Abstract
Increased lipid peroxidation is shown to be an early event of AD. However, it is not clear whether and how increased lipid peroxidation might lead to amyloidogenesis, a hallmark of AD. Gpx4 is an essential antioxidant defense enzyme that protects an organism against lipid peroxidation. Gpx4+/- mice show increased lipid peroxidation in brain, as evidenced by their elevated levels of 4-HNE. To understand the role of lipid peroxidation in amyloidogenesis, we studied secretase activities in Gpx4+/- mice as a function of age. Both young (6-month) and middle-aged (17- to 20- month) Gpx4+/- mice had higher levels of β-secretase activity than their age-matched wildtype controls, and the increased β-secretase activity in Gpx4+/- mice was a result of upregulation of BACE1 expression at the protein level. The high level of BACE1 protein led to increased endogenous Aβ1-40 in middle-aged Gpx4+/- mice. We further studied amyloidogenesis in APPGpx4+/- mice. Our data indicate that APPGpx4+/- mice had significantly increased amyloid plaque burdens and increased Aβ1-40 and Aβ1-42 levels compared to APPGpx4+/+ mice. Therefore, our results indicate that increased lipid peroxidation leads to increased amyloidogenesis through upregulation of BACE1 expression in vivo, a mechanism that may be important in pathogenesis of AD at early stages.
Keywords: Lipid peroxidation, Alzheimer’s disease, BACE1, glutathione peroxidase 4, Aβ, APP transgenic mice
Introduction
Oxidative stress in the central nervous system (CNS) predominantly manifests as lipid peroxidation because of the brain’s high metabolism and its high concentration of polyunsaturated fatty acids (PUFA) that are particularly susceptible to oxidation (Park and Floyd 1992;Mattson 2004). Accumulating data suggest that increased lipid peroxidation is an early event of Alzheimer’s disease (AD). For example, Pratico et al. (Pratico et al. 2002) reported that subjects with mild cognitive impairment (MCI) had significantly elevated levels of F2-isoprostanes in their cerebrospinal fluid, plasma and urine, and increased levels of 4-hydroxynonenal (4-HNE) and acrolein were also found in brain regions of subjects with MCI and patients with early AD by Williams et al. (2006) and Butterfield et al. (2006). However, the mechanistic role that increased lipid peroxidation plays in the pathogenesis of AD at early stages is still unclear.
β-amyloid (Aβ) plays a central role in AD pathogenesis (Hardy and Selkoe 2002), and oxidative stress is shown to increase the production of Aβ peptides in cell lines and AD animal models (Misonou et al. 2000;Paola et al. 2000;Li et al. 2004a). However, whether increased Aβ production is mediated by lipid peroxidation is unclear. β-site amyloid precursor protein cleavage enzyme 1 (BACE1) is a key rate limiting enzyme identified in the production of Aβ (Vassar 2004). The level and activity of BACE1 are shown to increase in AD brain and are proposed to drive Aβ overproduction in AD (Li et al. 2004b). Studies also show that BACE1 activity and amyloidogenesis are increased by traumatic brain injury, ischemia, and inhibition of mitochondria respiration, indicating that BACE1 expression is inducible by injury/stress (Blasko et al. 2004;Wen et al. 2004;Velliquette et al. 2005). Neuronal cells exposed to oxidizing agents such as H2O2 and 4-HNE also show increased BACE1 expression (Tamagno et al. 2002;Tamagno et al. 2005). However, although injury and stress could lead to increased lipid peroxidation, whether increased lipid peroxidation is responsible for upregulation of BACE1 expression in brain is not known.
Glutathione peroxidase 4 (Gpx4) is a ubiquitously expressed peroxidase that can directly reduce lipid hydroperoxides (LOOHs) in membrane (Girotti 1998). Because of its high affinity for membrane LOOHs, Gpx4 is an essential antioxidant defense enzyme that protects an organism against lipid peroxidation (Imai and Nakagawa 2003;Ran et al. 2003;Ran et al. 2004;Ran et al. 2006). The importance of Gpx4 in antioxidant defense is supported by the lethal phenotype of Gpx4 homozygous knockout mice (Yant et al. 2003;Imai et al. 2003). The Gpx4 heterozygous knockout (Gpx4+/-) mouse is a mouse model with deficiency in Gpx4 but without alterations in other major antioxidant defense enzymes such as Gpx1 and catalase (Ran et al. 2003;Ran et al. 2007). Thus, in the Gpx4+/- mouse, lipid peroxidation is the primary form of oxidative stress (Ran et al. 2003;Ran et al. 2007).
To understand the potential roles of increased lipid peroxidation in amyloidogenesis, we studied secretase activities and BACE1 regulation in Gpx4+/- mice and wildtype (Wt) mice as a function of age. Our results indicate that increased lipid peroxidation in Gpx4+/- mice results in upregulation of BACE1 expression in vivo, which in turn leads to increased level of endogenous Aβ1-40 in middle-aged Gpx4+/- mice. In addition, our results indicate that APP transgenic mice with deficiency in Gpx4 have increased amyloidogenesis, as evidenced by their increased amyloid plaque burden and increased levels of Aβ1-40 and Aβ1-42. Thus, our results indicate that increased lipid peroxidation leads to increased level of amyloidogenesis in vivo through upregulation of BACE1 expression.
Materials and Methods
Animals
Gpx4+/- mice, mice heterozygous for a targeted mutation in the Gpx4 gene, were originally generated in the 129 background (Yant et al. 2003). The mice used in this study were backcrossed 10 times to C57BL/6 mice. The Gpx4+/- mice were generated by breeding male Gpx4+/- mice to female C57BL/6 mice purchased from Jackson Laboratories (Bar Harbor, ME). The mice were genotyped at 4-5 weeks of age by PCR analysis of DNA obtained from tail clips as previously described (Yant et al. 2003). The mice were maintained under barrier conditions in a temperature-controlled environment. Male mice at ages 17 to 20 months were used as middle-aged mice, while 6-month-old male mice were used as young mice. Four groups of young Wt mice, young Gpx4+/- mice, middle-aged Wt mice and middle-aged Gpx4+/- mice were used in the study. The group size is 5 to 6 animals per group.
APP transgenic mice (Tg2576 mic) (Hsiao et al. 1996) were obtained from Taconic (Taconic, NY). Male Tg2576 mice were used to cross to female Gpx4+/- mice, and offspring were genotyped at weaning using tail DNA by a PCR based method. Male mice that were APP positive with two allele of functional Gpx4 gene (APPGpx4+/+) and mice that were APP positive with only one allele of functional Gpx4 (APPGpx4+/-) were included in this study. The ages of the mice were 11 to 12 months, and group size is 4 to 5 animals per group.
All procedures for handling the mice in this study were reviewed and approved by the IACUC (Institutional Animal Care and Use Committee) of University of Texas Health Science Center at San Antonio (UTHSCSA) and the IACUC of South Texas Veterans Health Care System, Audie Murphy VA Hospital.
