Abstract
The human immunodeficiency virus (HIV)-1 envelope glycoprotein gp120 has been implicated in mediating neuronal apoptosis, a hallmark feature of HIV-associated dementia (HAD). Mitigation of the toxic effects of gp120 could thus be a potential mechanism for reducing HIV toxicity in the brain. In this study we hypothesized that neurotrophic factor, such as platelet-derived growth factor (PDGF), could protect the neurons against gp120-mediated apoptosis. SH-SY5Y cells treated with gp120 exhibited increased cell death when measured by LDH and TUNEL assay with concomitant loss of neurites and increased cell rounding. Pre-treatment with PDGF-BB, however, reduced gp120-associated neurotoxicity and rescued the neurite outgrowth. Additionally, gp120-mediated activation of caspase-3 was also significantly reduced in cells pretreated with PDGF-BB. Anti-apoptotic effects of PDGF-BB were also confirmed by monitoring levels of anti and pro-apoptotic genes, Bcl-xL and Bax, respectively. Furthermore, PDGF-mediated protection against gp120 involved the PI-3 kinase/Akt pathway. Taken together these findings lead us to suggest that PDGF-BB could be considered as a therapeutic agent that can mitigate gp120-mediated neurotoxicity in HAD.
Keywords: PDGF, Gp120, Neurons, HIV Dementia
Introduction
It is estimated that almost 25% of untreated human immunodeficiency virus (HIV)-infected individuals and 7% of HIV-infected patients treated with highly active anti-retroviral therapy develop HIV-associated dementia (HAD) (Budka, 1991; McArthur et al, 1993; Sacktor et al, 2001; Spencer and Price, 1992), a neurodegenerative syndrome that is clinically characterized by progressive cognitive, motor, and behavioral abnormalities (Lipton and Gendelman, 1995; Price et al, 1991). Pathological manifestation of the syndrome, HIV-encephalitis (HIV-E), is accompanied by prominent microglial activation, formation of microglial nodules, perivascular accumulations of mononuclear cells, presence of virus-infected multinucleated giant cells, and neuronal damage and loss (Bell, 1998; Gendelman et al, 1994; Nath, 1999). The primary cell types infectedby HIV-1 in the brain are macrophages/microglia, and to a lesser extent, astrocytes, but not neurons (Kaul et al, 2001). One broad explanationfrequently advocated explaining the loss of neurons in thisdisease is that cellular and/or viral proteins released fromthe infected cells have a direct toxic effect on the neurons (Adamson et al, 1996; Brenneman et al, 1988; Chen et al, 2000; Dreyer et al, 1990; Hof et al, 1998; Kruman et al, 1998; New et al, 1997; Patel et al, 2000).
Amongst the HIV proteins that are shed in the brain from virus-infected cells, HIV envelope glycoprotein gp120 is shown to have neurotoxic effects both in vitro and in vivo (Bansal et al, 2000; Lipton et al, 1991; Meucci and Miller, 1996). In cell culture gp120 is able to induce apoptosis of neurons both in the presence and in the absence of microglia. Transgenic mice expressing gp120 in the brain also manifest neuropathological features similar to those observed in AIDS patients (Mucke et al, 1995). Several independent findings have shown that gp120-mediated neurotoxicity occurs via apoptosis through the seven-transmembrane domain chemokine receptor CXCR4 (Davis et al, 1997; Herbein et al, 1998; Hesselgesser et al, 1998a; Hesselgesser et al, 1998b; Klein et al, 1999; Kozak et al, 1999) involving the activation of proapoptotic caspase-3. Gp120-mediated caspase-3 activation has been specifically demonstrated in rat cerebellar granule cells (Campus et al, 2000), human embryonic kidney (Biard-Piechaczyk et al, 2000) and endothelial cells (Ullrich et al, 2000). Reciprocally, inhibition of caspase-3 has been shown to protect neurons from apoptosis. Hence, factors that inhibit caspase-3 activation could be valid candidates for rescuing neurons from gp120-toxicity.
Belonging in this category of caspase-inhibitors are various growth factors in the CNS that are neurotrophic and have been shown to block apoptosis, thereby serving as survival factors during normal nervous system development (Bachis and Mocchetti, 2005; Cameron et al, 1991; Cameron and Rakic, 1991; Sanders et al, 2000; Vogelbaum et al, 1998a; Vogelbaum et al, 1998b). In particular, brain-derived neurotrophic factor (BDNF), fibroblast growth factor (FGF), and insulin-like growth factor have been shown to protect neurons from gp120-mediated apoptosis (Bachis and Mocchetti, 2005; Kulik et al, 1997; Sanders et al, 2000). In the present study we explored the role of yet another neurotrophic factor, PDGF that has been documented to be critical for the development of brains of postnatal rats (Smits et al, 1991). PDGF is a family of five dimeric ligands (PDGF-AA, -AB, -BB, - CC, & -DD) assembled from four gene products (PDGF-A–D) that act via two classical receptor tyrosine kinases, PDGF-αR and PDGF-βR (Bergsten et al, 2001; Heldin et al, 2002; Li et al, 2000). Members of the PDGF family have multiple roles during embryogenesis and in a variety of pathological situations in the adult. The focus of this study was to examine whether PDGF could protect the neurons against gp120-mediated toxicity.
Materials and Methods
Materials
Human neuroblastoma cells (SH-SY5Y) were purchased from American Type Culture Collection (Manassas, VA). The rationale for choosing these cells was based on their ability to mimic the pathways involved in the neurodegenerative process observed in HIV-E (Everall et al, 2002; Sanders et al, 2000). Human recombinant PDGF-BB was purchased from R&D Systems (Minneapolis, MN, USA) and viral gp120 (IIIB strain) was obtained from the AIDS Research and Reference Reagent Program of National Institutes of Health.
Cell culture and Treatments
SH-SY5Y cells were plated at a density of 1×105/ml and cultured in a 1:1 mixture of Eagle’s Minimum Essential Medium containing non-essential amino acids (Gibco, Gaithersburg, MD) and F12 Medium (Gibco) supplemented with heat inactivated fetal bovine serum (10% v/v), 2mM glutamine at 37°C in 5% CO2. Confluent cells were replated at 1–5×105 cells/ml for different experiments and differentiated by treatment with 10μM retinoic acid (Sigma, St. Louis, MO) for 7 days with medium changes every two days.. For all of the experiments cells were serum starved for 24h in the presence of 10μM retinoic acid (Sigma) prior to treatment with PDGF-BB (20ng/ml; pre-determined dose) for 30 min followed by addition of gp120 IIIB (200ng/ml). In the experiment involving the phosphoinositide 3-OH kinase (PI3K) inhibitor (LY294002; Calbiochem, San Diego, CA) or MEK1/2 inhibitor (U0126; Calbiochem), SH-SY5Y cells were pretreated for 1h with 10μM inhibitor followed by treatment with PDGF-BB and/or gp120 as described above.
