) | 0.75 (0.23–2.45) | … |
| Biopsy factors | ||||
| Time from biopsy to LSM, months | 1.97 (0.5–5.5) | 1.78 (0.45–5.45) | 1.02 (0.88–1.17) | … |
| Biopsy specimen length, mm | 12 (11–14) | 12 (11–15) | 0.94 (0.79–1.13) | … |
| No. of portal tracts | 11 (8–13) | 9 (8–13) | 0.98 (0.82–1.16) | … |
| Total MHAI >5 | 23 (19.3) | 5 (26.3) | 1.49 (0.49–4.56) | … |
| Any steatosis | 40 (33.6) | 13 (68.4) | 4.28 (1.51–12.1) | 6.06 (1.46–25.1) |
| Any hepatic iron | 9 (7.5) | 6 (33.3) | 6.17 (1.87–20.3) | 22.4 (3.65–138) |
| Elastography factors | ||||
| Operator 1 | 42 (33.9) | 9 (45.0) | 1.0 | … |
| Operator 2 | 24 (19.4) | 2 (10.0) | 0.39 (0.08–1.95) | … |
| Operator 3 | 58 (46.8) | 9 (45.0) | 0.72 (0.27–1.98) | … |
| Period | ||||
| October 2005–March 2006 | 33 (26.6) | 4 (20.0) | … | |
| April 2006–August 2006 | 62 (50.0) | 12 (60.0) | 1.60 (0.48–5.34) | … |
| September 2006–January 2007 | 29 (23.4) | 4 (20.0) | 1.14 (0.26–4.96) | … |
| Median LSM:IQR 10.3 | 5 (4.0) | 2. (10.0) | 2.65 (0.48–14.7) | … |
| HIV-infected patients only | ||||
| HIV RNA level, per log10 copies/mL | 2.30 (2.30–3.85) | 2.30 (2.30–3.72) | 0.98 (0.54–1.77) | … |
| CD4 cell count, per 100 cells/mm3 increase | 362 (222–521) | 334 (178–606) | 0.94 (0.71–1.25) | … |
| CD4 cell count <350 cells/mm3 | 42/83 (50.6) | 7/13 (53.9) | 1.14 (0.35–3.68) | … |
NOTE. Data are no. (%) of patients for categorical variables and median value (interquartile range [IQR]) for continuous variables. Numbers and percentages of patients are inclusive of patients with available data. Multivariate models were adjusted for all other variables, with the presented estimated risk. Participants without histological evidence of cirrhosis were those with a fibrosis score of F0, F1, F2, or F3. Discordant was classified as a liver stiffness measurement (LSM) ≥12.3 kPa, and concordant was classified as an LSM <12.3 kPa). ALT, alanine aminotransferase; APRI, aspartate aminotransferase–to-platelet ratio; AST, aspartate aminotransferase; BMI, body mass index (calculated as the weight in kilograms divided by the square of the height in meters); JHHCC, Johns Hopkins University HIV Clinical Cohort; MHAI, modified hepatic activity index; ULN, upper limit of normal.
DISCUSSION
Among an urban, predominantly black study population of HCV-monoinfected persons and HIV-HCV–coinfected persons, liver stiffness measurement was well tolerated and had diagnostic accuracy for staging fibrosis and cirrhosis that was comparable to histologic methods. Because of the high acceptability, safety, and potential to repeat the test, the procedure holds promise to markedly expand liver disease screening and monitoring, particularly among IDUs.
For detection of fibrosis, elastography diagnostic performance in our study was comparable to prior validation studies involving HCV-monoinfected patients from Europe [10, 11, 23–25]. In early validation studies, the AUC-ROC for detecting significant fibrosis ranged from 0.79 to 0.83 [10, 11].
There are limited data on elastography for HIV-HCV–coinfected persons. In a multisite study from hospital-based hepatology clinics in France, de Ledinghen et al. [26] reported an AUC-ROC of 0.72 (95% CI, 0.60–0.84) for liver stiffness measurement in predicting fibrosis, compared with concurrent examination of a liver biopsy specimen, among 72 HIV-HCV–coinfected patients. Among 169 HIV-HCV–coinfected patients from 6 hospital-based infectious diseases clinics in Spain, the AUC-ROC for significant fibrosis was notably higher (0.87; 95% CI, 0.84–0.93) than that in the French study [27]. The diagnostic accuracy of liver stiffness measurement for fibrosis in our HIV-HCV–coinfected subset was higher than that in the French study and similar to that in the Spanish study.