Assay for 4-HNE adducts
Levels of 4-HNE protein adducts were measured by Western blots. Briefly, cerebral cortex tissues were homogenized in a RIPA buffer (20 mM Tris, pH7.4, 0.25 M NaCl, 1 mM EDTA, 0.5% NP-40, 50 mM sodium fluoride) supplemented with protease inhibitors. Equal amounts of total proteins (20 μg) were separated by 4-20% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked for 1 hour in 5% nonfat dry milk and were incubated for 2 hours at room temperature with an anti-4-HNE monoclonal antibody (MAB3249, R&D Systems, Inc., Minneapolis, MN). The membrane was further incubated with anti-mouse IgG. The bands were visualized using the ECL Kit (Amersham-Pharmacia, Piscataway, NJ). 4-HNE adducts bands were scanned and quantified using ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
RNA isolation and Real-Time PCR (RT-PCR)
Total RNA was isolated from cerebral cortex tissues using the TRIZOL method with tri-reagent (Invitrogen, Carlsbad, CA). Total RNA (1 μg) from individual preparations was reverse-transcribed using random hexamers and Multi-Scribe Reverse Transcriptase (Applied Biosystems, Foster City, CA). Quantitative PCR was performed with the SYBR Green PCR Master mix (Applied Biosystems) using an ABI Prism 7500 sequence detector and under thermal cycling conditions of pre-incubation (50°C, 2 min); DNA polymerase activation (95°C, 10 min); and 40 PCR cycles for 15 s at 95°C, and 1 min at 60°C. The following PCR primers used for quantification: BACE1 (forward: GGC AGT CTC TGG TAC ACA CC; reverse: ACT CCT TGC AGT CCA TCT TG), Gpx4 (forward: TGC ATC GTC ACC AAC GTG GC; reverse: CTT CAC CAC GCA GCC GTT CT), APP (forward: AGG CCT CAT CAT GTG TTC AA; reverse: CGG AGG TGT GTC ATA ACC TG). The levels of mRNA were normalized levels of beta-actin mRNA to control for input RNA. Levels of mRNA are presented as means ± SEM, relative to the data obtained from young Wt mice.
Measurement of secretase activities
Activity levels of γ-secretase and β-secretase were determined using γ-Secretase Activity Kit and β-Secretase Activity Kit from R&D Systems, Inc. (Minneapolis, MN). In these kits, secretase activities were determined using γ- and β- secretase-specific peptides conjugated to the reporter molecules EDANS and DABCYL. In the uncleaved form, the fluorescent emissions from EDANS are quenched by the physical proximity of the DABCYL moiety, which exhibits maximal absorption at the same wavelength (495 nm). Cleavage of the peptide by the secretases physically separates the EDANS and DABCYL, leading to the release of a fluorescent signal. The level of secretase enzymatic activity is proportional to the fluorometric reaction. Briefly, cortical tissues were homogenized in the buffers provided by the kits, and secretase activities in the tissue homogenates were determined according to the protocol provided by the manufacture. The secretase activities were expressed as fluorescent units per mg of total protein.
Assays for BACE1 protein, APP protein, and cell signaling proteins
Cerebral cortex tissues were homogenized in a RIPA buffer with protease inhibitors and phosphotases inhibitors. Equal amounts of total protein (40 μg) were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. The BACE1 protein levels were determined using a BACE1 specific monoclonal antibody (MAB5308, Chemicon International, Temecula, CA). The APP protein levels were measured using an APP-specific antibody from Zymed Laboratories, Inc. (South San Francisco, CA).
The levels of phosphorylated signaling proteins were determined using antibodies specific to phosphorylated proteins and total proteins obtained from Cell Signaling Technology, Inc. (Danvers, MA). The following antibodies were used: anti-phospho-SAPK/JNK (Thr183/Tyr185) (Cat# 9251); anti-SAPK/JNK (Cat# 9252); anti-phospho-p38 (Thr180/Tyr182) (Cat# 9211); anti-p38(Cat# 9212); anti-phospho-Akt (ser473) (Cat#4058); anti-Akt (Cat# 9272); anti-phospho-p44/42(Erk1/Erk2) (Thr202/Tyr204) (Cat#9102); anti-p44/42 (Erk1/Erk2) (Cat#9102).
Measurement of endogenous murine Aβ levels
Levels of endogenous mouse Aβ in the cerebral cortex of Gpx4+/- and Wt mice were measured using Aβ [1-40] ELISA KIT (MS, KMB3481) from Invitrogen (Carlsbad, CA). Briefly, 8x cold 5 M guanidine HCl / 50 mM Tris HCl buffer was added to the brain tissues, and the tissues were homogenized with a polytron tissue homogenizer. The tissue homogenates were mixed at room temperature for 3 - 4 hours before being diluted with cold Reaction Buffer provided in the kit. Aβ levels in the homogenates were subsequently measured using the protocol provided by the manufacture. A standard curve was generated using Aβ standards at the same time. The Aβ levels in the tissue homogenates were calculated based on the standard curve and expressed as pg per mg of total protein.
Measurement of amyloid plaque burden and Aβ in APP transgenic mice
Mice were overdosed with pentobarbital (100 mg/kg) intraperitoneally and perfused transcardially for 5 minutes with phosphate-buffered saline. One hemibrain was used for histology and the other was used for Aβ measurement.
For immunohistochemistry staining and image analysis, hemibrains were fixed by immersion in 4% paraformaldehyde in 0.1 M PBS (pH7.4) at 4°C overnight. After equilibrating, the hemibrains were sectioned using a frozen microtone (HM440E, Microm) at 50 micron-thick. The sections were pretreated with 50% formic acid for 5 min for antigen retrieval and 0.3% H2O2 in 50% methanol for 30 min to eliminate endogenous peroxidase activity in the tissues. The sections were permeablized with 0.3% Triton for 10 min. Subsequently, sections were incubated with an anti-Aβ antibody (6E10) (1:1,600 dilution) at 4°C overnight. Sections were incubated further with anti-mouse secondary antibody, reacted with horseradish-peroxidase-avidin-biotin complex (Vector Laboratories) and with DAB substrate (Vector Laboritories). The sections were subsequently stained with cresyl violet. Plaque burdens were determined as described by Lim et al (2001). Briefly, image analysis was performed on four similar coronal sections from APPGpx4+/+ and APPGpx4+/- mice. Light microscopic images of hippocampus were captured using a Nikon E400 microscope with Spot digital camera imaging system at 20× optical magnification. The area of Aβ immuoreactive structures and the area of brain region were analyzed using NIH Image software. The area of Aβ immuoreactive structures per hemibrain section was totaled, and plaque burden was calculated by dividing the total area of Aβ immuoreactive structures by the total area of brain region analyzed.