Rat Cortical Neuronal Cultures
Primary cultures of rat cortical neurons were prepared from 18-day-old fetuses of Sprague-Dawley rats as previously described (Brewer et al, 1993). Briefly, rat brain cortices were harvested by removal of brainstem and hippocampi followed by mechanical trituration. After suspending in Neurobasal medium (Gibco) supplemented with 2 mM glutamax, 2% B-27 supplement, and 1% antibiotic. , The cells were seeded at a density of 40,000 cells per well in 96 well plate or 5×105 cells per well in 6 well plate (all plates were pre-coated with poly-D-lysine) and maintained at 37°C with 5% CO2. At day 3, half the medium was changed and the cells were cultured for 3 more days. Cells were characterized with immunocytochemistry and were >95% pure.
Immunocyto/histochemistry
Immunohistochemical analysis of PDGF-B chain was carried out on archival paraffin-fixedtissue sections of brain from uninfected or simian human immunodeficiency virus (SHIV)-infected macaques with encephalitis, as described earlier (Sui et al, 2004). Sections from basal ganglia region of macaques were treatedwith rabbit polyclonal PDGF-B antibody (Santa Cruz, CA), followed by treatment with biotinylated goat anti-rabbit secondary antibody and peroxidase-conjugated streptavidin (Dako, Carpinteria, CA) and Nova Red substrate(Vector Laboratories, Burlingame, CA).
For immunocytochemical analysis of caspase-3, cells were treated with various agents as described above followed by fixation with 4% paraformaldehye for 10 min at room temperature. Fixed cells were then treated with 1:1000 diluted anti-cleaved caspase-3 antibody (Cell signaling, Danvers, MA) for 2h, followed by treatment with Alexa Fluor 488-conjugated anti-rabbit secondary antibody (1:500, Invitrogen, Carlsbad, CA) for 30 min at room temperature and mounted in Slow Fade anti-fade reagent with 4, 6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, OR). Images were captured using a inverted fluorescence microscope TE2000-E (Nikon, Tokyo, JAPAN).
Lactate Dehydrogenase (LDH) Assay
The LDH release assay was performed using a CytoTox96 non-radioactivecytotoxicity assay (Promega, Madison, MI). LDH released into the culture medium and total LDH of lysed cells were evaluated by this assay. Briefly, the SH-SY5Y cells were seeded at a density of 5× 104 cells per well on a 96-well microplate. Following treatment with PDGF-BB and/or gp120, 50μl ofmedium were moved to a new multi-well plate to evaluate the LDHreleased from cells into the medium, while the remaining mediumwas replaced with fresh serum-free medium. The cells were lysedby adding 15 μl of a lysis solution (9% Triton X-100 in water) and incubated for 45 min at 37°C. After incubation, the plate was centrifuged (250 g for 4 min) and then 50 μlof the supernatant were transferred to another plate. Thereafter,50 μl of Substrate Mix solution was added to each well (both supernatant and lysed cells) and incubated at room temperaturefor 30 min. The reaction was stopped with 50 μl of stop solution (1 M acetic acid) and the plates read at 490 nm. The ratio, released LDH: totalLDH, was then calculated as a measure of cell death.
TUNEL staining
SH-SY5Y cells were plated at a density of 1× 105 cells per well in a 24-well plate with cover slips for TUNEL staining. Following serum-starvation for 24h and pretreatment with PDGF-BB and/or gp120 for 16h at 37°C, cells were washed with PBS and fixed for 30 min with 4% paraformaldehyde at room temperature. The fixed cells were permeabilized with 1% triton X 100 for 30min followed by staining with TUNEL reaction mixture for 60 min according to the manufacturer’s instruction (Roche, Palo Alto, CA). Cover slips were mounted using Vectashield Mounting medium with DAPI (Invitrogen) as counterstaining and the slides were visualized under dark field using a fluorescence microscope. Six to eight images per treatment group were analyzed. Blind image analysis of tunnel positive cells in various treatments was done using Image J software (version 1.37, NIH, Bethesda, MD). Images from each slide were captured at 20x using a Nikon TE2000E microscope with a digital camera (Photometrics, Tucson, AZ). Threshold intensity for DAPI labeling was set to allow DAPI signals to be counted while eliminating false positive background staining. The number of DAPI positive cells was then quantified for all images. Similarly, threshold intensity for TUNEL labeling was set to allow TUNEL positive cells to be counted while eliminating false positive background staining. After quantifying the number of TUNEL positive cells for all the images, the percentage of TUNEL positive cells to the total number of DAPI positive cells was determined. The mean percentage (+/− SEM) of all images from each treatment group was reported.
Caspase-3 activity assay
Activity of caspase 3 was analyzed using the Caspase 3 Colorimetric Assay Kit from R&D Systems following manufacturer’s instructions. Briefly, SH-SY5Y cells were plated at 2×106 cells per well in 6 well plates. Following 6h treatment with PDGF and/or gp120, cells were lysed with 50μl lysis buffer for 10 min on ice. Following centrifugation (200g for 3–4min), 50μl of lysate was incubated with 50μl of 2X reaction buffer containing 0.5μl DTT and 5μl of the caspase-3 colorimetric substrate, DEVD-pNA. Following 2h of incubation at 37°C, caspase-3 protease activity was measured in a spectrophotometer at a wavelength of 405 nm. Absorbance was normalized to the protein concentration of each lysate, which was determined using the BCA Protein Assay Reagent from Pierce Chemical Co (Rockford, IL). Fold increase in caspase 3 activity in treated cells was calculated relative to the absorbance value obtained from the lysate of untreated cells. Each experiment consisted of seven replicates.
Western Blot Analyses
SH-SY5Y cells untreated or treated with PDGF and/or gp120 were lysed in lysis buffer (Sigma) containing protease inhibitors after 24 hrs post-treatment. Protein estimation in these samples was measured using the micro-BCA method (Pierce Chemical Co) protein assay kit. Western blot analyses were performed using primary antibodies against anti and pro-apoptotic proteins 1:200 Bcl-xL and Bax (Cell Signaling) on the same membrane, respectively. Western blots were also probed with antibodies recognizing phosphorylated forms of Akt (Cell Signaling, 1:500), Erk1/2 (Cell-signaling, 1:200) and β-actin (Sigma, 1:4000). The secondary antibodies used were horseradish peroxidase-conjugated anti rabbit (1:5000, Pierce Chemical Co) and detection was performed using the enhanced chemiluminescence system (Pierce Chemical Co). The ratio of Bcl-xL to Bax was then calculated following densitometric analyses of the intensity of bands.