Consistent with observations in HCV-monoinfected patients [10, 11, 24, 25], both prior studies of HIV-HCV–coinfected patients reported excellent ability to discriminate cirrhosis (AUC-ROC, >0.95) [26, 27]. In contrast, we did not observe substantially greater diagnostic accuracy of liver stiffness measurement for diagnosis of cirrhosis, compared with diagnosis of fibrosis. There are several probable reasons to explain observed differences in diagnostic accuracy between studies. First, the spectrum of liver disease stages within the study populations affects the diagnostic accuracy [28]. In our study, we had a relatively large proportion of participants with minimal fibrosis (59%) and a relatively small proportion with cirrhosis (25%). By comparison, the prevalence of minimal fibrosis was only 26%–36% in the HIV-uninfected study populations of Castera and Ziol [10, 11]. Likewise, the studies involving HIV-HCV–coinfected patients had less representation of patients with a fibrosis score of F0 or F1 (38%–39%) and an increased proportion of patients with cirrhosis (31%–38%) [26, 27]. The reduced prevalence of advanced disease in our population diminishes the predictive value for cirrhosis.
Technical differences in application of new technology such as elastography could affect the estimated accuracy of liver stiffness measurement. In our study, we had 3 trained operators perform elastography with use of a single machine at a single site and included only examinations that exceeded specified performance criteria. Of importance, we did not detect lower elastography performance measures in participants with liver stiffness measurement values that were discordant with results of examination of a biopsy specimen.
Discordant classification of participants by elastography could simply represent overdiagnosis of fibrosis, compared with examination of a biopsy specimen. However, the accuracy of the biopsy itself will impact the estimated diagnostic accuracy of liver stiffness measurement [29, 30]. One recognized error of staging fibrosis by liver biopsy testing is sampling error, resulting in an underrepresentation of fibrosis [4], which is most likely to occur with small samples of liver tissue [31]. Overestimation of fibrosis on examination of a biopsy sample occurs much less frequently, because it essentially occurs as a result of reader error. However, similar to prior findings [25, 27], biopsy sample quality did not appear to explain discordance in our study. Although we cannot precisely parse out the reasons for discrepancies among the available data, we consistently found that individuals classified as having significant fibrosis or cirrhosis by liver stiffness measurement but not by histologic examination displayed elevated markers of liver disease (increased AST level, ALT level, and AST-to-platelet ratio index) or other liver pathology (steatosis and hepatic iron). Additional studies will need to determine whether liver stiffness measurement may be better than liver biopsy for detection of fibrosis or whether liver stiffness measurement “overstaged” these discordant cases (figure 2), potentially reflecting the contribution of other disease processes, such as hepatic inflammation. Of note, in contrast to transient increases in liver stiffness measurement reported in persons with acute hepatitis or with acute liver damage [32, 33], all of our participants were chronically infected with HCV, with modest abnormalities of liver enzymes or synthetic function (table 2). Furthermore, compared with hepatology clinic–based studies, liver enzyme elevations in our community-recruited cohort were notably less frequent or less severe [34]. These findings suggest that clinicians should consider using multiple methods to stage liver disease, and as with other medical tests, they should interpret the findings in light of the pretesting disease probability and the inherent limitations of the tests.