The SDS soluble and SDS insoluble Aβ peptides were extracted using a protocol described by Kawarabayashi et al (2001). Briefly, the hemibrains were re-suspended in 2% SDS with protease inhibitors (100 mg/ml), sonicated, and centrifuged at 100,000 × g for 1h at 4°C. The supernatants containing SDS soluble Aβ peptides were removed. The pellets were resuspended in 70% formic acid, sonicated again and centrifuged at 100,000 × g for 1h at 4°C. The supernatants containing SDS insoluble Aβ (formic acid soluble) were removed afterwards. Aβ peptides (Aβ1-40 and Aβ1-42) levels in the supernatants of SDS and formic acid extraction were determined using human Aβ1-40 immunoassay kits and human Aβ1-42 immunoassay kits from BioSource International (Camarillo, CA). The supernatants containing SDS soluble Aβ were first diluted 1: 20 in SD buffer (sample dilution buffer) provided by the kits. Serial dilutions were subsequently made in SD buffer for Aβ measurement. The supernatants containing SDS insoluble Aβ were neutralized by 1:20 dilution in 1 M Tris (pH 11), and then diluted in SD buffer for Aβ measurement. The levels of Aβ peptides were expressed as picomolar per gram of wet weight brain tissue (pmol/g).
Statistical Analysis
Data are expressed as mean ± SEM as indicated in the figures. Results were statistically analyzed using two-way ANOVA or Student’s t test when appropriate. Statistical significance is set to a minimum of p<0.05.
Results
Increased lipid peroxidation in the brain of Gpx4+/- mice
To study the role of increased lipid peroxidation in amyloidogenesis as a function of age, we used Gpx4+/- mice and Wt mice that were 6 months of age and 17 to 20 months of age in this study. The age of 17 to 20 months was chosen because this is the age before a significant amount of death occurs in this strain of mice (Ran et al. 2007). Because the median lifespan of this strain of mice is about 34 months (Ran et al. 2007), we designated mice that were 17 to 20 months of age as middle-aged mice and mice that were 6 months of age as young mice.
To determine whether Gpx4 levels change with age, we measured Gpx4 mRNA levels in the cerebral cortex of young Wt mice, young Gpx4+/- mice, middle-aged Wt mice and middle-aged Gpx4+/- mice by quantitative RT-PCR. As shown in Figure 1A, both young and middle-aged Gpx4+/- mice showed reduced levels of Gpx4 mRNA. Young and middle-aged Gpx4+/- mice also had reduced Gpx4 protein (Figure 1B). However, no age-associated change in Gpx4 expression was observed in either the Wt mice or the Gpx4+/- mice.
Figure 1. Levels of lipid peroxidation in young and middle-aged Wt and Gpx4+/- mice.
A. Gpx4 mRNA levels in cerebral cortex tissues from young and middle-aged Gpx4+/- mice and control wildtype (Wt) mice were determined by quantitative RT-PCR. The mean value obtained from young Wt mice was artificially assigned as 1, and the relative values were presented as mean ± SEM. n=5. *: P<0.05.
B. Gpx4 protein levels in cerebral cortex tissues from young and middle-aged Gpx4+/- mice and Wt mice were determined by Western blots. The levels of β-actin protein were used as loading controls. Y-Wt: young Wt mice; Y-Gpx4+/-: young Gpx4+/- mice; M-Wt: middle-aged Wt mice; M-Gpx4+/-: middle-aged Gpx4+/- mice.
C. Total proteins from cerebral cortex tissues of middle-aged Wt mice (M-Wt) and middle-aged Gpx4+/- mice (M-Gpx4+/-) were separated by SDS-PAGE, transferred to nitrocellulose membrane and probed with an anti-4-HNE antibody. Arrows indicate major proteins with 4-HNE adducts.
D. 4-HNE adducts in cortex proteins from the four groups of young and middle-aged Wt and Gpx4+/- mice were determined by Western blots and quantified as described in Methods. The mean value of 4-HNE adducts obtained from young Wt mice was artificially assigned as 1, and the relative values are presented as mean ± SEM. *: P<0.05. n= 5.
We previously showed that old Gpx4+/- mice (26 to 29 months) had increased F4-neuroprostanes in their brains, which is indicative of increased lipid peroxidation (Ran et al. 2007). 4-HNE is an α,β unsaturated aldehyde produced during oxidation of ω-6 PUFA in membrane. 4-HNE can react with proteins to form 4-HNE adducts, and the level of 4-HNE adducts is also indicative of lipid peroxidation (Neely et al. 1999;Wataya et al. 2002;Cutler et al. 2004). To confirm if the young and middle-aged Gpx4+/- mice used in this study had increased lipid peroxidation in their brains, we measured levels of 4-HNE adducts in cerebral cortex proteins by Western blots. Figure 1C is a Western blot showing 4-HNE protein adducts in cortical protein from middle-aged Gpx4+/- mice and middle-aged Wt mice, and Figure 1D shows the quantified results of 4-HNE protein adducts levels in cortical proteins from the four groups of mice. Middle-aged Gpx4+/- mice had significantly increased 4-HNE adducts compared to both middle-aged Wt mice and young Gpx4+/- mice. In addition, young Gpx4+/- mice also had a significant increase in 4-HNE protein adducts over young Wt mice.