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance with a post hoc Student t test. Results were judged statistically significant if p < 0.05 by analysis of variance.
Results
PDGF-B chain is down-regulated in neurons in the brains of macaques with SHIV-encephalitis
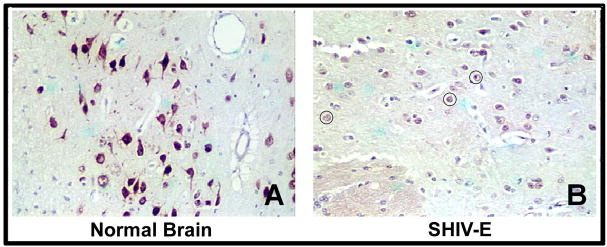
Before attempting to explore the role of PDGF-B chain in gp120-mediated toxicity, it was critical to first assess the expression of this factor in the brains of SHIV-infected macaques with and without encephalitis. We immunostained sections of basal ganglia region of brains of rhesus macaques with and without the CNS disease with the PDGF-B chain antibody. The basal ganglia was chosen for immunostaining due to the predilection of the virus for this region (Trotot and Gray, 1997). These studies presented in this report were focused on PDGF-B chain and not PDGF-A chain expression, since the former has been implicated as major player in neuronal fitness (Giacobini et al, 1993; Nikkhah et al, 1993). As shown in Figure. 1A, neurons in the brain sections from uninfected macaques stained strongly for the PDGF-B chain and maintained their architecture. In contrast, the corresponding encephalitic brain section demonstrated very weak staining for PDGF-B chain protein and this correlated with degeneration of neuronal processes (circled in Figure 1B).
Figure 1.
Decrease of PDGF-B chain in neurons of macaque brain with SHIV-encephalitis. Representative brain sections from the basal ganglia region of an uninfected (A) and SHIV-E (B) macaque immunostained with PDGF-B chain antibody. Red color represents PDGF-B chain positive cells. Degenerated neurons are shown in circle.
Gp120 down-regulates PDGF-B chain expression in neurons
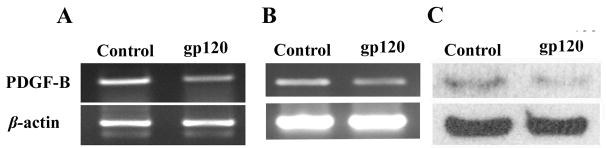
We next wanted to explore whether the down regulation of PDGF in neurons was result of direct effect of HIV viral protein, gp-120 a known neurotoxic agent (Bansal et al, 2000; Meucci and Miller, 1996). To test this we used a neuroblastoma cell-line, SH-SY5Y, differentiated into neurons by retinoic acid for 7–8 days followed by exposure to 200ng/ml of gp120. After 24hrs exposure, cells were collected for RNA and protein extraction to look for expression of PDGF-B chain by semi-quantitative RT-PCR and Western Blot analysis. As shown in figure 2A & C, treatment of cells with gp-120 resulted in inhibition of PDGF-B chain expression at both RNA and protein levels, respectively. Down regulation of PDGF-B chain by gp-120 was further confirmed in fetal rat primary neuronal cultures (Figure 2B).
Figure 2.
Down regulation of PDGF-B chain in neurons exposed to gp120. RT-PCR analysis for PDGF-B chain was done on total RNA extracted from SH-SY5Y cells (A) or primary rat neurons (B) exposed to 200ng/ml gp120 for 24h. C) Western blot analysis of PDGF-B chain on cell lysate obtained from SH-SY5Y cells treated with or without gp-120 (200ng/ml) for 24hrs. Figure shown is a representative of three different experiments.
Protective effect of PDGF-BB against gp120 mediated neurotoxicity
Since PDGF-B chain is produced by healthy neurons (Smits et al, 1991) and has neuroprotective properties (Hynds et al, 1997; Mohapel et al, 2005; Othberg et al, 1995; Sung et al, 2001; Tseng and Dichter, 2005), we hypothesized that pre-treatment of neurons with PDGF-BB protein could protect against gp120-mediated neurotoxicity.
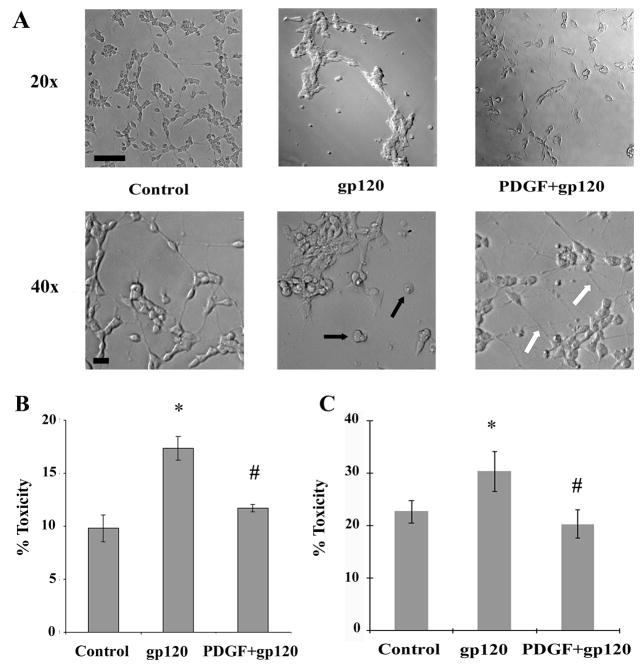
Treatment of SH-SY5Y cells with gp120 (200ng/ml) for 5 days resulted in increased cell death compared to untreated cells, as evidenced by rounding of cells with concomitant loss of the neurites (Figure. 3A). These findings were consistent with previous reports demonstrating neurotoxic role of gp120 (Bansal et al, 2000; Meucci and Miller, 1996). Pre-treatment of neuronal cells with PDGF (20ng/ml) for 30 min followed by exposure to gp120, however, resulted in protection of cells against gp120 toxicity (Figure. 3A).
Figure 3.
