Abstract
Statement of Translational Relevance
Stromal cells in the tumor microenvironment, such as macrophages, play an active role in tumor growth and angiogenesis. Lutgendorf et al., examined a series of ovarian cancer patients and demonstrated that negative behavioral factors such as depressive symptoms are strongly related to high matrix metalloproteinase-9 (MMP-9) levels in macrophages whereas positive factors such as social support are inversely related to MMP-9 and VEGF levels in tumor cells. These findings provide a new understanding of biobehavioral influences on the tumor microenvironment and may have implications for patient outcome and targeted pharmacological and/or behavioral interventions for ovarian cancer patients.
Purpose
Stromal cells in the tumor microenvironment, such as macrophages, play an active role in tumor growth and angiogenesis. However, little is known about relationships of biobehavioral factors with angiogenic cytokines and matrix metalloproteinases (MMPs) produced by stromal cells. This study examined distress, MMPs and angiogenic cytokines in ovarian cancer patients and in vitro.
Experimental Design
Patients suspected of ovarian cancer completed preoperative questionnaires. At surgery, 56 were confirmed to have epithelial ovarian cancer. Tumor samples were analyzed for macrophage (CD68+) and tumor cell levels of MMPs-2, -9, and vascular endothelial growth factor (VEGF). In vitro stimulation of isolated macrophage cells by the stress hormones norepinephrine (NE) and cortisol was performed to assess effects on MMP-9.
Results
Depressed patients demonstrated significant elevations of MMP-9 in CD68+ cells, adjusting for stage (p<0.0001). Patients with higher levels of current stress (p=0.01), life stress over the last 6 months (p=0.004), and general negative affect (p=0.007) also demonstrated significantly greater MMP-9 in CD68+ cells. In contrast, higher social support was associated with lower levels of MMP-9 (p=0.023) and VEGF (p=0.036) in tumor cells. In vitro analyses demonstrated that macrophage MMP-9 production could be directly enhanced (up to a 2-fold increase) by the stress hormones NE and cortisol.
Conclusions
Ovarian cancer patients with elevated depressive symptoms, chronic stress, and low social support demonstrated elevations in MMP-9 in tumor associated macrophages. Direct in vitro enhancement of stromal MMP-9 production by stress hormones was also demonstrated. These findings may have implications for patient outcomes in ovarian cancer.
Keywords: tumor associated macrophages, matrix metalloproteinases, ovarian cancer, angiogenesis, depression
INTRODUCTION
Recent reports have demonstrated that neuroendocrine stress hormones are able to stimulate the production of proangiogenic factors and matrix metalloproteinases (MMPs) from ovarian cancer cells, thereby enhancing the invasive and metastatic potential of these cells (1–3). Similar neuroendocrine induced enhancement of matrix metalloproteinases and VEGF has been observed from cell lines from other cancers (4, 5). Behavioral distress has been linked to higher levels of angiogenic cytokines such as vascular endothelial growth factor (VEGF) and interleukin-6 (IL-6) in ovarian cancer patients (6, 7). Stromal cells in the tumor microenvironment such as macrophages are known to play an active role in the promotion of tumor growth and angiogenesis (8). However, little is known about relationships of behavioral distress with angiogenic cytokines and MMPs produced by stromal cells, which is the focus of the current study.
Tumor associated macrophages (TAMs) are a major component of most, if not all, tumor microenvironments (9) and are recruited to tumors by a number of growth factors and chemotactic factors. In the presence of a proinflammatory tumor microenvironment, macrophages are induced to switch from their phagocytic phenotype to one that promotes production of factors that stimulate angiogenesis, breakdown of matrix, and tumor cell motility (8). Activated macrophages influence angiogenesis in several ways including production of matrix metalloproteinases 2 and 9 (MMP-2 and MMP-9) (8, 10). These MMPs are particularly relevant for promoting ovarian cancer growth and progression (11–13). In ovarian cancer, macrophages, rather than tumor cells, are thought to be the main source of MMP-9 (10). Macrophages also secrete angiogenic cytokines including VEGF (10, 14), a potent proangiogenic molecule that induces endothelial cells in nearby vascular microvessels to proliferate, migrate, and alter their pattern of gene expression in favor of angiogenesis (15). Macrophages contain both alpha (16) and beta-adrenergic receptors (17), which may mediate catecholamine stimulated production of proinflammatory cytokines by these cells (18, 19). Many other activities of monocytes are regulated by catecholamines (20). For example, catecholamines are known to promote the expression of MMP-9 in monocyte cell lines, and this effect is blocked by a beta antagonist (21). In the cardiovascular literature, behavioral factors such as distress and hostility have been related to macrophage activation in blood vessels (22, 23). However, it is not known whether these relationships with behavioral factors exist among macrophages in the tumor microenvironment.
