Abstract
The rapid advancement of neuroimaging methodology and availability has transformed neuroscience research. The answers to many questions that we ask about how the brain is organized depend on the quality of data that we are able to obtain about the locations, dynamics, fluctuations, magnitudes, and types of brain activity and structural changes. In this review, an attempt is made to take a snapshot of the cutting edge of a small component of the very rapidly evolving field of neuroimaging. For each area covered, a brief context is provided along with a summary of a few of the current developments and issues. Then, several outstanding papers, published in the past year or so, are described, providing an example of the directions in which each area is progressing. The areas covered include functional MRI (fMRI), voxel based morphometry (VBM), diffusion tensor imaging (DTI), electroencephalography (EEG), magnetoencephalography (MEG), optical imaging, and positron emission tomography (PET). More detail is included on fMRI, as subsections include: functional MRI interpretation, new functional MRI contrasts, MRI technology, MRI paradigms and processing, and endogenous oscillations in functional MRI.
Keywords: Functional MRI, fMRI, Voxel Based Morphometry, VBM, Diffusion Tensor Imaging, DTI, Diffusion Spectrum Imaging, DSI, Magnetoencephalography, MEG, Electroencephalography, EEG, Optical Imaging, Diffuse Optical Tomography, DOT, Endogenous oscillations, Default network, Resting state fluctuations, fMRI decoding, multivariate analysis, Positron emission tomography, PET
I. Introduction
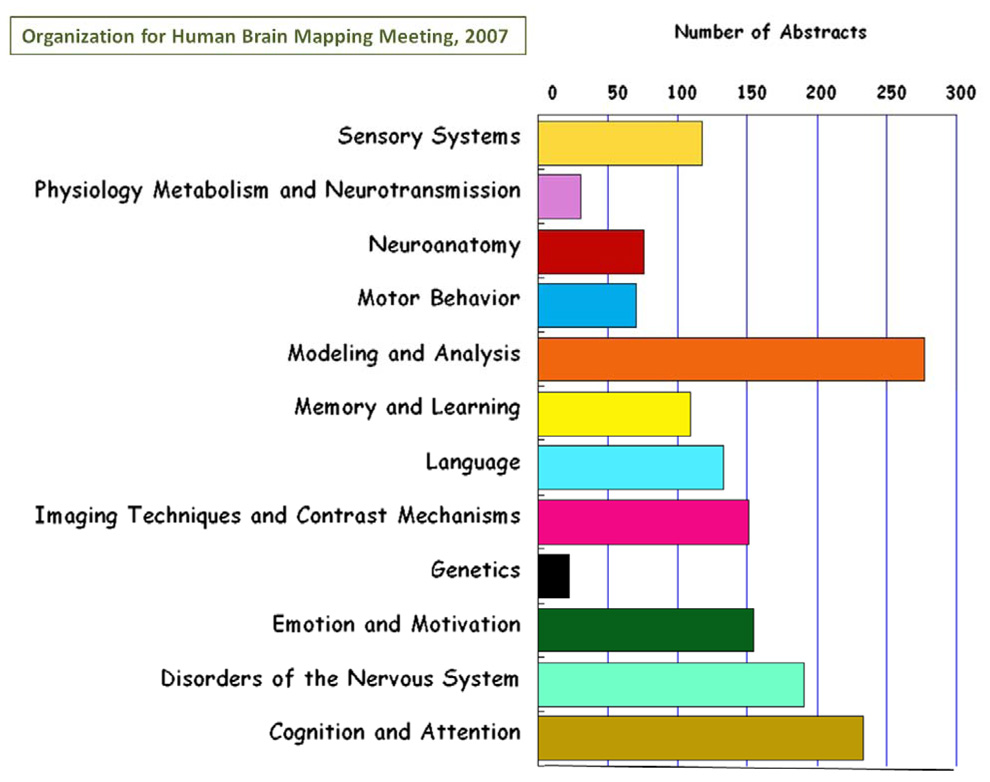
Many of the questions that we can ask about human brain structure and function are dependent on the sophistication of the neuroimaging technology that we use. Neuroimaging has grown into an essential tool for the neuroscientist seeking to understand the brain on the spatial scale ranging from neurons to systems and on a temporal scale ranging from milliseconds to decades. Recent years have seen rapid growth of neuroimaging methodology and, subsequently, insights into brain organization as well as burgeoning clinical applications. Improvements in technology, methodology, and interpretation continue to arise at an increasing rate. Correspondingly, the general level of sophistication with which neuroimaging tools are used by the wider community is continually being elevated. As thousands of papers per year are published in neuroimaging, it is impossible to keep up with the developments in any area other than one's own subspecialty. A representation of the rich diversity in the field is a breakdown of the number of scientific abstracts across neuroimaging research theme that were submitted to the Organization for Human Brain Mapping meeting in 2007. This is shown in Figure 1. From this breakdown of topics, neuroimaging methods account for at least one third of the total effort at the OHBM meeting. The focus of this review is neuroimaging methods as they have advanced in the past year or so. Descriptions of these developments are framed by discussions of historical context, specific ongoing issues, and general trends over longer time spans. The author's own subspecialty, functional MRI (fMRI), is the most thoroughly described, but the areas of diffusion tensor imaging (DTI), voxel based morphometry (VBM), magnetoencephalography (MEG), electroencephalography (EEG), and optical imaging are also touched upon. Many other areas of neuroimaging are unfortunately completely ignored. The goal of this review article is not to comprehensively cover all advancements in all aspects of neuroimaging methods in the past year, but highlight some of the ongoing themes and corresponding recent work in each of the areas that are described.
Figure 1.
Bar chart of the number of abstracts for each of the 12 categories in Neuroimaging presented at the 2007 Organization for Human Brain Mapping meeting. The focus of this current review article is on neuroimaging methods which mostly covers “modeling and analysis,” “ imaging techniques and contrast mechanisms,” and some “physiology metabolism and neurotransmission.” Overall, the topics covered in this article consist of, at most, about a third of the field of neuroimaging.
II. Functional MRI
Now in its seventeenth year, fMRI has become the tool of choice for the cognitive neuroscience community. It is essential for those interested understanding the functional correlates of behavior and disease in populations and, in a growing degree, individuals. Functional MRI has grown largely because of its noninvasiveness, relative ease of implementation, high spatial and temporal resolution, and importantly, signal fidelity. The fMRI signal is robust and for the most part, highly reproducible and consistent. Another development which further enabled the rapid spread of fMRI was the introduction in 1996 of echo planar imaging (EPI) as a standard capability on clinical scanners sold by most vendors.
Advancements in fMRI have been defined by higher resolution both in time and space, better understanding of the signal, more robust or sensitive processing methods, new applications, and new neuroscience or clinical findings with fMRI. What fMRI can do in terms of probing brain organization is growing rapidly and in some unanticipated directions. New insights into the limitations of fMRI - or rather, what fMRI cannot do, have been just as important as advances in what fMRI can do. A deepening collective understanding is shared that while fMRI can very effectively answer questions about what brain areas - at a specific spatial scale - are active in association with specific tasks, it cannot as effectively be used to address questions of mechanisms of cognition or provide insights into deep principals of how the brain is organized. It can perhaps be able to tell us what decisions you are going to make or what object you are looking at or even how you are feeling. it can also provide information regarding where in the brain processing related to (but not necessarily essential to) a specific processing task occurs. It might be able to provide sensitive probes, complementary to other clinical techniques to an array of diseases, and perhaps provide useful information for predicting future illnesses including psychological disorders or dementia.
Over the past year or so, several exciting advancements have come out - thus continuing to push the horizon of what fMRI can and might do. In addition, the emergence of a "standard practice" in fMRI for performing and reporting studies has come about(Poldrack et al., 2008). Specific cutting edge findings include insights into the mechanisms of blood oxygenation level dependent (BOLD) contrast, new hypotheses about hemodynamic contrast, new fMRI contrast mechanisms, higher resolution and increased sensitivity, and new paradigms and methods for analyzing and generating data. All of these have led to surprising new applications and findings. Below, these advancements are discussed in detail.
a. Functional MRI Interpretation
Over the years, fMRI has been defined by a certain ambiguity about the origins of the functional signal. It is based in hemodynamics and therefore is only an indirect measure of brain activation. Even though it is extremely robust and consistent, many assumptions have to be made if more in depth interpretation of the signal is desired. The field has benefitted from efforts to better understand not only the neuronal correlates of fMRI but the sources of fMRI signal variability. In the past year, these efforts have continued - as indicated by a large number of new fMRI contrast mechanism papers as well as some excellent review articles.
To begin, a recently published opinion piece by Greg Miller in Science (G. Miller, 2008) and a review article by Nikos Logothetis in Nature(Logothetis, 2008) do an excellent job in laying out some limits and misuses of fMRI while also discussing its potential. At the heart of a long-recognized problem with some fMRI studies is the issue of data interpretation. Data interpretation can be confounded on several levels. First, the fMRI signal is based on the complex interaction of neuronal activity, neuronal metabolism, blood flow and blood volume on a spatial scale that lumps together hundreds of thousands of neurons in each MRI voxel. These factors may vary not only across subject populations, individuals, and regions in the brain but also across voxels and even within voxels. This hemodynamic variability poses a severe limit on how fMRI can be used. On an individual level, this limit prevents an investigator from suggesting that one part of the brain shows "more" activity simply because the fMRI signal is greater. Among other things, this signal intensity is highly weighted by the blood volume in each voxel(Bandettini & Wong, 1997). Logothetis goes further in his review to suggest that the limits in our knowledge of precisely what neuronal activity (excitation, inhibition, sub threshold activity, top down or bottom up modulation) is manifest by the hemodynamic response limits the depth to which we can draw inferences even from parametrically modulated signal within a region. While most researchers have known these caveats all along, this particular one is a potentially significant confound in the interpretation of the many parametric studies that have been performed over the years.