PDGF protects SH-SY5Y cells against gp120-mediated toxicity. A) Phase contrast microscopy of SH-SY5Y cells treated with gp120 (200ng/ml) and/or PDGF-BB (20ng/ml) for 5 days. Cells treated with gp120 alone showed rounding (as shown by black arrows) with shortened neurites. Pre-treatment of gp120-treated neurons with PDGF-BB resulted in maintenance of cellular architecture with extended neuronal processes (indicated by white arrows). Scale bars, 50 μM. Neuronal viability in the presence of gp120 and/or PDGF-BB. SH-SY5Y cells (B) and rat primary neuronal culture (C) were exposed to gp120 (200ng/ml) with or without pre-treatment with PDGF-BB (20ng/ml). Supernatant fluids and cellular extracts were collected in 24h for LDH assay. Data are presented as mean ± SEM from three independent experiments. Statistical analysis was performed using Student’s t-test. (*p < 0.01 gp120 vs control, #p<0.01 gp120 vs PDGF+gp120).
To further corroborate these findings the release of a stable cytosolic enzyme, lactate dehydrogenase (LDH) used as an indicator of tissue or cell damage was examined in both SH-SY5Y and in fetal rat primary neuronal cultures treated with gp120 in presence or absence of PDGF-BB. Gp120 exposure induced significant damage of SH-SY5Y cells (p value < 0.05 versus control) as shown by increase in percentage of cell death compared to untreated cells (Figure 3B). When PDGF was examined for the protective effect against gp120 induced cell death, it was clear that pre-treatment of neurons with PDGF-BB was able to suppress the cell damage (Figure 3B). The protection of neurons by PDGF from gp120 toxicity was also observed in fetal rat primary cortical neurons (Figure 3C).
PDGF-BB prevents gp120-mediated apoptosis
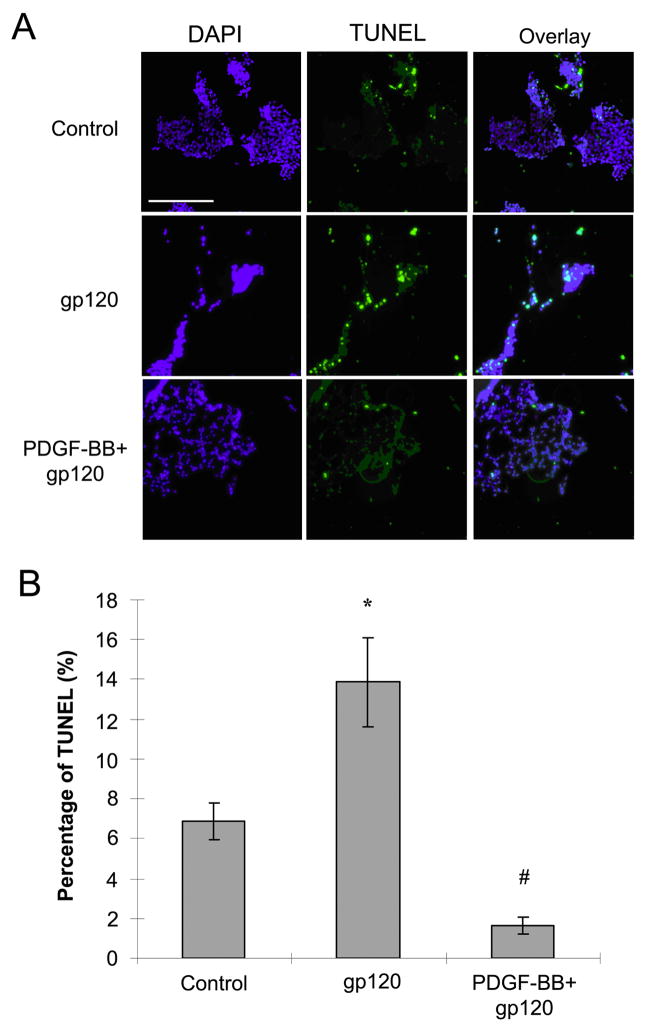
Since the primary mechanism by which gp120 induces neuronal death is in large part via the apoptotic pathway (Singh et al, 2004; Wright et al, 2007), it was hypothesized that PDGF protects neurons from gp120-mediated neurotoxicity by preventing apoptosis. TUNEL staining was done on SH-SY5Y cells treated or untreated with gp120 and/or PDGF-BB. As shown in figure 4, 15% of cells treated with gp120 were TUNEL positive at 18h and this increase in cell death was significantly reduced in the presence of PDGF-BB. Interestingly, the number of TUNEL positive cells treated with gp120 in presence of PDGF-BB was even less than the untreated cells (Figure 4).
Figure 4.
PDGF-BB mediates neuronal protection against gp120. A) SH-SY5Y cells treated with or without gp120 (200ng/ml) in the presence or absence of PDGF-BB (20ng/ml) for 18h were stained for TUNEL. Scale bar, 100 μM B) Histogram showing the percentage of TUNNEL positive SH-SY5Y cells relative to total number of neurons following various treatments. Six to eight images were randomly taken for counting positive signal in each experiment. Values are presented as mean ± SEM. (* p<0.01 gp120 vs control, #p<0.001 gp120 vs PDGF+gp120).
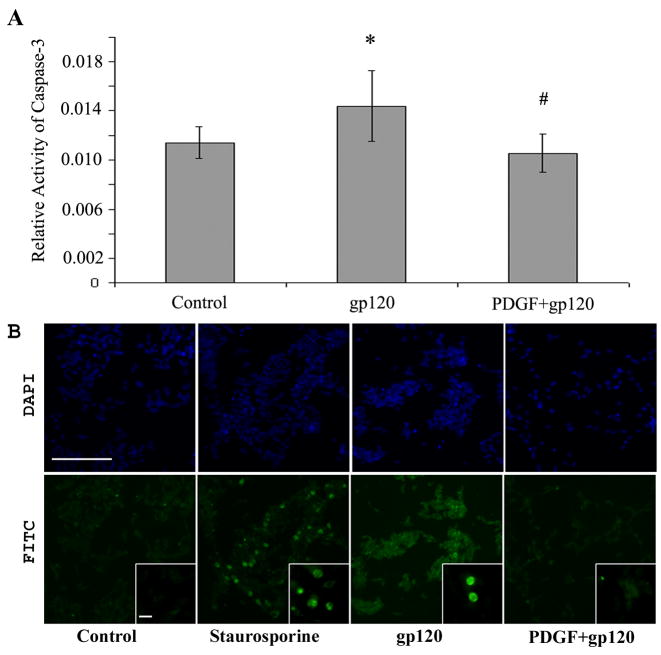
To corroborate the findings that PDGF-BB mediated protection involved abrogation of the apoptotic pathway, we monitored the activation of caspase-3 and stained the cells treated with gp120 and/or PDGF-BB with cleaved caspase-3, a protease essential for neuronal apoptosis (Wright et al, 2007). The activity of caspase-3 was measured by colorimetric assay in cell lysates treated with or without gp120 and/or PDGF-BB for 6h. In agreement with previous findings (Bansal et al, 2000; Meucci and Miller, 1996), gp120 treatment of SH-SY5Y cells resulted in a significant increase in active caspase-3 compared with untreated control cells (p value <0.05 versus control) (Figure 5A). Pre-treatment with PDGF-BB however, was able to abrogate gp120-mediated increase in caspase-3 activation. This was further confirmed by immunostaining for caspase-3. As shown in Figure 5B, cells treated with gp120 IIIB for 6 hrs demonstrated positive staining for activated caspase-3 whereas pretreatment with PDGF-BB resulted in reduction of gp120-induced caspase-3 activity.
