Abstract
Hearing loss during development leads to central deficits that persist even after the restoration of peripheral function. One key class of deficits is due to changes in central inhibitory synapses, which play a fundamental role in all aspects of auditory processing. This review focuses on the anatomical and physiological alterations of inhibitory connections at several regions within the central auditory pathway following hearing loss. Such aberrant inhibitory synaptic function may be linked to deficits in encoding binaural and spectral cues. Understanding the cellular changes that occur at inhibitory synapses following hearing loss may provide specific loci that can be targeted to improve function.
Keywords: auditory cortex, deafness, development, excitability, frequency tuning, inferior colliculus, LSO, MSO, sound localization
Rationale for studying the CNS
Hearing loss during early life may lead to permanent deficits in auditory perceptual skills, and difficulties in the acquisition of speech and language [1–7]. Although these effects are particularly severe following long periods of auditory deprivation, even the temporary elevation of thresholds can disrupt auditory processing [8–10]. Transient bouts of hearing loss due to ear canal blockade, infections or trauma are common during childhood, a time when the nervous system is still developing and is particularly vulnerable to the disruption of acoustic experience. It is possible that deficits may persist owing to alterations of synapses and circuits within the central auditory system even when peripheral hearing is restored. Thus, to better understand the cellular basis of these deficits, it is important to examine how developmental hearing loss modifies central synapses and circuits.
The proper establishment of central auditory connections is specified initially by genetic mechanisms. By contrast, later maturation depends upon spontaneous and acoustically driven activity. It is during this later period of development that hearing loss can affect neural properties. Perturbations of sensory experience have long been known to alter excitatory synapses; this principle extends to synaptic inhibition. In the CNS, activation of inhibitory neurons leads to the release of glycine or GABA, which generally open chloride channels. An influx of chloride ions causes the membrane potential of the postsynaptic neuron to hyperpolarize, making it less excitable. The discharge rates of postsynaptic cells thus depend upon the net integration between such inhibition and excitatory activity. Perturbations in inhibitory neural circuits may lead to excitotoxicity, epilepsy and psychiatric disorders, such as schizophrenia and autism [11]. Inhibition may also play a fundamental role in all aspects of central auditory processing. Therefore, it is imperative to understand how specific features of inhibitory neurons and synapses are altered by hearing loss, and how these changes impact the neural circuits that encode acoustic cues. In this review, we describe the alterations to inhibitory synapses throughout the central auditory pathway that attend moderate to profound hearing loss in animal models. We then propose how these alterations could be linked to behavioral deficits, and how this may direct us to novel therapeutic approaches.
Deficits associated with developmental sensorineural hearing loss & conductive hearing loss
There are many forms of hearing loss with different causes and degrees of severity, but they are generally categorized into either sensorineural hearing loss (SNHL) or conductive hearing loss (CHL). SNHL results from a disorder of the inner ear, the auditory nerve, or the central auditory nervous system, and can range from mild to profound elevation of hearing thresholds. Severe to profound SNHL is observed in six out of 1000 children [12,13]. This may be congenital or caused by environmental factors, including viral infection, noise-induced trauma or ototoxic drugs, such as certain antibiotics [14]. Most forms of SNHL are irreversible, and are treated either with hearing aids or cochlear prostheses [14]. The capacity of cochlear prostheses to restore auditory function depends on the age of implantation. Children who are implanted between 1 to 2years of age display significantly better speech perception and language development as compared with those children implanted between the ages of 2 and 3 years [15,16]. Since the majority of children receive implants between 2 and 6 years of age [17], this may result in a significant period during which the central auditory nervous system is deprived of activity.
Conductive hearing loss results in poor transmission of sound waves through the outer or middle ear, leading to elevated thresholds. One form of CHL, otitis media, is due to the inflammation of the middle ear and is the most frequently diagnosed disease in infants and children [18]. Otitis media generally leads to a 20–28 dB elevation of hearing thresholds, and severe cases can increase thresholds up to 50 dB [19–21]. By one estimate, 20% of children may experience unilateral or bilateral otitis media for more than half of their first three years of life [13]. Although normal hearing thresholds are usually restored by treating the infection, children who experience chronic or recurring ear infections may be at risk of developing with perceptual speech and language disabilities [1,2,7,22–25]. However, the long-term impact of otitis media is a controversial issue [7]. Despite the important clinical relevance of understanding the synaptic changes associated with developmental CHL, this has been examined in only a few studies. Therefore, this review is focused on SNHL, but will discuss the similarities between these two forms of hearing loss.
Both SNHL and CHL can result in deficits in the development of speech and language acquisition [1,25,26–31]. Deficiencies in auditory processing may also have implications for certain aspects of learning [32]. These complex deficits in language and learning may result, in part, from abnormal processing of basic auditory cues [33]. The goals of the basic research described below are to first understand how the CNS is altered following hearing and then determine how these cellular changes can explain deficits in auditory processing. Towards this end, we will focus on changes that occur at inhibitory synapses, and ask how these changes could impact sound localization and frequency discrimination.
Hearing loss affects sound localization: role of altered inhibitory connections
Role of experience in sound localization
The ability of most animals to locate the source of sound in space depends upon the comparison of acoustic cues at the two ears. This ability can be compromised by hearing loss, as demonstrated in humans with unilateral CHL due to a closed ear canal (i.e., atresia). Following surgery to correct the atresia, the ability of subjects to locate sounds in the free field remains impaired [9]. Sound localization is not only crucial for determining the source of a sound, but for segregating sounds in a multiple sound environment. For example, sound localization may be important for detecting the voice of a speaker in a crowded, noisy room. This is sometimes referred to as the ‘cocktail party effect’. Children aged between 6 and 12 who were diagnosed with recurrent otitis media prior to 5 years of age were tested with a laboratory version of this cocktail party effect, and their performance was significantly worse than control children, although it can return to normal over the course of two years [8,34].
Animal studies suggest that the effects of hearing loss on binaural percepts may be due, in part, to altered responses of central neurons that encode sound location. Animals localize sounds along the azimuth by computing the difference in intensity (interaural level difference [ILD]) or arrival time (interaural time difference [ITD]) of a sound between the two ears. Experimental studies demonstrate that CHL can disrupt the maturation of ILD and ITD coding [35–38]. For example, monaural CHL in barn owls results in changes of ILD coding in midbrain neurons. When one ear is first plugged, the sound level required to activate afferents from that ear is much greater than normal, distorting the ILD values. However, if the earplugs are kept in place during development, the inputs from each ear are adjusted such that ILD coding properties are reasonably normal in adulthood [38]. This ability of the CNS to adapt to early hearing experience is also evidenced by shifts in ITD and ILD tuning in owls chronically implanted with a filter in one ear to distort the interaural timing and intensity level of sounds [39–41]. However, when normal hearing is restored, these adjusted ILD and ITD responses are no longer accurate for sound localization. Thus, understanding the physiological adjustments that occur during development at the level of synapses and circuits may be a prerequisite for clinical intervention.
Examining synaptic inhibition in brain slices
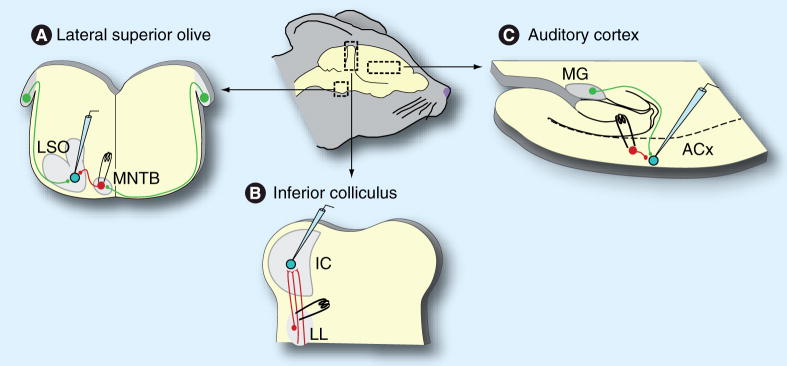
To evaluate the impact of hearing loss on synaptic inhibition, many of the studies presented in the following sections have used brain slice preparations. In these experiments, the brain is rapidly removed from an anesthetized animal. Slices are then cut and placed in a chamber with oxygenated artificial cerebral spinal fluid to keep the tissue alive. Intracellular recordings are then obtained from single cells in specific auditory regions, such as the lateral superior olive, the inferior colliculus and the auditory cortex (Figure 1). Using this method, the inhibitory inputs can be selectively assessed electrophysiologically and pharmacologically. Inhibitory neurons are stimulated with extracellular electrodes and the resulting inhibitory postsynaptic potentials (IPSPs) or currents (IPSCs) are recorded. To isolate inhibition, excitatory transmission is generally blocked with glutamate receptor antagonists. In some of the anatomical experiments described, single inhibitory neurons were filled with horseradish peroxidase to examine the morphology of these projections. Many of the studies presented in this review have used gerbils because their auditory system matures at a rapid pace [42,43] and their audiogram is similar to that of humans, especially within the low-frequency domain.
Figure 1. Experimental model to examine synaptic inhibition in the auditory system.
Schematic of a gerbil head representing the areas from which brain slices are generated (arrows). (A) Slice preparation showing the location of the LSO and MNTB. MNTB inhibitory projections (red) can be directly activated with an electrode while whole-cell recordings are obtained from an individual LSO neuron. (B) Slice preparation showing the location of the IC. Inhibitory afferents (red) arising from the LL can be directly activated while recordings are obtained from individual IC cells. (C) Thalamocortical slice preparation showing one half of the brain containing the MG and its excitatory projection (green) to the ACx. Intracortical inhibitory afferents (red) can be activated by a stimulating electrode while recordings are obtained from pyramidal cells.
ACx: Auditory cortex; IC: Inferior colliculus; LL: Lateral lemniscus; LSO: Lateral superior olivary nucleus; MG: Medial geniculate nucleus; MNTB: Medial nucleus of the trapezoid body.
Role of inhibition in sound localization circuits
In mammals, azimuthal sound location is first processed in two auditory brainstem nuclei, the lateral superior olivary (LSO) nucleus and the medial superior olivary (MSO) nucleus. In each of these nuclei, an inhibitory projection from the medial nucleus of the trapezoid body (MNTB) plays a critical role in the computation (Figure 2A).
Figure 2. Hearing loss affects development of medial nucleus of the trapezoid body inhibitory afferents to brainstem nuclei.
(A) (Top) Simplified schematic of a gerbil receiving two important sound cues for localization, ILD and ITD. (Bottom) Slice preparations showing the neural circuits that process these cues. ILD coding in the LSO nucleus is represented by the integration of excitatory drive from sound at the ipsilateral ear and MNTB inhibitory input driven by sound at the contralateral ear. ITD coding in the MSO nucleus is represented by the coincidental timing of excitatory drive from the two ears. MNTB inhibitory inputs to the MSO are involved in fine-tuning the ITD sensitivity of MSO neurons. (B) Hearing loss prevents the developmental refinement of inhibitory projections to the LSO and MSO. (Left) In the control LSO, inhibitory arborizations are spatially restricted along the frequency axis. The branching pattern of a single MNTB axon is shown within the LSO. Following contralateral SNHL, these arbors are significantly expanded, suggesting perturbed specificity [53]. (Right) In the control MSO, inhibitory projections are restricted to the soma. A schematic is shown of a single MSO neuron receiving MNTB inhibitory input with the red circles representing terminal boutons. Following contralateral SNHL, the inhibitory synapses remain distributed across the somatodendritic axis, and do not become localized to the soma [56].
ILD: Interaural level difference; ITD: Interaural time difference; LSO: Lateral superior olivary; MSO: Medial superior olivary; SNHL: Sensorineural hearing loss.