γ-secretase activity is augmented by age but not by lipid peroxidation
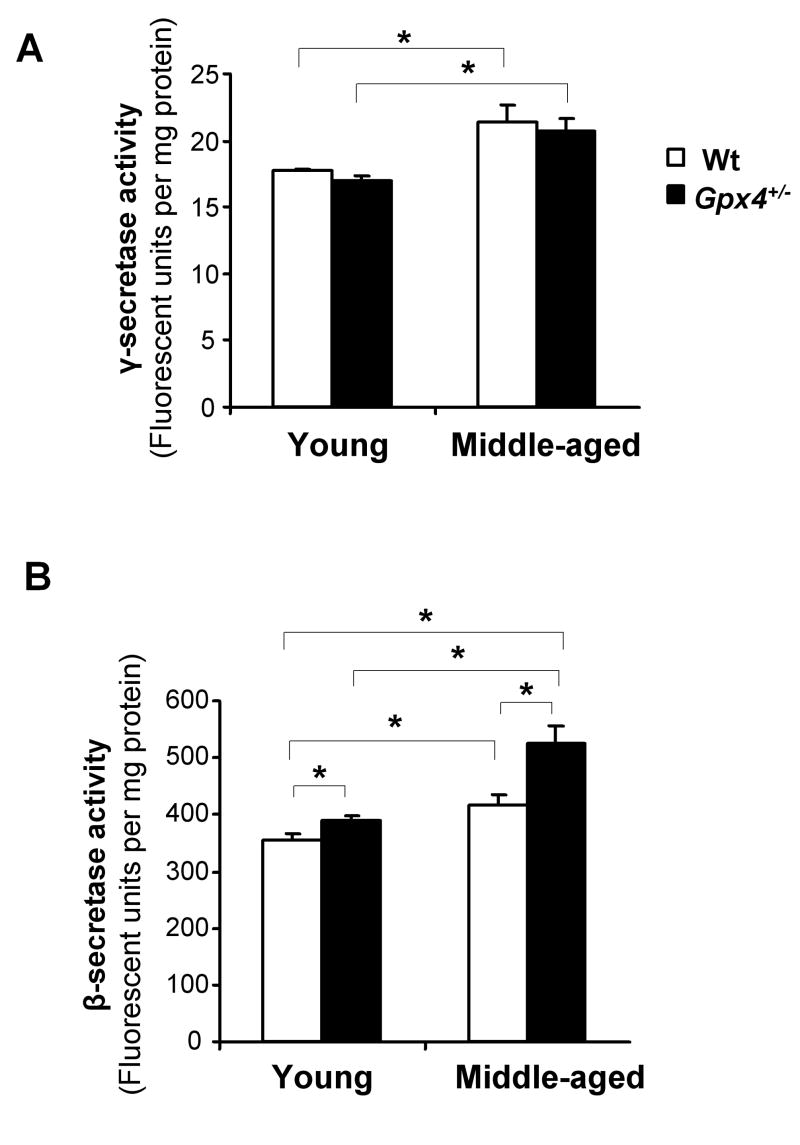
Aβ is derived from sequential cleavage of APP by β-secretase and γ-secretase. To determine whether lipid peroxidation and age affected γ-secretase activity, we measured γ-secretase activity in cortex tissues from the four groups of mice. As shown in Figure 2A, middle-aged Wt mice and middle-aged Gpx4+/- mice had higher γ-secretase activities than young Wt mice and young Gpx4+/- mice, respectively. However, no difference in γ-secretase activity was observed between middle-aged Wt mice and middle-aged Gpx4+/- mice or between young Wt mice and young Gpx4+/- mice. Therefore, γ-secretase activity increased with age, but was not affected by the level of lipid peroxidation.
Figure 2. γ- and β-secretase activities in cortex of young and middle-aged Wt and Gpx4+/- mice.
Total γ-secretase activity (A) and β-secretase activity (B) in cerebral cortex tissues from young and middle-aged Wt and Gpx4+/- mice were determined using secretase activity kits, as described in Methods. The data are presented as mean ± SEM. *: P<0.05. n= 5
β-secretase activity is augmented by lipid peroxidation
We also measured β-secretase activity in cortical tissues from young and middle-aged Wt and Gpx4+/- mice. As shown in Figure 2B, middle-aged Gpx4+/- mice had the highest level of β-secretase activity among the four groups of mice. In addition, both young and middle-aged Gpx4+/- mice had increased β-secretase activity compared to their age-matched Wt controls. Because young and middle-aged Gpx4+/- mice also had increased 4-HNE levels compared to their age-matched Wt controls (Figure 1D), this data indicate that β-secretase activity was augmented by increased lipid peroxidation.
Upregulation of BACE1 protein by lipid peroxidation
BACE1 has been identified as the β-secretase in Aβ production (Vassar 2004). To determine whether increased β-secretase activity in Gpx4+/- mice was a result of elevated BACE1 expression, we measured BACE1 proteins by Western blots. As shown in Figure 3A, due to post-translational processing (Bennett et al. 2000;Benjannet et al. 2001), BACE1 protein in brain appeared in several sizes from 55 KD to 72 KD on the Western blot. Figure 3B is a graph showing quantified results of BACE1 protein levels. As shown in Figure 3A and 3B, middle-aged Gpx4+/- mice had the highest levels of BACE1 protein among the four groups of mice. Both middle-aged and young Gpx4+/- mice had increased BACE1 protein compared to their aged-matched Wt controls, indicating that increased β-secretase activity in Gpx4+/- mice was conferred by elevated BACE1 protein. The BACE1 protein level data for the four groups of mice in Figure 3B directly correlate with the 4-HNE adducts levels (Figure 1D), suggesting that upregulation of BACE1 expression was a direct result of increased lipid peroxidation.
Figure 3. BACE1 levels in young and middle-aged Wt and Gpx4+/- mice.
A. Total proteins from cerebral cortex tissues of Wt mice and Gpx4+/- mice were separated by SDS-PAGE, transferred to nitrocellulose membrane and probed with an anti-BACE1 antibody. The levels of β-actin protein were used to control loading.
B. Levels of BACE1 protein in cortex from young and middle-aged Wt and Gpx4+/- mice. The relative values are presented as mean ± SEM. *: P<0.05. n=3. Y-Wt: young Wt mice; Y-Gpx4+/-: young Gpx4+/- mice; M-Wt: middle-aged Wt mice; M-Gpx4+/-: middle-aged Gpx4+/- mice.
C. Total BACE1 mRNA levels in cerebral cortex tissues were determined by quantitative RT-PCR. The relative values are presented as mean ± SEM. n=5.
To determine whether increased BACE1 protein was a result of increased transcription, we measured BACE1 mRNA levels in the four groups of mice by quantitative RT-PCR. As shown in Figure 3C, no differences in BACE1 mRNA were detected among the four groups of mice, indicating that lipid peroxidation-mediated upregulation of BACE1 expression occurs at protein level.
Altered cell signaling associated with upregulation of BACE1 protein
Studies of NT2 cells indicate that activation of stress-activated protein kinase (SAPK) pathways is important in oxidative stress induced-upregulation of BACE1 expression at the transcription level (Tamagno et al. 2002;Tamagno et al. 2005). To determine whether the same pathways played a role in mediating the upregulation of BACE1 protein induced by increased lipid peroxidation, we compared levels of phosphorylated JNK (Figure 4A) and phosphorylated p38 (Figure 4B) between middle-aged Gpx4+/- mice and middle-aged Wt mice by Western blots. The quantified results of phosphorylated levels of JNK and p38 are presented in Figure 4E. As shown in Figures 4A, 4B, and 4E, there were no significant differences in phosphorylated levels of JNK and p38 between middle-aged Gpx4+/- mice and middle-aged Wt mice, suggesting that SAPK signaling did not play a significant role in upregulation of the BACE1 protein induced by lipid peroxidation in vivo. Previous studies indicated that the PI-3K/Akt pathway and extracellular signal regulated MAP kinase (ERK) pathway could be important in protein regulation in neurons (Gingras et al. 2001;Lenz and Avruch 2005). To determine whether the PI-3K/Akt and ERK pathways might play a role in lipid peroxidation-induced BACE1 protein upregulation, we measured the levels of phosphorylated Akt (Figure 4C) and phosphorylated ERK1/2 (Figure 4D) in middle-aged Gpx4+/- mice and middle-aged Wt mice. As shown in Figures 4C, 4D, and 4E, in cerebral cortex tissues from middle-aged Gpx4+/- mice, there were significantly increased levels of phosphorylated Akt and phosphorylated ERK1/2, suggesting that activation of the PI3K/Akt and ERK pathways might be important in regulating BACE1 expressing at protein level induced by increased lipid peroxidation.
