Synopsis
Incidents of cancer, particularly in elderly patients living in developing countries, are predicted to raise significantly as the general population rises to an estimated 8.9 billion by the year 2050. Immune cells with specific functions and abilities to localize are vital to cancer prevention. Incidents of multiple myeloma, which is caused by transformed plasma cells, will increase in the coming decades. Combinational treatments prolong the survival of myeloma patients, in turn, escalating the management costs. Even though there have been many accomplishments made in the areas of immune cells and the biology of myeloma, there are still many obstacles in the way of conceptualizing the inter-relationships between immune cells and tumor cells. In order to provide better understanding of these concepts and to move towards improved therapies for myeloma, cell-based therapeutic approaches should be developed. In the following chapter, we will attempt to further illuminate these processes and concepts.
Keywords: Myeloma, immunotherapy, idiotype, dendritic cells
Novel targeted therapies are achieving responses in over 90% of newly diagnosed multiple myeloma (MM) patients; with one third of these patients achieving complete or near complete responses. However, patients still experience disease progression and curative outcomes are rare. This has led to evaluation of novel therapeutic interventions including, immunes-therapy in MM. Allogeneic transplant has provided the rational for development of vaccination strategies. Development of successful immune therapy in MM has been directed at two aspects; first, to develop a successful vaccine that is able to target MM cells with therapeutic efficacy; and second, to improve the immune function in MM patients in order to allow robust responses to immune-based intervention1.
In this chapter, we will provide a brief summary of the status of the immune system in myeloma, and a detailed account of the clinical trials performed with myeloma related antigens including idiotype (Id) alone or in conjugation with other proteins and pulsed with dendritic cells (DCs). Future immunotherapy strategies for the improvement of treatment of multiple myeloma and monoclonal gamapathy of undetermined significance (MGUS) will also be covered.
Obstacles to effective anti-myeloma immunity
With the advancements in immunology, there is better understanding of the interrelationships between MM cells and immune cells. In order to develop effective cancer vaccine, one has to strike a balance between triggering autoimmunity and generating tumor immunity. A low-avidity auto-reactive T cells are present and can be used to mount tumor immunity2. In order to generate robust immune responses even against foreign pathogenic antigens, dendritic cells orchestrates an appropriate inflammatory cytokine milieu3. In light of the above two requirements, identifying tumor antigens, which are important in eliciting anti-tumor immunity rather than triggering auto-immunity, has been a major obstacle in developing immune-therapy for MM. The approaches pursued are first, to identify patient-specific self antigens that are randomly mutated over time due togenetic instability4. These types of antigens do not generally induce auto-immunity and tolerance; however, this approach may be less practical for large-scale applications. The second approach involves use of shared non-mutated self-antigens, which may be prone to develop tolerance when generating anti-tumor immunity. In this context, TERT (telomerase reverse transcriptase) has been targeted to vaccinate cancer patients to reduce tumor evasion5,6. Additionally, there is increased realization of the importance of micro-environmental components including stromal cells and cytokines in supporting the tumor cell survival as well as the antigenic determinants that needs to be considered for effective immune-therapy strategy7. Finally, in order to optimize immune therapy strategies, multivariable trials evaluating immunological end points along with efficacy should take the place of the traditional clinical trial design for testing cytotoxic drugs8.
Interactions between immune cells and MM cells
For decades, tumor vaccines have concentrated on generating CTLs (Cytotoxic T Lymphocytes) in order to kill tumor cells9. However, growing literature provides convincing evidence that CD4 cells and antibody production increases the efficacy of immune-therapy approaches10-13. Further more, one has to take advantage of homeostatic proliferation14,15 in a lymphopenic host towards developing anti-tumor immunity. Additional role of immune regulation by FOXP3+ (Forkhead box protein 3) CD4 cells16-23, the influence of cytokine milieu to generate TH17 cells24-26 and stress-related NKG2D (Natural-Killer group 2 memberD) ligands (MICA and MICB, MHC-class-I-polypeptide-related sequence A & B)27,28 in modulating immune responses is being defined. The regulatory T cells that are positive for FOXP3 (natural, expanded & induced) play a critical role in immune homeostasis and are capable of modulating immunity, autoimmunity or tolerance. They predominantly express CD4 and CD2529 in addition to FOXP3, CTLA-4 (CTL-associated protein 4) and GITR (Glucocorticoid-induced tumor necrosis factor receptor). Their functions are mediated by TGF-β (Transforming growth factor-beta), and vitamin A derivatives30; and associated with other transcription factors including NFAT (Nuclear factor of activated T cells), AP1 (Activator protein 1), Runx1 (Runt-related transcription factor 1) and NFkB (Nuclear factor-kappa B) 22,31,32. Naïve CD4 cells can be differentiated to FOXP3+ cells in the presence of TGF-β and can be differentiated into TH17 cells in the presence of TGF-β +IL (Interleukin) -633,34 and IL-1β or IL-2324,26. On the other hand, IL-6 suppresses the induction of FOXP334,35. Complex networks of cytokines play a crucial role in the differentiation of FOXP3 and/or TH17cells during which antigen-specific immune responses are generated. With reduced FOXP3+ cell number and function, autoimmunity may be observed, while their increased activity may lead to immune-suppression in instead of immunity.