Because of the variability of the timing of hemodynamics, we also have well understood limits (on the order of seconds) on the inferences of the relative timing of brain activity between regions. Without a clear understanding of the non-neuronal sources of this spread in latency, inferences about causality based on relative timing (below 2 second differences) are inherently weak and likely to be so weighted by the underlying vasculature-influenced timing that they are incorrect. A growing area in fMRI method development is that of hemodynamic calibration - to measure and subsequently account for the hemodynamic variability over space(Ances et al., 2008; Chiarelli, Bulte, Wise, Gallichan, & Jezzard, 2007; Hoge et al., 1999a; Kida, Rothman, & Hyder, 2007; Thomason, Foland, & Glover, 2007). Nevertheless, the problem of parsing excitatory and inhibitory brain activity, for example, remains unable to be "calibrated" with fMRI.
Another perhaps more common problematic level of interpretation in fMRI is not that of inferring the level, degree, or timing of BOLD signal change but that of drawing unsubstantiated inferences about mental states or conditions from assessment of brain activation maps. Functional MRI is not immune to the problem of researchers creating the "just so" story that can explain any complicated pattern of activation shown in the data. The best fMRI studies are those with testable hypotheses, tightly controlled paradigms, appropriate calibrations, and careful inferences. It is typically correct to say "this area is associated with processing visual stimuli." It is much more risky (but not necessarily impossible) to say "Because there is more activation in this area, this subject prefers this political candidate more."
Regarding fMRI interpretation, a surprising amount of progress has been made in past year. Several papers are worth mentioning. First, an exciting study by Schummers et al(Schummers, Yu, & Sur, 2008) describes in detail the spatial and temporal behavior of astrocyte activation relative to neuronal activation, and convincingly demonstrates that it is astrocytes that signal blood flow to increase with activation. Interestingly, this work shows that the spatial tuning of visual cortex astrocytes to orientation column stimulation is sharper than that of neurons. In addition, the activation timing of astrocytes is delayed about 4 seconds from that of neurons. To show astrocyte influence on hemodynamics, astrocyte activation was selectively modulated using isoflurane, which has less of an effect on neurons. When astrocyte activity was suppressed, the measured hemodynamic response to stimulation was also shown to be suppressed. These findings certainly shed light on the mechanism of fMRI contrast mechanisms and will cause us to refine our models of neurovascular coupling.
As is well known, considerable controversy continues as to the physiologic origin of several specific aspects of BOLD contrast, including BOLD nonlinearity, pre-undershoot, post-undershoot, and fluctuations. Many papers published this year have provided provocative data perhaps shedding light on several of these unknowns. In particular, Devor et al. (Devor et al., 2007) provides a unique angle on decreased BOLD signal. Devor et al. shows, in a rat model, that when given somatosensory stimulation, a concentric pattern of neuronal depolarization (and corresponding increase in oxygenation) surrounded by hyperpolarization (and corresponding decrease in oxygenation) was created. The most intriguing part of this study was that, in the areas of hyperpolarization (and deoxygenation), which was up to 3 mm away from the central depolarization, there was a clear vasconstriction. This appears to imply that inhibitory synaptic activity, rather than a decrease in firing rate, drives vasoconstriction. These data appear to contradict the current understanding, supported by a study by Shmuel et al. (Shmuel et al., 2002) that shows a clear decrease in spiking that spatially corresponds to a decrease in BOLD signal. If this turns out to be a general finding, this may not only explain negative signal changes (inhibitory synaptic activity in distinct locations in space) but perhaps also the post stimulus undershoot (inhibitory synaptic activity at distinct times following activation). This group also published an intriguing hemodynamic model called the vascular anatomical network model (VAN) (Boas, Jones, Devor, Huppert, & Dale, 2008) that goes beyond the Balloon model(Buxton, Uludag, Dubowitz, & Liu, 2004) in that it is based on physically measurable parameters and models effects both in time and over space. It has been able to accurately predict, based on low level input parameters, blood pressure in vascular compartments, and dynamics of blood pressure and hemoglobin saturation.
Regardless of these pieces of interesting data and insightful modeling, controversy still reigns. One clear cut example, touched on above, is that of the post stimulus undershoot. As is well understood, BOLD signal increases with an increase in blood oxygenation or decrease in blood volume. Three popular hypotheses for the post undershoot exist: The first is that there is a perseveration of increased blood volume, after blood flow and oxygenation have returned to baseline. The second is that there is a perseveration of oxidative metabolic rate after blood flow and volume have returned to baseline, thus reducing blood oxygenation. The third is that there is a post stimulus decrease in flow, causing blood oxygenation to decrease since the time for oxygen to be delivered to active tissue increases, thus enhancing delivery. The work by Devor et al (Devor et al., 2007) , mentioned above, may suggest the latter explanation. Other groups have experimental evidence for a blood volume perseveration (Silva, Koretsky, & Duyn, 2007), while yet another set of researchers have experimental evidence for oxidative metabolic rate perseveration (H. Z. Lu, Golay, Pekar, & van Zijl, 2004) or just against blood volume perseveration (Frahm et al., 2008). There exist different assumptions, experimental conditions, and spatial scales with all of these studies. As with most fMRI contrast mechanism work, the explanation for an effect, when discovered, is always more complicated than we expect it to be. To most people outside of the subspecialty of fMRI contrast mechanism studies, this seems like a somewhat esoteric discussion since the post stimulus undershoot is typically not even used as a source of contrast. Understanding it fully is nevertheless extremely important as this understanding may lend insight into other aspects of BOLD contrast and potentially lead to an enhanced capability of using BOLD contrast to understand neuronal activity.
Turning to negative signal changes, a common network showing negative signal changes in response to a wide range of cognitive tasks has been termed the default mode network(McKiernan, D'Angelo, Kucera-Thompson, Kaufman, & Binder, 2002; Raichle et al., 2001). An ongoing debate continues with regard to precisely what these negative signal changes imply, and what the functional role of this network is. One recent study combined spectroscopy and fMRI to further probe these areas(Northoff et al., 2007). Specifically, relative concentrations of GABA (an inhibitory neurotransmitter) were measured in the anterior cingulate cortex (ACC) - one node of this negative signal change network - during a task which caused a decrease in signal there. The findings demonstrated that a clear negative relationship exists between GABA concentrations and the magnitude of BOLD signal decrease in the ACC. Both the previous study and this seem to be aspects of a similar evolving story - that inhibitory activity apparently leads to negative signal changes in the area showing the inhibitory activity. Another study combined EEG and fMRI data to probe the default mode(Scheeringa et al., 2008). When EEG power at theta frequencies (2 to 9 Hz) was used a regressor for resting state fMRI signal, only negative correlations were found which spatially corresponded to the default mode network. The conclusion of this study was that increased frontal theta activity can be seen as an indicator of default mode network activity. There is more on theta activity in the MEG/EEG section of this review.
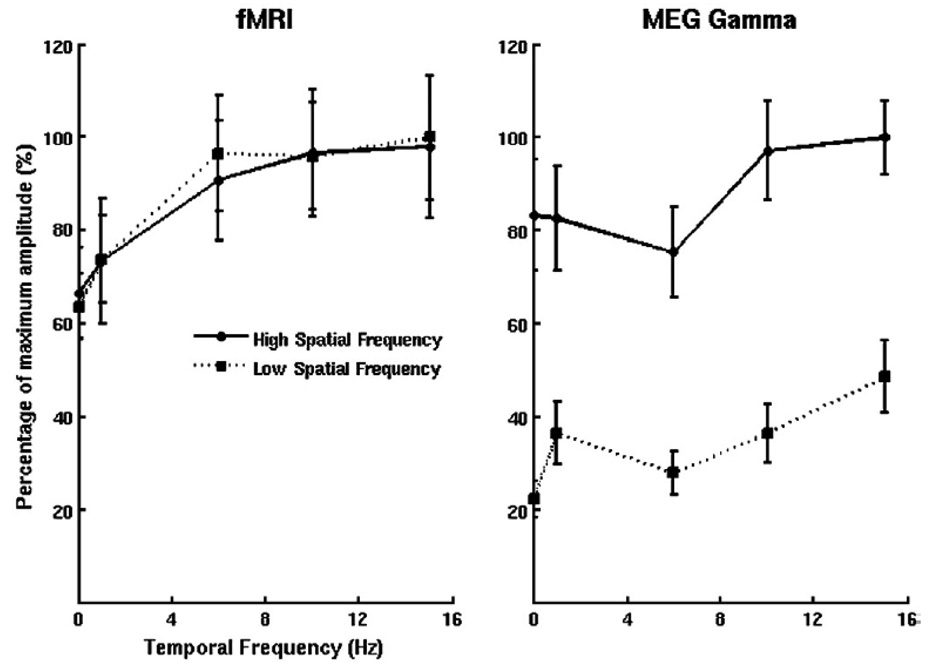
Functional MRI contrast continues to be compared with that of other modalities, with occasionally curious and surprising results. Spatial location, timing, and magnitude have been studied as they relate to EEG, MEG, optical imaging, and electrophsyiologic measures. Generally, these have been confirmatory studies, showing a general agreement in space, magnitude, and timing between modalities. A recent eye-opening report is not quite so confirmatory. In their study, Muthukumaraswamy and Singh compared fMRI and MEG Gamma frequency (40 to 60 Hz) responses to a visual stimulus which was varied in spatial frequency and temporal frequency(Muthukumaraswamy & Singh, 2008). While the spatial overlap between brain activation measured with the two modalities was substantial, the parametric dependence of the signals varied between the two. This is shown in Figure 2. For fMRI, nearly identical temporal frequency dependence curves were generated for both spatial frequencies. With MEG, high spatial frequency showed much higher power across temporal frequency than low spatial frequency. In addition, both temporal frequency dependence curves were generally flatter for MEG than for fMRI. These results strongly suggest that the two measures are tuned to different aspects of neuronal activity.