Lateral superior olivary neurons compute the position of a high-frequency sound based on the difference in sound levels at the two ears owing to attenuation of sound by the head [44]. The LSO receives a glycinergic inhibitory projection from the MNTB and an excitatory projection from the cochlear nucleus (CN) [44]. When the sound source is located ipsilateral to a LSO, the ipsilateral excitatory pathway from the CN is maximally activated, while the contralateral inhibitory pathway is minimally activated owing to sound attenuation. In this case, the LSO neuron discharge rate is maximal. When the sound source is contralateral to a LSO, the inhibitory pathway is maximally activated and the LSO neuron discharge rate is low. ILD coding is quite dynamic during development, owing to natural modifications of the inhibitory pathway after the onset of hearing [42,45]. This implies that the proper maturation of inhibition is essential for ILD coding and could be disrupted by early hearing loss.
For ITD processing, the MSO computes the microsecond difference in the arrival times of a low-frequency sound at the two ears. In this circuit, MSO cells receive bilateral excitatory projections from the CN and act as coincident detectors, firing maximally when the excitatory inputs arrive simultaneously. MNTB inhibitory afferents also project to the MSO, and are involved in the ITD computation [46–48]. The MNTB inhibitory projections to both MSO and LSO are modified during normal development, and early hearing loss profoundly impacts this maturation, as discussed below.
Development of MNTB projections to LSO is perturbed by hearing loss
To understand the influence of early hearing loss on inhibitory structure and function, it is essential to first study the development under normal circumstances. In the LSO, single MNTB terminal arborizations become physically restricted during postnatal development [49,50]. Furthermore, the number of MNTB afferents that innervate each LSO neuron declines two-fold during the first three postnatal weeks, and the remaining inhibitory afferents become much stronger [50,51]. The idea that inhibitory synapses are refined along the LSO axis during postnatal development is supported by the observation that glycine receptor expression is modified over the same ages [45]. Thus, it is evident that inhibitory terminals are quite dynamic during development, and this leads to a high degree of inhibitory synapse specificity within the LSO that is necessary for ILD coding.
Is this normal developmental pruning of the MNTB afferents in the LSO affected by hearing loss? In the gerbil and other small rodents, airborne sound can first elicit a response from the cochlea at approximately postnatal day (P) 12 [43,52]. Therefore, the influence of experience was assessed in gerbils by ablating one cochlea at P7, and thus functionally deafferenting one MNTB. Pups with this unilateral SNHL were raised for approximately 1 week after hearing onset and compared with controls that had developed with normal auditory experience during this time. Following unilateral SNHL, MNTB arborizations failed to attain the normal level of specificity within the LSO. As shown in Figure 2B, the arbors were more spread out, similar to those in younger animals [53]. Consistent with this finding, bilateral SNHL prevented the distribution of glycine receptors from attaining the adult pattern [54]. Together, these studies suggest that the refinement of inhibitory MNTB arbors within LSO is delayed or prevented by SNHL. If similar changes were to accompany moderate forms of SNHL, this could compromise the ability of LSO neurons to encode the range of ILD cues essential for sound localization. LSO neurons would not integrate afferents that carry the same frequency information, because contralateral inhibition fails to target a specific region along the LSO tonotopic axis.
Development of MNTB projections to MSO is perturbed by hearing loss
The postnatal development of MNTB projections to the MSO is also dramatically affected by hearing loss. Single MNTB arborizations undergo developmental refinement across the tonotopic axis of MSO, analogous to that which occurs in the LSO. Furthermore, the refinement of inhibitory arbors occurs at individual MSO cells. Inhibitory terminals are gradually eliminated from the dendrites, becoming confined to the cell body [55]. Changes in the distribution patterns of glycine-containing boutons and glycine receptor clusters on MSO neurons confirm that both pre- and post-synaptic elements of these inhibitory synapses are eliminated [56].
The effect of SNHL was assessed by ablating one cochlea before hearing onset. As illustrated in Figure 2B, SNHL animals displayed inhibitory terminals distributed on the dendrites, similar to neonates. Furthermore, this immature inhibitory innervation pattern persisted into adulthood [56]. Rearing developing animals in omnidirectional white noise, which reduces ITD cues, also disrupted the refinement of inhibitory projections to the cell body [55,56]. Furthermore, single MNTB axonal arbors in these noise-reared animals displayed a significant increase in spread across the MSO tonotopic axis [55]. Since ITD coding is impaired in these animals [57], it is possible that the unrefined inhibitory synaptic contacts are responsible. Specifically, deficient inhibitory somatic innervation after SNHL or noise rearing may diminish the role of these inputs in the integration of ipsilateral and contralateral excitation. This may affect the range of MSO responses to physiological ITDs [46,47]. As in the LSO, the lack of specificity of inhibition along the tonotopic axis of the MSO after hearing loss may disrupt integration of inputs carying the same frequency information.
Synaptic mechanisms of altered MNTB inhibitory projections
What synaptic mechanisms underlie the refinement of MNTB neurons during development? In an independent set of experiments, it was discovered that MNTB synapses display an activity-dependent form of long-term depression (LTD) that could support synapse elimination. Low-frequency stimulation of MNTB afferents produces a profound LTD of the evoked inhibitory synaptic potentials or currents. This inhibitory LTD is age-dependent; it is prominent during the period of synapse elimination and declines during the third postnatal week [58].
The discovery that early synapses in the MNTB–LSO circuit release GABA led to the hypothesis that GABA is necessary for the induction of inhibitory LTD. Classically, inhibitory transmission within the LSO was thought to be exclusively glycinergic [59–62]. However, by a combination of electrophysiological and electron microscopic immunocytochemical assays, it was revealed that inhibitory synapses from the MNTB primarily release GABA during the first postnatal week, but gradually switch to glycine by the third week [63,64]. In an elegant study, Nabekura and coinvestigators demonstrated colocalization and corelease of GABA and glycine from individual inhibitory terminals [65]. The early release of GABA appears to be important for inhibitory LTD. Repetitive focal application of GABA to individual LSO neurons resulted in depression of inhibitory potentials, while similar glycine applications did not produce any depression [66]. GABA binding to the G-protein-coupled GABAB receptor was found to mediate this LTD [66,67]. These results led to the hypothesis that the activity-dependent refinement of inhibitory terminals is supported by a LTD mechanism. Inhibitory synaptic LTD actually declines with age, while LTP becomes more prominent [58,68]. If the developmental pruning of MNTB arbors does depend on LTD, then it is possible that SNHL perturbs LTD itself. While this has not been tested, hearing loss was found to disrupt long-term excitatory synaptic plasticity in the auditory cortex [69].
Other examples of experience-dependent changes in inhibitory projections
Several studies on the auditory pathway and other sensory modalities support activity-dependent refinement of inhibitory innervation. For example, inhibitory afferents form a striping pattern in the rat auditory midbrain, resembling the ocular dominance columns produced by thalamic afferents in the visual cortex. These ‘stripes’ emerge from a diffuse projection pattern during the first two postnatal weeks; the segregation of inhibitory stripes is prevented by unilateral or bilateral SNHL [70,71]. Moreover, inhibitory interneurons in the auditory, somatosensory and visual cortices show changes in innervation patterns following developmental sensory deprivation. In contrast to the effects on MNTB inhibitory afferents, deprivation may result in decreased projections and synapses of cortical inhibitory interneurons. For example, in the somatosensory cortex, symmetrical, presumably inhibitory, synapses are reduced by 52% following neonatal sensory deprivation induced by whisker trimming from birth [72]. Similarly, following monocular deprivation during a critical developmental period, the innervation of cortical inhibitory interneurons to the soma of excitatory pyramidal cells was reduced by 36% in the deprived visual cortex as compared with the nondeprived [73]. In the auditory cortex, SNHL induced by bilateral cochlear ablation before hearing onset leads to a significant reduction in the number of inhibitory terminals identified by glutamic acid decarboxylase (GAD65/67) immunoreactivity [74]. Thus, several independent and compelling lines of evidence have demonstrated the significance of sensory experience on the establishment of inhibitory projections.
Hearing loss affects frequency discrimination: role of diminished inhibitory gain
Role of experience on frequency discrimination
Difficulties with speech and language acquisition after developmental hearing loss have been associated with an impaired ability to perceive frequency differences between sounds. For example, children with mild to moderate SNHL showed poor performance on a frequency discrimination task compared with control children for both high and low frequencies [75]. This result is consistent with reports of impaired frequency discrimination in children with a history of otitis media [76], as well as adults with mild or moderate hearing loss [77].
Animal studies demonstrate that these behavioral effects of hearing loss on frequency discrimination may be due to changes in frequency tuning of individual cells within the central auditory system. Auditory regions such as the LSO, inferior collicus (IC), auditory thalamus and auditory cortex are tonotopically organized, such that the cells are tuned to specific characteristic frequencies (CFs) and are spatially mapped along an axis from high to low CFs. Hearing loss can broaden tuning curves of single cells, shift CFs and lead to global changes in tonotopy. For example, frequency tuning curves of IC neurons become broader following noise- and drug-induced hearing loss [78–82] and in mice with congenital SNHL [83]. Partial hair cell damage within regions of the cochlea also leads to a reorganization of the cortical tonotopy [81,84–88]. Together, these studies show that central correlates of frequency discrimination are disrupted by hearing loss. Once again, changes in synaptic inhibition may offer a cellular explanation for some of these changes.
Role of inhibition in frequency tuning
Although outweighed by excitatory synapses in number, inhibitory synapses can profoundly influence the activity of networks because of their distinct anatomical and functional properties [89–92]. Manipulations that strengthen or weaken inhibition are known to change frequency receptive fields of auditory cortical neurons. Activation of inhibitory circuits leads to the sharpening of excitatory receptive fields, while inactivation broadens receptive fields [93–98]. This principal applies generally to most regions of the central auditory pathway. For example, direct pharmacological manipulations have demonstrated that GABAergic inhibition refines frequency tuning in the IC [99].
In the cortex, as in the brainstem, inhibition may act to narrow tuning by suppressing responses to stimuli that are spectrally far from the CF [100,101]. Single neurons in the auditory cortex receive information from a broad range of the audible spectrum, evidenced by recent in vivo experiments showing subthreshold responses that extend well beyond the classical spiking receptive fields [100–106]. Enhancing inhibition by cortical application of the GABAA receptor agonist, muscimol, fully suppresses long-latency responses, which are thought to be elicited by intracortical horizontal pathways [102]. In addition, it has been proposed that inhibitory cells, themselves, have broader spiking tuning curves than excitatory cells, resulting in relatively stronger inhibitory responses to stimuli far from the CF [105–109], although this issue remains unresolved [101,103,110,111].
The important role of inhibition in frequency tuning implies that these connections may be involved following hearing loss. Pharmacological blockade of inhibition in the IC produces effects that closely resemble those induced by hearing loss. Both lead to an expansion of the tuning curve, particularly at higher intensities [80]. The idea that inhibition is compromised following hearing loss is further supported by studies showing that neurons become significantly more excitable in the IC [80,112–114] and cortex [115–118]. For example, one study evaluated response thresholds of neurons within the auditory cortex of deaf adult cats with cochlear implants. Thresholds were determined by the minimum electrical current required to evoke spikes. A group of cats deafened at birth was compared with a group acutely deafened hours before the recording. The cats with long-term deafness showed lower response thresholds than the acutely deafened controls. Moreover, the cortical area activated by this threshold current was expanded in the long-term group, reflecting a disruption of tonotopy [119]. In another form of hearing loss induced by partial cochlear damage, the efficacy of surround inhibition was diminished, resulting in broadening of excitatory receptive fields [87,120]. These studies lead to the hypothesis that weakened inhibition may contribute to the compromised frequency discrimination following hearing loss. The following sections will review data from brain slice preparations showing that at every relay station examined, inhibitory transmission is downregulated following hearing loss. Interestingly, the mechanisms by which inhibitory gain is regulated at these synapses appear to be diverse, and include both pre- and post-synaptic sites.