Immuno-biology in myeloma
Multiple myeloma exhibits a number of immune deficiencies in various compartments of the immune system. One has to appreciate the complex nature of the network that prevents the generation of robust immune responses in myeloma patients as represented in Figure 1.
Figure 1.
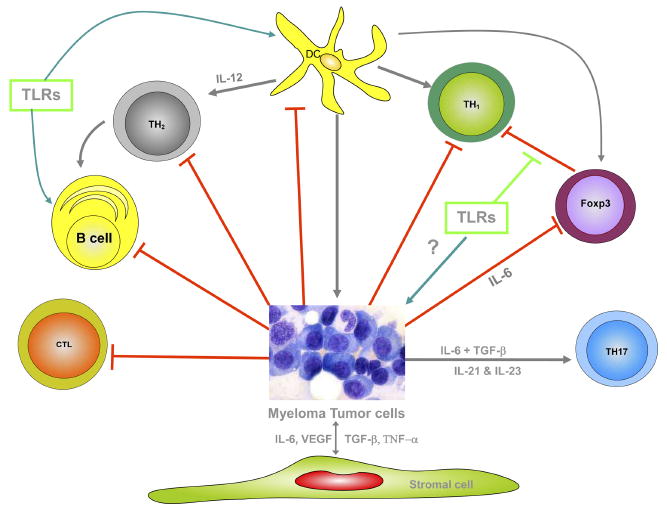
Cell-mediated responses
Myeloma patients have hyperactive T cell responses and deficient antibody responses mediated by B cells1. In terms of the expression of various surface markers and functional ability, both CD4 and CD8 T cells seems to be impaired in both MGUS and myeloma 36,37,38, however, the basis for these deficiencies remains unclear. An impairment in the diversity of T cell receptors by defective V-beta repertoire is observed in myeloma following high dose chemotherapy39. Even though, specific-cellular and humoral responses against both viral and tumor antigens have been reported in myeloma40, protective antibody production against pneumonia-causing bacteria is lacking in more than 80% of myeloma patients41,42. In addition, myeloma patients showed poor cytotoxic T cell responses against viral antigens in general40. A number of studies have shown that idiotype-specific responses can be generated in myeloma patients following vaccination indicating at least partly competent immune system with the ability to generate anti-tumor immune responses is feasible.
Antigen-presenting cells
Antigen-presenting cells (APCs) are generally equipped with antigen processing and presentation as well as co-stimulatory machinery and human leukocyte antigens (HLA) molecules, to generate specific immune responses. We and others have shown in earlier studies that APC are functional in myeloma and can be used for the immune-based clinical studies43. In our laboratory, different classes of myeloma proteins pulsed with DCs were utilized in generating in vivo anti-myeloma immune responses 44. However, others have shown impaired DC function for example in one study, DCs failed to up-regulate necessary CD80 expression which may be associated with myeloma progression37. This might be due to exposure to cytokines like TGF-β and IL-10. CD34+ DC progenitor cell development is influenced by IL-6 and can be corrected by the treatment with anti-IL-6 antibodies45. In addition, in a recent study46, monocyte-derived DCs from myeloma patients expressed lower levels of important activation surface markers including, CD40, CD80 and HLA-DR and presented recall antigens poorly to T cells. This might be due to a number of cytokines elevated in the tumor microenvironment as well as serum including IL-6, IL-10, TGF-β and vascular endothelial growth factor (VEGF). It is now feasible to improve the defective DC functions in myeloma47 by in vitro DC development techniques and by using innate immune stimulants like CpGs48,49.
Regulatory T cells
Dysregulation of naturally occurring CD4+CD25+ T regulatory cells in myeloma at initial diagnosis has been reported50. This study indicated lower numbers of Foxp3+ regulatory T cells in PBMC (Peripheral blood mono nuclear cells) of myeloma patients compared to normal healthy volunteers; this was additionally associated with impaired inhibitory responses in MM. Generally speaking, regulatory T cells contribute in establishing homeostasis following specific-immunity and in keeping auto-antigen-based immune responses under control or with a very minimal level of pathological consequences. High IL-6 levels in myeloma may down-regulate regulatory T cell suppressive activity34,51. However, in another study, a higher number of regulatory T cells were reported in myeloma in purified CD4+CD25high population, capable of suppressive activity at 1:2 ratio in a three-way mixed lymphocyte reaction (MLR) following 24 hours of in vitro activation52. These regulatory T cells were activated away from the tumor microenvironment cytokine milieu prior to evaluation of their functional capacity; and under these conditions, regulatory T cells may have expanded or induced.