Figure 2.
Obtained from (Muthukumaraswamy & Singh, 2008). This shows the temporal frequency tuning curves for peak responses in fMRI and MEG Gamma frequency (40×60Hz) data in primary visual cortex, clearly indicating a difference between fMRI and MEG responses. No spatial frequency dependence is seen in fMRI, while with MEG, as strong spatial frequency dependence is demonstrated. In addition, with MEG, the tuning curves are show a more flat responsivity than the fMRI curves.
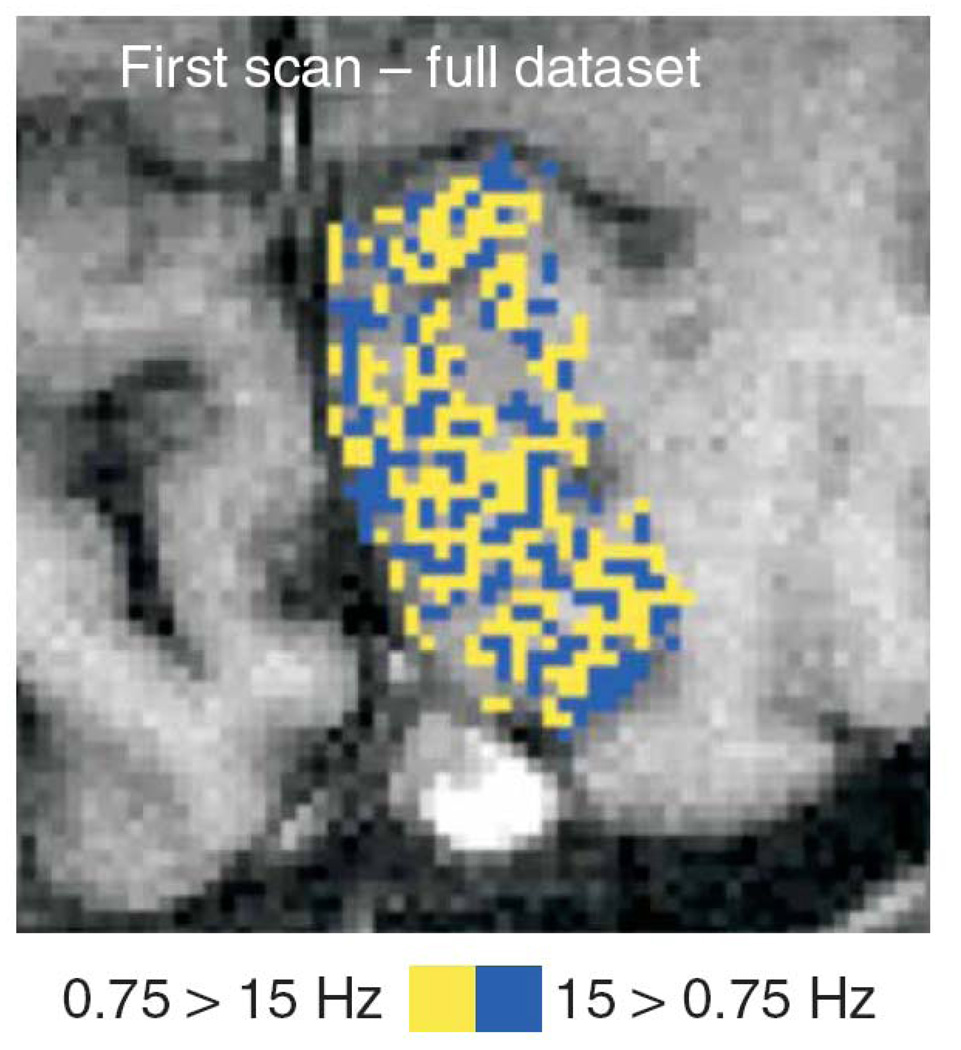
Taking the investigation of temporal frequency dependence further with high resolution BOLD contrast, Sun et al. demonstrate in a recent study (Sun et al., 2007) a dramatic columnar organization in V1 of visual temporal frequency preference. This map is shown in Figure 3. So the story of frequency dependence is complicated by this fine grained structure of temporal frequency responsiveness.
Figure 3.
Obtained from (Sun et al., 2007). This is NOT an ocular dominance column map. It is an fMRI-derived temporal frequency domain map, showing the fine structure of areas in primary visual cortex that are either selective to high temporal frequency visual stimuli (blue) or low temporal frequency visual stimuli (yellow). This is the first demonstration of spatial organization of cortex based on temporal frequency.
b. New Functional MRI Contrasts
As the imaging community comes to terms with the limits of fMRI, many are busy trying to develop an MRI-based functional contrast that may somehow be a more direct, sensitive, quantitative measure of neuronal activity. Indeed, many other functional contrasts have been put forward as possible. These include the non-invasive measures of activation induced changes in perfusion (Detre, Leigh, Williams, & Koretsky, 1992), blood volume(H. Lu, Golay, Pekar, & Van Zijl, 2003), diffusion(Le Bihan, Urayama, Aso, Hanakawa, & Fukuyama, 2006; A.W. Song et al., 2003), CMRO2 (Davis, Kwong, Weisskoff, & Rosen, 1998; Hoge et al., 1999b), temperature (Yablonskiy, Ackerman, & Raichle, 2000), and presumably magnetic field changes in the vicinity of active neurons(Bandettini, Petridou, & Bodurka, 2005). These results have always been met with extreme interest by the imaging community but none have proven superior to BOLD - because the techniques are more technically challenging or, more often, the effect size produced using the specific contrast sensitivity is considerably less than BOLD contrast. Perfusion imaging has perhaps come the closest to BOLD in terms of utility and functional contrast. While it generally has less brain coverage, lower temporal resolution, and lower functional contrast to noise than BOLD, it does have the advantages of higher specificity, baseline information, and less baseline drift. The latter advantage is significant when probing very slow brain activation timing(Aguirre, Detre, Zarahn, & Alsop, 2002).
Progress in an area may not always mean that a technique grows in utility or validity but rather that papers are published that increase our understanding of the results. In the past year, some progress has been made with regard to two types of new MRI-based brain activation contrast: The measurement of decreases in diffusion coefficient with brain activation and direct measurement of neuronal activity as indicated by MR phase or magnitude changes.
Depending on the degree of sensitization to diffusion, brain activation causes either an increase or decrease in measured diffusion coefficient. If a very small diffusion weighting is used - sensitizing the signal to extremely rapid random motion of spins - specifically that of capillary flow, then, hypothetically, if brain activation causes an increase in flow, this diffusion coefficient will increase and will be manifest as a signal decrease(A.W. Song, Woldorff, Gangstead, Mangun, & McCarthy, 2002). If the diffusion weighting is much larger, the sensitization is weighted towards changes in diffusion in very slow moving spins. Hypothetically, with brain activation, a small amount of cell swelling occurs, increasing the proportion of presumably slower diffusing intracellular spins. Therefore, with brain activation and when using a large diffusion weighting, the measured diffusion coefficient will decrease, resulting in an increase in this highly diffusion weighted signal(Le Bihan et al., 2006). It is hypothesized that this contrast is a more direct measure of neuronal activity. In recent years, this technique has received a considerable amount of publicity but has been difficult to replicate. Last year, two papers were published which contradicted the hypothesis that the origins of this diffusion coefficient change were in tissue(Jin & Kim, 2008; K. L. Miller et al., 2007). These papers suggested that the origin of these activation-related diffusion coefficient decreases was in the vasculature. Currently, the story remains to be fully resolved.
A second perhaps more popular potential functional contrast is that which allows detection of neuronal currents directly. Following a few results in phantoms (Bodurka & Bandettini, 2002; Konn, Gowland, & Bowtell, 2003), preliminary (and non-replicated) results in humans (Xiong, Fox, & Gao, 2003), and some studies with cell cultures (Petridou et al., 2006) and in intact systems (Park, Lee, & Park, 2004) - all suggesting that direct detection of neuronal currents in humans might be possible, researchers in the field are taking a second, more in depth look at what hypothetically they are looking for and what the prospects realistically are for detection. The hypothesis for this effect is as follows: Neuronal activity produces a transient current, presumably in dendrite clusters, setting up a transient, small in extent, and very subtle magnetic fields superimposed on the existing magnetic field. The precise timing, extent, and magnitude of these fields is a source of debate and study. These small field inhomogeneities contribute to NMR phase shifts or phase dispersion, depending on the geometry. If the fields are larger and of a single orientation, a predominant phase shift would occur. If they are smaller and more random in orientation, phase dispersion occurs. Optimal contrast weighting for these so far has been long TE (TE=T2* of tissue) gradient echo, which unfortunately, is also the optimal contrast weighting for BOLD contrast. One of the reasons why clear detection is so difficult is because any hint of BOLD contrast has to be clearly ruled out.
In the past year, several outstanding papers have contributed to this effort to image and understand neuronal current effects in MRI. These include some extremely detailed models of magnetic fields produced by neuronal activity (Cassara, Hagberg, Bianciardi, Migliore, & Maraviglia, 2008; Paley, Chow, Whitby, & Cook, 2008), very carefully executed but unfortunately, negative findings (Tang, Avison, Gatenby, & Gore, 2008), some highly innovative pulse sequences that may be more sensitive to periodic neuronal currents (Buracas, Liu, Buxton, Frank, & Wong, 2008), and some novel ideas regarding the potential to detect neuronal currents using extremely low magnetic field strength scanners (Cassara & Maraviglia, 2008; Kraus Jr, Volegov, Matlachov, & Espy, 2008). At extremely low fields (i.e. fields such that the Larmor frequency is on the same order of magnitude as predominant brain frequencies as measured with MEG), the hypothesis is that brain activity at specific frequencies (i.e. 10 Hz to 60 Hz) would enhance spin relaxation for spins precessing at a corresponding frequency. This is an intriguing hypothesis that is certainly worth exploring.