Figure 5.
Neuronal apoptosis in SH-SY5Y cells exposed to gp120 with or without PDGF-BB pretreatment. Cells treated with or without gp120 IIIB in presence or absence of PDGF-BB pre-treatment were monitored for: A) caspase-3 activity assay in cell lysates and B) active caspase-3 staining by immunocytochemistry using the anti-cleaved caspase-3 antibody. The immunochemistry images showing nuclear (upper panel) and active caspase-3 staining (lower panel) were captured on Nikon inverted fluorescence microscope TE2000-E with a 20x and 100x (inset) objective (scale bar, 100 μM). Data for caspase-3 activity represents the mean ± SEM from seven independent experiments (*P<0.05 gp120 vs control, #P<0.01 gp120 vs PDGF+gp120).
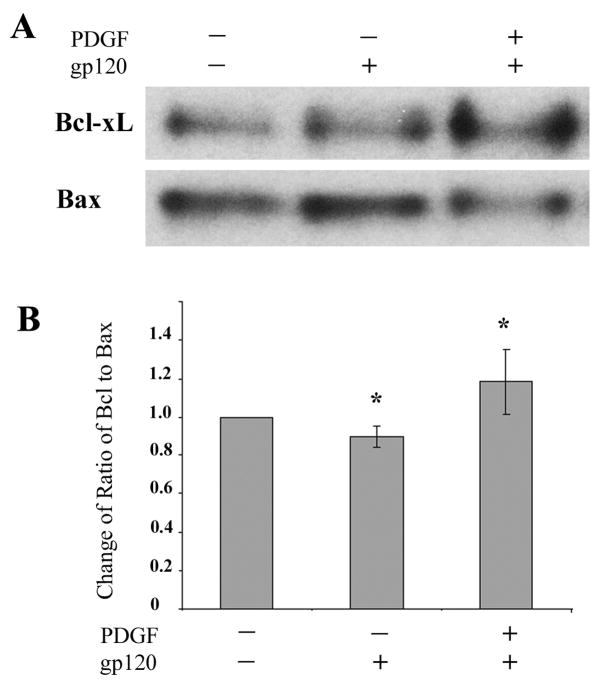
Reduction in neuronal injury in cells pre-treated with PDGF-BB was also confirmed by Western blot analyses of the anti and pro-apoptotic proteins Bcl-xL and Bax, respectively. As shown in Figure 6, neurons challenged with HIV-1 protein gp120 demonstrated a reduced band density ratio of Bcl-xL to Bax. Pre-treatment of gp120-exposed neurons to PDGF-BB, however, resulted in an increase in the anti-apoptotic gene expression, as demonstrated by an increase in Bcl-xL to Bax ratio.
Figure 6.
Ratio of anti- and pro-apoptotic markers in PDGF-mediated neuroprotection of SH-SY5Y cells. A) Cells treated with or without gp120 in the absence or presence of PDGF-BB pre-treatment were lysed and assayed for levels of anti- and pro-apoptotic markers Bcl-xL and Bax, respectively by Western blot analysis in the same membrane. B) Densitometry scan of the ratio of band intensities of Bcl-xL/Bax from three different western blot experiments (*p<0.05, treatment vs control).
Role of PI3Kinase/Akt pathway in PDGF-BB-mediated protection of neurons against gp120-toxicity
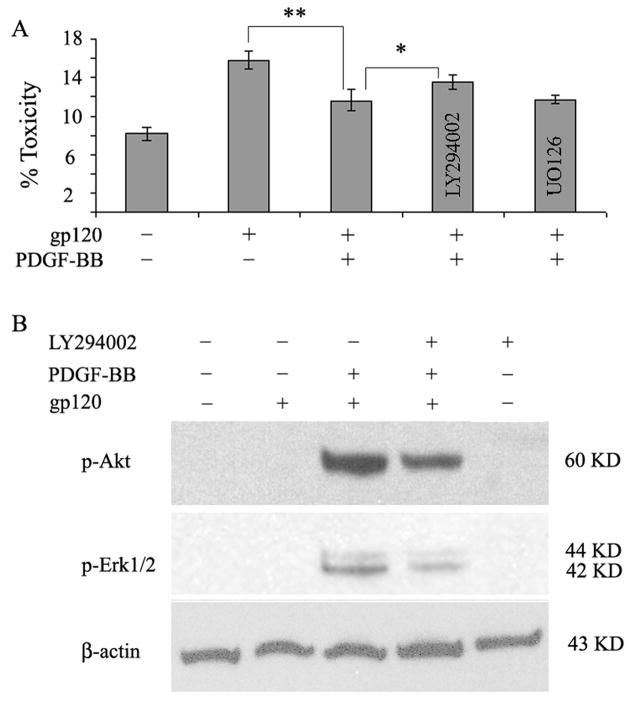
PI3K/Akt pathway has been implicated in regulating several important cellular processes, including survival, proliferation, and metabolism (Hanai et al, 2006; Romashkova and Makarov, 1999; Simakajornboon et al, 2001; Zhang et al, 2003). PI3 kinase pathway plays a critical role in PDGF signaling upstream of PDK1/2 and Akt/PKB Kinases (Ullrich and Schlessinger, 1990). Therefore it was hypothesized that activation of PI3K/Akt pathway by PDGF-BB may be involved in neuronal protection against gp120 toxicity. In order to confirm this, SH-SY5Y cells were pretreated with or without the inhibitor for PI3 Kinase for 1h prior to exposure of cells with PDGF-BB and/or gp120 as described above followed by analysis of cell death by LDH assay. As shown in figure 7, pre-treatment of cells with PI3K inhibitor partially abrogated PDGF-mediated protection against gp120 toxicity (Figure 7A P value < 0.05) compared with cells treated with PDGF-BB plus gp120. These findings led to the suggestion that PI3 Kinase plays a role in PDGF-mediated protection of neurons against gp120 toxicity.