Hearing loss decreases inhibitory gain in the CN and MNTB
In the MNTB of congenitally deaf mice, glycinergic miniature inhibitory currents are reduced [121]. These results are consistent with a downregulation of glycinergic inhibition in the CN of animals deafened as adults either by unilateral cochlear ablation or by neomycin application. In these studies, deafness reduced glycine receptor binding [122] and the number of glycinergic presynaptic terminals [123,124]. In a similar set of studies, both SNHL induced by cochlear ablation and CHL induced by middle ear ossicle removal, led to a comparable decrease in glycine release and increase in glycine uptake in the CN [125,126]. In addition to hearing loss-induced changes in excitatory transmission [127] and intrinsic properties [128], such reduced glycinergic inhibition within the CN and MNTB may underlie the altered tonotopy [129].
Hearing loss decreases inhibitory gain in the LSO
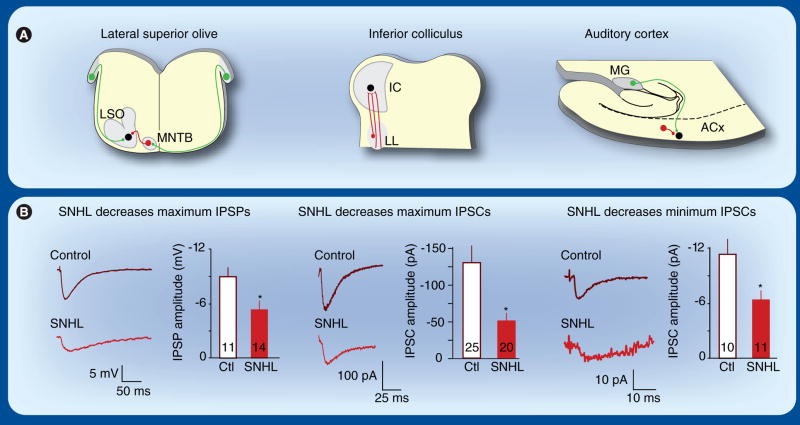
As discussed above, MNTB projections failed to attain a normal level of anatomical specificity to the LSO in gerbils with SNHL induced before hearing onset. In addition, the amplitude of MNTB-evoked IPSPs declines significantly (Figure 3) [130]. This is consistent with decreased glycinergic terminals in the LSO after adult animals were deafened with neomycin [124]. Thus, in conjunction with the disorganized projection pattern, synaptic inhibition becomes weaker following hearing loss, and this could affect the tonotopy of the LSO.
Figure 3. Hearing loss weakens inhibitory synaptic strength.
(A) Schematics of the LSO (left), IC (middle) and ACx (right) show inhibitory projections respectively arising from the MNTB, the LL and within the cortex. (B) Recordings of evoked IPSPs or IPSCs in Ctl and SNHL neurons. Bar graphs (mean ± SEM) summarize the decrease of inhibitory synaptic strength following hearing loss (*p < 0.05) [130,132,138]. The number of recorded neurons is shown within each bar.
ACx: Auditory cortex; Ctl: Control; IC: Inferior colliculus; IPSC: Inhibitory postsynaptic current; IPSP: Inhibitory postsynaptic potential; LL: Lateral lemniscus; LSO: Lateral superior olivary nucleus; MG: Medial geniculate nucleus; MNTB: Medial nucleus of the trapezoid body; SNHL: Sensorineural hearing loss.
Hearing loss decreases inhibitory gain in the IC
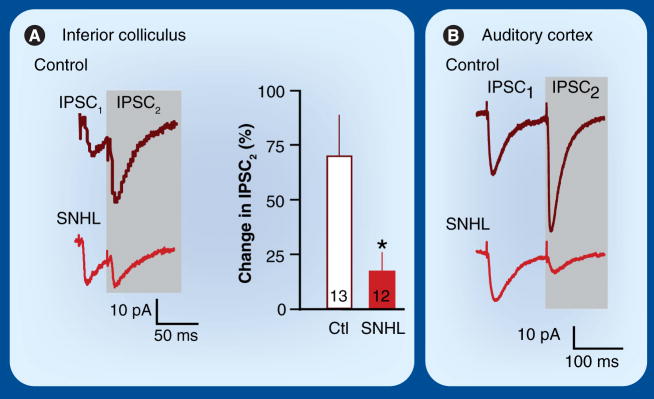
The IC is an obligatory relay in the ascending pathway that receives glycinergic and GABAergic inhibitory projections from several brainstem nuclei [131]. In a transverse brain slice preparation, much of the ascending inhibitory pathway can be activated with a stimulating electrode placed just ventral to the IC. Using this preparation, it was found that bilateral SNHL induced before hearing onset led to a significant reduction in the size of evoked IPSCs (Figure 3) [132]. Moreover, SNHL led to a change in short-term synaptic plasticity (i.e., the dynamic change in strength that is observed during repeated stimulation [133,134]). To assess short-term plasticity of IPSCs, paired stimulus pulses were delivered to the inhibitory pathway, and the interval between pulses was varied. In control neurons, the IPSCs exhibited paired-pulse facilitation at most intervals, with the second response being 30 to 70% greater than the first. This facilitation was nearly eliminated in SNHL animals (Figure 4A) [132,135]. Thus, hearing loss decreases the strength of synaptic inhibition in the IC, and this effect may become more profound during prolonged periods of stimulation.
Figure 4. Hearing loss changes inhibitory short-term plasticity.
(A) Representative IPSCs from control and SNHL inferior colliculus neurons evoked by paired stimuli applied to the lateral lemniscus (ISI = 33 ms). In Ctl neurons, the second IPSC is larger than the first (paired-pulse facilitation). However, following SNHL, this facilitation is significantly reduced. Bar graphs (mean ± SEM) summarize the percent change in the amplitude of IPSC2 ([IPSC2–IPSC1]/IPSC1) for Ctl and SNHL animals (*p < 0.05) [135]. The number of recorded neurons is shown within each bar. (B) Representative IPSCs evoked by paired stimuli applied to cortical layer 4 and recorded in L2/3 pyramidal cells (ISI = 120 ms) show that SNHL leads to paired-pulse depression (*p < 0.05) [139].
Ctl: Control; IPSC: Inhibitory postsynaptic current; SNHL: Sensorineural hearing loss.
Hearing loss decreases inhibitory gain in the auditory cortex
To determine whether hearing loss reduces inhibitory synapse strength in the cortex, inhibitory input to cortical pyramidal neurons was recorded in a brain slice preparation that preserves the projection from the auditory thalamus, the medial geniculate nucleus [136,137]. IPSPs and IPSCs were evoked by intracortical stimulation. Consistent with findings in the brainstem, the strength of inhibition decreased in animals with bilateral SNHL induced before hearing onset. Spontaneous IPSCs, minimum-evoked IPSCs and maximum-evoked IPSPs were all significantly reduced in SNHL animals (Figure 3) [137,138]. Moreover, the prolonged IPSCs recorded in SNHL animals at P18–21 resembled those from animals aged before hearing onset (P8–11), suggesting an arrest in the maturation of these synapses [138]. Finally, SNHL had an effect on cortical short-term plasticity similar to that observed in the IC. IPSCs recorded in control pyramidal neurons generally displayed paired-pulse facilitation, but this was significantly reduced in neurons from both SNHL and CHL animals (Figure 4B) [139]. Thus, decreased inhibitory synapse strength accumulates as one ascends the auditory pathway, suggesting a larger net effect than would be evident in any one region alone.
Synaptic mechanisms of diminished inhibitory gain
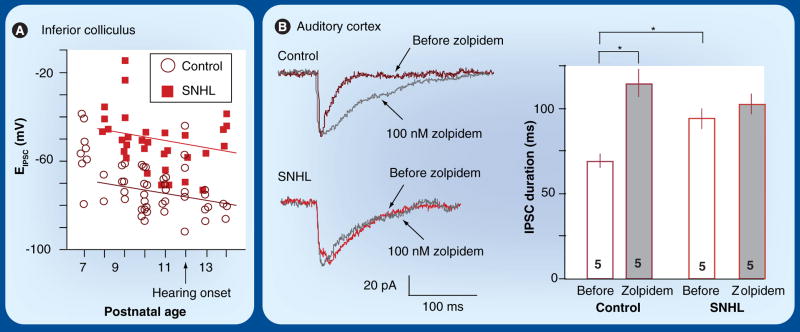
What synaptic mechanisms underlie the smaller and slower inhibitory currents that result from hearing loss? Beyond the structural alterations discussed above, there is evidence to suggest that changes occur at both pre- and post-synaptic loci. First, a depolarization in the IPSC reversal potential (EIPSC) may contribute to decreased amplitudes. In the LSO, an 8 mV depolarizing shift of EIPSC was observed in animals with unilateral SNHL induced before hearing onset [130]. A similar phenomenon was observed in IC neurons using perforated patch recordings, which do not disrupt the intracellular chloride concentration. In the IC, EIPSC depolarizes by 24 mV following bilateral SNHL (Figure 5A) [132]. Since EIPSC is determined by the intracellular chloride concentration [140], it is possible that chloride transport is improperly regulated after SNHL. In most adult neurons, low intracellular chloride is maintained by a K–Cl cotransporter (KCC2) that extrudes chloride [141–144]. To assess whether chloride transport was altered after hearing loss, the effects of three agents that reduce chloride transport were evaluated. All three manipulations depolarized EIPSC in control neurons, but did not have an effect on SNHL neurons. Since KCC2 is expressed at normal levels in SNHL neurons, it appears that the decline in inhibitory synaptic gain is partly due to diminished KCC2 function [145].
Figure 5. Mechanisms leading to disruption of inhibitory synapse strength and kinetics.
(A) Reduced strength may result from a shift in the inhibitory reversal potential (EIPSC). Distribution and regression analysis of EIPSC of control (circles) and SNHL (squares) neurons recorded in the inferior colliculus. Note that SNHL leads to a 24 mV depolarization in the mean E IPSC (p = 0.0001) [132]. (B) Altered kinetics may result from a disruption of GABAA-receptor subunit composition. SNHL sIPSCs are significantly prolonged as compared with controls (compare before zolpidem traces between control and SNHL; *p < 0.05). An α1 subunit-specific agonist (zolpidem) prolongs sIPSC durations in control, but not in SNHL neurons, suggesting that the kinetic difference is due to failed α1 function following SNHL. Bar graphs show that zolpidem is only effective at prolonging durations for control neurons (mean ± SEM; *p < 0.05) [138]. The number of recorded neurons is shown within each bar.
sIPSC: Spontaneous inhibitory postsynaptic current; SNHL: Sensorineural hearing loss.
A second hearing-loss-induced change occurs to the postsynaptic inhibitory receptor. Both the strength and kinetics of inhibitory postsynaptic currents have been shown to depend on the number of postsynaptic GABAA receptors and the specific array of subunits [146–154]. The prolonged IPSCs in the cortex suggest a change in the type of GABAA receptor, which is composed of several subunits. Two subunits, the α1 and β2/3, are associated with faster IPSC kinetics when they are upregulated during development. To determine if the function of these subunits was altered by SNHL, the sensitivities of a specific α1 subunit agonist, zolpidem, and a specific β2/3 subunit agonist, loreclezole, were tested. In controls, each agonist enhanced the duration of spontaneous IPSCs. However, this effect was absent in SNHL neurons (Figure 5B, α1 function). Changes in the functional expression of the β2/3 subunit is supported by an immunocytochemical study showing that the proportion of β2/3 subunits at the postsynaptic membrane declined significantly in neurons from SNHL animals [74]. Furthermore, as in the SNHL animals, IPSCs from animals aged before hearing onset did not show sensitivity to the specific GABAA receptor agonists. This is consistent with studies in other systems showing profound changes in developmental expression of specific subunits [155] that correlate with the developmental modification in IPSC kinetics [156–161]. Together, these studies strongly imply that the decreased and prolonged inhibition after SNHL is partly due to an arrest in the normal development of GABAA receptor subunit expression.