TH17 cells
The role of TH17 cells in tumor immunity is not well-established53, however, two cytokines, IL-6 and TGF-β, important in their development, are highly expressed in myeloma. A recent study has reported elevated levels of IL-17 in myeloma compared to normal donor sera. This particular inflammatory cytokine may promote tumor growth via promotion of angiogenesis54. Similarly, IL-21, a IL-17 associated cytokine, has been reported to increase myeloma growth and block IL-6 dependent apoptosis in myeloma cell-lines55.
In spite of the abnormalities and deficiencies observed in myeloma in various compartments of the immune system, anti-tumor immune responses are observed in MM and an ongoing effort is to devise strategies to induce and augment specific responses that may then have clinically relevant effects.
Myeloma clinical trials
As a potential target for immunotherapy, idiotype protein, the immunoglobulin produced in large quantities by myeloma patients, has been extensively studied. These antibody molecules are generated by rearrangement of variable, diversity and joining regions of heavy and light chains. In the past decade, a number of studies have demonstrated a TH1 type of immunity can be generated using idiotype-pulsed DCs in an HLA-restricted fashion. Thus, these studies demonstrate feasibility of developing idiotype-specific T cell-mediated anti-myeloma responses.
In vivo experimentations
In early 70s, animal experimentations 56 showed that immunizations with purified idiotype proteins were able to produce anti-idiotype antibodies in mice. Further more, when tumor cells that were producing immunized idiotype were transferred to naïve and un-immunized animals, only 11% of animals developed tumors. However, when tumor cells only producing light chains were transferred to naïve animals such protection was not observed.
Pre-clinical studies
Yi et al57 reported that T cells from myeloma patients were capable of responding to autologous idiotype in vitro. Specific-idiotype mediated IFN-γ (Interferon-gamma) and IL-2 production was observed in 66% and 76% of T cells respectively, from myeloma patients. Idiotype-specific proliferation was observed in 36% of the patients tested. These results indicated that the T cells against self-idiotype can be used to generate T cell responses and provided the rational to use the idiotype as a tumor-specific target for vaccination studies. In addition, an another study by King et al58 showed that mice immunized with DNA vaccine consisting of idiotype and fragment C of tetanus toxin, 70% of animals survived for more than two months after challenge with tumor cells compared to vehicle control animals. This particular study indicated that DNA fusion vaccines could be effective in generating protective immunity against tumors. Stritzke et al59 have reported that when animals were vaccinated with tumor cells along with GM-CSF, followed by IL-2 administration, the tumor recurrence was delayed and survival improved compared to control animals. This indicated that NK cells and CD8 T cells were important in generating tumor-specific immunity to render the protection in these animals tested.
Idiotype-based clinical results
In order to vaccinate patients with a given antigen to generate immune responses, one has to have a large quantity of clinical grade antigen and that is specific to the tumor. It is easy to purify large amount of monoclonal para-protein or idiotype from the serum of myeloma patients. As shown in Table 1, a number of clinical trials using idiotype alone or in combination with cytokines as tumor antigen to vaccinate myeloma patients have been performed. Bergenbrant et al60 used myeloma para protein alone to repeatedly immunize five myeloma patients. T cells following vaccination produced higher levels of IFN-γ in response to idiotype protein. However, with repeated immunizations, T cell responses appeared to be rare and patients did not achieve clinical responses following vaccination. Osterborg et al61 immunized five myeloma patients with autologous idiotype protein along with GM-CSF and although in vitro studies showed that both CD4 and CD8 cells produced IFN-γ and IL-2 following vaccination, significant T cell proliferative and DTH responses were not observed. Changes in para-protein levels were also not reported in these immunized patients. Rasmussen et al62 have administered seven vaccinations to six myeloma patients with idiotype and IL12 with or without GM-CSF (Granulocyte-macrophage colony-stimulating factor). Immediately following vaccination, clonal B cells went down and most of the patients showed specific-T cell responses. However, after 30 weeks post-vaccination, T cell responses were diminished and para protein levels were elevated. In another study by Bertinetti et al63, three auto SCT myeloma patients were given four immunizations of idiotype with GM-CSF in addition to hepatitis B vaccine. Even though partial clinical remission was observed in vaccinated patients, it did not correlate with T cells responses and hepatitis B antibodies were not detected following vaccination. In a long-term study, Massaia et al64 and Coscia et al65 vaccinated 12 myeloma patients with idiotype with KLH (Keyhole limpet hemocyanin) and GM-CSF following high dose chemotherapy and autoSCT (Auto-stem cell transplant) in first CR (Complete response). Even though, a majority of the patients (85%) showed DTH (Delayed-type hypersensitivity) response, T cell responses and anti-idiotype antibodies were not significantly increased following vaccinations and no clinical benefit was observed following vaccinations. Using allogeneic transplantation settings, we have vaccinated66 HLA-matched sibling donors first with Idiotype and KLH in addition to GMCSF prior to bone-marrow collection and transplant. The rationale for vaccinating BMT (Bone-marrow transplant) donors prior to transplantation is to transfer tumor-specific immune components with the graft. After BMT, patients received three booster vaccinations. Three out of five patients after vaccination showed idiotype-specific T cell responses, improved clinical response from partial remission prior to BMT and Idiotype vaccinations to CR and maintenance of response up to 5-8 years. In autologous transplant setting, we have conducted a large study with three different cohorts and a total of fifty myeloma patients have been enrolled. In this study, patients following double auto transplant received three or six vaccinations with idiotype coupled with KLH with GM-CSF in first two cohorts. A third cohort was vaccinated before and after transplantation. The majority of the patients (58%) following vaccinations produced idiotype-specific TH1 type of responses and had an elevated proliferative response in addition to DTH responses (42%).