Figure 4. Cell signaling in middle-aged Wt and Gpx4+/- mice.
Levels of phosphorylated JNK protein and total JNK protein (A), phosphorylated p38 protein and total p38 protein (B), phosphorylated Akt protein and total Akt protein (C) and phosphorylated ERK1/2 protein and total ERK1/2 protein (D) in cortex tissues of middle-aged Wt mice and middle-aged Gpx4+/- mice were measured by Western blots. The ratios of phosphorylated proteins in total proteins were determined from the Western blots as described in Methods (E). The relative values were presented as mean ± SEM. *P< 0.05. n=5
Increased endogenous Aβ level in middle-aged Gpx4+/- mice
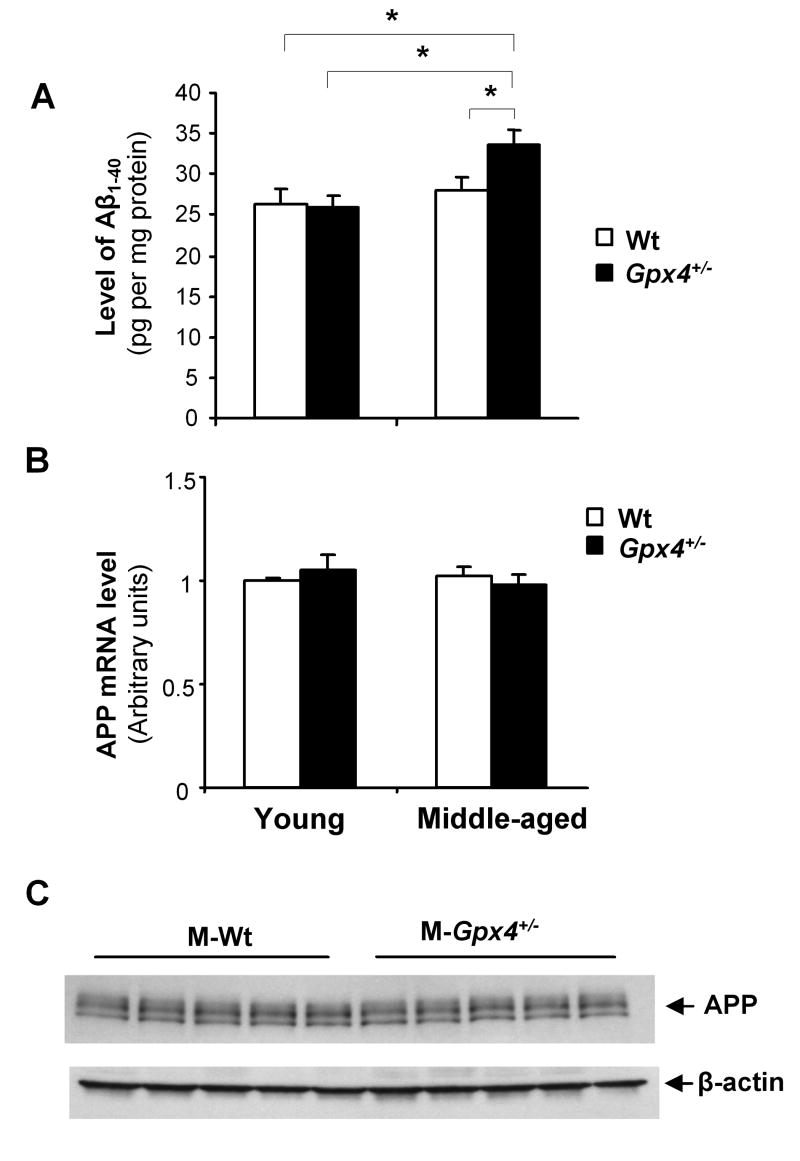
To determine whether increased lipid peroxidation could result in elevated amyloidogenesis, we next measured mouse endogenous Aβ levels in cerebral cortex tissues from the four groups of mice. Aβ1-40 is the major species of Aβ peptides. As shown in Figure 5A, middle-aged Gpx4+/- mice, which had the highest levels of 4-HNE and BACE1 protein, showed significantly increased level of Aβ1-40 compared to middle-aged Wt mice, young Gpx4+/- mice and young Wt mice. We did not observe any difference in endogenous Aβ1-42 among the four groups of mice (data not shown), likely due to difficulty in quantification of the extreme low level of endogenous Aβ1-42. To determine whether APP expression contributed to increased Aβ1-40 levels in middle-aged Gpx4+/- mice, we measured APP mRNA levels in the four groups of mice by quantitative RT-PCR. As shown in Figure 5B, no differences in APP mRNA levels were detected among the four groups of mice. We also compared APP protein levels in middle-aged Gpx4+/- mice and middle-aged Wt mice and observed no difference (Figure 5C). Therefore, upregulation of the BACE1 protein induced by high level of lipid peroxidation appears to be responsible for the increased Aβ1-40 level in middle-aged Gpx4+/- mice.
Figure 5. Aβ levels in young and middle-aged Wt and Gpx4+/- mice.
A. Total mouse endogenous Aβ in cerebral cortex tissues from young and middle-aged Wt and Gpx4+/- mice were extracted and Aβ1-40 levels were determined using Aβ ELISA kits, as described in Methods. The data are presented as mean ± SEM. *: P<0.05. n= 5.
B. Total APP mRNA levels in cerebral cortex tissues were determined by quantitative RT- PCR. The relative values are presented as mean ± SEM. n=5.
C. Total APP protein levels in cerebral cortex tissues of middle-aged Wt and Gpx4+/- mice were determined by Western blots. The levels of β-actin protein were used as loading controls.