Figure 7.
Involvement of PI3k/Akt pathway in PDGF-BB mediated protection against gp120 toxicity. A) LDH assay of SH-SY5Y cells pretreated with or without PI3K inhibitor (LY294002) or MEK1/2 inhibitor (U0126) followed by PDGF and/or gp120 exposure. Pre-treatment of cells with PI3K inhibitor resulted in significant (P value <0.05 PDGF+gp120+LY vs PDGF+gp120) reduction of PDGF-mediated protection. Pre-treatment with Erk1/2 inhibitor did not reverse the protective effect of PDGF. Data represents mean ± SEM from three independent experiments. B) Western blot analysis of lysates of phosphorylated Akt and Ek1/2 in SH-SY5Y cells pretreated with or without PI3K inhibitor (10uM LY294002) prior to PDGF and/or gp120 exposure. Blots were reprobed with antibody specific for β-actin for normalization.
Since Akt lies downstream of PI3Kinase, the next set of experiments were undertaken to examine whether the protection mediated by PDGF also involves Akt activation. Using western blot analyses it was demonstrated that SH-SY5Y cells pre-treated with the PI3K inhibitor followed by exposure to PDGF and/or gp120 had decreased activation of Akt compared with cells not treated with the inhibitor (Figure 7B).
Another downstream pathway employed by PDGF during wound healing and mitotic activity (Rubinfeld and Seger, 2005) is the Erk ½ MAP kinase. This kinase can be activated by PDGF both via PI3 Kinase dependent pathway and by the Ras/Raf MEK pathway. To examine the role of Erk1/2 in PDGF mediated protection against gp120 toxicity, cell lysates from SH-SY5Y cells pre-treated with PDGF followed by gp120 were assessed for phosphorylated Erk1/2 by western blot analysis. As shown in figure 7B, PDGF pre-treatment of gp120 treated SH-SY5Y cells resulted in increased activation of Erk1/2 compared with cells not treated with PDGF. To confirm that PDGF-mediated activation of Erk1/2 is via the PI3 Kinase pathway, pharmacological inhibitor of PI3K was used to pre-treat cells prior to PDGF and/or gp120 exposure. Cells treated with the inhibitor demonstrated a decrease in Erk1/2 activation (figure 7B). Despite the reduction in Erk1/2 activation by PI3K inhibitor, the ability of PDGF to mediate neuroprotection against gp120 remained unchanged in the presence of PI3K inhibitor (Figure 7A), thus suggesting that Erk1/2 activity is not involved in the PDGF mediated neuroprotection against gp120 toxicity.
Discussion
HIV-associated dementia (HAD) is the most severe form of HIV-related neuropsychiatric impairment. The syndrome is characterized by motor and behavioral dysfunctions leading to seizures, coma and death within months (Navia et al, 1986) and is associated with neuropathology involving HIV-1 proteins and activation of proinflammatory cytokine circuits. Although the incidence of HAD has decreased considerably in the era of antiretroviral therapy (ART), its prevalence is onthe rise with the emergence of a more subtle form of minor cognitive motor disorder (McArthur et al, 2003). HIV-E, the pathologic correlate of HAD reveals a broad spectrum of pathological changes including multifocal and sub-acute encephalitis, focal accumulation of macrophages & multinucleated giant cells, widespread reactive astrogliosis, cerebral cortical atrophy, loss of specific neuronal subpopulations, and diffuse white matter pallor (Bell, 1998; Everall et al, 1991; Gendelman et al, 1994; Nath, 1999; Price et al, 1988). Neuronal dysfunction/loss associated with HAD is often exemplified by loss of synapses, shortening of neurites and appearance of dendritic abnormalities (McArthur et al, 2005). Interestingly, unlike most other viral encephalopathies, neuronal loss associated with HIV-E is not due to direct viral infection of the neurons. One broad explanationfrequently advocated to explain this indirect killing of neurons is attributed to the toxicity mediated by the viral proteins including gp120 that are released fromvirus-infected cells in the CNS (Adamson et al, 1996; Brenneman et al, 1988; Chen et al, 2000; Dreyer et al, 1990; Hof et al, 1998; Kruman et al, 1998; New et al, 1997; Patel et al, 2000).
The HIV envelope glycoprotein gp120 has been demonstrated to have neurotoxic effects both in vitro and in vivo (Bachis and Mocchetti, 2006; Bansal et al, 2000; Kaul et al, 2001; Lipton et al, 1991; Meucci and Miller, 1996). Gp120 is able to induce apoptosis of neurons in cell culture, both in the presence and in the absence of microglia. Transgenic mice expressing gp120 in the brain manifest neuropathological features similar to those observed in AIDS patients (Mucke et al, 1995). Hence, it is both direct and indirect mechanisms that are responsible for the neuronal death associated with HIV-1 infection.
The broad spectrum of behavioral and neuropathological abnormalities observed in patients with HAD suggests a sensitive balance between neuroprotective and neurotoxic factors in the CNS. In the CNS neurotrophic factors are critical for protection of neurons during cellular injury/stress. PDGF, an important neurotrophic factor is known to attenuate excitotoxic death in cultured hippocampal neurons (Tseng and Dichter, 2005) and induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions (Mohapel et al, 2005). In this present study we report that PDGF neurotrophic activity can also be extended to neuroprotection against the HIV envelope gp120.
Dramatically reduced expression of PDGF-B chain was observed in neurons in the basal ganglia region of infected macaques with encephalitis compared with similar brain regions of macaques without the disease. In the diseased brains, not only was there a reduction in the expression of PDGF-B chain by neurons but there was also a concomitant alteration in the neuronal morphology that was, visible as rounded cells with retracted neurites.
Treatment of SH-SY5Y with viral neurotoxin gp120 resulted in decrease in PDGF-B chain expression with corresponding changes in cell morphology involving rounding of cells and loss of neurites. Interestingly, pre-treatment of neuronal cells with PDGF-BB abrogated neuronal retraction and resulted in reduced cell death compared with cells treated with gp120 alone. PDGF is known to mediate its neuroprotective effect through the induction of neurite outgrowth, as demonstrated in a variety of in vitro neuronal systems, including fetal rat and human dopaminergic neurons (Othberg et al, 1995), SH-SY5Y cells (Hynds et al, 1997) and hippocampal HiB5 cells (Sung et al, 2001). Other neurotrophic factors such as FGF and BDNF have earlier been documented to play a role in rescuing cells against gp120-mediated toxicity (Bachis and Mocchetti, 2005; Hashimoto et al, 2002).