Table 1.
Idiotype-based clinical trails in Myeloma
| Vaccine | Patients | Cellular Responses | Clinical response | Author (Ref #) | |
|---|---|---|---|---|---|
| T cell | B cell | ||||
| Id alone Repeated Vaccines | 5 | 3/5 | + | No Response | Bergenbrant et al. (60) |
| Id + GM-CSF 6 Vaccines | 5 | 1/5 | - | Para protein levels unchanged | Osterborg et al. (61) |
| Id +KLH + GM-CSF (+IL-2) AutoSCT at Remission |
12 T cell HD Chemo | 2/11 | - | Para protein levels unchanged | Massaia et al., Coscia et al. (64,65) |
| Id + IL-12 +/- GM-CSF 7 Vaccines | 6 | 5/6 | - | No change in Para protein | Rasmussen et al. (62) |
| Id +GM-CSF 4 Vaccines + HepB Vac | 3 autoSCT | 1/3 | - | No abs to HepB | Bertinetti et al. (63) |
| Tumor cell + GM-CSF-K562 8 Vaccines after AutoSCT |
16 | 3/5 | + | 3/16 rise in Para Protein | Borrello et al. (ASH presented) |
| Id + KLH +GM-CSF, 6 vaccines | 5, AlloSCT | 4/5 | + | 3/5 Stable CR | Neelapu et al. (66) |
| Id + KLH + GM-CSF, 3 or 6 vaccines | 50 autoSCT | 28/48 | + | No response | Munshi et al. (ASH presented) |
Abbreviations: Id, Idiotype; KLH, keyhole limpet haemocyanin; AutoSCT, Autologous Stem Cell Transplantation; GM-CSF, Granulocyte-Macrophage Colony Stimulating Factor; IL-12, Interleukin-12; HD, High Dose; HepB, Hepatitis B vaccine; CR, Complete Response.
In summary, these studies demonstrated that myeloma patients do respond to vaccination and that idiotype is a weak immunogen, however, the responses are not robust, do not persist for prolonged periods of time and clinically meaningful outcomes have not been observed. This has prompted investigation of novel approaches and antigens for immunotherapy.
Idiotype-pulsed DC trials in myeloma
The majority of clinical trials conducted using idiotype-pulsed DCs showed immune response, yet significant clinical responses are lacking (Table 2). Lim et al67 showed that following three idiotype-pulsed DC vaccinations, both idiotype-specific-T cell and anti-idiotypic-antibody responses were observed, however, none of the vaccinated patients showed clinical improvements. Titzer et al68 vaccinated eleven myeloma patients with idiotype-pulsed with CD34-derived DCs. T cells from only four out of ten patients were able to show antigen-specific production of cytokines following vaccination and clinical responses were not observed following vaccination.
Table 2.