A unique paper, tangentially related to fMRI contrast mechanisms, published this year is worth mentioning. Moore and Cao have put forth what they call the "Hemo-Neural" hypothesis stating that activation induced hyperemia (increase in blood flow) has a neuromodulatory function (Moore & Cao, in press). In other words, an increase in neuronal activity increases blood flow, which, in turn further increases the gain of local cortical circuits, perhaps influencing detection and discrimination of sensory stimuli. They propose the following mechanism for this effect: The delivery of blood-borne messengers, mechanical modulation, thermal modulation, or hemodynamic modulation of astrocytes exerts an influence on neuronal activity. Evidence for some of these mechanisms is provided in their study. In general, this highly novel paper is certainly worth taking a look at, as, in the context of creating models of neuronal activity, if hemodynamic changes up regulate neuronal excitability significantly then their effects need to be taken into account.
c. MRI Technology
Just about every significant step in the advancement in fMRI applications has been made as a direct result of an advancement in MRI technology. MRI technology includes: magnetic field, rf coil conFiguration, and acquisition and/or image reconstruction strategies. Advancements in MRI technology include increases in field strength, increases in image signal to noise, increases in resolution, and increases in imaging speed. In the past year, several dramatic improvements in MRI technology have been published. These are discussed below.
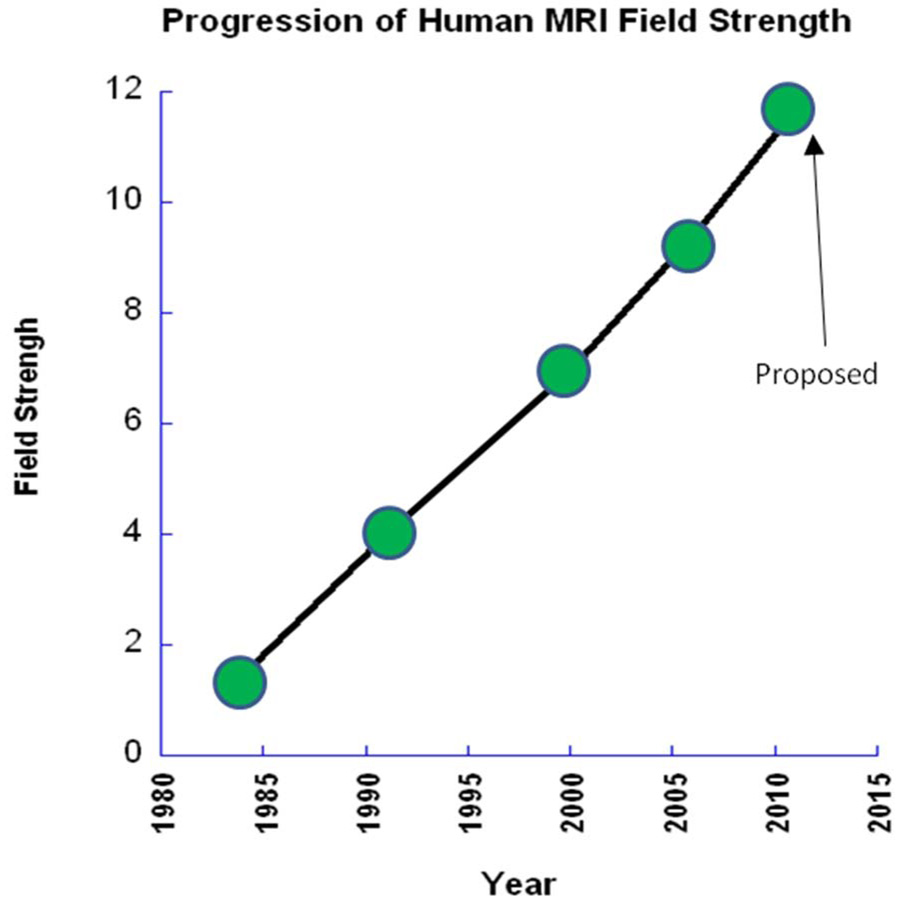
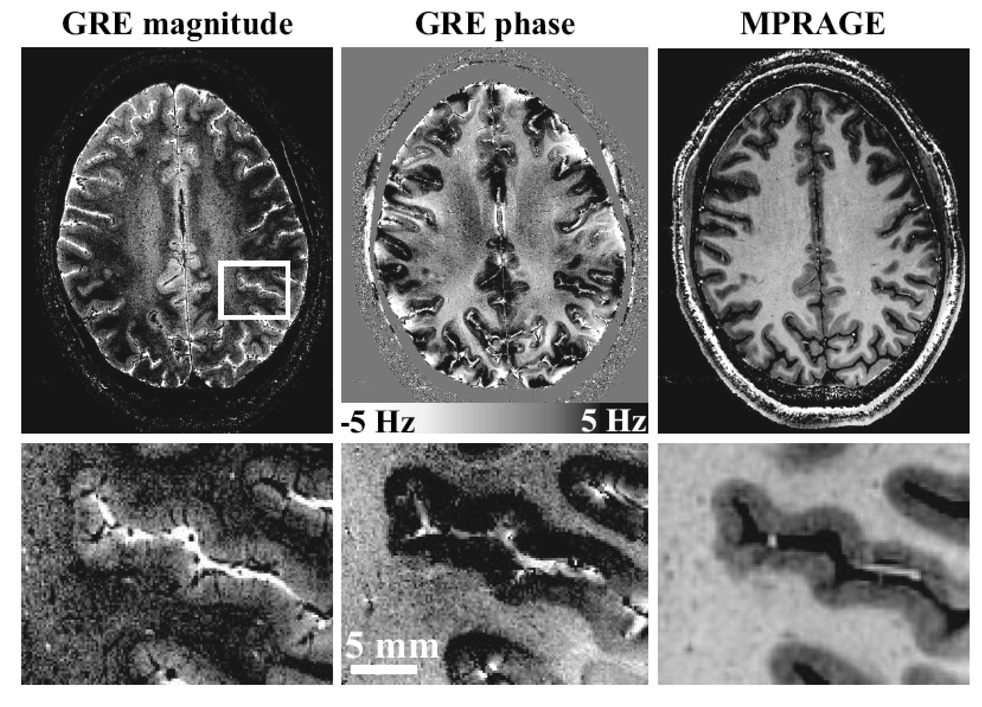
A look at the field strength used for scanning humans over the years reveals a surprising linear trend, as shown in Figure 4. A new MRI Center, NeuroSpin, has proposed developing a human 11.7 T scanner by 2012 - thus maintaining the linear trend. In addition, at the time of writing this review article, there are over 28 human 7T scanners in operation. A summary of the countries where the 7T scanners reside is shown in Figure 5. The push for higher field strength is driven not only by a direct proportionality of sensitivity to field strength, but by two other related factors: With this increased sensitivity comes the ability to collect high SNR functional and anatomical images faster and at higher resolution - allowing investigation of more subtle signal changes or anatomic features or allowing scanning of volunteers or patients in a faster time. Also, the images at 7T are not simply higher signal to noise or higher resolution, but qualitatively different in terms of anatomical contrast. Qualitatively new anatomic contrast is apparent in 7T anatomical images. An example of the qualitative difference in contrast at 7T is a study by Duyn et al (Duyn et al., 2007), which demonstrates that MRI phase is a highly sensitive contrast at 7T, revealing a 10 x improvement in gray matter-white matter delineation over magnitude contrast at lower field strengths, as well as cortical layers, and perhaps even white matter tracts. The authors speculate that the phase shifts are mostly due to differences in susceptibility effects, but other hypothesis have been put forward (Zhong, Leupold, von Elverfeldt, & Speck, 2008) suggesting that macromolecules play a role. Examples of detailed, high contrast images at 7T are shown in Figure 6.
Figure 4.
Plot of the highest current MRI field strength used for human imaging as it has increased over the years, showing a surprising linear trend.
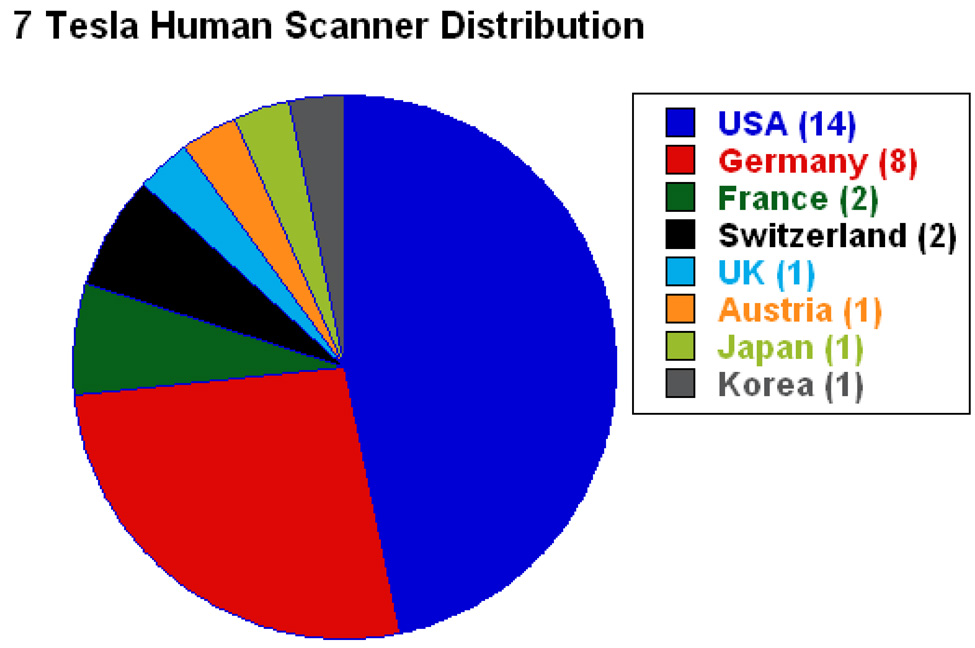
Figure 5.