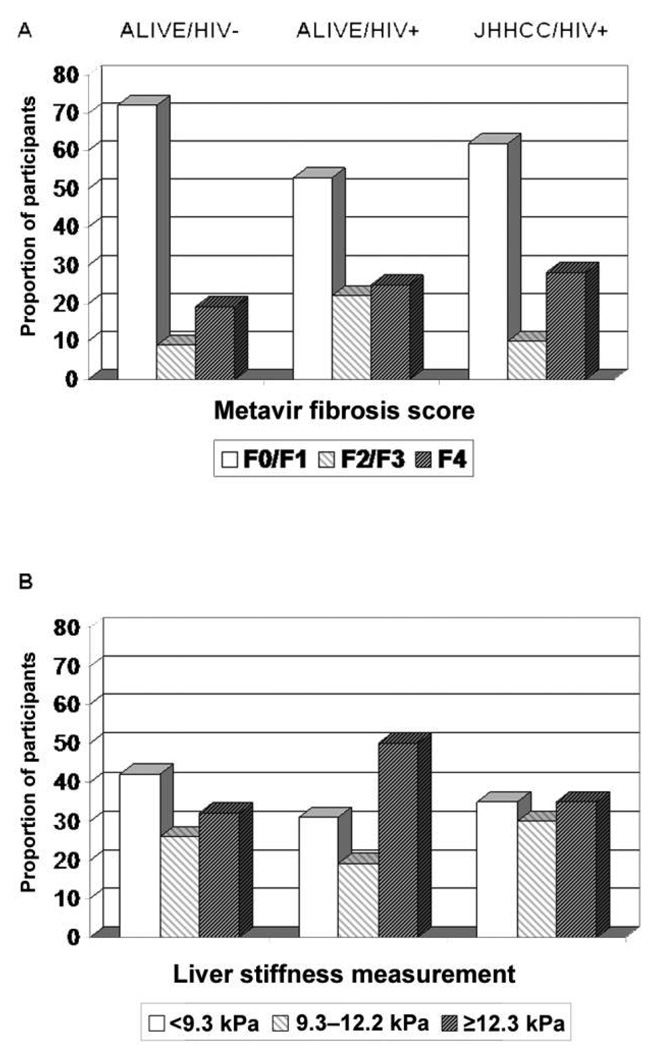
Figure 2.
Distribution of the severity of liver fibrosis, based on histologic findings (by Metavir fibrosis score; A) and on liver stiffness measurements (B), stratified by study group and HIV status.
Of importance, inaccuracies related to fibrosis staging will be amplified in patient subgroups in which the true disease prevalence is high. In subgroup analyses, we observed diminished liver stiffness measurement accuracy, compared with accuracy of examination of a biopsy sample, among individuals with HIV infection, specifically among community-based, out-of-treatment ALIVE participants with low CD4 cell counts. To our knowledge, no other study has directly compared elastography accuracy among HIV-infected patients and HIV-uninfected patients with chronic HCV infection. However, because HIV-related CD4 cell count decreases are associated with increased odds of having significant fibrosis, lower apparent accuracy may merely reflect more frequent underrepresentation of fibrosis by histologic findings among this subset with a higher prevalence of disease. Likewise, greater discordance was observed among patients with higher AST-to-platelet ratio index scores.
As a reflection of the aforementioned limitations, diagnostic accuracy will also be reduced when greater proportions of persons have mid-stage disease, compared with when greater proportions of patients have low-stage or high-stage disease [28]. This principle might also explain differences in apparent accuracy noted within subsets of our 2 cohorts. ALIVE HIV-infected participants with more advanced immunosuppression demonstrated a more advanced spectrum of underlying liver disease (figure 2A), compared with the more bimodal distribution in the ALIVE HIV-infected group or with JHHCC participants.
Racial differences have been detected for some HCV clinical outcomes, such as natural recovery from HCV infection and IFN-α responsiveness [17, 35]. Although we did not observe any racial differences, our assessment of race was limited because we included relatively few participants who were not black. We are not aware of other studies that have assessed elastography performance by race.
In summary, elastography is a safe method for detection of fibrosis and cirrhosis in HCV-monoinfected persons and HIV-HCV–coinfected persons. Additional investigations will need to establish elastography effectiveness among populations with varying disease severity and in the presence of other disease processes. The broad acceptability of elastography makes the test potentially attractive for use in large-scale clinical research studies, especially those including IDUs. In clinical practice, liver stiffness measurement results, liver biopsy examination results, and all medical test results should be interpreted on the basis of a full understanding of their accuracy and limitations, as well as on the basis of the level of suspicion for the outcome (pretesting probability). In liver disease staging in particular, caution should be used when assuming that disease stage is low on the basis of a single test result, especially when the probability of significant disease is high (such as in HIV-HCV–coinfected patients with advanced immunosuppression).