Clinical trails using DCs pulsed with idiotype of Myeloma patients
| Vaccines | Patients | Clinical Outcome | Authors (Ref#) |
|---|---|---|---|
| 7 Vaccines (Id + KLH +DCs) (2-iv Id + DCs & 5- Id + KLH) |
12 AutoSCT High Dose Chemotherapy |
Stable | Reichardt et al. (71,72) |
| 3 Vaccines (Id +DCs) | 6 | Progressed | Lim et al. (67) |
| 7 Vaccines (2-Id + DCs & 5-Id+ KLH) | 26, High dose chemotherapy, AutoSCT | 17 Live/ Stable | Liso et al. (69) |
| 4 Vaccines (1-Id + DCs & 3-Id+GMCSF) | 11 | Progressed | Titzer et al. (68) |
| 3 Vaccines (Id +DCs + IL-2) | 5 High dose Chemotherapy at Stable PR | 4 Stable/1 Relapsed | Yi et al. (70) |
| 4-7 Vaccines (Id + KLH + GMCSF) |
4 AlloSCT | 3 Progressed | Bendandi et al. (73) |
Abbreviations: Id, Idiotype; KLH, keyhole limpet haemocyanin; DCs, Dendritic cells; AutoSCT, Autologous Stem Cell Transplantation; GMCSF, Granulocyte-Macrophage Colony Stimulating Factor; PR, Partial response, & IL2, Interleukin 2
Liso et al69 immunized twenty-six patients following high dose chemotherapy with idiotype-pulsed DCs with or without KLH. Only four out of twety-six patients showed specific-proliferative responses, however, seventeen patients are alive at 30 months post-vaccination. Yi et al70 vaccinated five patients at remission following high dose chemotherapy. Three idiotype-pulsed monocyte-derived DC vaccinations were administered followed by IL-2 therapy for five days. Four out of five patients showed Idiotype specific-T cell cytokines production and one patient achieved partial response (PR) following vaccination. Reichardt et al71 vaccinated twelve patients post autoSCT with idiotype-pulsed DCs. In terms of T cell responses, only two out of twelve demonstrated idiotype-specific proliferation and one of three patients showed specific CTL-mediated killing. No clinical improvement was reported following vaccinations72. Recently, Bendandi et al73 conducted a clinical trial using DCs loaded with idiotype in four myeloma patients following reduced intensity conditioning alloSCT. Anti-idiotype antibody responses were not seen. T cell responses by production of TH1 cytokines were noted in two out of 4 patients. Three patients in this study had transient responses and one patient stable disease. Recently, sixteen myeloma patients have been vaccinated following autoSCT with irradiated autologous myeloma plasma cells and genetically modified K562 cells to produce GM-CSF. Of the sixteen patients that have completed the study, six showed CR and five with PR after autoSCT and vaccination without noticeable toxicities. Both cellular and antibody responses have been observed in addition to DTH responses.
In summary, generating anti-tumor immunity is feasible, yet convincing clinical efficacy is lacking in myeloma even with DC-pulsed idiotypic vaccinations. Since intravenous DC vaccinations lead to its sequestration74,75, one can improve DC migration patterns by subcutaneous administration to generate protective TH1 type responses 76. Since immature DCs are unstable when necessary cytokines are withdrawn77, the use of mature DC derived from peripheral blood monocytes would be superior in presenting antigens to T cells. Even though idiotype is a weak immunogen, one can generate adequate immunity against this type of tumor-specific antigen both post-autologous transplantation as well as donor vaccinated post-allogeneic stem cell transplantation.
Future directions
Targeting a single tumor-specific antigen will allow tumor cells to become resistant by mutation of a particular gene and by evading immunity. In order to avoid this obstacle, one has to use approaches directed at multiple antigenic targets. One example is pulsing DCs with whole cell-myeloma lysates or fusing DCs with myeloma cells. A pre-clinical study using animal cells and human cells have confirmed the feasibility of presenting a wide array of myeloma-related antigens by fusing myeloma cells with DCs for the development of effective CTLs78. A clinical study of MM/DC fusion cell vaccination with GM-CSF is ongoing. Eleven patients have been enrolled in this study and demonstrated that adequate number of functional DCs can be obtained to vaccinate patients three or more times without toxicity. The majority of the patients were stabilized with tumor-specific T cell responses by increased production of IFN-γ following vaccination.
As production of patient specific vaccination is difficult, there is an ongoing effort to identify a cocktail of antigens, which can be used in universal fashion for all the patients. A number of investigators have identified novel myeloma-specific antigens by screening myeloma cDNA expression libraries using the SEREX (Serological expression cloning) technique for eventual development of an antigen cocktail. With this technology, myeloma–specific antigens such as XBP1, OFD1 (Orofaciodigital 1), BCMA (B-cell maturation receptor) and ROCK1 (Rho-associated kinase 1) are identified. Recently, immune responses directed at an embryonic stem cell marker, SOX2 expressed in the CD138- compartment in MGUS patients have been reported. Such responses may help in predicting clinical out-comes based on the SOX2–induced immunity79. Furthermore, other trans-membrane proteins, including MUC1, previously reported to be expressed in glandular epithelial cells80, have been shown to be over-expressed in myeloma cell-lines and primary myeloma cells81,82. Malignancy-associated cancer-testis antigens including, MAGE family: BAGE, GAGE, PRAME, NY-ESO-1, and Sperm protein1783, generally, not expressed in normal tissues, have been shown to be expressed in myeloma. These have been targeted for active investigation for cancer immunotherapy because of their tumor-specific expression and the ability to induce tumor-specific immunity. However, the vaccinations with these cellular antigens generating significant clinical response have yet to be seen. In addition, since most of these cellular antigens are present in the late phase of myeloma and associated with only a subset of myeloma cells, using them for vaccination may not translate into clinical efficacy.