To address this knowledge gap, we examined relationships between a panel of behavioral measures and levels of MMP-2, MMP-9, and VEGF in TAMs and autologous tumor cells in ovarian cancer patients. We hypothesized that higher levels of chronic life stress, depression, and perceived stress would be associated with higher levels of TAMs and tumor cells expressing MMP-2, MMP-9, and VEGF. It was also hypothesized that social support, as a buffer to psychological stress, would be associated with lower levels of TAMs and tumor cells expressing these factors. In vitro molecular analyses examined whether macrophage production of MMPs could be directly enhanced by the stress hormones norepinephrine (NE) and cortisol.
MATERIALS AND METHODS
Participants
Women over 18 years of age with a new diagnosis of a pelvic or abdominal mass suspected to be ovarian cancer were recruited for the study. Inclusion was confirmed by histologic diagnosis with primary invasive epithelial ovarian, primary papillary peritoneal, or primary fallopian tube cancer. Patients were excluded for primary cancer of another organ site, a non-epithelial ovarian tumor, an ovarian tumor of low malignant potential, a history of systemic corticosteroid medication use in the last 4 months or comorbidities known to alter the immune response such as an immunomodulatory or inflammatory disease. Of 139 patients who met inclusion criteria and were approached for participation, 21 declined. Of the 118 patients who agreed to participate, 40 patients had benign disease, 9 had tumors of low malignant potential, and 5 had other cancers, 4 withdrew prior to surgery and 1 chose not to have surgery. Slides were not available for 3 patients. Thus a total of 56 ovarian cancer patients were included. This research was approved by the Institutional Review Boards at the University of Iowa, the University of Miami, and University of Texas MD Anderson Cancer Center. Patients completed psychosocial questionnaires between their initial pre-operative appointment and surgery.
Behavioral Measures
Depression
The Center for Epidemiological Studies Depression Scale (CES-D) is a 20-item measure designed to assess depressive symptomatology (24) and has frequently been used in studies of cancer patients. Scores of 16 or higher are associated with clinical depression (24, 25).
Perceived Stress
The Perceived Stress Scale (PSS) is a 14-item measure that examines the degree to which situations in one’s life over the past month are perceived as stressful (26). The instrument provides a global measure of stress, focusing on the degree to which one’s life is perceived as unpredictable, uncontrollable, and overwhelming. This measure has been associated with vulnerability to development of infectious disease in healthy individuals (26).
Impact of Event Scale
The Impact of Event Scale (IES) is a 15-item measure that examines one’s subjective stress response to a specific stressful event (27). The scale measures both intrusive stress responses (e.g., having waves of strong feelings about the event) and avoidance stress responses (e.g., trying not to think about it). This measure has been frequently been used to assess stress of cancer patients, and has been associated with impaired immunologic functioning in breast cancer patients between surgery and the start of treatment (28). Patients were asked to complete the measure in response to their potential diagnosis of ovarian cancer.
Chronic Stress
A modified version of the Life Experiences Survey (LES) (29) was utilized to assess the number of major life changes each patient experienced over the past year and the degree to which each life change was experienced as stressful. The LES lists specific major life changes (e.g., death of a close family member, loss of a job) which are rated for occurrence/non-occurrence and severity. These major life changes have been shown to correlate with adverse health outcomes (30).
Negative Affect
The Profile of Mood States Short Form (POMS-SF) lists 37 mood-related adjectives to which subjects respond according to their mood over the past week. These are rated on a 5-point scale from 0 (not at all) to 4 (extremely), with factor-validated subscales of anxiety, depression, anger, fatigue, confusion, and vigor. A composite score, total mood disturbance (TMD), is created by summing all factors except vigor and subtracting vigor from the total score (31, 32).
Social Support
The Social Provisions Scale (SPS) is a 24-item self-report scale measuring the degree to which an individual perceives their social relationships as supportive (33). The scale has demonstrated adequate reliability and validity in a number of studies with different populations (33, 34). In this study, the total social support score was used.
Demographic and clinical information
Patients completed information about demographic characteristics. Clinical and histopathologic information was obtained from medical records. To control for possible confounds, health behaviors such as hours of sleep during the last night and last week, smoking status, and intake of alcohol and coffee during the 7 days before surgery were assessed.
Confocal Microscopy
Formalin-fixed, paraffin embedded tissues were used for immunofluorescent staining to colocalize macrophages (CD68) with either MMP-2, MMP-9, or VEGF. Sections were deparaffinized via graded xylenes and alcohols then rehydrated in PBS, as previously described.(35) Antigen retrieval using the DAKO Target solution was performed by microwaving for 10 minutes at 95 °C. Sections were incubated overnight at 4 °C in primary antibodies in the following combinations: CD68 + MMP-2, CD68 + MMP-9, CD68 + VEGF (CD68, 1:1000 dilution; MMP-2, 1:500 dilution, Millipore AB807; MMP-9, 1:500, Millipore AB13458; VEGF, 1:50). Sections were then washed with PBS and incubated in the appropriate fluorescent secondary antibody at 1:500 dilutions (CD68: Texas red labeled anti-mouse; MMP-2, MMP-9, and VEGF: Alexa-488 conjugated anti-rabbit) for 1 hour at room temperature.