Pie chart showing the current distribution (as of the summer of 2008), by country, of 7 Tesla human scanners. (Data is courtesy of Hellmut Merkle, NINDS)
Figure 6.
Obtained from (Duyn et al., 2007). This is an illustration of the image quality of magnitude and phase images obtained at 7T. The GRE data, left and center, had a resolution of 240 × 240 um with a scan duration of 6.5 min, whereas the MP-RAGE data on the right had a resolution of 480 × 480 um and a scan time of 20 min. The scale bar shows the frequency shifts corresponding to the phase changes.
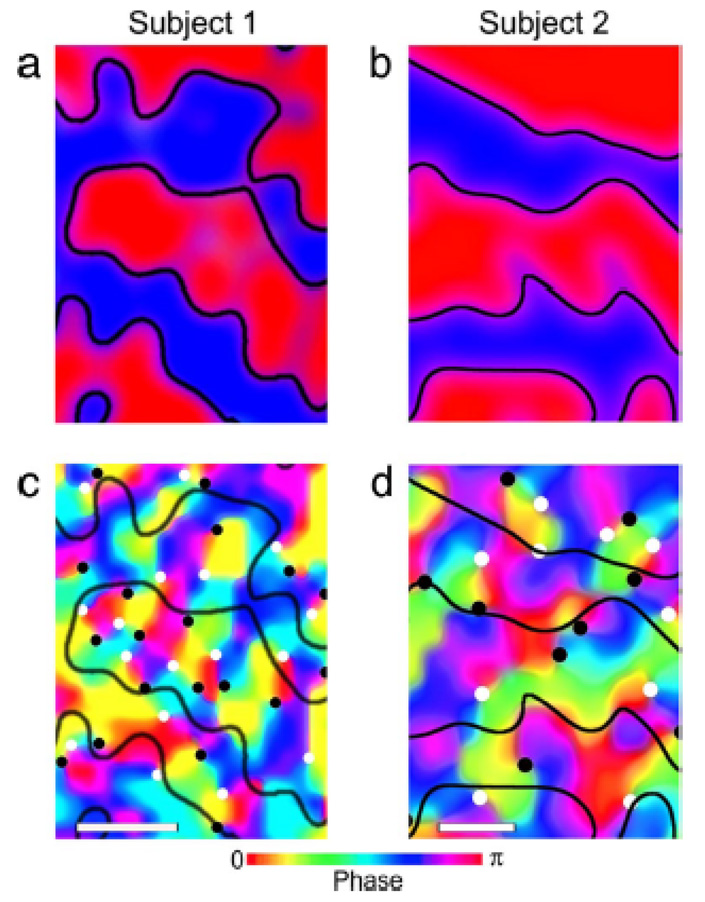
Performance of fMRI at high field is technically extremely challenging. Challenges include magnetic field inhomogeneity, RF power deposition in some sequences, RF excitation inhomogeneity, acoustic noise, increased physiologic noise, and patient discomfort, among others. Nevertheless the benefits are beginning to manifest themselves in fMRI. A recent publication by Yacoub et al (Yacoub, Harel, & Ugurbil, 2008) has shown that activation of human orientation columns (about a third of the size of ocular dominance columns) can be imaged. These results are shown in Figure 7. To achieve this, scanning was performed at 7T on a Siemens/Varian console using a four shot spin-echo sequence, resulting in an in-plane resolution of 0.5 × 0.5 mm2. A novel finding from this study is that there is a bias toward processing orientations around the vertical direction. An important point also to be taken from this study is that at 7T, the intravascular contribution is reduced such that spin-echo sequences do not suffer from large vessel intravascular effects - as occurs at lower field strengths. This enables spin-echo sequences to have a smaller functional point spread function than gradient echo sequences(Shmuel, Yacoub, Chaimow, Logothetis, & Ugurbil, 2007; Yacoub, Shmuel, Logothetis, & Uğurbil, 2007), thus being critical for the imaging of orientation columns.
Figure 7.
Obtained from (Yacoub et al., 2008). This shows ocular dominance columns for two subjects in panels a and b. Red and blue represent voxels that showed preference to right and left eye stimulation, respectively. Maps shown in panels c and d, show maps in the same cortical areas, of orientation preference. The black and white circles on the orientation preference maps show where multiple preferences converge. These form “pinwheel center.” White circles indicate clockwise pinwheels and black circles indicate counterclockwise pinwheels. The white bars at the base of the images are 0.5 mm in length.
Other technological innovations have been in the direction of faster image acquisition. Currently, almost all functional MRI data involves the collection of a stack of 2D images which takes about 2 seconds. With higher bandwidth receivers, parallel RF coil arrays, stronger gradients, and novel image reconstruction strategies, the goal of obtaining a relatively high resolution volume of data in a single excitation or with a single acquisition is drawing closer. In the past year, two papers have described novel echo volume imaging (EVI) techniques. The first paper, by Lindquest et al (Lindquist, Zhang, Glover, & Shepp, 2008), describes a method by which a central cubic portion of 3D raw data space is sampled in 100 ms, leading to a volume of low resolution (25 × 25 × 17 matrix size to 46 × 46 × 17 matrix size) but rapidly obtained data. This approach was then used to study transient dynamics of the hemodynamic response. First the pre-undershoot, presumably associated with a rapid increase in oxidative metabolic rate before an increase in flow, was observed. Secondly, in a task that involved motor cortex activation cued by a visual stimulus, a difference in onset time of the pre-undershoot was observed. The second paper, by Rabrait et al (Rabrait et al., 2008), demonstrates how parallel acquisition, outer volume suppression, and SENSE reconstruction can be combined to obtain a 20 × 20 × 24 matrix size with a 120 × 120 × 144 mm3 FOV to create isotropic 6 mm voxel sizes. It is important to note that, in typical fMRI studies with voxel sizes of about 3 mm3, spatial smoothing, normalization, and multi-subject averaging reduces the effective resolution to about this. In fact it is more optimal to acquire images initially at this lower resolution then to acquire at a higher resolution (and lower SNR) and then spatially smooth. It appears that in the near future, EVI will be a new standard in image acquisition for those researchers performing multi-subject averaging or interesting in studying subtle dynamics of the response across the brain.
Lastly, Lin et al (Lin et al., 2008) and Hennig et al (Hennig, Zhong, & Speck, 2007) have taken parallel acquisition to a new level in minimizing the need for time-consuming spatial encoding gradients. Lin et al, using a 32 channel parallel array with the coil configuration resembling EEG or MEG sensor geometries, apply similar source localization algorithms to reconstruct these images. They have named this approach magnetic resonance Inverse Imaging (InI). Hennig et al, have reduced the need for spatial encoding gradients even further with their one-voxel-one-coil (OVOC) imaging method. While the obvious advantage of these methods is the extreme rapidity with which useful volumes are obtained, another advantage is the presumably silent acquisition. This avenue of technology development appears to be just beginning. It's easy to imagine improvement in coil configurations optimized for specific brain areas or more efficient sampling schemes. Higher bandwidth acquisition rates will also lead to further improvements in resolution. Perhaps in the next several years, relatively silent, whole brain fMRI will be the standard.
d. fMRI Paradigms and Processing
The powerful approach of analyzing fMRI that involves extraction of subtle voxel-wise activation patterns rather than mapping blobs of activation has, in the past few years, seen a tremendous surge of interest due to some of the dramatic results that it has produced (Carlson, Schrater, & He, 2003; Cox & Savoy, 2003; Davatzikos et al., 2005; Eger, Ashburner, Haynes, Dolan, & Rees, 2008; Hanson, Matsuka, & Haxby, 2004; Haxby et al., 2001; Haynes & Rees, 2005a, 2005b, 2006; Haynes et al., 2007; Kamitani & Tong, 2005; Kriegeskorte & Bandettini, 2007a, 2007b; Kriegeskorte, Formisano, Sorger, & Goebel, 2007; Kriegeskorte, Goebel, & Bandettini, 2006; LaConte, Strother, Cherkassky, Anderson, & Hu, 2005; Mitchell et al., 2004; Mourao-Miranda, Bokde, Born, Hampel, & Stetter, 2005; O'Toole, Jiang, Abdi, & Haxby, 2005; Pessoa & Padmala, 2005, 2007; Shinkareva et al., 2008; Strothert et al., 2002) (Friston et al., 2008) This new class of techniques is generally known as multivariate pattern recognition, classification, or decoding: (i.e. aiming to identify a perceptual representation, activation, or cognitive state on the basis of multivoxel regional fMRI signals.) When a perceptual representation can be decoded from the activity pattern, the brain region studied contains information about the stimulus. In general, such “information-based” analysis requires multivariate techniques, but not necessarily decoding. In many instances "decoding" has been performed using univariate techniques.
Multivariate techniques in neuroimaging were introduced over a decade ago (Worsley, Poline, Friston, & Evans, 1997), but the current interest in the information-based approach is derived from a the idea that brain activation patterns reveal information carried by a neuronal population code at the scale of imaging voxels. This idea motivates multivariate analysis in single subjects without smoothing of the data. An information-based analysis determines whether there is a statistical dependency (i.e. mutual information) between the experimental conditions and the regional spatiotemporal activity patterns. Information undetected by activation-based mapping is often present in neuroimaging data. If the information resides in the fine-scale pattern of the activity, the spatial average may be similar between conditions, so no effect may be found by conventional methods with the data spatially averaged for ROI analysis or smoothed for statistical mapping. Information-based analysis can be applied to predefined ROIs. Alternatively, a continuous information-based mapping can be performed with a multivariate searchlight in order to discover regions carrying a particular type of information (Kriegeskorte et al., 2006).