Finally, there may be various factors underlying the SNHL-induced change in inhibitory short-term plasticity. Paired-pulse responses are generally associated with dynamic changes to presynaptic transmitter release [133,134]. The decrease in paired-pulse facilitation may be associated with an increase in release probability. There are clear indications that hearing loss increases release probability at cortical inhibitory terminals. In SNHL animals, the frequency of spontaneous IPSCs recorded in cortical pyramidal cells was over twice the rate recorded in controls [138]. Further, an EM-immunocytochemical finding shows that the key GABA synthesizing enzyme, GAD65/67, is increased by 47% in single inhibitory terminals following SNHL [74]. Additional factors that may mediate the SNHL-induced change in short-term plasticity include changes in postsynaptic GABAA receptor desensitization, presynaptic GABAB autoreceptor function, the efficiency of GABA re-uptake, or vesicle depletion [133,134,162–165]. However, these issues remain to be explored.
Dimished inhibitory gain may underlie compromised frequency discrimination
Given the robust reduction of inhibitory gain that occurs throughout the ascending auditory pathway, many auditory percepts are likely affected. Frequency discrimination is one such percept that is expected to depend on inhibition. Synaptic inhibition is recruited by excitatory afferents at the CF of the neuron, and by afferents carrying non-CF information. After hearing loss, the inhibitory synapses may have less efficacy to suppress spiking of normally subthreshold events elicited by non-CF afferents. Consistent with this, IPSPs are less effective in blocking current-evoked action potentials after hearing loss. In control IC neurons, IPSPs blocked 97% of current-evoked action potentials and the duration of inhibition lasted for 81 ms, but in deafened neurons, only 43% of action potentials were blocked and the duration of inhibition was only 27 ms [145]. If inhibitory input elicited by CF afferents is also weak, then the threshold to activate the neuron could also decline. This has been observed in deafened cats implanted with cochlear electrodes [119]. Understanding the synaptic mechanisms that underlie such decreased inhibition may be essential for alleviating various perceptual deficits that attend hearing loss, including compromised frequency discrimination.
Other examples of experience-dependent downregulation of inhibitory strength
Partial or total loss of activity in the visual and somatosensory systems leads to downregulation of GABAergic transmission, consistent with findings in the auditory system. For example, blocking action potentials of cultured neurons from the visual cortex by chronic exposure to tetrodotoxin leads to a significant decline in miniature IPSC amplitudes due to a decrease in the average number of open GABA-gated channels and their inappropriate clustering at the postsynaptic membrane [166]. Similarly, weakened connections between fast spiking GABAergic interneurons and pyramidal neurons are observed in the visual cortex in animals raised with monocular deprivation [167]. In the somatosensory cortex, deprivation by whisker trimming during a developmental period leads to a downregulation of GABAA receptors [168]. Thus, reduced inhibitory gain following deprivation is a general principle, and the resulting enhanced excitability may have consequences for many aspects of sensory processing.
Similarities between SNHL & CHL
Studies directly comparing the synaptic effects of SNHL and CHL suggest that these two distinct forms of hearing loss produce similar changes in synaptic transmission [125,169,170]. For example, unilateral SNHL and CHL have a similar effect on glucose uptake in the contralateral IC [171,172]. As compared with most SNHL models, CHL can be induced reversibly by ear plugs or atresia. These studies suggest that some of the neural effects of unilateral CHL are reversed when normal hearing is restored, although this depends on the age at which hearing is restored [173,174]. This is consistent with the effects observed after reversible unilateral SNHL was induced by tetrodotoxin application to the round window. Following a brief period of SNHL, there was a reduction of cell size within the CN and MNTB, which fully recovered within a week [175,176]. Past investigations to assess the electrophysiological ramifications of hearing loss using brain slice preparations were limited to young animals. However, now that it is feasible to examine synaptic transmission in brain slices obtained from adult animals [177], it will be possible to examine the long-term effects of both permanent and transient forms of hearing loss. Understanding the modifications in the inhibitory circuits during recovery from SNHL or CHL may advance our understanding on the role of inhibition in normal hearing.
Similarities between developmental & age-related hearing loss
This review discusses the structural and physiological alterations at inhibitory synapses triggered by two major forms of hearing loss induced prior to the onset of hearing. Many synaptic features following hearing loss, such as IPSC kinetics, bear a close resemblance to an immature phenotype [138]. Therefore, such modifications may be largely attributed to the arrest of certain developmental mechanisms that require sound-driven activity. Alternatively, some of the effects observed after developmental hearing loss happen too quickly to be due to a developmental delay. For example, the shift in EIPSC of IC neurons happens within one day of the in vivo manipulation [132]. It is possible that these robust effects only occur during critical developmental periods when the mechanisms for plasticity are optimal to respond to the sensory environment. In support of this, the plasticity of cortical frequency representations in response to pure-tone exposure is limited to a few days following hearing onset in rats [178]. However, it is also clear that the auditory system retains a degree of plasticity throughout adulthood, suggesting that the capacity of the inhibitory circuits to adapt persists [179]. For example, similar rapid effects on inhibition are observed following acute unilateral ablation in adult animals [180]. Therefore, it is possible that studies on early hearing loss reflect some homeostatic changes that are independent of developmental mechanisms [181]. In support of this, there are parallels between developmental and age-related hearing loss.
The reduction of inhibitory transmission following developmental hearing loss may be similar to that observed in presbycusis (age-related hearing loss). In fact, the first set of reliable observations showing that inhibitory properties were use-dependent came from studies on very old animals. This work demonstrated a down-regulation of inhibitory synaptic gain in both the IC and cortex [182–186]. Moreover, in the CN, age-related hearing loss decreased the number of glycine-immunoreactive neurons [187]. Such diminished glycinergic transmission can have a functional consequence on auditory processing in aging animals. For example, the cells in the dorsal CN display an altered pattern of discharge rates to CF tones and changed temporal properties [188].
Some of the mechanisms that underlie this downregulation of inhibition may be similar to those discussed in this review. For example, as in developmental hearing loss, aging may lead to an altered subunit composition of the inhibitory receptors [189–192]. Consistent with developmental SNHL, age-related changes may be associated with a downregulation of the α1 subunit of the GABAA receptor [191]. However, there are also differences between developmental and age-related hearing loss. For example, aging is associated with a decrease in GABA-positive neurons and a decrease in GABA content and release [182,184,185,193–195]. By contrast, existing data support an increase in GABA release after developmental SNHL [74,138]. Therefore, the precise changes that occur may rely on the age at hearing loss, as well as the magnitude and duration of the loss [125,196–197]. Despite these differences, it remains useful to draw parallels between studies of developmental and age-related hearing loss. Between 25 and 40% of the population aged 60 years and older is hearing impaired [198]. Furthermore, age-related hearing loss is often associated with tinnitus, a condition in which patients hear ‘ringing in the ears’ in the absence of sound. One possible cause of tinnitus is thought to be a decrease in GABAergic inhibition in the auditory cortex following hearing loss [199,200]. In support of this, drugs that are believed to enhance inhibition by increasing GABA release may alleviate tinnitus [201]. Finding common mechanisms between different types of hearing loss may lead to broad therapeutic solutions for both children and adults suffering with hearing-loss-related dysfunctions in the nervous system.
Future perspective
During critical periods of development, the brain clearly has the capacity to adjust to changes in the sensory environment. The adaptive advantage of this is evident: the brain can gear processing towards relevant sensory stimuli. However, the risk is that the circuits of the brain are vulnerable to transient periods of abnormal developmental experience and become rewired such that the range of sound cues will no longer be faithfully encoded. This review has emphasized how readjustments in inhibitory synapse gain may contribute to such changes in coding properties. First, we provided evidence that inhibitory circuits and synapses are vulnerable to a common developmental sensory perturbation, hearing loss. Second, we suggested how altered inhibitory synapses may be involved in perceptual deficits associated with hearing loss, such as sound localization and frequency discrimination. Of course, reduced synaptic inhibition may further affect brain circuits by gating changes in excitatory synapses. For example, pharmacological blockade of glycinergic inhibition in the LSO leads to a profound change in LSO dendrites and the excitatory synapses to these cells [63,202]. In the visual cortex, inhibition has been advanced as the mechanism that regulates the onset and termination of the critical period [203–205]. Together, these studies point to the inhibitory system as a critical therapeutic target. Restoring normal inhibitory function may alleviate some of the behavioral deficits in sensory perception and may even initiate new changes in the brain circuits to readjust to the sensory environment.
It is possible that central neural circuits may be restored simply by stimulation of the peripheral afferents. For example, in congenitally deaf or neonatally deafened cats, electrical stimulation with cochlear implants restored the ultrastructure of auditory nerve synapses [206] and cell size [207] within the CN. Moreover, some studies have shown that electrical stimulation partially restores functional properties, such as temporal resolution and response latency [208–210]. However, electrical stimulation can also lead to a degradation of neural function. For example, chronic stimulation at one location within the cochlea may lead to a less precise cochleotopic map [211]. This degradation may be prevented by alternately stimulating two adjacent intracochlear channels, suggesting that the effects of cochlear implants depend upon the timing and location of the electrical stimuli [212]. Consistent with this, it has been recently shown that chronic stimulation with environmentally derived electrical stimuli can partially restore the cochleotopic map in auditory cortex [213]. Given that both spatial cochleotopy and temporal properties can be partially restored by chronic electrical stimulation, it may be possible to restore the disrupted spatiotemporal patterns of cortical activity observed after deafness, although this has not yet been tested [214]. Despite these compelling studies that some aspects of neuronal structure and function can be restored with stimulation by cochlear implants, less is known about how inhibition is affected. One recent study suggests that inhibitory synapses are restored by cochlear prostheses. In adult rats with unilateral SNHL, chronic electrical stimulation reversed the decrease in inhibition within the IC, evidenced by a recovery of normal GAD67 mRNA and protein levels and glycinergic receptor (GlyRα1) expression [215]. It is not clear whether this outcome would occur following developmental hearing loss. In future studies, it will be important to determine whether a similar restoration of inhibitory synaptic transmission by electrical stimulation occurs after developmental SNHL or CHL, and if this restoration occurs throughout the auditory system.
The cellular mechanisms discussed in this review may offer clues to the design of pharmacological strategies for ameliorating the effects of early hearing loss. For example, one fundamental change that leads to ineffective inhibitory synaptic transmission is the loss of a chloride battery [145]. Therefore, it would be reasonable to search for agents that selectively increase the function of KCC2. A second set of changes that leads to weak inhibition with slow kinetics is the loss of GABAA receptor trafficking and a failure to express the adult subunit isoforms [74,138]. Since benzodiazepine-like drugs, such as zolpidem, are commonly used as prescription medications, drugs of this sort might also be examined as a means of restoring inhibitory function [216]. This strategy has been used to improve the sensitivity of visual cortex neurons in aging primates and auditory processing in an animal model of presbycusis [217,218]. Other candidates raising inhibitory strength currently prescribed to humans include selective GABA reuptake inhibitors, such as tiagabine [219] and the GABAB receptor agonist, baclofen [220].
By understanding how manipulations of the inhibitory system shape both inhibitory and excitatory synapses, we will gain insight into the conditions that make the circuits of the brain most labile to sensory experience. For children and adults with hearing loss, this may translate into novel therapeutic treatments designed to make the circuits of the brain receptive to change after restoration of peripheral hearing. It is possible that pharmacological intervention to scale up inhibitory gain may be one means to restore deficits associated with hearing loss.
Executive summary
Rationale for studying the CNS
▪ Hearing loss during development may lead to deficits that persist even after restoration of peripheral function.
▪ Using brain slice preparations, it is possible to examine effects of hearing loss on central synapse function.
▪ This review is focused on central inhibitory synapses, which play a fundamental role in all aspects of auditory processing and are profoundly altered by hearing loss.
Hearing loss affects sound localization: role of altered inhibitory connections
▪ In humans and animals, the ability to localize sounds can be compromised after hearing loss.
▪ The inhibitory projections from the medial nucleus of the trapezoid body (MNTB) to two brainstem nuclei (lateral superior olivary and medial superior olivary nuclei) that encode sound location undergo anatomical refinement during development. This refinement may depend on synaptic long-term depression.
▪ Following hearing loss, inhibitory MNTB projections do not become properly refined, which may impact sound localization.