Images of co-stained slides were taken at 200X magnification on a Zeiss Axioplan2 microscope. Immunofluorescent intensity was measured using the Image-Pro Plus software, version 5.1 (Media Cybernetic, Inc; Bethesda, MD). Using the “Line Profile” command (“Measure” menu), staining foci were selected by positioning the cursor over a region of the image with specific staining pattern of interest (e.g. co-staining with red and green fluorescence indicates CD68 positivity) and adjusting the size of the measured area. Once the region of desired staining was defined, the intensity values in the light spectrum of interest (green, blue, or red) were measured and displayed as a histogram. For each patient, 10 fields showing immunofluorescent staining were imaged and measured for intensity. The technician was blind to the behavioral data.
In Vitro Stimulation of Macrophages
To determine whether hormones released in vivo during stress might act directly on macrophages to alter MMP production, monocyte-derived macrophages were treated with 1 μM NE or 0.1 μM cortisol (hydrocortisone; both from Sigma, St. Louis, MO) and supernatants were harvested at 2, 4, 6, 12, and 24 hrs for assessment of MMP-9 levels by enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, MN). Macrophages were derived by isolating CD14+ cells from ficoll-separated whole blood using immunomagnetic positive selection (Miltenyi Biotec, Auburn CA), and culturing cells for 6 days at 2.5 × 105/ml in RMPI (Mediatech, Manassas, VA) supplemented with 10% human AB serum (Omega Scientific, Tarzana CA) and 2 ng/ml recombinant human Macrophage Colony Stimulating Factor (M-CSF; R & D Systems). Two independent experiments were conducted and each sample was assayed in duplicate.
Statistical Analyses
Version 15.0 of SPSS (Statistical Program for the Social Sciences, Chicago, IL) was used to analyze data. All distributions were examined for outliers and non-normality. Log transformations were applied to normalize distributions where necessary. Pearson product moment correlations were calculated to assess relationships between outcome variables and disease stage, age, and health behaviors. General linear model (GLM) procedures were used to compare outcome measures for depressed and non-depressed individuals, adjusting for stage. To examine relationships of biobehavioral factors with macrophage and tumor-cell populations, multivariate hierarchical linear regressions were performed, adjusting for stage of disease as a conservative measure. Overall tests of cell stimulation were performed using a repeated measures ANOVA with time as the within-subjects variable and stimulation condition (either hydrocortisone or NE) as the between subjects measure. Cell stimulation in comparison to the control at 2 and 6 hours was examined using independent t-tests. Alpha was set at p < .05 for all analyses.
RESULTS
Patient Characteristics
The mean age of participants was 63.5 years (range, 39 - 87). The majority of patients (82.1%) had advanced-stage disease (stages III and IV) with predominantly high-grade tumors (Table 1). There were no significant associations between age, health behaviors (smoking/not smoking, alcohol use, caffeine use, sleep last week or last night) and dependent variables (all P values > 0.08). Although stage was not significantly correlated with dependent variables, it was used as a covariate in all equations as a conservative measure. Mean MMP and VEGF levels in CD68+ and tumor cells are shown in Table 2.
TABLE 1.
Patient Characteristics
| Measure | Cancer Patients (N = 56) |
|---|---|
| Age in years | |
| Mean (Standard Deviation) | 63.51 (11.01) |
| Education | % of Patients |
| Some high school | 1.9 |
| High school graduate | 42.6 |
| Trade school/some college | 35.2 |
| College graduate | 14.8 |
| Postgraduate | 5.6 |
| Marital Status | |
| Single | 9.3 |
| Divorced | 14.8 |
| Widowed | 18.5 |
| Married/living with partner | 57.4 |
| Stage | |
| I | 17.9 |
| II | 0.0 |
| III | 71.4 |
| IV | 10.7 |
| Grade | |
| 1 | 9.8 |
| 2 | 21.6 |
| 3 | 66.7 |
| 4 | 2.0 |
| Tumor Histology | |
| Serous | 89.3 |
| Endometrioid | 7.1 |
| Clear Cell | 1.8 |
| Mucinous | 1.8 |
| Residual Disease/Debulking | |
| Suboptimal | 71.9 |
| Optimal | 28.9 |
TABLE 2.
Macrophage and Tumor Expression of MMP-2, MMP-9, and VEGF
| Cells | MMP-2+ |
MMP-9+ |
VEGF+ |
|||
|---|---|---|---|---|---|---|
| M | S.D. | M | SD | M | SD | |
| Macrophage | 55.80 | 7.83 | 60.37 | 21.04 | 61.72 | 7.94 |
| Tumor | 44.15 | 6.77 | 47.49 | 8.04 | 46.76 | 8.18 |
M = Mean; SD = Standard Deviation
Data is expressed in Fluorescence Intensity (arbitrary units).