It should be emphasized that this approach to neuroimaging, while, on the surface, appears to approach the brain as a black box from which to extract information about what the subject is doing, can be used as a highly sensitive probe to understand which aspects of a response are most informative, and therefore most relevant to behavior. In addition, as researchers begin to explore and understand precisely why certain classification algorithms work better in the context of extracting information from messy biological systems, perhaps this information will also be useful in lending insight into brain function that could not be probed any other way. Ultimately, the technique may, at the moment, appear to be a flashy way of doing tricks with fMRI, but in fact, this approach has tremendous potential to pose and answer extremely subtle questions about how the brain is organized.
In the past year, two outstanding studies have further pushed the limits of decoding mental content from brain activity. In previous decoding studies, the experiments have been divided between a training set in which the spatial pattern of brain activity was associated with an object (or object category), orientation, or position; and an experiment set in which the same stimuli used in the training set were used. The training set and test set was the same or highly similar stimuli. The two studies described below extend fMRI decoding to allow the accurate identification of brain activation associated completely novel stimuli or tasks.
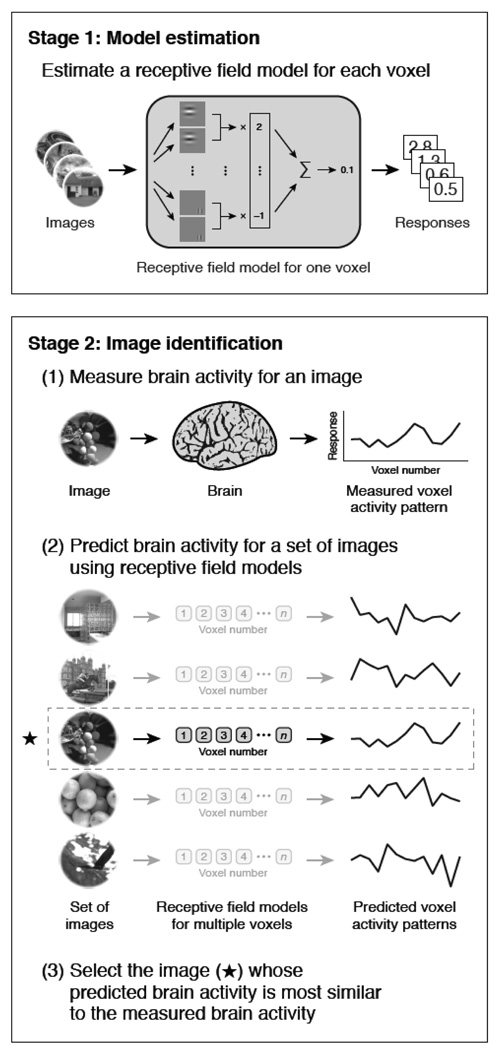
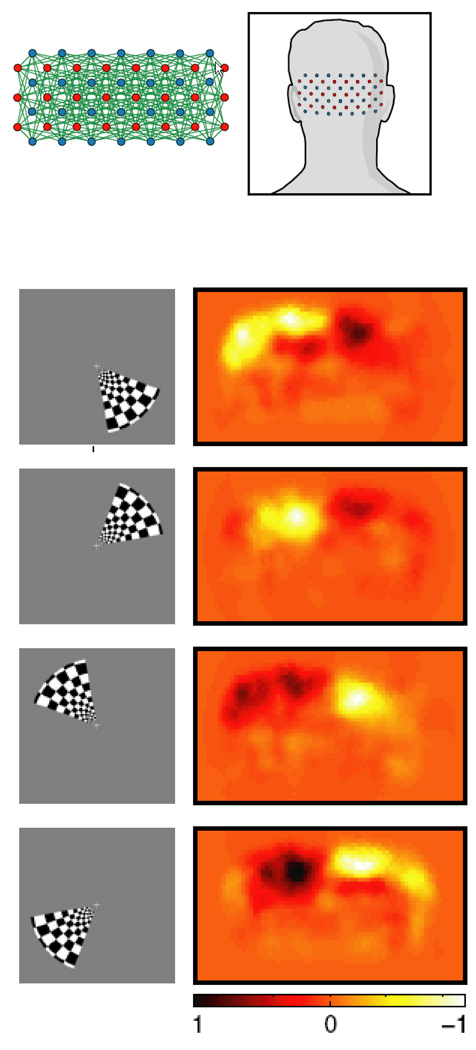
An exciting paper by Kay et. al (Kay, Naselaris, Prenger, & Gallant, 2008) has demonstrated the ability for fMRI patterns to be used to identify completely novel images that the subject was viewing. A schematic diagram of the principle steps of this study is shown in Figure 8. This study consisted of a training set in which subjects viewed 1750 natural images. From this training set a quantitative receptive field model for each voxel was created using Gabor wavelets, characterizing tuning dimensions in space, orientation, and spatial frequency. Subjects then viewed 120 completely novel images. The corresponding brain activity patterns were compared with the receptive field models obtained from the training set. Accuracy ranged from 72% to 92%. In addition, the decrease of accuracy was extremely small as the size of the test set increased, providing hope that with the appropriate training set size and tuning dimensions, activation for just about any object that exists may be characterized with a high level of accuracy.
Figure 8.
Obtained from (Kay et al., 2008). This is a schematic diagram showing the steps in the fMRI decoding experiment. In the first stage, fMRI data were recorded as subjects viewed a large collection of natural images. From these data, receptive field models for each voxel were created, based on a Gabor wavelet pyramid. These describe tuning along the dimensions of space, orientation, and spatial frequency. In the next stage, fMRI data were recorded while each subject viewed a collection of novel natural images. For each brain map, an attempt is made to identify the image that was seen using the receptive field models.
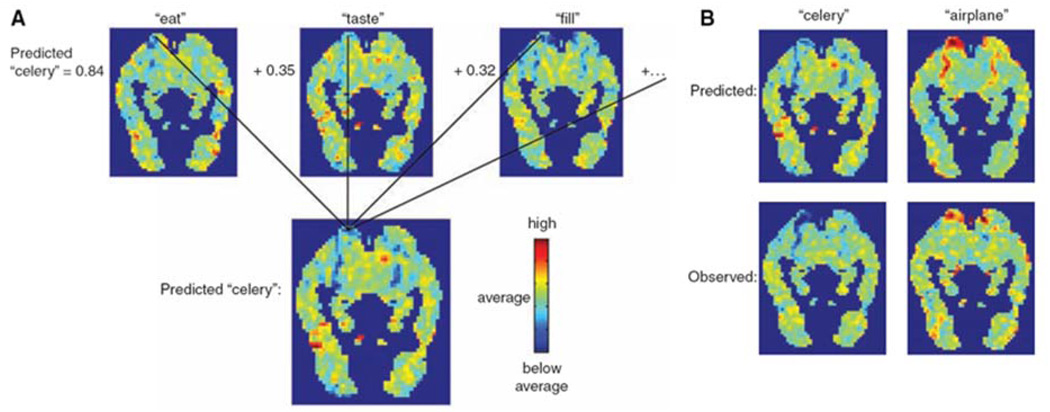
In the second study, Mitchell et al (Mitchell et al., 2008) describe a method by which whole-brain patterns associated with statistical relationships between words representing abstract semantic concepts are determined and used to predict mental states not present in the training set. The essence of this method is shown in Figure 9. These results establish a direct predictive relationship between the statistics of word occurrences in text and the activation patterns associated with processing word meanings. A new insight into how the brain processes concrete nouns is also put forth in this study. Essentially, the meaningful fMRI patterns for these nouns are distributed across the brain (i.e. also in prefrontal regions) rather than only existing in typical sensory-motor regions.
Figure 9.
Obtained from (Mitchell et al., 2008). This figure shows the process by which predicted fMRI maps were created for specific stimulus words. The top three maps in A. are learned spatial coefficients for 3 of the 25 semantic features related to “celery” (“eat,” “taste,” and “fill”). The co-occurrence in normal speech of each of these features is shown on the left of the images. These weighted images are summed to create the predicted image. In B. are two examples of predicted maps (above), showing a clear difference between the two, with observed maps (below) showing a relatively high level of similarity between predicted and observed.
Both of these studies represent an important paradigm shift in fMRI decoding - that of using a large training set focused on more elemental aspects of a brain state (i.e. from visual stimulus or word set) to predict the brain activation pattern associated with novel stimuli or brain states associated with processing novel semantic content. Not only do these studies pave the way for extensive applications of fMRI for "brain reading" but also lend a unique insight into how the brain is processing information by being able to identify the "most informative" voxels and regions. Similar insights regarding the significance of tuning functions and whole brain activation patterns would not likely have been uncovered using standard univariate mapping techniques. These studies represent the very beginning of an extremely powerful new approach to fMRI in reading brains and in understanding brain organization.
A related study by Williams et. al (Williams, Dang, & Kanwisher, 2007) addresses a previously overlooked aspect of pattern information - that which regards which aspects of a specific pattern in the brain are actually used in the processing of a visual stimulus or word and which are nonessential epiphenomenon. In their study, subjects were scanned while observing novel objects that belonged in one of three categories. They were asked to decide which category each viewed object fell into. The corresponding fMRI activation patterns in retinotopic and lateral occipital cortex (LOC) were shown to contain category information associated with objects, but only in the LOC were the patterns stronger for correct than for incorrect trials. Put another way, in trials in which the subjects did not correctly categorize the stimulus, the correct stimulus information was present in retinotopic cortex but not in LOC. This work represents an alternative direction in fMRI pattern effect imaging - that of determining pattern representations and then comparing with corresponding behavior to further classify cortical areas as they influence behavior.