Hearing loss affects frequency discrimination: role of diminished inhibitory gain
▪ Deficits in frequency discrimination can result from hearing loss. Broadened frequency tuning of single cells within regions of the auditory system may explain these perceptual deficits.
▪ Inhibition sharpens frequency tuning in auditory regions, including the inferior colliculus and the auditory cortex.
▪ Following hearing loss, inhibitory gain decreases profoundly in many regions of the ascending central auditory system, which may underlie deficits in frequency discrimination.
▪ Such weakening in inhibitory strength may depend on various synaptic mechanisms, including changes in the inhibitory reversal potential, subunit composition of postsynaptic GABAA receptors, and presynaptic release properties.
Similarities between sensorineural hearing loss & conductive hearing loss
▪ Sensorineural hearing loss and conductive hearing loss may produce similar synaptic effects. However, little is known about how conductive hearing loss alters inhibitory synapses.
Similarities between developmental & age-related hearing loss
▪ Developmental and age-related hearing loss exhibit some similar cellular characteristics, including a decrease of inhibitory gain.
Future perspective
▪ Cochlear implants may restore some aspects of neuronal structure and function. It is essential to determine whether inhibitory gain can also be restored.
▪ Pharmacological agents that boost the gain of inhibitory synapses are interesting candidates to restore some deficits associated with hearing loss.
Acknowledgments
Financial & competing interests disclosure
Supported by DC006864 (Dan H Sanes and Vibhakar C Kotak) and DC008920 (Anne E Takesian). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Contributor Information
Anne E Takesian, Center for Neural Science, New York, University, NY 10003, USA, Tel.: +1 212 998 3914, Fax: +1 212 995 4011, aet241@nyu.edu.
Vibhakar C Kotak, Center for Neural Science, New York, University, NY 10003, USA, Tel.: +1 212 998 3916, Fax: +1 212 995 4011, vck1@nyu.edu.
Dan H Sanes, Center for Neural Science & Department of Biology, New York, University, NY 10003, USA, Tel.: +1 212 998 3924, Fax: +1 212 998 4348, sanes@cns.nyu.edu.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Mody M, Schwartz RG, Gravel JS, Ruben RJ. Speech perception and verbal memory in children with and without histories of otitis media. J Speech Lang Hear Res. 1999;42:1069–1079. doi: 10.1044/jslhr.4205.1069. [DOI] [PubMed] [Google Scholar]
- 2.Vernon-Feagans L. Impact of otitis media on speech, language, cognition, and behavior. In: Rosenfield RM, Blueston CD, editors. Evidence-Based Otitis Media. Decker; St Louis, MO, USA: 1999. pp. 353–373. [Google Scholar]
- 3.Psarommatis IM, Goritsa E, Douniadakis D, Tsakanikos M, Kontrogianni AD, Apostolopoulos N. Hearing loss in speech-language delayed children. Int J Pediatr Otorhinolaryngol. 2001;58:205–210. doi: 10.1016/s0165-5876(01)00430-x. [DOI] [PubMed] [Google Scholar]
- 4.Kidd G, Jr, Arbogast TL, Mason CR, Walsh M. Informational masking in listeners with sensorineural hearing loss. J Assoc Res Otolaryngol. 2002;3:107–119. doi: 10.1007/s101620010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emmorey K, Allen JS, Schenker N, Damasio H. A morphometric analysis of auditory brain regions in congenitally deaf adults. Proc Natl Acad Sci USA. 2003;100:10049–10054. doi: 10.1073/pnas.1730169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iverson P. Evaluating the function of phonetic perceptual phenomena within speech recognition: an examination of the perception of/d/-/t/by adult cochlear implant users. J Acoust Soc Am. 2003;113:1056–1064. doi: 10.1121/1.1531985. [DOI] [PubMed] [Google Scholar]
- 7.Moore DR. Auditory processing disorders: acquisition and treatment. J Commun Disord. 2007;40(4):295–304. doi: 10.1016/j.jcomdis.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Hogan SC, Meyer SC, Moore DR. Binaural unmasking returns to normal in teenagers who had otitis media in infancy. Audiol Neurootol. 1996;1:104–111. doi: 10.1159/000259189. [DOI] [PubMed] [Google Scholar]
- 9.Wilmington D, Gray L, Jahrsdoerfer R. Binaural processing after corrected congenital unilateral conductive hearing loss. Hear Res. 1994;74:99–114. doi: 10.1016/0378-5955(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin Genet. 2007;72:1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- 12.Bergstrom L, Hemenway WG, Downs MP. A high risk registry to find congenital deafness. Otolaryngol Clin North Am. 1977;4:369–399. [PubMed] [Google Scholar]
- 13.Billings KR, Kenna MA. Causes of pediatric sensorineural hearing loss. Arch Otolaryngol Head Neck Surg. 1999;125:517–521. doi: 10.1001/archotol.125.5.517. [DOI] [PubMed] [Google Scholar]
- 14.Kenna MA. Medical management of childhood hearing loss. Pediatric Ann. 2004;33(12):822–832. doi: 10.3928/0090-4481-20041201-08. [DOI] [PubMed] [Google Scholar]
- 15▪.Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol. 2004;9:224–233. doi: 10.1159/000078392. Evidence that cochlear implantation in deaf children before the age of 2 years has significant advantages for the acquisition of language and speech perception as compared with implantation after that age. This study supports the existence of a sensitive period for auditory-mediated language development. [DOI] [PubMed] [Google Scholar]
- 16.Nicholas JG, Geers AE. Effects of early experience on the spoken language of deaf children at 3 years of age. Ear Hear. 2007;27(3):286–298. doi: 10.1097/01.aud.0000215973.76912.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NIDCD statistics, NIH Publication No. 00–479
- 18.Dhooge IJ. Risk factors for the development of otitis media. Curr Allergy Asthma Rep. 2003;3(4):321–325. doi: 10.1007/s11882-003-0092-8. [DOI] [PubMed] [Google Scholar]
- 19.Paradise JL. Otitis media during early life: how hazardous to development? A critical review of the evidence. Pediatrics. 1997;68:869–873. [PubMed] [Google Scholar]
- 20.Fria TJ, Cantekin EI, Eichler JA. Hearing acuity of children with otitis media with effusion. Arch Otolaryngol. 1985;111:10–16. doi: 10.1001/archotol.1985.00800030044003. [DOI] [PubMed] [Google Scholar]
- 21.Roberts J, Hunter L, Gravel J, et al. Otitis media, hearing loss, and language learning: controversies and current research. J Dev Behav Pediatr. 2004;25(2):110–122. doi: 10.1097/00004703-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Hogan SC, Stratford KJ, Moore DR. Duration and recurrence of otitis media with effusion in children from birth to 3 years: prospective study using monthly otoscopy and tympanometry . BMJ. 1997;314(7077):350–353. doi: 10.1136/bmj.314.7077.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan SC, Moore DR. Impaired binaural hearing in children with middle ear disease . J Assoc Res Otol. 2003;4:123–129. doi: 10.1007/s10162-002-3007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall AJ, Munro KJ, Heron J. Developmental changes in word recognition threshold from two to five years of age in children with different middle ear status. Int J Audio. 2007;46(7):355–361. doi: 10.1080/14992020701331570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zumach A, Gerrits E, Chenault MN, Anteunis LJ. Otitis media and speech-in-noise recognition in school-aged children . Audiol Neurootol. 2008;14(2):121–129. doi: 10.1159/000162664. [DOI] [PubMed] [Google Scholar]
- 26.Reichman J, Healey WC. Learning disabilities and conductive hearing loss involving otitis media . J Learn Disabil. 1983;16(5):272–278. doi: 10.1177/002221948301600506. [DOI] [PubMed] [Google Scholar]
- 27.Bennett FC, Furukawa CT. Effects of conductive hearing loss on speech, language, and learning development . Clin Rev Allergy. 1984;2(4):377–385. [PubMed] [Google Scholar]
- 28.Schlieper A, Kisilevsky H, Mattingly S, Yorke L. Mild conductive hearing loss and language development, a one year follow-up study. J Dev Behav Pediatr. 1985;6(2):65–68. [PubMed] [Google Scholar]
- 29.Teele DW, Steward IA, Teele JH, Smith DK, Tergonning SJ. Acoustic reflectometry for assessment of hearing loss in children with middle ear effusion . Pediatr Infect Dis J. 1990;9(12):870–872. doi: 10.1097/00006454-199012000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Schönweiler R, Ptok M, Radü HJ. A cross-sectional study of speech and language abilities of children with normal hearing, mild fluctuating hearing loss, or moderate to profound sensorineural hearing loss . Int J Pediatr Otorhinolaryngol. 1998;44(3):251–258. doi: 10.1016/s0165-5876(98)00075-5. [DOI] [PubMed] [Google Scholar]
- 31.Psillas G, Psifidis A, Antoniadou-Hitoglou M, Kouloulas A. Hearing assessment in pre-school children with speech delay. Auris Nasus Larynx. 2006;33(3):259–263. doi: 10.1016/j.anl.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Pittman AL, Lewis DE, Hoover BM, Stelmachowicz PG. Rapid word-learning in normal-hearing and hearing-impaired children: effects of age, receptive vocabulary, and high-frequency amplification . Ear Hear. 2005;26:619–629. doi: 10.1097/01.aud.0000189921.34322.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moeller MP, Tomblin JB, Yoshinaga-Itano C, Connor CM, Jerger S. Current state of knowledge: language and literacy of children with hearing impairment. Ear Hear. 2007;28:740–753. doi: 10.1097/AUD.0b013e318157f07f. [DOI] [PubMed] [Google Scholar]
- 34.Hall JW, 3rd, Grose JH, Pillsbury HC. Long-term effects of chronic otitis media on binaural hearing in children . Arch Otolaryngol Head Neck Surg. 1995;121(8):847–852. doi: 10.1001/archotol.1995.01890080017003. [DOI] [PubMed] [Google Scholar]
- 35.Silverman MS, Clopton BM. Plasticity of binaural interaction I Effect of early auditory deprivation . J Neurophysiol. 1977;40:1266–1274. doi: 10.1152/jn.1977.40.6.1266. [DOI] [PubMed] [Google Scholar]
- 36.Moore DR, Irvine DR. Plasticity of binaural interaction in the cat inferior colliculus . Brain Res. 1981;208:198–202. doi: 10.1016/0006-8993(81)90632-6. [DOI] [PubMed] [Google Scholar]
- 37.Mogdans J, Knudsen EI. Adaptive adjustment of unit tuning to sound localization cues in response to monaural occlusion in developing owl optic tectum . J Neurosci. 1992;12:3473–3484. doi: 10.1523/JNEUROSCI.12-09-03473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mogdans J, Knudsen EI. Early monaural occlusion alters the neural map of interaural level differences in the inferior colliculus of the barn owl . Brain Res. 1993;619(1–2):29–38. doi: 10.1016/0006-8993(93)91593-h. [DOI] [PubMed] [Google Scholar]
- 39.Miller GL, Knudsen EI. Early auditory experience induces frequency-specific, adaptive plasticity in the forebrain gaze fields in the barn owl . J Neurophysiol. 2001;85:2184–2194. doi: 10.1152/jn.2001.85.5.2184. [DOI] [PubMed] [Google Scholar]
- 40.Gold JI, Knudsen EI. Abnormal auditory experience induces frequency-specific adjustments in auditory spatial tuning in the optic tectum of young owls . J Neurophysiol. 2000;82:2197–2209. doi: 10.1152/jn.1999.82.5.2197. [DOI] [PubMed] [Google Scholar]
- 41.Gold JI, Knudsen EI. Adaptive adjustment of connectivity in the inferior colliculus revealed by focal pharmacological inactivation . J Neurophysiol. 2001;85:1575–1584. doi: 10.1152/jn.2001.85.4.1575. [DOI] [PubMed] [Google Scholar]
- 42.Sanes DH, Rubel EW. The ontogeny of inhibition and excitation in the gerbil lateral superior olive . J Neurosci. 1988;8:682–700. doi: 10.1523/JNEUROSCI.08-02-00682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woolf NK, Ryan AF. The development of auditory function in the cochlea of the mongolian gerbil . Hear Res. 1984;13:277–283. doi: 10.1016/0378-5955(84)90081-9. [DOI] [PubMed] [Google Scholar]
- 44.Tollin DJ. The lateral superior olive: a functional role in sound source localization . Neuroscientist. 2003;9(2):127–143. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- 45.Sanes DH, Wooten GF. Development of glycine receptor distribution in the lateral superior olive of the gerbil . J Neurosci. 1987;7:3803–3811. doi: 10.1523/JNEUROSCI.07-11-03803.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grothe B, Sanes DH. Synaptic inhibition influences the temporal coding properties of medial superior olivary neurons: an in vitro study. J Neurosci. 1994;14(3 Pt 2):1701–1709. doi: 10.1523/JNEUROSCI.14-03-01701.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417(6888):543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- 48.Pecka M, Brand A, Behrend O, Grothe B. Interaural time difference processing in the mammalian medial superior olive: the role of glycinergic inhibition. J Neurosci. 2008;28:6914–6925. doi: 10.1523/JNEUROSCI.1660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanes DH, Siverls V. Development and specificity of inhibitory terminal arborizations in the central nervous system. J Neurobiol. 1991;22:837–854. doi: 10.1002/neu.480220805. [DOI] [PubMed] [Google Scholar]