Gp120-mediated toxicity of neurons involves apoptotic cell death (Singh et al, 2004). Neuronal cells pre-treated with PDGF-BB exhibited reduced apoptosis as was evident by TUNEL assay. Further corroboration of this phenomenon was also reflected in caspase-3 activity in cells treated with the neurotrophic factor prior to exposure to gp120. Our findings confirmed that gp120 activates caspase-3 and that PDGF-BB abrogated this effect, thus rescuing neurons from the apoptotic effects of gp120. These finds were further confirmed by assessing the relative levels of the anti- (Bcl-xL) and pro-apoptotic (Bax) gene products.
Many studies have implied the involvement of PDGF-mediated PI3K/Akt signaling pathway in cell survival (Simakajornboon et al, 2001; Wang et al, 2005; Zhang et al, 2005; Zhang et al, 2003). Using pharmacological inhibitors, our findings also suggest that PDGF exerts its protection against gp120 toxicity by the PI3K/Akt pathway. Erk1/2 that lies downstream of PI3K did not seem to be involved in the neuroprotection function of PDGF.
Further studies aimed at exploring the detailed signaling pathways and their downstream transcription factors that are involved in the neuroprotective role of PDGF are warranted. Delivery of PDGF in the CNS could have implications in the development of therapeutic strategies against HAD.
Acknowledgments
This work was supported by grants DA020392-01, MH62969-01, RR016443, MH-068212, and MH072355 from the National Institutes of Health.
We gratefully acknowledge the technical assistance of Fenglan Jia in preparation of this manuscript.
Reference List
- Adamson DC, Dawson TM, Zink MC, Clements JE, Dawson VL. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Mol Med. 1996;2:417–428. [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. Brain-derived neurotrophic factor is neuroprotective against human immunodeficiency virus-1 envelope proteins. Ann N Y Acad Sci. 2005;1053:247–257. doi: 10.1196/annals.1344.022. [DOI] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. Semisynthetic sphingoglycolipid LIGA20 is neuroprotective against human immunodeficiency virus-gp120-mediated apoptosis. J Neurosci Res. 2006;83:890–896. doi: 10.1002/jnr.20780. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Bell JE. The neuropathology of adult HIV infection. Rev Neurol (Paris) 1998;154:816–829. [PubMed] [Google Scholar]
- Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3:512–516. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- Biard-Piechaczyk M, Robert-Hebmann V, Richard V, Roland J, Hipskind RA, Devaux C. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120) Virology. 2000;268:329–344. doi: 10.1006/viro.1999.0151. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1991;1:163–175. doi: 10.1111/j.1750-3639.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Cliffer KD, Dougherty PM, Willis WD, Carlton SM. Changes in lectin, GAP-43 and neuropeptide staining in the rat superficial dorsal horn following experimental peripheral neuropathy. Neurosci Lett. 1991;131:249–252. doi: 10.1016/0304-3940(91)90625-4. [DOI] [PubMed] [Google Scholar]
- Cameron RS, Rakic P. Glial cell lineage in the cerebral cortex: a review and synthesis. Glia. 1991;4:124–137. doi: 10.1002/glia.440040204. [DOI] [PubMed] [Google Scholar]
- Campus G, Lumbau A, Bachisio SL. Caries experience and streptococci and lactobacilli salivary levels in 6–8-year-old Sardinians. Int J Paediatr Dent. 2000;10:306–312. doi: 10.1046/j.1365-263x.2000.00206.x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Huang Y, Zhao X, Skulsky E, Lin D, Ip J, Gettie A, Ho DD. Enhanced infectivity of an R5-tropic simian/human immunodeficiency virus carrying human immunodeficiency virus type 1 subtype C envelope after serial passages in pig-tailed macaques (Macaca nemestrina) J Virol. 2000;74:6501–6510. doi: 10.1128/jvi.74.14.6501-6510.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CB, Dikic I, Unutmaz D, Hill CM, Arthos J, Siani MA, Thompson DA, Schlessinger J, Littman DR. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer EB, Kaiser PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, Masliah E. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21:493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Lipton SA, Tardieu M, Bukrinsky MI, Nottet HS. The neuropathogenesis of HIV-1 infection. J Leukoc Biol. 1994;56:389–398. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- Giacobini MM, Almstrom S, Funa K, Olson L. Differential effects of platelet-derived growth factor isoforms on dopamine neurons in vivo: -BB supports cell survival, -AA enhances fiber formation. Neuroscience. 1993;57:923–929. doi: 10.1016/0306-4522(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Hanai Y, Tokuda H, Ohta T, Matsushima-Nishiwaki R, Takai S, Kozawa O. Phosphatidylinositol 3-kinase/Akt auto-regulates PDGF-BB-stimulated interleukin-6 synthesis in osteoblasts. J Cell Biochem. 2006;99:1564–1571. doi: 10.1002/jcb.21007. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Sagara Y, Langford D, Everall IP, Mallory M, Everson A, Digicaylioglu M, Masliah E. Fibroblast growth factor 1 regulates signaling via the glycogen synthase kinase-3beta pathway. Implications for neuroprotection. J Biol Chem. 2002;277:32985–32991. doi: 10.1074/jbc.M202803200. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398:284–290. doi: 10.1006/abbi.2001.2707. [DOI] [PubMed] [Google Scholar]
- Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O’Brien WA, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Liang M, Hoxie J, Greenberg M, Brass LF, Orsini MJ, Taub D, Horuk R. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J Immunol. 1998a;160:877–883. [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998b;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Hof PR, Lee PY, Yeung G, Wang RF, Podos SM, Morrison JH. Glutamate receptor subunit GluR2 and NMDAR1 immunoreactivity in the retina of macaque monkeys with experimental glaucoma does not identify vulnerable neurons. Exp Neurol. 1998;153:234–241. doi: 10.1006/exnr.1998.6881. [DOI] [PubMed] [Google Scholar]
- Hynds DL, Burry RW, Yates AJ. Gangliosides inhibit growth factor-stimulated neurite outgrowth in SH-SY5Y human neuroblastoma cells. J Neurosci Res. 1997;47:617–625. doi: 10.1002/(sici)1097-4547(19970315)47:6<617::aid-jnr7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol. 1999;163:1636–1646. [PubMed] [Google Scholar]