Lastly, every so often a paper captures the attention of those in the field simply because of the cleverness and elegance of a paradigm. A recent study by Hassan et. al (Hasson, Yang, Vallines, Heeger, & Rubin, 2008) does just that. The study sets out to identify brain regions responsive to sensory information accumulated over different temporal windows. The paradigm simply involves viewing of a movie played either forward, backward, or in a scrambled manner. Low level processing regions such as primary visual (V1) cortex and motion sensitive area (MT+) showed minimal dependence on the direction in which the movie was played, while higher regions such as the superior temporal sulcus (STS), precuneus, posterior lateral sulcus (LS), temporal parietal junction (TPJ), and frontal eye field (FEF) were highly affected by how the movie was played. In addition LS, TPJ, and FEF activation depended on information accumulated over time periods of about 36 sec while STS and precuneus activation depended on information accumulated over about 12 sec. Using a simple, natural paradigm involving movie viewing, temporal processing scales were elegantly and effectively probed. The summary of this study is shown in Figure 10.
Figure 10.
Obtained from (Hasson et al., 2008). This shows a summary of their clever experiment testing which cortical areas are sensitive and insensitive to time reversals in movie sequences. Plots are from area MT+. A. Representative frames from the silent films used. Average time courses for B. two forward, and C. two backward presentations. D. Superimposed plots of a time-reversed version of the backwards movie time course and the forward time course (both time course shifted appropriately to account for the hemodynamic delay). E. Cross correlations of various iterations of the two forward time courses with themselves (black), backward with themselves (red), reversed backward with forward (green), and backward with forward (in blue - as expected showing minimal correlation) showing that at least in MT+, temporal order is certainly not critical for activation.
e. Endogenous Oscillations in Functional MRI
One area of rapid development in fMRI is that of using endogenous or "resting state" fluctuations or oscillations to explore resting state networks. The basic observation is that when subjects are not performing any task in the scanner, the time series signal consists of fluctuations that are not simply thermal noise of the scanner. Components of these fluctuations include cardiac pulsations of blood and CSF (inflow effects), breathing oscillations (changes in Bo field from chest expansion), bulk motion, scanner instability, vasomotion, BOLD and flow fluctuations that occur with slow changes in end tidal CO2 variations, and BOLD and flow fluctuations that occur with spontaneous neuronal activity. The last component is of course what's most interesting to neuroscientists. The predominant frequency component of these "neuronally related" fluctuations is at or below 0.1 Hz.
Research related to this phenomenon has been along determining three avenues: 1. How to most efficiently and robustly extract and map these correlated resting state fluctuations. 2. How to best interpret these fluctuations, as well as to best separate neuronal from physiologic fluctuations, and 3. How to best apply the phenomenon of resting state fluctuations towards neuroscience questions and towards understanding patient populations. Hundreds of papers have covered these topics in only the past 5 years. In the past year, the trend of an explosive interest in endogenous oscillations continues. A recent special issue of the journal Human Brain Mapping (Volume 29, Issue 7, July 2008) was completely devoted to the publication of studies about endogenous oscillations. Several excellent review articles have also been written in the past year.
Regarding each of the three areas of endogenous oscillations research, the following are some notable advancements in the past year.
1. Endogenous oscillation processing
The current problem in resting state processing is as follows: Given at about 60,000 voxels, and 60,000 × 300 = 18 million time points in a single 5 minute volumetric fMRI scan, the task of determining the correlation of every voxel time series with every other voxel in the brain is computationally prohibitive. Two alternative solutions have been popular. The first is to chose "eed voxel" regions (Biswal, Yetkin, Haughton, & Hyde, 1995) (M. D. Greicius, Krasnow, Reiss, & Menon, 2003) and the second is to use relatively unsupervised model-free approaches such as independent component analysis (ICA) (Damoiseaux et al., 2006). Each of the methods has a limitation. With the seed voxel approach, the results are highly dependent on the choice of ROI or voxel from which the "seed" reference time series is chosen from. Within this chosen time series, much of the energy in the signal driving the correlation may also be artifactual. In addition, many correlated areas are of course, missed, depending on how many "seeds" are chosen. With the ICA approach, it's difficult to sort individual components and to subsequently further interpret what each component means (i.e. What frequency components are correlated? What about phase offsets over space?). As ICA methodology improves, it appears that the problem of robustly extracting a stable set of networks for comparison and clinical use is being solved. Papers that have discussed how to process resting state data last year include the following: (Calhoun, Kiehl, & Pearlson, 2008; Cohen et al., 2008; Duff et al., 2008; Esposito et al., 2008; Salvador et al., 2008; van den Heuvel, Mandl, & Hulshoff Pol, 2008; Wink, Bullmore, Barnes, Bernard, & Suckling, 2008; Zhu et al., 2008).
2. Endogenous oscillation interpretation and separation
A central issue is whether or not the extracted networks actually mean anything neuronal, and if they do contain neuronally-relevant fluctuations, how are these separated robustly from data that contain non-neuronal fluctuations? In the past year, several excellent multi-modal studies have been carried out to demonstrate the neuronal basis of fMRI-based endogenous oscillations (de de Munck et al., 2008; Hamandi et al., 2008; Helmut Laufs, 2008; Shmuel & Leopold, 2008). In addition, several studies have recently addressed the issue of how physiologic processes such as breathing and cardiac function can influence fluctuations (Birn, Murphy, & Bandettini, 2008; Birn, Smith, Jones, & Bandettini, 2008; Shmueli et al., 2007). Other studies have shown functionally correlated endogenous oscillations in the generally "cleaner signal" perfusion data (Chuang et al., 2008). While identification of potentially confounding artifacts is relatively straightforward, a clear assessment in each subject of what regions show artifactual correlation and which do not is much more difficult. It is problematic to cleanly "regress out" respiration or other variations. Most would agree that there is sufficient evidence that spontaneous and correlated neuronal activity certainly does contribute to signal fluctuations. Work is ongoing, and will be ongoing for quite a few years, to minimize any false positives in the identification of these networks.
One other point that is not discussed as much as other aspects of endogenous oscillations is in regard to what the "purposes" or precise mechanisms of these neuronally mediated oscillations in flow and BOLD are? Are they only an epiphenomenon of regions being physically connected or functionally related, or do they serve a specific critical function to how we interact with the world? Four interesting studies have come out in the past year which speak to a functional role - or at least an effect on behavior - of endogenous oscillations.
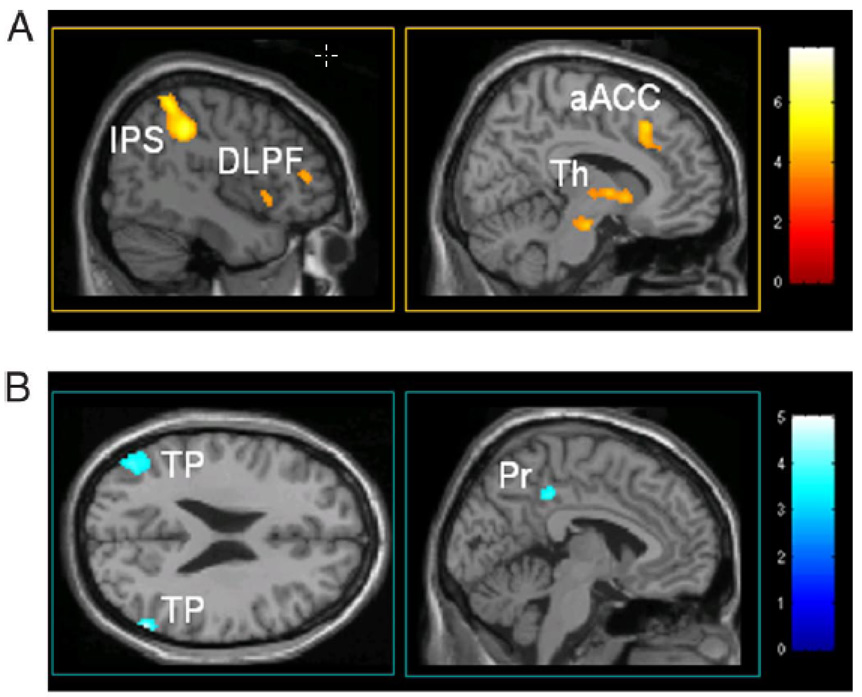
The first study, carried out by Boly et. al (Boly et al., 2007), starts with the observation that baseline signal magnitude in the brain spontaneously varies. They then demonstrate that a temporal variance in perception of identical stimuli is positively correlated with pre-trial activity in medial thalamus and lateral frontoparietal network (thought to be associated with vigilance and external monitoring) and negatively correlated with posterior cingulate/precuneus and temporoparietal cortices, which are hypothesized to be related to introspection and incidentally, the primary areas associated with what has been identified as the "default network." These results are summarized in Figure 11.
Figure 11.
Obtained from (Boly et al., 2008). Maps of baseline activity that predicts conscious perception of subsequent somatosensory stimuli. A. Increased activity of the medial thalamus (Th), dorsolateral prefrontal cortex (DLPF), intraparietal sulcus/posterior parietal cortex (IPS), and anterior cingulate cortex (aACC) 3 sec before stimulus presentation predicts perception of low-intensity sensory stimuli. B. Decreased baseline activity in the default brain network - posterior cingulate/precuneus (Pr), bilateral temporal/parietal junctions (TP) - exerts an enhancing effect on perception of subsequent somatosensory stimuli.