- 50▪.Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. Demonstration that inhibitory projections to the lateral superior olive (LSO) undergo a profound refinement before the onset of hearing. The number of functional medial nucleus of the trapezoid body (MNTB) inputs declines and the remaining connections show a large increase in synaptic strength. [DOI] [PubMed] [Google Scholar]
- 51.Sanes DH. The development of synaptic function and integration in the central auditory system . J Neurosci. 1993;13:2627–2637. doi: 10.1523/JNEUROSCI.13-06-02627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McFadden SL, Walsh EJ, McGee J. Onset and development of auditory brainstem responses in the Mongolian gerbil (Meriones unguiculatus) Hear Res. 1996;100:68–79. doi: 10.1016/0378-5955(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 53.Sanes DH, Takács C. Activity-dependent refinement of inhibitory connections. Eur J Neurosci. 1993;5:570–574. doi: 10.1111/j.1460-9568.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 54.Koch U, Sanes DH. Afferent regulation of glycine receptor distribution in the gerbil LSO . Microsc Res Tech. 1998;41(3):263–269. doi: 10.1002/(SICI)1097-0029(19980501)41:3<263::AID-JEMT9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 55.Werthat F, Alexandrova O, Grothe B, Koch U. Experience-dependent refinement of the inhibitory axons projecting to the medial superior olive. Dev Neurobiol. 2008;68(10):1454–1462. doi: 10.1002/dneu.20660. [DOI] [PubMed] [Google Scholar]
- 56▪.Kapfer C, Seidl AH, Schweizer H, Grothe B. Experience-dependent refinement of inhibitory inputs to auditory coincidence-detector neurons. Nat Neurosci. 2002;5:247–253. doi: 10.1038/nn810. Reveals that inhibitory synapses are gradually eliminated from the dendrites of individual medial superior olivary (MSO) neurons during postnatal development. This refinement is disrupted both in pups with unilateral sensorineural hearing loss (SNHL) and in pups raised in omnidirectional white noise, showing a role for auditory experience. [DOI] [PubMed] [Google Scholar]
- 57.Seidl AH, Grothe B. Development of sound localization mechanisms in the Mongolian gerbil is shaped by early acoustic experience. J Neurophysiol. 2005;94:1028–1036. doi: 10.1152/jn.01143.2004. [DOI] [PubMed] [Google Scholar]
- 58.Kotak VK, Sanes DH. Long-lasting inhibitory synaptic depression is age- and calcium- dependent . J Neurosci. 2000;20:5820–5826. doi: 10.1523/JNEUROSCI.20-15-05820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moore MJ, Caspary DM. Strychnine blocks binaural inhibition in lateral superior olivary neurons . J Neurosci. 1983;3:237–247. doi: 10.1523/JNEUROSCI.03-01-00237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanes DH, Geary WA, Wooten GF, Rubel EW. Quantitative distribution of the glycine receptor in the auditory brain stem of the gerbil . J Neurosci. 1987;7:3793–3802. doi: 10.1523/JNEUROSCI.07-11-03793.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wenthold RJ, Huie D, Altschuler RA, Reeks KA. Glycine immunoreactivity localized in the cochlear nucleus and superior olivary complex . Neuroscience. 1987;22:897–912. doi: 10.1016/0306-4522(87)92968-x. [DOI] [PubMed] [Google Scholar]
- 62.Wenthold RJ, Altschuler RA, Hampson DR. Immunocytochemistry of neurotransmitter receptors . J Electron Microscop Tech. 1990;15:81–96. doi: 10.1002/jemt.1060150108. [DOI] [PubMed] [Google Scholar]
- 63.Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system . J Neurosci. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korada S, Schwartz IL. Development of GABA, glycine and their receptors in the auditory brainstem of gerbil: a light and electron microscopic study . J Comp Neurol. 1999;409:664–681. doi: 10.1002/(sici)1096-9861(19990712)409:4<664::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 65.Nabekura J, Katsurabayashi S, Kakazu Y, et al. Developmental switch from GABA to glycine release in single central synaptic terminals . Nat Neurosci. 2004;7:17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- 66.Chang EH, Kotak VC, Sanes DH. Long-term depression of synaptic inhibition is expressed postsynaptically in the developing auditory system. J Neurophysiol. 2003;90:1479–1488. doi: 10.1152/jn.00386.2003. [DOI] [PubMed] [Google Scholar]
- 67.Kotak VC, DiMattina C, Sanes DH. GABAB and Trk receptor signaling mediates long-lasting inhibitory synaptic depression. J Neurophysiol. 2001;86:536–540. doi: 10.1152/jn.2001.86.1.536. [DOI] [PubMed] [Google Scholar]
- 68.Kotak VC, Sanes DH. Gain adjustment of inhibitory synapses in the auditory system . Biol Cybern. 2003;89(5):363–370. doi: 10.1007/s00422-003-0441-7. [DOI] [PubMed] [Google Scholar]
- 69.Kotak VK, Breithaupt AD, Sanes DH. Developmental hearing loss eliminates long-term potentiation in the auditory cortex . Proc Natl Acad Sci USA. 2007;104(9):3550–3555. doi: 10.1073/pnas.0607177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gabriele ML, Brunso-Bechtold JK, Henkel CK. Plasticity in the development of afferent patterns in the inferior colliculus of the rat after unilateral cochlear ablation . J Neurosci. 2000;20:6939–6949. doi: 10.1523/JNEUROSCI.20-18-06939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71▪.Franklin SR, Brunso-Bechtold JK, Henkel CK. Bilateral cochlear ablation in postnatal rat disrupts development of banded pattern of projections from the dorsal nucleus of the lateral lemniscus to the inferior colliculus. Neuroscience. 2008;154:346–354. doi: 10.1016/j.neuroscience.2008.02.011. Study showing that the banded pattern of labeled inhibitory projections from a lemniscal nucleus to the inferior colliculus is disrupted in rats with bilateral cochlear ablations. The results suggest that spontaneous activity is necessary for the segregation of inhibitory terminals through inhibitory synapse elimination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadaka Y, Weinfeld E, Lev DL, White EL. Changes in mouse barrel synapses consequent to sensory deprivation from birth . J Comp Neurol. 2003;457(1):75–86. doi: 10.1002/cne.10518. [DOI] [PubMed] [Google Scholar]
- 73.Chattopadhyaya B, Di Cristo G, Higashiyama H, et al. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period . J Neurosci. 2004;24(43):9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪.Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABA-A receptors in the auditory cortex. Cereb Cortex. 2008;18(12):2855–2867. doi: 10.1093/cercor/bhn044. Presents quantitative electron microscopic immunocytochemical evidence for pre- and post-synaptic changes at inhibitory synapses following hearing loss. SNHL reduces the number of GABAergic axon terminals, increases the key GABA synthesizing enzyme (GAD65/67), and leads to decreased trafficking of the GABA A receptor subunit β2/3 to the postsynaptic membrane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75▪.Halliday LF, Bishop DV. Frequency discrimination and literacy skills in children with mild to moderate sensorineural hearing loss. J Speech Lang Hear Res. 2005;48(5):1187–1203. doi: 10.1044/1092-4388(2005/083). Demonstrates that children with SNHL show deficits in frequency discrimination for both high and low frequencies. However, the study suggests that such impairments do not necessarily result in language or reading deficits. [DOI] [PubMed] [Google Scholar]
- 76.Cranford JL, Thompson N, Hoyer E, Faires W. Brief tone discrimination by children with histories of early otitis media. J Am Acad Audio. 1997;8(2):137–141. [PubMed] [Google Scholar]
- 77.Nelson DA, Freyman RL. Psychometric functions for frequency discrimination from listeners with sensorineural hearing loss . J Acoust Soc Am. 1986;79(3):799–805. doi: 10.1121/1.393470. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Salvi RJ, Powers N. Plasticity of response properties of inferior colliculus neurons following acute cochlear damage. J Neurophysiol. 1996;75:171–183. doi: 10.1152/jn.1996.75.1.171. [DOI] [PubMed] [Google Scholar]
- 79.Eggermont JJ, Komiya H. Moderate noise trauma in juvenile cats results in profound cortical topography. Hear Res. 2000;142(1–2):89–101. doi: 10.1016/s0378-5955(00)00024-1. [DOI] [PubMed] [Google Scholar]
- 80.Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage . Hear Res. 2002;168:238–249. doi: 10.1016/s0378-5955(02)00360-x. [DOI] [PubMed] [Google Scholar]
- 81.Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82(3):601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- 82.Irvine DR, Rajan R, Smith R. Effects of restricted cochlear lesions in adult cats on the frequency organization of the inferior colliculus. J Comp Neurol. 2003;467(3):354–374. doi: 10.1002/cne.10921. [DOI] [PubMed] [Google Scholar]
- 83.Barsz K, Wilson WW, Walton JP. Reorganization of receptive fields following hearing loss in inferior colliculus neurons . Neuroscience. 2007;147(2):532–545. doi: 10.1016/j.neuroscience.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness . J Comp Neurol. 1989;282(3):456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- 85.Harrison RV, Nagasawa A, Smith DW, Stanton S, Mount RJ. Reorganization of auditory cortex after neonatal high frequency cochlear hearing loss . Hear Res. 1991;54(1):11–19. doi: 10.1016/0378-5955(91)90131-r. [DOI] [PubMed] [Google Scholar]
- 86.Irvine DR, Rajan R. Injury-induced reorganization of frequency maps in adult auditory cortex: the role of unmasking of normally-inhibited inputs. Acta Otolaryngol Suppl. 1997;532:39–45. doi: 10.3109/00016489709126143. [DOI] [PubMed] [Google Scholar]
- 87.Rajan R. Receptor organ damage causes loss of cortical surround inhibition without topographic map plasticity. Nat Neurosci. 1998;1:138–143. doi: 10.1038/388. [DOI] [PubMed] [Google Scholar]
- 88.Irvine DR, Rajan R, Brown M. Injury- and use-related plasticity in adult auditory cortex. Audiol Neurootol. 2001;6(4):192–195. doi: 10.1159/000046831. [DOI] [PubMed] [Google Scholar]
- 89.Shuz A, Palm G. Density of neurons and synapses in the cerebral cortex of the mouse. J Comp Neurology. 1989;286(4):442–455. doi: 10.1002/cne.902860404. [DOI] [PubMed] [Google Scholar]
- 90.Chagnac-Amitai Y, Connors BW. Horizontal spread of synchronized activity in neocortex and its control by GABA-mediated inhibition . J Neurophysiol. 1989;61:747–758. doi: 10.1152/jn.1989.61.4.747. [DOI] [PubMed] [Google Scholar]
- 91.DeFelipe J, Farinas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992;39(6):563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- 92.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 93.Müller CM, Scheich H. Contribution of GABAergic inhibition to the response characteristics of auditory units in the avian forebrain. J Neurophysiol. 1988;59:1673–1689. doi: 10.1152/jn.1988.59.6.1673. [DOI] [PubMed] [Google Scholar]
- 94.Chen QC, Jen PH. Bicuculline application affects discharge patterns, rate-intensity functions, and frequency tuning characteristics of bat auditory cortical neurons . Hear Res. 2000;150:161–174. doi: 10.1016/s0378-5955(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex . Neuroreport. 2000;11(5):1137–1140. doi: 10.1097/00001756-200004070-00045. [DOI] [PubMed] [Google Scholar]
- 96.Wang J, McFadden SL, Caspary D, Salvi R. γ-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;19:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- 97.Foeller E, Vater M, Kössl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. J Assoc Res Otolaryngol. 2001;2:279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- 99.Yang L, Pollak GD, Resler C. GABAergic circuits sharpen tuning curves and modify response properties in the mustache bat inferior colliculus. J Neurophysiol. 1992;68:1760–1774. doi: 10.1152/jn.1992.68.5.1760. [DOI] [PubMed] [Google Scholar]