Behavioral Factors and Expression of MMPs and VEGF by Macrophages and Tumor Cells
Depressed mood and macrophages
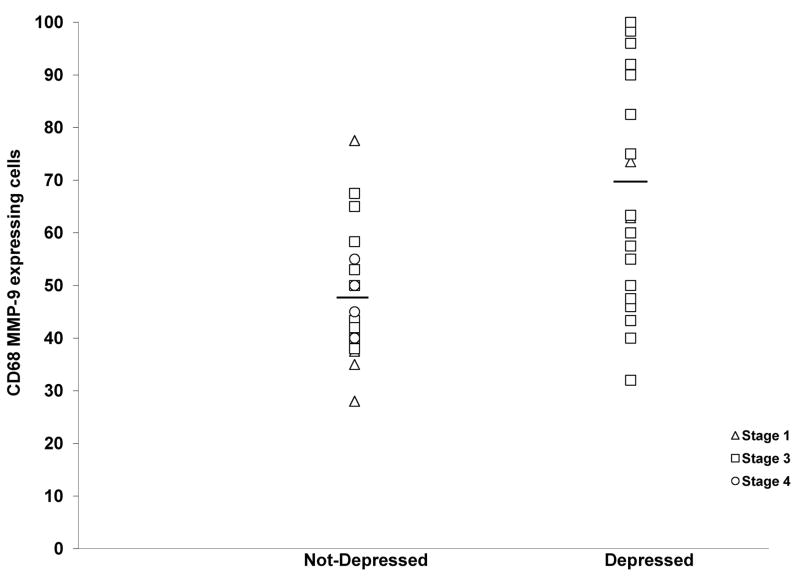
Fifty-eight percent of patients had depression scores on the CES-D within the range of clinical depression. Based on the known production of VEGF, MMP-2 and MMP-9 by macrophages as well as the responsiveness of these factors to stress hormones in ovarian cancer cells, we next asked whether there would be associations between behavioral stress or depression and macrophage production of these factors. As seen in Figure 1, depressed patients demonstrated significant elevations of MMP-9 in CD68+ cells (M= 69.89, S.D. 19.02) as compared to those who were not depressed (M= 48.36, S.D. 19.03; F [1,46]=15.34, P < 0.0001). There were no significant differences in MMP-2 (P=0.64) or VEGF expression in CD68+ cells (P=0.06). All analyses adjusted for stage. Representative images of co-localized expression of MMP-9, MMP-2, and VEGF with CD68+ cells are shown in Figure 2.
Figure 1.
Tumor associated macrophages (CD68 +cells) expressing MMP-9 among depressed (CESD ≥ 16; (M= 69.89, S.D. 19.02) and non-depressed (CESD < 16; (M= 48.36, S.D. = 19.03) ovarian cancer patients (P < 0.0001) adjusting for stage. MMP-9 data is expressed in Fluorescence Intensity (arbitrary units). Bars represent the means of each group.
Figure 2.
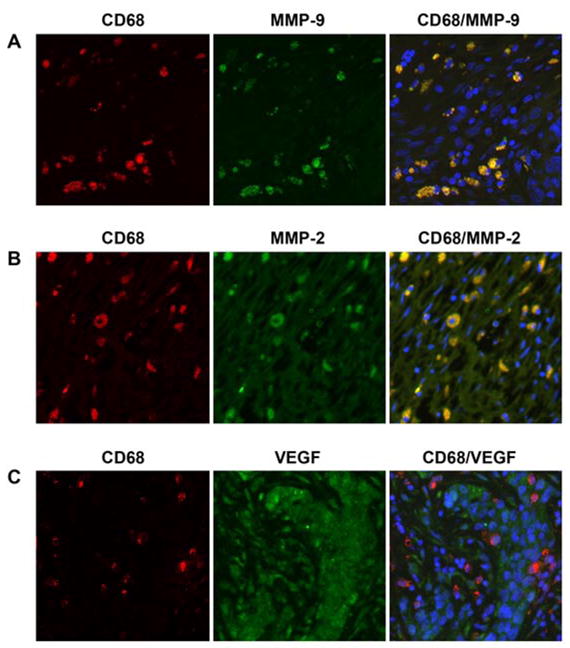
Representative images of immunofluorescence labeling of human epithelial ovarian cancer samples with CD68 (red) for macrophages, Hoechst (blue) for nuclei, and either: A) MMP-9 (green), B) MMP-2 (green), or C) VEGF (green). All pictures were taken at original magnification, X200.
Stress, social support, and macrophages
Multiple regression analyses examined relationships between stress, negative mood, social support and physiological variables, adjusting for stage. Patients reporting higher levels of current stress, greater severity and number of stressful life events over the last 6 months, and higher general negative affect had significantly higher levels of MMP-9 in CD68+ cells (IES: β=.44, p=0.002; PSS: β=.48, p=.001; chronic life stress severity: β =.39, p=.006; number of stressful life events: β=.41, p=0.004; and overall distress [POMS TMD], β=0.52, p < 0.001). In contrast, no relationships were observed between these stress variables and expression of MMP-2 or VEGF in CD68+ cells (all p values > 0.21). Additionally, no relationship was found between social support and MMP or VEGF expression in CD68+ cells (all p values > 0.16).