The ongoing future approach to obtain clinically effective vaccination includes methods to increase the immunogenicity of DCs, to apply methods to extend tumor immunity following vaccinations, to develop approaches to improve immune status in myeloma patient, to utilize homeostatic proliferation to generate efficient tumor immunity, to establish regulatory T cells homeostatic functions to the normal thresh-hold level, and to abrogate TH17-mediated immunopathology. Eventually, a combination of vaccination with tumor-specific antigens, adoptive transfer of T cells cultured with immunomodulatory agents will provide the robust and sustained immune responses with clinical efficacy.
Acknowledgments
The National Institutes of Health and VA support this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Munshi NC. Immunoregulatory mechanisms in multiple myeloma. Hematol Oncol Clin North Am. 1997;11:51–69. doi: 10.1016/s0889-8588(05)70415-9. [DOI] [PubMed] [Google Scholar]
- 2.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 5.Shammas MA, Shmookler Reis RJ, Li C, et al. Telomerase inhibition and cell growth arrest after telomestatin treatment in multiple myeloma. Clin Cancer Res. 2004;10:770–776. doi: 10.1158/1078-0432.ccr-0793-03. [DOI] [PubMed] [Google Scholar]
- 6.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 7.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 8.Simon RM, Steinberg SM, Hamilton M, et al. Clinical trial designs for the early clinical development of therapeutic cancer vaccines. J Clin Oncol. 2001;19:1848–1854. doi: 10.1200/JCO.2001.19.6.1848. [DOI] [PubMed] [Google Scholar]
- 9.Zeis M, Siegel S, Wagner A, et al. Generation of cytotoxic responses in mice and human individuals against hematological malignancies using survivin-RNA-transfected dendritic cells. J Immunol. 2003;170:5391–5397. doi: 10.4049/jimmunol.170.11.5391. [DOI] [PubMed] [Google Scholar]
- 10.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 11.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 13.Smith CM, Wilson NS, Waithman J, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 14.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe N, Hanabuchi S, Soumelis V, et al. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5:426–434. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- 16.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 17.Pennington DJ, Silva-Santos B, Silberzahn T, et al. Early events in the thymus affect the balance of effector and regulatory T cells. Nature. 2006;444:1073–1077. doi: 10.1038/nature06051. [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 19.Gavin MA, Rasmussen JP, Fontenot JD, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 20.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 21.Marson A, Kretschmer K, Frampton GM, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 25.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007 doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 26.Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007 doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 27.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern-Ginossar N, Elefant N, Zimmermann A, et al. Host immune system gene targeting by a viral miRNA. Science. 2007;317:376–381. doi: 10.1126/science.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 30.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 31.Ono M, Yaguchi H, Ohkura N, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–689. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 32.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 33.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 35.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Massaia M, Bianchi A, Attisano C, et al. Detection of hyperreactive T cells in multiple myeloma by multivalent cross-linking of the CD3/TCR complex. Blood. 1991;78:1770–1780. [PubMed] [Google Scholar]
- 37.Brown RD, Pope B, Murray A, et al. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001;98:2992–2998. doi: 10.1182/blood.v98.10.2992. [DOI] [PubMed] [Google Scholar]
- 38.Xie J, Wang Y, Freeman ME, 3rd, Barlogie B, Yi Q. Beta 2-microglobulin as a negative regulator of the immune system: high concentrations of the protein inhibit in vitro generation of functional dendritic cells. Blood. 2003;101:4005–4012. doi: 10.1182/blood-2002-11-3368. [DOI] [PubMed] [Google Scholar]
- 39.Mariani S, Coscia M, Even J, et al. Severe and long-lasting disruption of T-cell receptor diversity in human myeloma after high-dose chemotherapy and autologous peripheral blood progenitor cell infusion. Br J Haematol. 2001;113:1051–1059. doi: 10.1046/j.1365-2141.2001.02871.x. [DOI] [PubMed] [Google Scholar]