- 100.Metherate R, Kaur S, Kawai H, Lazar R, Liang K, Rose HJ. Spectral integration in auditory cortex: mechanisms and modulation . Hear Res. 2005;206(1–2):146–158. doi: 10.1016/j.heares.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 101.Oswald AM, Schiff ML, Reyes AD. Synaptic mechanisms underlying auditory processing . Curr Opin Neurobiol. 2006;16(4):371–376. doi: 10.1016/j.conb.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 102.Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol. 2004;91:2251–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- 103.Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex . Neuron. 2003;47(3):437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 104.Liu BH, Wu GK, Arbuckle R, Tao HW, Zhang LI. Defining cortical frequency tuning with recurrent excitatory circuitry . Nat Neurosci. 2007;10(12):1594–1600. doi: 10.1038/nn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu GK, Arbuckle R, Liu BH, Tao HW, Zhang LI. Lateral sharpening of cortical frequency tuning by approximately balanced inhibition . Neuron. 2008;58(1):132–143. doi: 10.1016/j.neuron.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Atencio CA, Schreiner CE. Spectrotemporal processing differences between auditory cortical fast-spiking and regular-spiking neurons . J Neurosci. 2008;28(15):3897–3910. doi: 10.1523/JNEUROSCI.5366-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suga N, Manabe T. Neural basis of amplitude-spectrum representation in auditory cortex of the moustached bat. J Neurophysiol. 1982;47(2):225–255. doi: 10.1152/jn.1982.47.2.225. [DOI] [PubMed] [Google Scholar]
- 108.Shamma SA. Speech processing in the auditory system II Lateral inhibition and the central processing of speech evoked activity in the auditory nerve . J Acoust Soc Am. 1985;78(5):1622–1632. doi: 10.1121/1.392800. [DOI] [PubMed] [Google Scholar]
- 109.Calford MB, Semple MN. Monaural inhibition in cat auditory cortex . J Neurophysiol. 1995;73(5):1876–1891. doi: 10.1152/jn.1995.73.5.1876. [DOI] [PubMed] [Google Scholar]
- 110.Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex . Nature. 2003;424(6945):201–205. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]
- 111.Tan AY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons . J Neurophysiol. 2004;92(1):630–643. doi: 10.1152/jn.01020.2003. [DOI] [PubMed] [Google Scholar]
- 112.Kitzes LM, Semple MN. Single-unit responses in the inferior colliculus: effects of neonatal unilateral cochlear ablation . J Neurophysiol. 1985;53(6):1483–1500. doi: 10.1152/jn.1985.53.6.1483. [DOI] [PubMed] [Google Scholar]
- 113.Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N. Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hear Res. 1990;50:245–258. doi: 10.1016/0378-5955(90)90049-u. [DOI] [PubMed] [Google Scholar]
- 114.Szczepaniak WS, Møller AR. Evidence of decreased GABAergic influence on temporal integration in the inferior colliculus following acute noise exposure: a study of evoked potentials in the rat . Neurosci Lett. 1995;196:77–80. doi: 10.1016/0304-3940(95)11851-m. [DOI] [PubMed] [Google Scholar]
- 115.Qiu C, Salvi R, Ding D, Burkard R. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hear Res. 2000;139(1–2):153–171. doi: 10.1016/s0378-5955(99)00171-9. [DOI] [PubMed] [Google Scholar]
- 116.Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer specific manner. Cereb Cortex. 2000;10:714–726. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- 117.Noreña AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma . J Neurophysiol. 2003;90:2387–2401. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- 118.Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss . Hear Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 119.Raggio MW, Schreiner CE. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation III Activation patterns in short- and long-term deafness . J Neurophysiol. 1999;82(6):3506–3526. doi: 10.1152/jn.1999.82.6.3506. [DOI] [PubMed] [Google Scholar]
- 120.Rajan R. Plasticity of excitation and inhibition in the receptive field of primary auditory cortical neurons after limited receptor organ damage. Cereb Cortex. 2001;11(2):171–182. doi: 10.1093/cercor/11.2.171. [DOI] [PubMed] [Google Scholar]
- 121.Leao RN, Oleskevich S, Sun H, Bautista M, Fyffe RE, Walmsley B. Differences in glycinergic mIPSCs in the auditory brain stem of normal and congenitally deaf neonatal mice. J Neurophysiol. 2004;91(2):1006–1012. doi: 10.1152/jn.00771.2003. [DOI] [PubMed] [Google Scholar]
- 122.Suneja SK, Benson CG, Potashner SJ. Glycine receptors in adult guinea pig brain stem auditory nuclei: regulation after unilateral cochlear ablation . Exp Neurol. 1998;154(2):473–488. doi: 10.1006/exnr.1998.6946. [DOI] [PubMed] [Google Scholar]
- 123.Asako M, Holt AG, Griffith RD, Buras ED, Altshuler RA. Deafness-related decrease in glycine-immunoreactive labeling in the rat cochlear nucleus . J Neurosci. 2005;81(1):102–109. doi: 10.1002/jnr.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Buras ED, Holt AG, Griffith RD, Asako M, Altschuler RA. Changes in glycine immunoreactivity in the rat superior olivary complex following deafness . J Comp Neurol. 2006;494(1):179–189. doi: 10.1002/cne.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suneja SK, Potashner SJ, Benson CG. Plastic changes in glycine and GABA release and Uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation . Exp Neurol. 1998;151(2):273–288. doi: 10.1006/exnr.1998.6812. [DOI] [PubMed] [Google Scholar]
- 126.Potashner SJ, Suneja SK, Benson CG. Altered glycinergic synaptic activities in guinea pig brain stem auditory nuclei after unilateral cochlear ablation . Hear Res. 2000;147(1–2):125–136. doi: 10.1016/s0378-5955(00)00126-x. [DOI] [PubMed] [Google Scholar]
- 127.Oleskevich S, Walmsley B. Synaptic transmission in the auditory brainstem of normal and congenitally deaf mice. J Physiol. 2002;540(2):447–455. doi: 10.1113/jphysiol.2001.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Francis HW, Manis PB. Effects of deafferentation on the electrophysiology of ventral cochlear nucleus neurons . Hear Res. 2000;149(1–2):91–105. doi: 10.1016/s0378-5955(00)00165-9. [DOI] [PubMed] [Google Scholar]
- 129.Zhang JS, Kaltenbach JA. Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sounds . Neurosci Lett. 1998;250(3):197–200. doi: 10.1016/s0304-3940(98)00482-0. [DOI] [PubMed] [Google Scholar]
- 130.Kotak VC, Sanes DH. Developmental influence of glycinergic transmission: regulation of NMDA receptor-mediated EPSPs . J Neurosci. 1996;16:1836–1843. doi: 10.1523/JNEUROSCI.16-05-01836.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pollak GD, Burger RM, Klug A. Dissecting the circuitry of the auditory system. Trends Neurosci. 2003;26(1):33–39. doi: 10.1016/s0166-2236(02)00009-7. [DOI] [PubMed] [Google Scholar]
- 132.Vale C, Sanes DH. Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain . J Neurosci. 2000;20(5):1912–1920. doi: 10.1523/JNEUROSCI.20-05-01912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zucker RS, Regehr WG. Short-term synaptic plasticity . Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 134.Stevens C. Neurotransmitter release at central synapses. Neuron. 2003;40:381–388. doi: 10.1016/s0896-6273(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 135.Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus . Eur J Neurosci. 2002;16(12):2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- 136.Cruikshank SI, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87(1):361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- 137▪.Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25(15):3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. Demonstrates that hearing loss enhances excitability in the auditory cortex via various intrinsic and synaptic mechanisms, including a decrease in the magnitude of inhibitory synaptic potentials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138▪.Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex. 2008;18(9):2098–2108. doi: 10.1093/cercor/bhm233. Changes in the subunit composition of the postsynaptic GABA A receptor may underlie a downregulation in inhibitory transmission in the auditory cortex following developmental hearing loss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takesian AE, Kotak VC, Sanes DH. Unpublished observations. [Google Scholar]
- 140.Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and γ-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Delpire E, Rauchman MI, Beier DR, Hebert SC, Gullans SR. Molecular cloning and chromosome localization of a putative basolateral Na+-K+–2Cl− cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J Biol Chem. 1994;269:25677–25683. [PubMed] [Google Scholar]
- 142.Payne JA. Functional characterization of the neuronal-specific K–Cl cotransporter: implications for K+o regulation. Am J Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 143.Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain . J Biol Chem. 1996;27:16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 144.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development, and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 145▪.Vale C, Schoorlemmer J, Sanes DH. Deafness disrupts chloride transporter function and inhibitory synaptic transmission. J Neurosci. 2003;23(20):7516–7524. doi: 10.1523/JNEUROSCI.23-20-07516.2003. Presents evidence for decreased function of the K–Cl cotransporter in the inferior colliculus following SNHL, providing one cellular explanation for diminished inhibitory synaptic currents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of γ-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci USA. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABA A receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- 148.Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA A receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- 149.Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric γ-amobutyric acid type A receptors . J Biol Chem. 1996;271:89–96. doi: 10.1074/jbc.271.1.89. [DOI] [PubMed] [Google Scholar]
- 150.Baumann SW, Baur R, Sigel E. Individual properties of the two functional agonist sites in GABA A receptors. J Neurosci. 2003;23:11158–11166. doi: 10.1523/JNEUROSCI.23-35-11158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABA A receptor subunit mRNAs in the rat brain I Telencephalon, diencephalons, mesencephalon. J Neurosci. 1992;12(3):1063–1076. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital . Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- 153.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19(4):139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 154.Möhler H. GABAA receptor diversity and pharmacology. Cell Tiss Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 155.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain III Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABAA receptor α1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Banks MI, Hardie JB, Pearce RA. Development of GABAA receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol. 2002;88:3097–3107. doi: 10.1152/jn.00026.2002. [DOI] [PubMed] [Google Scholar]
- 158.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 159.Bosman LW, Heinen K, Spijker S, Brussaard AB. Mice lacking the major adult GABAA receptor subtype have normal number of synapses, but retain juvenile IPSC kinetics until adulthood. J Neurophysiol. 2005;94:338–346. doi: 10.1152/jn.00084.2005. [DOI] [PubMed] [Google Scholar]
- 160.Huntsman MM, Huguenard JR. Fast IPSCs in rat thalamic reticular nucleus require the GABAA receptor β1 subunit . J Physiol. 2006;572:459–475. doi: 10.1113/jphysiol.2006.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ing T, Poulter MO. Diversity of GABAA receptor synaptic currents on individual pyramidal cortical neurons. Eur J Neurosci. 2007;25:723–734. doi: 10.1111/j.1460-9568.2007.05331.x. [DOI] [PubMed] [Google Scholar]