- Kozak SL, Kuhmann SE, Platt EJ, Kabat D. Roles of CD4 and coreceptors in binding, endocytosis, and proteolysis of gp120 envelope glycoproteins derived from human immunodeficiency virus type 1. J Biol Chem. 1999;274:23499–23507. doi: 10.1074/jbc.274.33.23499. [DOI] [PubMed] [Google Scholar]
- Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Backstrom G, Hellstrom M, Bostrom H, Li H, Soriano P, Betsholtz C, Heldin CH, Alitalo K, Ostman A, Eriksson U. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- Meucci O, Miller RJ. gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. J Neurosci. 1996;16:4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132:767–776. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Campbell IL. Transgenic models to assess the neuropathogenic potential of HIV-1 proteins and cytokines. Curr Top Microbiol Immunol. 1995;202:187–205. doi: 10.1007/978-3-642-79657-9_13. [DOI] [PubMed] [Google Scholar]
- Nath A. Pathobiology of human immunodeficiency virus dementia. Semin Neurol. 1999;19:113–127. doi: 10.1055/s-2008-1040830. [DOI] [PubMed] [Google Scholar]
- Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- New DR, Ma M, Epstein LG, Nath A, Gelbard HA. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol. 1997;3:168–173. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- Nikkhah G, Odin P, Smits A, Tingstrom A, Othberg A, Brundin P, Funa K, Lindvall O. Platelet-derived growth factor promotes survival of rat and human mesencephalic dopaminergic neurons in culture. Exp Brain Res. 1993;92:516–523. doi: 10.1007/BF00229041. [DOI] [PubMed] [Google Scholar]
- Othberg A, Odin P, Ballagi A, Ahgren A, Funa K, Lindvall O. Specific effects of platelet derived growth factor (PDGF) on fetal rat and human dopaminergic neurons in vitro. Exp Brain Res. 1995;105:111–122. doi: 10.1007/BF00242187. [DOI] [PubMed] [Google Scholar]
- Patel CA, Mukhtar M, Pomerantz RJ. Human immunodeficiency virus type 1 Vpr induces apoptosis in human neuronal cells. J Virol. 2000;74:9717–9726. doi: 10.1128/jvi.74.20.9717-9726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Brew B, Sidtis J, Rosenblum M, Scheck AC, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Price RW, Sidtis JJ, Brew BJ. AIDS dementia complex and HIV-1 infection: a view from the clinic. Brain Pathol. 1991;1:155–162. doi: 10.1111/j.1750-3639.1991.tb00655.x. [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31:151–174. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, Becker JT, Cohen B, McArthur JC. HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- Sanders VJ, Everall IP, Johnson RW, Masliah E. Fibroblast growth factor modulates HIV coreceptor CXCR4 expression by neural cells. HNRC Group. J Neurosci Res. 2000;59:671–679. doi: 10.1002/(SICI)1097-4547(20000301)59:5<671::AID-JNR10>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Simakajornboon N, Szerlip NJ, Gozal E, Anonetapipat JW, Gozal D. In vivo PDGF beta receptor activation in the dorsocaudal brainstem of the rat prevents hypoxia-induced apoptosis via activation of Akt and BAD. Brain Res. 2001;895:111–118. doi: 10.1016/s0006-8993(01)02054-6. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by human immunodeficiency virus-1 Tat and gp120: Differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits A, Kato M, Westermark B, Nister M, Heldin CH, Funa K. Neurotrophic activity of platelet-derived growth factor (PDGF): Rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci U S A. 1991;88:8159–8163. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer DC, Price RW. Human immunodeficiency virus and the central nervous system. Annu Rev Microbiol. 1992;46:655–693. doi: 10.1146/annurev.mi.46.100192.003255. [DOI] [PubMed] [Google Scholar]
- Sui Y, Potula R, Dhillon N, Pinson D, Li S, Nath A, Anderson C, Turchan J, Kolson D, Narayan O, Buch S. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;164:1557–1566. doi: 10.1016/S0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JY, Lee SY, Min DS, Eom TY, Ahn YS, Choi MU, Kwon YK, Chung KC. Differential activation of phospholipases by mitogenic EGF and neurogenic PDGF in immortalized hippocampal stem cell lines. J Neurochem. 2001;78:1044–1053. doi: 10.1046/j.1471-4159.2001.00491.x. [DOI] [PubMed] [Google Scholar]
- Trotot PM, Gray F. Diagnostic Imaging Contribution in the Early Stages of HIV Infection of the Brain. Neuroimaging Clin N Am. 1997;7:243–260. [PubMed] [Google Scholar]
- Tseng HC, Dichter MA. Platelet-derived growth factor-BB pretreatment attenuates excitotoxic death in cultured hippocampal neurons. Neurobiol Dis. 2005;19:77–83. doi: 10.1016/j.nbd.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Ullrich CK, Groopman JE, Ganju RK. HIV-1 gp120- and gp160-induced apoptosis in cultured endothelial cells is mediated by caspases. Blood. 2000;96:1438–1442. [PubMed] [Google Scholar]
- Vogelbaum MA, Tong JX, Higashikubo R, Gutmann DH, Rich KM. Transfection of C6 glioma cells with the bax gene and increased sensitivity to treatment with cytosine arabinoside. J Neurosurg. 1998a;88:99–105. doi: 10.3171/jns.1998.88.1.0099. [DOI] [PubMed] [Google Scholar]
- Vogelbaum MA, Tong JX, Rich KM. Developmental regulation of apoptosis in dorsal root ganglion neurons. J Neurosci. 1998b;18:8928–8935. doi: 10.1523/JNEUROSCI.18-21-08928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gang Zhang Z, Lan Zhang R, Chopp M. Activation of the PI3-K/Akt pathway mediates cGMP enhanced-neurogenesis in the adult progenitor cells derived from the subventricular zone. J Cereb Blood Flow Metab. 2005;25:1150–1158. doi: 10.1038/sj.jcbfm.9600112. [DOI] [PubMed] [Google Scholar]
- Wright KM, Vaughn AE, Deshmukh M. Apoptosome dependent caspase-3 activation pathway is non-redundant and necessary for apoptosis in sympathetic neurons. Cell Death Differ. 2007;14:625–633. doi: 10.1038/sj.cdd.4402024. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou R, Xiang G. Stepholidine protects against H2O2 neurotoxicity in rat cortical neurons by activation of Akt. Neurosci Lett. 2005;383:328–332. doi: 10.1016/j.neulet.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Zhang SX, Gozal D, Sachleben LR, Jr, Rane M, Klein JB, Gozal E. Hypoxia induces an autocrine-paracrine survival pathway via platelet-derived growth factor (PDGF)-B/PDGF-beta receptor/phosphatidylinositol 3-kinase/Akt signaling in RN46A neuronal cells. Faseb J. 2003;17:1709–1711. doi: 10.1096/fj.02-1111fje. [DOI] [PubMed] [Google Scholar]