Stress, social support and ovarian tumor cells
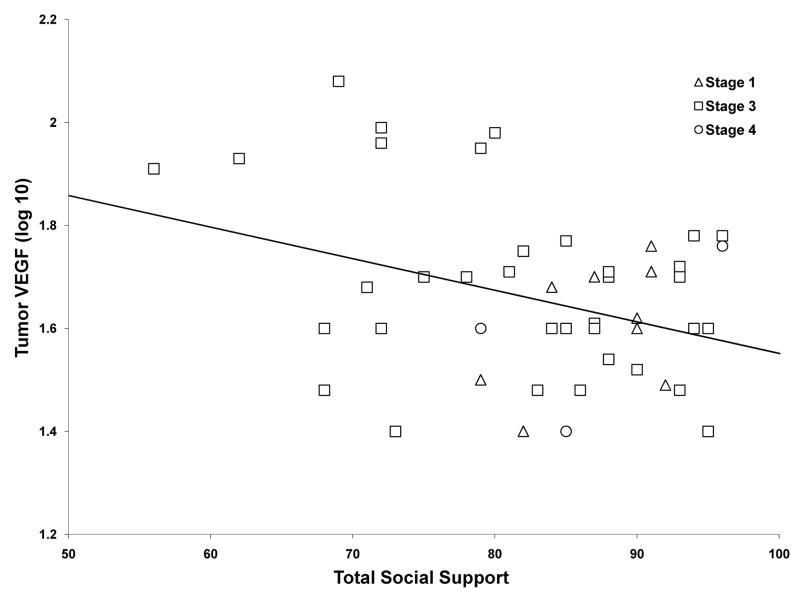
Next we examined relationships of behavioral variables and expression of MMPs and VEGF in tumor cells of these patients. As seen in Figure 3, patients with higher levels of total social support had significantly lower levels of VEGF expression in tumor cells (β = −.32, p=0.027). In examining types of social support that contributed to this finding, it was specifically patients reporting higher levels of attachment with others (β = −.42, p=0.003) that had lower expression of tumor VEGF. In addition, patients reporting higher levels of social relationships in which they were nurturing others had lower tumor expression of MMP-9 (β = −.32, p=0.024). Paralleling these findings, total social support (β = −.212) and attachment (β = −.202), were also negatively associated with tumor expression of MMP-9, but these findings did not reach significance (p values < 0.175). Otherwise, no relationships were found between stress factors and tumor cells expressing these factors (all p values > .16).
Figure 3.
Tumor production of vascular endothelial growth factor (VEGF) as a function of social support (Social Provisions scale), adjusting for clinical stage (β = −.31, p = .036). VEGF data is expressed in Fluorescence Intensity (arbitrary units). The fitted line represents the slope of this relationship and is fitted representing Stage III ovarian cancer.
In Vitro stimulation of Macrophages
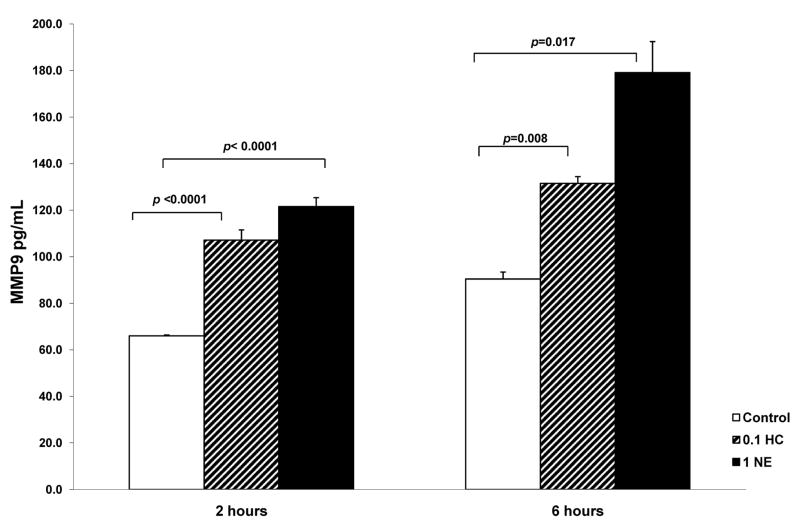
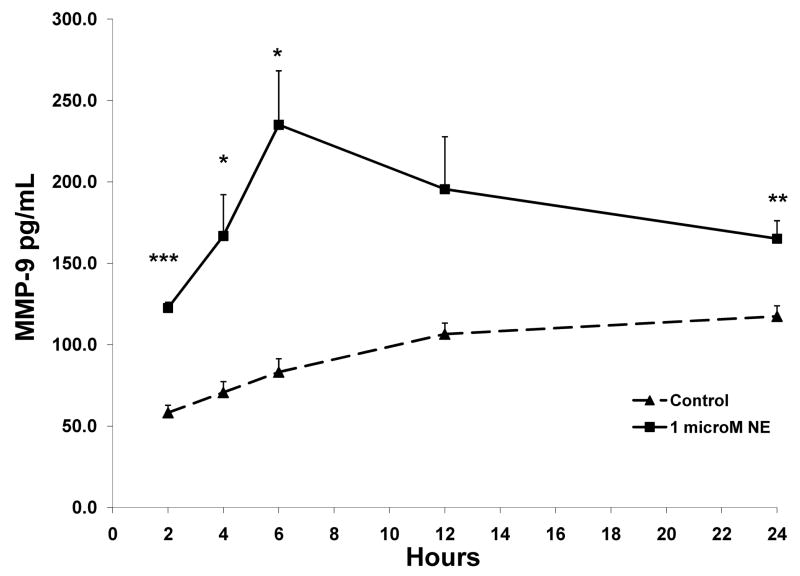
Stress and depression are known to activate the sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) axis (36). To determine whether the neuroendocrine mediators released by these systems might directly regulate macrophage production of MMP9, we stimulated primary monocyte-derived macrophages with cortisol (hydrocortisone) or norepinephrine (NE) concentrations representative of those induced by stress in vivo. As shown in Figure 4, incubation with 0.1 μM hydrocortisone induced an approximately 2-fold increase in macrophage MMP-9 expression at 2 hours (p< 0.0001) and smaller but significant increases at 6 hours (p=0.008). NE 1 μM also induced an approximately 2-fold increase in MMP-9 production at 2 and 6 hours (p<0.0001; p=0.017, respectively). A repeated-measures ANOVA indicated that there was a significant dose by time interaction for NE (p=0.001), and a significant main effect for stimulation condition (1 μM NE vs. control) (p=0.005), with effects of NE peaking at 6 hours. (Figure 5). For cortisol, there was a significant main effect for stimulation condition (p<0.0001) with a slow but consistent rise of MMP-9 over time.