- 40.Maecker B, Anderson KS, von Bergwelt-Baildon MS, et al. Viral antigen-specific CD8+ T-cell responses are impaired in multiple myeloma. Br J Haematol. 2003;121:842–848. doi: 10.1046/j.1365-2141.2003.04375.x. [DOI] [PubMed] [Google Scholar]
- 41.Zinneman HH, Hall WH. Recurrent pneumonia in multiple myeloma and some observations on immunologic response. Ann Intern Med. 1954;41:1152–1163. doi: 10.7326/0003-4819-41-6-1152. [DOI] [PubMed] [Google Scholar]
- 42.Robertson JD, Nagesh K, Jowitt SN, et al. Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br J Cancer. 2000;82:1261–1265. doi: 10.1054/bjoc.1999.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raje N, Gong J, Chauhan D, et al. Bone marrow and peripheral blood dendritic cells from patients with multiple myeloma are phenotypically and functionally normal despite the detection of Kaposi's sarcoma herpesvirus gene sequences. Blood. 1999;93:1487–1495. [PubMed] [Google Scholar]
- 44.Butch AW, Kelly KA, Munshi NC. Dendritic cells derived from multiple myeloma patients efficiently internalize different classes of myeloma protein. Exp Hematol. 2001;29:85–92. doi: 10.1016/s0301-472x(00)00619-6. [DOI] [PubMed] [Google Scholar]
- 45.Ratta M, Fagnoni F, Curti A, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–237. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Hong S, Yang J, et al. Optimizing immunotherapy in multiple myeloma: Restoring the function of patients' monocyte-derived dendritic cells by inhibiting p38 or activating MEK/ERK MAPK and neutralizing interleukin-6 in progenitor cells. Blood. 2006;108:4071–4077. doi: 10.1182/blood-2006-04-016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dauer M, Obermaier B, Herten J, et al. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–4076. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 48.West MA, Wallin RP, Matthews SP, et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 49.Hermans IF, Silk JD, Gileadi U, et al. Dendritic cell function can be modulated through cooperative actions of TLR ligands and invariant NKT cells. J Immunol. 2007;178:2721–2729. doi: 10.4049/jimmunol.178.5.2721. [DOI] [PubMed] [Google Scholar]
- 50.Prabhala RH, Neri P, Bae JE, et al. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006;107:301–304. doi: 10.1182/blood-2005-08-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 52.Beyer M, Kochanek M, Giese T, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–3949. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 53.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 54.Alexandrakis MG, Pappa CA, Miyakis S, et al. Serum interleukin-17 and its relationship to angiogenic factors in multiple myeloma. Eur J Intern Med. 2006;17:412–416. doi: 10.1016/j.ejim.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Brenne AT, Ro TB, Waage A, Sundan A, Borset M, Hjorth-Hansen H. Interleukin-21 is a growth and survival factor for human myeloma cells. Blood. 2002;99:3756–3762. doi: 10.1182/blood.v99.10.3756. [DOI] [PubMed] [Google Scholar]
- 56.Lynch RG, Graff RJ, Sirisinha S, Simms ES, Eisen HN. Myeloma proteins as tumor-specific transplantation antigens. Proc Natl Acad Sci U S A. 1972;69:1540–1544. doi: 10.1073/pnas.69.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi Q, Osterborg A, Bergenbrant S, Mellstedt H, Holm G, Lefvert AK. Idiotype-reactive T-cell subsets and tumor load in monoclonal gammopathies. Blood. 1995;86:3043–3049. [PubMed] [Google Scholar]
- 58.King CA, Spellerberg MB, Zhu D, et al. DNA vaccines with single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nat Med. 1998;4:1281–1286. doi: 10.1038/3266. [DOI] [PubMed] [Google Scholar]
- 59.Stritzke J, Zunkel T, Steinmann J, Schmitz N, Uharek L, Zeis M. Therapeutic effects of idiotype vaccination can be enhanced by the combination of granulocyte-macrophage colony-stimulating factor and interleukin 2 in a myeloma model. Br J Haematol. 2003;120:27–35. doi: 10.1046/j.1365-2141.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- 60.Bergenbrant S, Yi Q, Osterborg A, et al. Modulation of anti-idiotypic immune response by immunization with the autologous M-component protein in multiple myeloma patients. Br J Haematol. 1996;92:840–846. doi: 10.1046/j.1365-2141.1996.419959.x. [DOI] [PubMed] [Google Scholar]
- 61.Osterborg A, Yi Q, Henriksson L, et al. Idiotype immunization combined with granulocyte-macrophage colony-stimulating factor in myeloma patients induced type I, major histocompatibility complex-restricted, CD8- and CD4-specific T-cell responses. Blood. 1998;91:2459–2466. [PubMed] [Google Scholar]