- 162.Browning ND, Bureau M, Dudek EM, Olsen RW. Protein kinase C and cAMP-dependent protein kinase phosphorylate the β subunit of the purifed γ-amimobutyric acid A receptor . Proc Natl Acad Sci USA. 1990;87:1315–1318. doi: 10.1073/pnas.87.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 164.Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 165.Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res Rev. 2004;45:196–212. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 166.Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC postsynaptic GABAA amplitude by decreasing the number of receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation . Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- 168.Fuchs JL, Salazar E. Effects of whisker trimming on GABAA receptor binding in the barrel cortex of developing and adult rats. J Comp Neurol. 1998;395(2):209–216. [PubMed] [Google Scholar]
- 169.Potashner SJ, Suneja SK, Benson CG. Regulation of D-aspartate release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation . Exp Neurol. 1997;148(1):222–235. doi: 10.1006/exnr.1997.6641. [DOI] [PubMed] [Google Scholar]
- 170.Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex . J Neurosci. 2007;27(35):9417–9426. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tucci DL, Cant NB, Durham D. Conductive hearing loss results in a decrease in central auditory system activity in the young gerbil. Laryngoscope. 1999;109(9):1359–1371. doi: 10.1097/00005537-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 172.Hutson KA, Durham D, Imig T, Tucci DL. Consequences of unilateral hearing loss: cortical adjustment to unilateral deprivation . Hear Res. 2008;237(1–2):19–31. doi: 10.1016/j.heares.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Knudsen EI, Knudsen PF, Esterly SD. A critical period for the recovery of sound localization accuracy following monaural occlusion in the barn owl . J Neurosci. 1984;4(4):1012–1020. doi: 10.1523/JNEUROSCI.04-04-01012.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hutson KA, Durham D, Tucci DL. Reversible conductive hearing loss: restored activity in the central auditory system . Audiol Neurootol. 2009;14(2):69–77. doi: 10.1159/000158535. [DOI] [PubMed] [Google Scholar]
- 175.Pasic TR, Rubel EW. Cochlear nucleus cell size is regulated by auditory nerve electrical activity . Otolaryngol Head Neck Surg. 1991;104(1):6–13. doi: 10.1177/019459989110400103. [DOI] [PubMed] [Google Scholar]
- 176.Pasic TR, Moore DR, Rubel EW. Effect of altered neuronal activity on cell size in the medial nucleus of the trapezoid body and ventral cochlear nucleus of the gerbil . J Comp Neurol. 1994;348(1):111–120. doi: 10.1002/cne.903480106. [DOI] [PubMed] [Google Scholar]
- 177.Kawai H, Lazar R, Metherate R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nat Neurosci. 2007;10(9):1168–1175. doi: 10.1038/nn1956. [DOI] [PubMed] [Google Scholar]
- 178.De Villers-Sidano E, Change EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat . J Neurosci. 2007;27(1):180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dahmen JC, King AJ. Learning to hear: plasticity of auditory cortical processing . Curr Opin Neurobiol. 2007;17(4):456–464. doi: 10.1016/j.conb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 180.McAlpine D, Martin RL, Mossop JE, Moore DR. Response properties of neurones in the inferior colliculus of the monaurally-deafened ferret to acoustic stimulation of the intact ear . J Neurophysiol. 1997;78:767–779. doi: 10.1152/jn.1997.78.2.767. [DOI] [PubMed] [Google Scholar]
- 181.Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 182▪.Caspary DM, Raza A, Lawhorn Armour BA, Pippin J, Arneric SP. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implications for neural presbycusis. J Neurosci. 1990;10:2363–2372. doi: 10.1523/JNEUROSCI.10-07-02363.1990. Reveals a profound loss of GABA within the inferior colliculus in aged rats compared with young adult rats. An immunocytochemical assay shows an age-related reduction in the number of GABA-positive neurons. Moreover, a neurochemical assay demonstrates a decrease in both basal and K+-evoked release of GABA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Milbrandt JC, Albin RL, Caspary DM. Age-related decrease in GABAB receptor binding in the Fischer 344 rat inferior colliculus. Neurobiol Aging. 1994;15:699–703. doi: 10.1016/0197-4580(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 184.Raza A, Milbrandt JC, Arneric SP, Caspary DM. Age-related changes in brainstem auditory neurotransmitters: measures of GABA and acetylcholine function . Hear Res. 1994;77:221–230. doi: 10.1016/0378-5955(94)90270-4. [DOI] [PubMed] [Google Scholar]
- 185.Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus . Exp Gerontol. 1995;30(3–4):349–360. doi: 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- 186.Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system . J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Willott JF, Milbrandt JC, Bross LS, Caspary DM. Glycine immunoreactivity and receptor binding in the cochlear nucleus of C57BL/6J and CBA/CaJ mice: effects of cochlear impairment and aging . J Comp Neurol. 1997;385(3):405–414. [PubMed] [Google Scholar]
- 188.Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs . J Neurosci. 2005;25(47):10952–10959. doi: 10.1523/JNEUROSCI.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gutiérrez A, Khan ZU, De Blas AL. Immunocytochemical localization of γ 2 short and γ 2 long subunits of the GABAA receptor in the rat brain. J Neurosci. 1994;14:7168–7179. doi: 10.1523/JNEUROSCI.14-11-07168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Milbrandt JC, Hunter C, Caspary DM. Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 244 rat inferior colliculus. J Comp Neurol. 1997;379(3):455–465. doi: 10.1002/(sici)1096-9861(19970317)379:3<455::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 191.Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABAA receptor subunit composition and function in rat auditory system. Neuroscience. 1999;93:307–312. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- 192.Krenning J, Hughes LF, Caspary DM, Helfert RH. Age-related glycine receptor subunit changes in the cochlear nucleus of Fischer-344 rats . Laryngoscope. 1998;108:443–465. doi: 10.1097/00005537-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 193.Bledsoe SC, Jr, Nagase S, Miller JM, Altschuler RA. Deafness-induced plasticity in the mature central auditory system . Neuroreport. 1995;7:225–229. [PubMed] [Google Scholar]
- 194.Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex . Neuroscience. 2005;132:1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 195.Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol. 2009;44(3):161–169. doi: 10.1016/j.exger.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 196.Holt AG, Asako M, Lomax CA, et al. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity . J Neurochem. 2005;93:1069–1086. doi: 10.1111/j.1471-4159.2005.03090.x. [DOI] [PubMed] [Google Scholar]
- 197.Argence M, Saez I, Sassu R, Vassias I, Vidal PP, de Waele C. Modulation of inhibitory and excitatory synaptic transmission in rat inferior colliculus after unilateral cochleectomy: an in situ and immunofluorescence study. Neuroscience. 2006;141:1193–1207. doi: 10.1016/j.neuroscience.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 198.Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care: scientific review . JAMA. 2003;289(15):1976–1985. doi: 10.1001/jama.289.15.1976. [DOI] [PubMed] [Google Scholar]
- 199.Kral A, Majernik V. On lateral inhibition in the auditory system. Gen Physiol Biophys. 1996;15(2):109–127. [PubMed] [Google Scholar]
- 200.Eggermont JJ, Roberts LE. The neuroscience of tinnitus . Trends Neurosci. 2004;27(11):676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 201.Bauer CA, Brozoski TJ. Effect of gabapentin on the sensation and impact of tinnitus . Laryngoscope. 2006;116(5):675–681. doi: 10.1097/01.MLG.0000216812.65206.CD. [DOI] [PubMed] [Google Scholar]
- 202.Sanes DH, Chokshi P. Glycinergic transmission influences the development of dendritic shape. Neuroreport. 1992;3(4):323–326. doi: 10.1097/00001756-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 203.Kirkwood A, Bear MF. Elementary forms of synaptic plasticity in the visual cortex . Biol Res. 1995;28(1):73–80. [PubMed] [Google Scholar]
- 204.Hensch TK. Critical period mechanisms in developing visual cortex . Curr Top Dev Biol. 2005;69:215–237. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- 205.Möhler H, Fritschy JM, Crestani F, Hensch T, Rudolph U. Specific GABA A circuits in brain development and therapy. Biochem Pharmacol. 2004;68(8):1685–1690. doi: 10.1016/j.bcp.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 206.Ryugo DK, Kretzmer EA, Niparko JK. Restoration of auditory nerve synapses in cats by cochlear implants . Science. 2005;310(5753):1490–1492. doi: 10.1126/science.1119419. [DOI] [PubMed] [Google Scholar]
- 207.Lustig LR, Leake PA, Snyder RL, Rebscher SJ. Changes in the cat cochlear nucleus following neonatal deafening and chronic intracochlear electrical stimulation . Hear Res. 1994;74(1–2):29–37. doi: 10.1016/0378-5955(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 208.Snyder R, Leake P, Rebscher S, Beitel R. Temporal resolution of neurons in cat inferior colliculus to intracochlear electrical stimulation: effects of neonatal deafening and chronic stimulation. J Neurophysiol. 1995;73:449–467. doi: 10.1152/jn.1995.73.2.449. [DOI] [PubMed] [Google Scholar]
- 209.Klinke R, Kral A, Heid S, Tillein J, Hartmann R. Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. Science. 1999;285:1729–1733. doi: 10.1126/science.285.5434.1729. [DOI] [PubMed] [Google Scholar]
- 210.Vollmer M, Leake PA, Beitel RE, Rebscher SJ, Snyder RL. Degradation of temporal resolution in the auditory midbrain after prolonged deafness is reversed by electrical stimulation of the cochlea . J Neurophysiol. 2005;93(6):3339–3355. doi: 10.1152/jn.00900.2004. [DOI] [PubMed] [Google Scholar]
- 211.Snyder RL, Rebscher SJ, Cao KL, Leake PA, Kelly K. Chronic intracochlear electrical stimulation in the neonatally deafened cat. I: Expansion of central representation . Hear Res. 1990;50(1–2):7–33. doi: 10.1016/0378-5955(90)90030-s. [DOI] [PubMed] [Google Scholar]
- 212.Leake PA, Snyder RL, Rebscher SJ, Moore CM, Vollmer M. Plasticity in central representations in the inferior colliculus induced by single- vs. two-channel electrical stimulation by a cochlear implant after neonatal deafness. Hear Res. 2000;147(1–2):221–241. doi: 10.1016/s0378-5955(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 213.Fallon JB, Irvine DR, Shepherd RK. Cochlear implant use following neonatal deafness influences the cochleotopic organization of the primary auditory cortex in cats . J Comp Neurol. 2009;512:101–114. doi: 10.1002/cne.21886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kral A, Tillein J, Hubka P, et al. Spatiotemporal patterns of cortical activity with bilateral cochlear implants in congenital deafness . J Neurosci. 2009;29(3):811–827. doi: 10.1523/JNEUROSCI.2424-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Argence M, Vassias I, Kerhuel L, Vidal PP, de Waele C. Stimulation by cochlear implant in unilaterally deaf rats reverses the decrease of inhibitory transmission in the inferior colliculus. Eur J Neurosci. 2008;28:1589–1602. doi: 10.1111/j.1460-9568.2008.06454.x. [DOI] [PubMed] [Google Scholar]
- 216.Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300(1):2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 217.Levanthal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300(5620):812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- 218▪.Gleich O, Hamann I, Klump GM, Kittel M, Strutz J. Boosting GABA improves impaired auditory temporal resolution in the gerbil. Neuroreport. 2003;14:1877–1880. doi: 10.1097/00001756-200310060-00024. Study suggests that pharmacological elevation of GABA levels may improve auditory temporal resolution in aged gerbils. Elevated gap-detection thresholds in aged gerbils were restored to normal values after treatment with Sabril, which increases the concentration of GABA in the brain. [DOI] [PubMed] [Google Scholar]
- 219.Bauer J, Cooper-Mahkorn D. Tiagabine: efficacy and safety in partial seizures – current status. Neuropsychiatr Dis Treat. 2008;4(4):731–736. doi: 10.2147/ndt.s833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Addolorato G, Leggio L, Agabio R, Colombo G, Gasbarrini G. Baclofen: a new drug for the treatment of alcohol dependence. Int J Clin Pract. 2006;60(8):1003–1008. doi: 10.1111/j.1742-1241.2006.01065.x. [DOI] [PubMed] [Google Scholar]