Figure 4.
(A) MMP-9 production by monocyte-derived macrophages incubated with 0 and 0.1 μM hydrocortisone and 1 μM norepinephrine with supernatants harvested at 2 and at 6 hours. MMP-9 levels (pg/mL) were analyzed by ELISA. (B) MMP-9 production by monocyte-derived macrophages incubated with 0 and 1 μM norepinephrine with supernatants harvested at 2, 4, 6, 12 and 24 hours. MMP-9 levels (pg/mL) were analyzed by ELISA. Repeated measures ANOVA indicated a significant effect for condition over time (p=0.001). All data points represent the mean of 2 experiments. Significant differences as compared to the control wells (with media alone added to the cells) are indicated. Error bars represent standard error of the mean.
DISCUSSION
The key findings of this study are that stress-related biobehavioral factors were associated with both stromal and tumor expression of factors supporting angiogenesis and invasion in the tumor microenvironment of ovarian cancer patients. Specifically, depressed patients and patients reporting higher levels of chronic stress, current stress, and negative affect showed higher MMP-9 expression in tumor-associated macrophages. In contrast, patients with higher levels of social support had lower levels of VEGF and MMP-9 expression in tumor cells. MMP-2 expression by macrophages or tumor cells was not significantly associated with any of the biobehavioral factors examined. To the best of our knowledge, this is the first clinical study to demonstrate significant associations between biobehavioral factors and stromal macrophage production of matrix metalloproteinases.
These findings extend previous experiments demonstrating that chronic stress and social isolation increase expression of VEGF and MMP-9 by human ovarian tumors implanted orthotopically in mice (1). The current findings also extend our previous report of relationships between greater social support and lower serum VEGF in ovarian cancer patients (37) and demonstrate that similar relationships exist within the tumor microenvironment. The findings are also consistent with previously reported associations between elevated distress, poor social support, and elevations in the pro-angiogenic cytokine interleukin-6 (IL-6) in ascites and plasma of ovarian cancer patients (38).
Mechanisms underlying these associations likely involve stress hormones such as norepinephrine (NE) and cortisol (hydrocortisone). In vitro stimulation of isolated human macrophages with NE and hydrocortisone enhanced production of MMP-9, thus supporting the presence of causal pathways between stress and stimulation of this MMP. These findings extend previous in vitro (2, 6) and in vivo (1) studies of ovarian carcinoma showing that NE induces an increase in MMP-9, MMP-2, and VEGF expression by ovarian tumor cells via β-adrenergic receptor signaling. Specific doses of cortisol have also been shown to enhance VEGF production by ovarian cancer cell lines (6).
Macrophages are known to express β-adrenergic (17) as well as α-adrenergic receptors (16). Some data suggest that NE ligation of α1- and β-adrenergic receptors on human monocytes can activate nuclear factor κ-B (NF-κB), which mediates the expression of several other angiogenic factors including VEGF and MMP-9 (39) as well as IL-6 (40). As elevated levels of stress (41, 42), depression (43–45) and social isolation (46, 47) have been associated with elevations in NE and cortisol (36, 48, 49), it is not surprising that these factors are associated with increased expression of MMP-9 by tumor associated macrophages and MMP-9 and VEGF by tumor cells in ovarian cancer patients (50). Stress also induces an increase in NE in animal models (1).
It is not clear why an association was seen between distress and macrophage-produced MMP-9 but not with tumor-produced MMP-9 or VEGF, which were associated with social support/isolation. Variations in the neuroendocrine factors in the tumor microenvironment and receptors associated with depression and social support/isolation have not been well characterized. Furthermore, the predominant source of MMP-9 in ovarian tumors is known to be from macrophages (10); however, there may be compensatory pathways operating as well. The lack of a significant association between biobehavioral factors and MMP-2 was unanticipated. Additional studies will be required to fully define the mechanisms by which biobehavioral factors might influence MMPs within the tumor microenvironment.