Increased amyloidogenesis in APP transgenic mice with reduced Gpx4 (APPGpx4+/-mice)
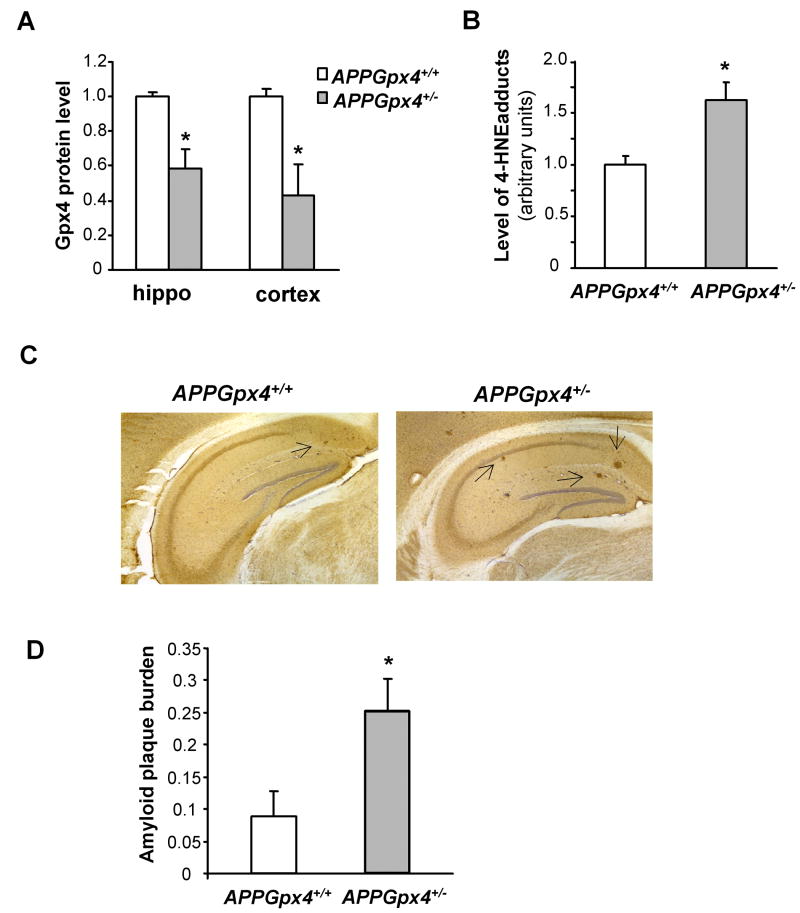
We further investigated the effect of increased lipid peroxidation on amyloidogenesis using APP transgenic mice. We generated APP transgenic mice in Gpx4 heterozygous knockout background (APPGpx4+/- mice) and APP transgenic mice in Gpx4 wildtype background (APPGpx4+/+ mice) by crossing the APP transgenic mice (Tg2576 mice) to Gpx4+/- mice. Both APPGpx4+/- mice and APPGpx4+/+ mice had the “Swedish double mutation” form of APP transgene; however, APPGpx4+/- mice had only one functional allele of Gpx4 gene, while APPGpx4+/+ mice had two functional alleles of Gpx4 gene. As a result, APPGpx4+/- mice had reduced Gpx4 protein levels in cerebral cortex and hippocampus compared to APPGpx4+/+ mice, as indicated in Figure 6A. To determine if reduced Gpx4 resulted in increased lipid peroxidation in APPGpx4+/- mice, we also measured the level of 4-HNE protein adducts in cerebral cortex from APPGpx4+/- and APPGpx4+/+ mice. As indicated in Figure 6B, the level of 4-HNE protein adducts in brains from APPGpx4+/- mice was significantly higher than APPGpx4+/+ mice, indicating that APPGpx4+/- mice had increased level of lipid peroxidation.
Figure 6. Amyloid plaque burden in APPGpx4+/+ and APPGpx4+/- mice.
A. Gpx4 protein levels in APPGpx4+/- and APPGpx4+/+ mice. The data in the graph are presented as mean ± SEM of Gpx4 protein levels. *: P<0.05. n=4-5
B. Levels of 4-HNE protein adducts in APPGpx4+/- and APPGpx4+/+ mice. The data in the graph are presented as mean ± SEM. *: P<0.05. n=4
C. Representative pictures of hippocampus from APPGpx4+/+ mice and APPGpx4+/- mice stained with anti-Aβ 6E10 antibody. The arrows indicate amyloid plaques.
D. Amyloid plaque burdens in hippocampus of APPGpx4+/+ and APPGpx4+/- mice were determined as described in Methods and expressed as mean ± SEM. *: P<0.05. n=4.
To determine whether increased lipid peroxidation resulted in increased amyloidogenesis in APPGpx4+/- mice, we compared amyloid plaque burden in APPGpx4+/- mice and APPGpx4+/+ mice that were 11 to 12 months of age. Figure 6C shows representative pictures of hippocampus regions of APPGpx4+/- mice and APPGpx4+/+ stained with 6E10 antibody. As shown, there were only small plaques sparsely populated in the hippocampus region of APPGpx4+/+ mice; however, in APPGpx4+/- mice, there were well-developed amyloid plaques. The amyloid plaque burdens of APPGpx4+/+ mice and APPGpx4+/- mice were quantified as described in Methods and presented in Figure 6C. Compared to APPGpx4+/+ mice, APPGpx4+/- mice had significantly increased amyloid plaque burdens in their hippocampus.
We also compared levels of Aβ in APPGpx4+/- and APPGpx4+/+ mice that were 11 to 12 months of age. SDS soluble and SDS insoluble Aβ1-40 and Aβ1-42 in hippocampus from two groups of APPGpx4+/- and APPGpx4+/+ mice were extracted as reported previously (Kawarabayashi et al. 2001) and measured using Aβ sandwich ELISA kits. Although there were no difference in SDS soluble Aβ1-40 between APPGpx4+/- and APPGpx4+/+ mice (Figure 7A), APPGpx4+/- mice had significantly elevated levels of SDS insoluble Aβ1-40 compared to APPGpx4+/+ mice (Figure 7B). The total level of Aβ1-40 (SDS soluble plus SDS insoluble) in APPGpx4+/- mice (768.3 ± 180.5 pmol/g) is also significantly higher than that in APPGpx4+/+ mice (348.7 ± 19.1 pmol/g). The levels of SDS soluble (Figure 7C) and insoluble Aβ1-42 (Figure 7D) were significantly higher in APPGpx4+/- mice than in APPGpx4+/+ mice. The total level of Aβ1-42 (SDS soluble plus SDS insoluble) in APPGpx4+/- mice (348.2 ± 78.9 pmol/g) is also significantly higher than APPGpx4+/+ mice (145.5 ± 9.5 pmol/g). Aβ1-40 /Aβ1-42 ratio is known to be affected by alterations in γ-secretase activity but not by alterations in β-secretase activity (Borchelt et al. 1996). Although APPGpx4+/- mice have increased Aβ1-40 and Aβ1-42, the Aβ1-40 /Aβ1-42 ratio is not altered in these mice (data not shown). Thus, the data of increased amyloidogenesis in APPGpx4+/- mice is consistent with data from middle-aged Gpx4+/- mice showing that increased amyloidogenesis is mediated by lipid peroxidation- induced BACE1 upregulation.
Figure 7. Levels of Aβ peptides in APPGpx4+/+ mice and APPGpx4+/- mice.