Acknowledgments
We thank the study participants for their important contributions to this ongoing work.
Financial support. National Institute of Drug Abuse (R01s DA16078, DA13806, DA12568, and DA04334); American Cancer Society (MRSG-07-284-01-CCE to G.D.K.), and Johns Hopkins General Clinical Research Center, funded by the National Center for Research Resources (UL1 RR025005).
Footnotes
Potential conflicts of interest. N.A. has served as a consultant and has received grant support from EchoSens. All other authors: no conflicts.
References
- 1.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 2.Goedert JJ, Eyster ME, Lederman MM, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood. 2002;100:1584–1589. [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 6.Strathdee SA, Latka M, Campbell J, et al. Factors associated with interest in initiating treatment for hepatitis C virus (HCV) infection among young HCV-infected injection drug users. Clin Infect Dis. 2005;40 Suppl 5:S304–S312. doi: 10.1086/427445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta SH, Lucas GM, Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–2369. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- 9.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 12.Vlahov D, Anthony JC, Munoz A, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr. 1991;109:75–100. [PubMed] [Google Scholar]
- 13.Rai R, Wilson LE, Astemborski J, et al. Severity and correlates of liver disease in hepatitis C virus–infected injection drug users. Hepatology. 2002;35:1247–1255. doi: 10.1053/jhep.2002.33151. [DOI] [PubMed] [Google Scholar]
- 14.Wilson LE, Torbenson M, Astemborski J, et al. Progression of liver fibrosis among injection drug users with chronic hepatitis C. Hepatology. 2006;43:788–795. doi: 10.1002/hep.21091. [DOI] [PubMed] [Google Scholar]
- 15.Sulkowski MS, Mehta SH, Torbenson MS, et al. Rapid fibrosis progression among HIV/hepatitis C virus–co-infected adults. AIDS. 2007;21:2209–2216. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 16.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17 Suppl 1:S38–S41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 18.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 19.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 20.Sulkowski MS, Mehta SH, Torbenson M, et al. Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS. 2005;19:585–592. doi: 10.1097/01.aids.0000163935.99401.25. [DOI] [PubMed] [Google Scholar]
- 21.Vlahov D, Graham N, Hoover D, et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users: plasma viral load and CD4+ cell count. JAMA. 1998;279:235–240. doi: 10.1001/jama.279.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 23.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganne-Carrie N, Ziol M, de Ledinghen V, et al. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511–1517. doi: 10.1002/hep.21420. [DOI] [PubMed] [Google Scholar]
- 26.de Ledinghen V, Douvin C, Kettaneh A, et al. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus–coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–179. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 27.Vergara S, Macias J, Rivero A, et al. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis. 2007;45:969–974. doi: 10.1086/521857. [DOI] [PubMed] [Google Scholar]
- 28.Irwig L, Bossuyt P, Glasziou P, Gatsonis C, Lijmer J. Designing studies to ensure that estimates of test accuracy are transferable. BMJ. 2002;324:669–671. doi: 10.1136/bmj.324.7338.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poynard T, Munteanu M, Imbert-Bismut F, et al. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344–1355. doi: 10.1373/clinchem.2004.032227. [DOI] [PubMed] [Google Scholar]
- 30.Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. J Hepatol. 2009;50:36–41. doi: 10.1016/j.jhep.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–244. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 32.Arena U, Vizzutti F, Corti G, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380–384. doi: 10.1002/hep.22007. [DOI] [PubMed] [Google Scholar]
- 33.Sagir A, Erhardt A, Schmitt M, Haussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592–595. doi: 10.1002/hep.22056. [DOI] [PubMed] [Google Scholar]
- 34.Mehta SH, Netski D, Sulkowski MS, Strathdee SA, Vlahov D, Thomas DL. Liver enzyme values in injection drug users with chronic hepatitis C. Dig Liver Dis. 2005;37:674–680. doi: 10.1016/j.dld.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Muir AJ, Bornstein JD, Killenberg PG. Peginterferon α–2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]