These findings are significant in that they demonstrate another mechanism by which stress factors may facilitate tumor growth and invasion, that is, by stimulation of stromal cells such as macrophages in the tumor microenvironment. Under conditions of behavioral distress or low social support, it is plausible that elevated levels of stress hormones such as catecholamines and glucocorticoids may 1) stimulate TAM production of MMP-9, which promotes an environment supportive of angiogenesis and invasion and 2) directly stimulate tumor production of MMP-9 and VEGF. The net result is that the downstream effects of stress hormones on tumor growth would be amplified by both direct and indirect influences. Future studies examining levels of NE and/or cortisol within ovarian tumor samples would provide valuable insights into the role of adrenergic and glucocorticoid mechanisms in clinical tumor biology.
Limitations
It should be noted that the clinical findings are correlational and thus limit causal inferences. However, in vitro data are consistent with an interpretation of stress-induced enhancement of macrophage production of MMP-9. Small sample size may also have resulted in under reporting of associations between distress and tumor production of MMP-9.
Conclusion
Depression and stress were associated with expression of MMP-9 by tumor associated macrophages and MMP-9 and VEGF by tumor cells in ovarian cancer patients. These findings identify new biological mechanisms that may underlie stress-induced progression of cancer, and could have implications for survival and treatment.
Acknowledgments
This research was funded in part by grants #CA88293 and #CA104825 to SKL and NIH grants (CA109298 and CA110793) and the U.T. MD Anderson Cancer Center SPORE in ovarian cancer (P50CA083639) to AKS.
References
- 1.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 2.Sood AK, Bhatty R, Kamat AA, et al. Stress hormone mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–75. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsson MB, Armaiz-Pena G, Takahashi R, et al. Stress hormones regulate IL-6 expression by human ovarian carcinoma cells through a SRC-dependent mechanism. J Biol Chem. 2008 doi: 10.1074/jbc.M611539200. in press. [DOI] [PubMed] [Google Scholar]
- 4.Yang E, Bane CM, MacCallum RC, Kiecolt-Glaser JK, Malarkey WB, Glaser R. Stress-related modulation of matrix metalloproteinase expression. J Neuroimmunol. 2002;133:144–50. doi: 10.1016/s0165-5728(02)00270-9. [DOI] [PubMed] [Google Scholar]
- 5.Yang EV, Sood AK, Chen M, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357–64. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 6.Lutgendorf SK, Cole S, Costanzo E, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–21. [PubMed] [Google Scholar]
- 7.Lutgendorf S, Weinrib AZ, Penedo F, Russell A, DeGeest K, Costanzo ES, Henderson P, Sephton SE, Rohleder N, Lucci JA, Cole S, Sood AK, Lubaroff D. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. doi: 10.1200/JCO.2007.14.1978. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 9.Balkwill F, Mantovani A. Inflammation and Cancer: Back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Van Arsdall M, Tedjarati S, et al. Contributions of Stromal Metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 94:1134–42. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 11.Kamat AA, Fletcher MS, Gruman L, et al. The clinical relevance of stromal matrix metalloproteinase (MMP) expression in ovarian cancer. Clin Cancer Res. 2006;12:1707–14. doi: 10.1158/1078-0432.CCR-05-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sood AK, Fletcher MS, Coffin JE, et al. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am J Obstet Gynecol. 2004;190:899–909. doi: 10.1016/j.ajog.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagemann T, Robinson SC, Schulz M, Trümper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteinases. Carcinogenesis. 2004;25:1543–9. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 15.Brown L, Detmar M, Claffey K, et al. Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. EXS. 1997;79:233–69. doi: 10.1007/978-3-0348-9006-9_10. [DOI] [PubMed] [Google Scholar]
- 16.Szelenyi J, Kiss JP, Puskas E, Szelenyi M, Vizi ES. Contribution of differently localized alpha 2- and beta-adrenoceptors in the modulation of TNF-alpha and IL-10 production in endotoxemic mice. Ann NY Acad Sci. 2000;917:145–53. doi: 10.1111/j.1749-6632.2000.tb05378.x. [DOI] [PubMed] [Google Scholar]
- 17.Van Miert A. Present concepts on the inflammatory modulators with special reference to cytokines. Vet Res Commun. 2002;26:111–26. doi: 10.1023/a:1014043601287. [DOI] [PubMed] [Google Scholar]
- 18.Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 19.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and anti-inflammatory cytokines, and autoimmunity. Ann NY Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 20.Garcia JJ, del Carmen Saez M, De la Fuente M, Ortega E. Regulation of phagocytic process of macrophages by noradrenaline and its end metabolite 4-hydroxy-3-metoxyphenyl-glycol. Role of alpha- and beta-adrenoreceptors. Mol Cell Biochem. 2003;254:299–304. doi: 10.1023/a:1027345820519. [DOI] [PubMed] [Google Scholar]