Aβ1-40 and Aβ1-42 peptides were extracted from hippocampus of APPGpx4+/+ mice and APPGpx4+/- mice, as described in Methods. Levels of SDS soluble Aβ1-40 (A), SDS insoluble Aβ1-40 (B), SDS soluble Aβ1-42 (C) and SDS insoluble Aβ1-42 (D) were determined by ELISA methods, as described in Methods. *: P<0.05. n=4-5
Discussion
Recent studies suggest that increased lipid peroxidation is an early event of AD (Pratico et al. 2002;Williams et al. 2006;Butterfield et al. 2006). To gain insights into the mechanistic role of increased lipid peroxidation in amyloidogenesis, we studied secretase activities in young and middle-aged Gpx4+/- and Wt mice in this study. The Gpx4+/- mouse is a mouse model with reduced expression of Gpx4, an essential antioxidant defense enzyme. Because no alterations of other major antioxidant defense enzymes such as catalase, Gpx1, MnSOD, and Cu/ZnSOD are found in the Gpx4+/- mouse model (Yant et al. 2003;Ran et al. 2003;Ran et al. 2007), increased lipid peroxidation is the primary form of oxidative stress in the Gpx4+/- mouse, thereby making this mouse a useful model in dissecting the specific role of increased lipid peroxidation in amyloidogenesis. Our results showed that γ-secretase activities increased with age; however, lipid peroxidation did not play a major role in age-associated increase in γ-secretase activity. In contrast, our results from young and middle-aged Gpx4+/- mice showed that β-secretase activity was significantly elevated by increased lipid peroxidation. Our data further indicated that the increased β-secretase activity was a result of increased BACE1 protein. Because the levels of BACE1 protein in young and middle-aged Gpx4+/- mice were directly correlated with levels of 4-HNE adducts, our results indicate that increased lipid peroxidation was responsible for upregulation of BACE1 expression. Previous studies showed that BACE1 expression was inducible by injury and stress (Blasko et al. 2004;Wen et al. 2004;Velliquette et al. 2005;Tamagno et al. 2002;Tamagno et al. 2005). Although injury and stress could result in increased lipid peroxidation, whether BACE1 induction was a result of increased lipid peroxidation is not clear from those studies. In this study, using a mouse model (Gpx4+/- mice) in which lipid peroxidation is the primary form of oxidative stress, we showed that levels of lipid peroxidation directly correlated with BACE1 levels. Therefore, our results indicate that increased lipid peroxidation can lead to upregulation of BACE1 expression in vivo.
Our results also showed that high levels of lipid peroxidation resulted in increased BACE1 protein without altering BACE1 mRNA levels, indicating that lipid peroxidation-induced upregulation of BACE1 expression occurs at the protein level. Studies by several groups show that, in AD brains, BACE1 protein and activities are increased but that there is no change in the BACE1 mRNA level (Holsinger et al. 2002;Preece et al. 2003;Yasojima et al. 2001;Yang et al. 2003;Fukumoto et al. 2002). Thus, the upregulation of BACE1 at the protein level appears to be an important mechanism in AD. BACE1 expression is regulated at both the transcriptional level and the translational level (Rossner et al. 2006), and Tesco et al (2007) showed recently that BACE1 protein stability control is also an important mechanism in regulating BACE1 protein level. Previous studies show that alteration in lipid environment such as the composition of lipid rafts affects BACE1 activity (Cordy et al. 2003;Ehehalt et al. 2003;Abad-Rodriguez et al. 2004). Our results indicate that upregulation of BACE1 expression at protein level is a major mechanism responsible for augmentation of β-secretase activity induced by increased lipid peroxidation. However, whether increased lipid peroxidation alters BACE1 activity is unclear at present.
The SAPK, PI-3K/ Akt and extracellular signal regulated MAP kinase (ERK1/2) pathways are important in mediating this intracellular stress response (Mielke and Herdegen 2000). Studies in cell lines indicate that the activation of SAPK (JNK and p38) signaling is important in mediating oxidative stress-induced upregulation of BACE1 expression (at protein and mRNA levels) (Tamagno et al. 2005;Tamagno et al. 2008). However, despite the significant differences in levels of lipid peroxidation and BACE1 protein levels between middle-aged Gpx4+/- mice and middle-aged Wt mice, we observed no differences in phosphorylated levels of JNK and p38 between these two groups of mice. Therefore, our results suggest that the roles of SAPK pathway in the upregulation of the BACE1 expression are different in vivo from in vitro. Our results showed that upregulation of BACE1 protein in middle-aged Gpx4+/- mice is correlated with increased levels of phosphorylated Akt and ERK1/2, suggesting that the activation of PI-3K/Akt pathway and ERK could be important in regulating BACE1 protein in vivo.
The source of increased lipid peroxidation that led to upregulation of BACE1 protein in Gpx4+/- mice is not known at present. Interestingly, Velliquette et al. (Velliquette et al. 2005) showed that inhibition of mitochondrial energy metabolism could induce the upregulation of BACE1 expression at the protein level. Because the inhibition of mitochondrial electron transfer could lead to increased mitochondrial reactive oxygen species (ROS) production, and because Gpx4 plays an important role in the protection against mitochondrial damage (Liang et al. 2007), mitochondrial ROS might be a source of increased lipid peroxidation in Gpx4+/- mice.
Our results showed that middle-aged Gpx4+/- mice had increased endogenous Aβ1-40. In order to confirm this result, we further studied the effect of increased lipid peroxidation on amyloidogenesis in APP transgenic mice. Our results show that APPGpx4+/- mice had increased lipid peroxidation due to deficiency in Gpx4. Our results further showed that, compared to APPGpx4+/+ mice, APPGpx4+/- mice had increased amyloid plaque burdens and significantly elevated levels of both Aβ1-40 and Aβ1-42. Therefore, our results from both middle-aged Gpx4+/- mice and APPGpx4+/- mice show that increased lipid peroxidation leads to increased amyloidogenesis.
It is shown that increased lipid peroxidation is an early event in AD (Pratico et al. 2002;Williams et al. 2006;Butterfield et al. 2006); therefore, the observation that increased lipid peroxidation leads to increased amyloidogenesis could be important to the pathogenesis of AD at earlier stages. Because lipid peroxidation could also cause damage to neurons, manipulations aiming to reduce lipid peroxidation and ROS production might be especially beneficial for subjects with MCI and early-stage AD patients by suppressing Aβ production and ameliorating damage to neurons caused by increased lipid peroxidation. In conclusion, our results demonstrated that increased lipid peroxidation can lead to amyloidgenesis through upregulation of BACE1 expression in vivo, suggesting that interventions to prevent/suppress lipid peroxidation may be promising in reducing amyloidogenesis at the early stages of AD.
Acknowledgments
The study was supported by a Merit Award (Q.R.) from the Department of Veteran Affairs and a K01AG022014 Award (Q.R.) from NIH/NIA.