- 62.Rasmussen T, Hansson L, Osterborg A, Johnsen HE, Mellstedt H. Idiotype vaccination in multiple myeloma induced a reduction of circulating clonal tumor B cells. Blood. 2003;101:4607–4610. doi: 10.1182/blood-2002-06-1925. [DOI] [PubMed] [Google Scholar]
- 63.Bertinetti C, Zirlik K, Heining-Mikesch K, et al. Phase I trial of a novel intradermal idiotype vaccine in patients with advanced B-cell lymphoma: specific immune responses despite profound immunosuppression. Cancer Res. 2006;66:4496–4502. doi: 10.1158/0008-5472.CAN-05-4233. [DOI] [PubMed] [Google Scholar]
- 64.Massaia M, Borrione P, Battaglio S, et al. Idiotype vaccination in human myeloma: generation of tumor-specific immune responses after high-dose chemotherapy. Blood. 1999;94:673–683. [PubMed] [Google Scholar]
- 65.Coscia M, Mariani S, Battaglio S, et al. Long-term follow-up of idiotype vaccination in human myeloma as a maintenance therapy after high-dose chemotherapy. Leukemia. 2004;18:139–145. doi: 10.1038/sj.leu.2403181. [DOI] [PubMed] [Google Scholar]
- 66.Neelapu SS, Munshi NC, Jagannath S, et al. Tumor antigen immunization of sibling stem cell transplant donors in multiple myeloma. Bone Marrow Transplant. 2005;36:315–323. doi: 10.1038/sj.bmt.1705057. [DOI] [PubMed] [Google Scholar]
- 67.Lim SH, Bailey-Wood R. Idiotypic protein-pulsed dendritic cell vaccination in multiple myeloma. Int J Cancer. 1999;83:215–222. doi: 10.1002/(sici)1097-0215(19991008)83:2<215::aid-ijc12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 68.Titzer S, Christensen O, Manzke O, et al. Vaccination of multiple myeloma patients with idiotype-pulsed dendritic cells: immunological and clinical aspects. Br J Haematol. 2000;108:805–816. doi: 10.1046/j.1365-2141.2000.01958.x. [DOI] [PubMed] [Google Scholar]
- 69.Liso A, Stockerl-Goldstein KE, Auffermann-Gretzinger S, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood progenitor cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2000;6:621–627. doi: 10.1016/s1083-8791(00)70027-9. [DOI] [PubMed] [Google Scholar]
- 70.Yi Q, Desikan R, Barlogie B, Munshi N. Optimizing dendritic cell-based immunotherapy in multiple myeloma. Br J Haematol. 2002;117:297–305. doi: 10.1046/j.1365-2141.2002.03411.x. [DOI] [PubMed] [Google Scholar]
- 71.Reichardt VL, Okada CY, Liso A, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma--a feasibility study. Blood. 1999;93:2411–2419. [PubMed] [Google Scholar]
- 72.Reichardt VL, Milazzo C, Brugger W, Einsele H, Kanz L, Brossart P. Idiotype vaccination of multiple myeloma patients using monocyte-derived dendritic cells. Haematologica. 2003;88:1139–1149. [PubMed] [Google Scholar]
- 73.Bendandi M, Rodriguez-Calvillo M, Inoges S, et al. Combined vaccination with idiotype-pulsed allogeneic dendritic cells and soluble protein idiotype for multiple myeloma patients relapsing after reduced-intensity conditioning allogeneic stem cell transplantation. Leuk Lymphoma. 2006;47:29–37. doi: 10.1080/10428190500272473. [DOI] [PubMed] [Google Scholar]
- 74.Eggert AA, Schreurs MW, Boerman OC, et al. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res. 1999;59:3340–3345. [PubMed] [Google Scholar]
- 75.Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- 76.Fong L, Brockstedt D, Benike C, Wu L, Engleman EG. Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol. 2001;166:4254–4259. doi: 10.4049/jimmunol.166.6.4254. [DOI] [PubMed] [Google Scholar]
- 77.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587–4595. [PubMed] [Google Scholar]
- 78.Gong J, Koido S, Chen D, et al. Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12. Blood. 2002;99:2512–2517. doi: 10.1182/blood.v99.7.2512. [DOI] [PubMed] [Google Scholar]
- 79.Spisek R, Kukreja A, Chen LC, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–840. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ho SB, Niehans GA, Lyftogt C, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–651. [PubMed] [Google Scholar]
- 81.Treon SP, Maimonis P, Bua D, et al. Elevated soluble MUC1 levels and decreased anti-MUC1 antibody levels in patients with multiple myeloma. Blood. 2000;96:3147–3153. [PubMed] [Google Scholar]
- 82.Takahashi T, Makiguchi Y, Hinoda Y, et al. Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. J Immunol. 1994;153:2102–2109. [PubMed] [Google Scholar]
- 83.Zendman AJ, Ruiter DJ, Van Muijen GN. Cancer/testis-associated genes: identification, expression profile, and putative function. J Cell Physiol. 2003;194:272–288. doi: 10.1002/jcp.10215. [DOI] [PubMed] [Google Scholar]