- 21.Speidl WS, Toller WG, Kaun C, et al. Catecholamines potentiate LPS-induced expression of MMP-1 and MMP-9 in human monocytes and in the human monocytic cell line U937: possible implications for peri-operative plaque instability. FASEB J. 2004;18:603–5. doi: 10.1096/fj.03-0454fje. [DOI] [PubMed] [Google Scholar]
- 22.Suarez EC, Lewis J, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimujlation are associated with hostility and severity ofr depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:119–128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Fricchione G, Bilfinger T, Hartman A, Liu Y, Stefano G. Neuroimmunologic implications in coronary artery disease. Adv Neuroimmunol. 1996;6:131–142. doi: 10.1016/0960-5428(96)00012-5. [DOI] [PubMed] [Google Scholar]
- 24.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401. [Google Scholar]
- 25.Ensel WM. Measuring depression: The CES-D scale. New York: Academic Press; 1986. [Google Scholar]
- 26.Cohen S, Kamarck T, Mermelstein R. Global measure of perceived stress. J Health Hum Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 27.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: A measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Andersen B, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30–6. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saranson I, Johnson J, Siegel J. Assessing the impact of life changes: Development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46:932–46. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 30.Leserman J, Whetten K, Lowe K, Stangl D, Swartz MS, Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosom Med. 2005;67:500–7. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- 31.Curran SL, Andrykowski MA, Studts JL. Short form of the profile of mood states (POMS-SF): Psychometric information. Psychol Assess. 1995;7:80–3. [Google Scholar]
- 32.Sacham SA. Shortened version of the Profile of Mood States. J Pers Assess. 1983;47:305–6. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 33.Russell D, Cutrona C, Rose J, Yurko K. Social and emotional loneliness: an examination of Weiss’s Typology of loneliness. J Pers Soc Psychol. 1984;6:1313–21. doi: 10.1037//0022-3514.46.6.1313. [DOI] [PubMed] [Google Scholar]
- 34.Cutrona CE, Russell DW. Advances in Personal Relationships. JAI Press; 1987. The provisions of social relationships and adaptation to stress; pp. 37–67. [Google Scholar]
- 35.Thaker PH, Yazici S, Nilsson MB, et al. Antivascular therapy for orthotopic human ovarian carcinoma through blockade of the vascular endothelial growth factor and epidermal growth factor receptors. Clin Cancer Res. 2005;11:4923–33. doi: 10.1158/1078-0432.CCR-04-2060. [DOI] [PubMed] [Google Scholar]
- 36.Weiner H. Perturbing the organism: the biology of stressful experience. Chicago: University of Chicago Press; 1992. [Google Scholar]
- 37.Lutgendorf SK, Johnsen EL, Cooper B, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–15. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- 38.Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305–13. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Bierhaus A, Nawroth PP. Modulation of the vascular endothelium during infection--the role of NF-kappa B activation. Contrib Microbiol. 2003;10:86–105. doi: 10.1159/000068133. [DOI] [PubMed] [Google Scholar]
- 41.Morrell EM, Hollandsworth JG. Norepinephrine alterations under stress conditions following the regular practice of meditation. Psychosom Med. 1986;48:270–7. doi: 10.1097/00006842-198603000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Mausbach BT, Dimsdale JE, Ziegler MG, et al. Depressive symptoms predict norepinephrine response to a psychological stressor task in Alzheimer’s caregivers. Psychosom Med. 2005;67:638–42. doi: 10.1097/01.psy.0000173312.90148.97. [DOI] [PubMed] [Google Scholar]
- 43.Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res. 2004;57:353–8. doi: 10.1016/j.jpsychores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Veith RC, Lewis N, Linares OA, et al. Sympathetic nervous system activity in major depression. Basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch Gen Psychiatry. 1994;51:411–22. doi: 10.1001/archpsyc.1994.03950050071008. [DOI] [PubMed] [Google Scholar]
- 45.Esler M, Turbott J, Schwarz R, et al. The peripheral kinetics of norepinephrine in depressive illness. Arch Gen Psychiatry. 1982;39:295–300. doi: 10.1001/archpsyc.1982.04290030035006. [DOI] [PubMed] [Google Scholar]
- 46.Seeman T, Berkman L, Blazer D, Rowe J. Social ties and support and neuroendocrine function: The MacArthur studies of successful aging. Ann Behav Med. 1994;16:95–106. [Google Scholar]
- 47.Seeman T, McEwen B. Impact of social environment characteristics on neuroendocrine regulation. Psychosom Med. 1996;58:459–71. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Deuschle M, Weber B, Colla M, Depner M, Heuser I. Effects of major depression, aging and gender upon calculated diurnal free plasma cortisol concentrations: A re-evaluation study. Stress. 1998;2:281–7. doi: 10.3109/10253899809167292. [DOI] [PubMed] [Google Scholar]
- 49.Tse WS, Bond AJ. Relationship between baseline cortisol, social functioning and depression: a mediation analysis. Psychiatry Res. 2004;126:197–201. doi: 10.1016/j.psychres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]