Abbreviations
- AD
Alzheimer’s disease
- Gpx4
glutathione peroxidase 4
- BACE1
β-site amyloid precursor protein cleavage enzyme 1
- 4-HNE
4-hydroxy-2-nonenal
- Gpx4+/- mice
Gpx4 heterozygous knockout mice
- APPGpx4+/- mice
APP transgenic mice with deficiency in Gpx4
- APPGpx4+/+ mice
APP transgenic mice wildtype for Gpx4
Reference List
- Abad-Rodriguez J, Ledesma MD, Craessaerts K, Perga S, Medina M, Delacourte A, Dingwall C, De Strooper B, Dotti CG. Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol. 2004;167:953–960. doi: 10.1083/jcb.200404149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjannet S, Elagoz A, Wickham L, Mamarbachi M, Munzer JS, Basak A, Lazure C, Cromlish JA, Sisodia S, Checler F, Chretien M, Seidah NG. Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J Biol Chem. 2001;276:10879–10887. doi: 10.1074/jbc.M009899200. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Denis P, Haniu M, Teplow DB, Kahn S, Louis JC, Citron M, Vassar R. A furin-like convertase mediates propeptide cleavage of BACE, the Alzheimer’s beta -secretase. J Biol Chem. 2000;275:37712–37717. doi: 10.1074/jbc.M005339200. [DOI] [PubMed] [Google Scholar]
- Blasko I, Beer R, Bigl M, Apelt J, Franz G, Rudzki D, Ransmayr G, Kampfl A, Schliebs R. Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer’s disease beta-secretase (BACE-1) J Neural Transm. 2004;111:523–536. doi: 10.1007/s00702-003-0095-6. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Perluigi M, De Marco C, Coccia R, Cini C, Sultana R. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci Lett. 2006;397:170–173. doi: 10.1016/j.neulet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Imai H, Hirao F, Sakamoto T, Sekine K, Mizukura Y, Saito M, Kitamoto T, Hayasaka M, Hanaoka K, Nakagawa Y. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem Biophys Res Commun. 2003;305:278–286. doi: 10.1016/s0006-291x(03)00734-4. [DOI] [PubMed] [Google Scholar]
- Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint BM, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem. 2004a;89:1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H, Wong P, Price D, Shen Y. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2004b;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Van Remmen H, Frohlich V, Lechleiter J, Richardson A, Ran Q. Gpx4 protects mitochondrial ATP generation against oxidative damage. Biochem Biophys Res Commun. 2007;356:893–898. doi: 10.1016/j.bbrc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke K, Herdegen T. JNK and p38 stresskinases--degenerative effectors of signal-transduction-cascades in the nervous system. Prog Neurobiol. 2000;61:45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- Misonou H, Morishima-Kawashima M, Ihara Y. Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochemistry. 2000;39:6951–6959. doi: 10.1021/bi000169p. [DOI] [PubMed] [Google Scholar]
- Neely MD, Sidell KR, Graham DG, Montine TJ. The lipid peroxidation product 4-hydroxynonenal inhibits neurite outgrowth, disrupts neuronal microtubules, and modifies cellular tubulin. J Neurochem. 1999;72:2323–2333. doi: 10.1046/j.1471-4159.1999.0722323.x. [DOI] [PubMed] [Google Scholar]
- Paola D, Domenicotti C, Nitti M, Vitali A, Borghi R, Cottalasso D, Zaccheo D, Odetti P, Strocchi P, Marinari UM, Tabaton M, Pronzato MA. Oxidative stress induces increase in intracellular amyloid beta-protein production and selective activation of betaI and betaII PKCs in NT2 cells. Biochem Biophys Res Commun. 2000;268:642–646. doi: 10.1006/bbrc.2000.2164. [DOI] [PubMed] [Google Scholar]
- Park J, Floyd RA. Lipid Peroxidation Products Mediate The Formation of 8-Hydroxydeoxyguanosine In DNA. Free Radic Biol Med. 1992;12:245–250. doi: 10.1016/0891-5849(92)90111-s. [DOI] [PubMed] [Google Scholar]
- Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Preece P, Virley DJ, Costandi M, Coombes R, Moss SJ, Mudge AW, Jazin E, Cairns NJ. Beta-secretase (BACE) and GSK-3 mRNA levels in Alzheimer’s disease. Brain Res Mol Brain Res. 2003;116:155–158. doi: 10.1016/s0169-328x(03)00233-x. [DOI] [PubMed] [Google Scholar]
- Ran Q, Gu M, Van Remmen H, Strong R, Roberts JL, Richardson A. Glutathione peroxidase 4 protects cortical neurons from oxidative injury and amyloid toxicity. J Neurosci Res. 2006;84:202–208. doi: 10.1002/jnr.20868. [DOI] [PubMed] [Google Scholar]
- Ran Q, Liang H, Gu M, Qi W, Walter CA, Roberts LJ, Herman B, Richardson A, Van Remmen H. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J Biol Chem. 2004;279:55137–55146. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- Ran Q, Liang H, Ikeno Y, Qi W, Prolla TA, Roberts LJ, Wolf N, VanRemmen H, Richardson A. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J Gerontol A Biol Sci Med Sci. 2007;62:932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- Ran Q, Van Remmen H, Gu M, Qi W, Roberts LJ, Prolla T, Richardson A. Embryonic fibroblasts from Gpx4+/- mice: a novel model for studying the role of membrane peroxidation in biological processes. Free Radic Biol Med. 2003;35:1101–1109. doi: 10.1016/s0891-5849(03)00466-0. [DOI] [PubMed] [Google Scholar]
- Rossner S, Sastre M, Bourne K, Lichtenthaler SF. Transcriptional and translational regulation of BACE1 expression--implications for Alzheimer’s disease. Prog Neurobiol. 2006;79:95–111. doi: 10.1016/j.pneurobio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O, Smith MA, Perry G, Tabaton M. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis. 2002;10:279–288. doi: 10.1006/nbdi.2002.0515. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Guglielmotto M, Giliberto L, Vitali A, Borghi R, Autelli R, Danni O, Tabaton M. JNK and ERK1/2 pathways have a dual opposite effect on the expression of BACE1. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA, Perry G, Tabaton M. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R. BACE1: the beta-secretase enzyme in Alzheimer’s disease. J Mol Neurosci. 2004;23:105–114. doi: 10.1385/JMN:23:1-2:105. [DOI] [PubMed] [Google Scholar]
- Velliquette RA, O’Connor T, Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer’s disease pathogenesis. J Neurosci. 2005;25:10874–10883. doi: 10.1523/JNEUROSCI.2350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wataya T, Nunomura A, Smith MA, Siedlak SL, Harris PL, Shimohama S, Szweda LI, Kaminski MA, Avila J, Price DL, Cleveland DW, Sayre LM, Perry G. High molecular weight neurofilament proteins are physiological substrates of adduction by the lipid peroxidation product hydroxynonenal. J Biol Chem. 2002;277:4644–4648. doi: 10.1074/jbc.M110913200. [DOI] [PubMed] [Google Scholar]
- Wen Y, Onyewuchi O, Yang S, Liu R, Simpkins JW. Increased beta-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004;1009:1–8. doi: 10.1016/j.brainres.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, Beach T, Sue L, Wong P, Price D, Li R, Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- Yant LJ, Ran Q, Rao L, Van Remmen H, Shibatani T, Belter JG, Motta L, Richardson A, Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- Yasojima K, McGeer EG, McGeer PL. Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res. 2001;919:115–121. doi: 10.1016/s0006-8993(01)03008-6. [DOI] [PubMed] [Google Scholar]