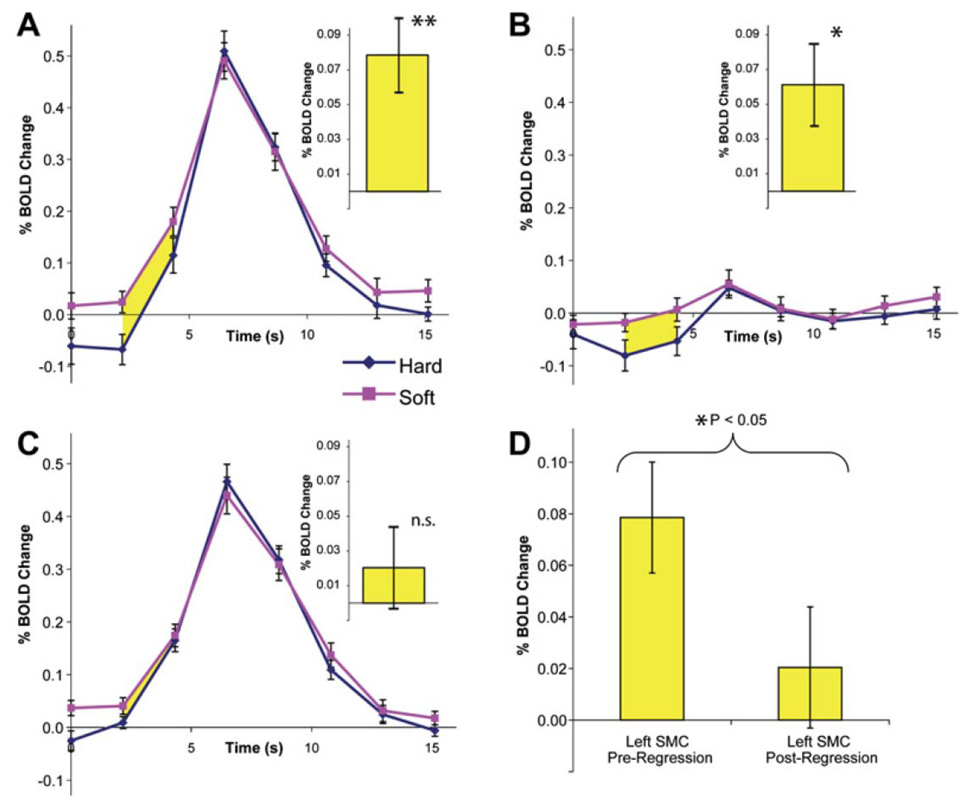
The second study, by Fox et. al (Fox, Snyder, Vincent, & Raichle, 2007) demonstrates not only that endogenous oscillations persist during tasks and that they contribute to a significant fraction of the trial to trial variance of the BOLD response in supplementary motor cortex with a simple finger tapping task, but, amazingly they also affect motor performance. The performance of the task itself (force of the button press) is affected by when the task is performed relative to the phase of the endogenous oscillation. It appears that when the oscillations are at the peak of their cycle at the beginning of activation-induced BOLD changes, the force of the finger tap force is lightest, and vice versa…when the trough is at the beginning of the BOLD-signal change, the finger tap force is greatest. About 74% of the behavioral (finger tapping force) variance is attributed to ongoing endogenous oscillations in supplementary motor cortex. These results are shown in Figure 12.
Figure 12.
Obtained from (Fox et al., 2007). This is a demonstration that coherent spontaneous activity in the motor cortex influences the force at which subjects press a button. A. Average left SMC BOLD time courses for the hard (blue) and soft (red) button presses. Yellow indicates significant differences between them. The same time courses for the right SMC also shows a BOLD-behavior effect. C. After spontaneous fluctuations were regressed out, the effect disappears. D. Summary of the comparison of A and C.
The third study, by Mason et al, (Mason et al., 2007) suggests that the default network is most active during mind wandering. In a clever study, they demonstrate a positive correlation between the percent signal change in specific nodes of the default network and the frequency of stimulus independent thoughts (or mind wandering), suggesting a conscious introspection role of this network. They conclude with a few speculations: Stimulus independent thoughts may maintain the brain in an optimal state of arousal, thus facilitating performance on mundane tasks. Stimulus independent thoughts maintain a sense of coherence with one's past and future or may be a epiphenomenon of the brain's ability to manage multiple tasks.
Fourth, a comparison study of the resting state networks (default, oculomotor, somatomotor, and visual) in anaesthetized monkeys was carried out by Vincent et al (Vincent et al., 2007). Not only did they show that the same "default" network was activated in humans and monkey but provided anatomical connectivity and evoked response pattern support for the oculomotor network. This work strongly argues that the "default" network is not dependent on the level of consciousness, as suggested in the study by Mason, mentioned above, nor is it a uniquely human system.
All four of these studies provide intriguing evidence that endogenous oscillations influence how we perceive the world and interact with it. What to make of this information with regard to understanding the functional significance of endogenous oscillations remains to be fully worked out.
3. Applications of neuronal fluctuation assessment
A surprisingly large number of applications of studies involving endogenous oscillations have been reported in a very short time. In the past year, these have included children and infant studies (Liu, Flax, Guise, Sukul, & Benasich, 2008; Thomason et al., 2008), populations with multiple sclerosis (Lowe et al., 2008), intelligence (M. Song et al., 2008), acupuncture (Dhond, Yeh, Park, Kettner, & Napadow, 2008), sleep (Horovitz et al., 2008), sedation (M. Greicius et al., 2008), seizure activity (Lui et al., 2008), Alzheimer's (Y. Liu, K. Wang et al., 2008), schizophrenia (Y. Liu, M. Liang et al., 2008; Zhou et al., 2008), chronic pain (Baliki, Geha, Apkarian, & Chialvo, 2008), ADHD (Castellanos et al., 2008), depression (M. D. Greicius et al., 2007), and consciousness (Boly et al., 2008). For future clinical studies, this is the paradigm that clinicians are looking to with hope: fMRI signal changes that do not require a probe task but that are extremely sensitive to patient population and conscious state.
III. Gray and While Matter Imaging: VBM and DTI
a. Voxel Based Morphometry
There is growing evidence that the brain structure changes on a temporal scale that is faster than previously thought - and potentially as a result of a large number of influences. These influences include normal development, aging, drug abuse, psychiatric disorders, stressful or enriching environmental factors, learning, and chronic health problems. Evidence is growing that the brain may exhibit identifiable structural changes within weeks or months in correspondence with these influences. The current method for comparing changes in gray matter is with voxel based morphometry (VBM). VBM is the comparison of local gray matter concentration between groups of subjects. Typically, high resolution MRI scans are compared after segmentation and spatial normalization. This technique started in the mid 1990's (Wright et al., 1995) and has since then increased tremendously in popularity, doubling in the number of publications every two years. The prevalent unknowns in VBM are the following: The relationship between gray matter concentration and MRI signal intensity is not clearly established. Secondly, the mechanism and timing by which gray matter concentration changes with learning, experience, or disease has never been established. Some recent papers suggest that it happens quite rapidly (May et al., 2007). Two excellent review articles (Draganski & May, 2008; Johansen-Berg, 2007) discuss some possible mechanism of neuronal plasticity and very clearly lay out the problem, but don't provide any conclusive answers regarding the relationship between experience and neuronal re-wiring, and then MRI-detectable changes in gray matter density. A third unknown is more related to practical aspects of the fidelity of VBM comparison data. Many variables cause highly nonlinear distortions in MR images making them extremely difficult to register. Any small registration error will lead to errors in VBM comparison data. The field appears to have a need a comprehensive assessment of the variability in VBM data. When a comparison is made where no changes would be expected, how frequently do changes show up?
In spite of these unknowns, researchers have charged ahead with tremendous optimism and energy in the past 8 years. The field itself has received a considerable boost by the seminal work of Ashburner and Friston, laying out a clear set of methods for performing VBM (Ashburner & Friston, 2000). A list of only a small fraction of the applications include: Schizophrenia (Wright et al., 1995), aging (Good et al., 2001b), dementia (Mummery et al., 2000; Rosen, Gorno-Tempini et al., 2002), sex and handedness (Good et al., 2001a), headache (May, 2006), sleep apnea (Macey et al., 2002), cocaine abuse (Franklin et al., 2002), mild cognitive impairment (Chetelat et al., 2002), back pain (Apkarian et al., 2004), epilepsy (Woermann, Free, Koepp, Sisodiya, & Duncan, 1999), post traumatic stress disorder (Yamasue et al., 2003), inherited speech and language disorder (Watkins et al., 2002), Huntington's disease (Thieben et al., 2002), developmental stuttering (Sommer, Koch, Paulus, Weiller, & Büchel, 2002), Parkinson's disease (Burton, McKeith, Burn, Williams, & O'Brien, 2004), intelligence (Haier, Jung, Yeo, Head, & Alkire, 2004; Wilke, Sohn, Byars, & Holland, 2003), autism (Boddaert et al., 2004; Salmond, De Haan, Friston, Gadian, & Vargha-Khadem, 2003), bipolar disorder (Lyoo et al., 2004; Wilke, Kowatch, Delbello, Mills, & Holland, 2004), borderline personality disorder (Rusch et al., 2003), chronic smoking (Brody et al., 2004), herpes (Gitelman, Ashburner, Friston, Tyler, & Price, 2001), symphony orchestra musicians (Sluming et al., 2002), marijuana abuse (Matochik, Eldreth, Cadet, & Bolla, 2005), aphasia (Rosen, Kramer et al., 2002), aphasia and apraxia (Josephs et al., 2006), ecstasy (MDMA) abusers (Cowan et al., 2003), multiple sclerosis (Pelletier, Garrison, & Henry, 2004), tinnitus (Muhlau et al., 2006), and panic disorder (Massana et al., 2003).
VBM is clearly exploding in terms of findings. A reasonable thing to do with regard to just about any difference in behavior, genetics, development, or experience is to compare the brain structure with a control group using high resolution MRI. While all of the comparisons are fascinating, some of the most attention-grabbing results in recent years have been functional as well as structural changes due to learning or with training (Draganski & May, 2008; Kelly & Garavan, 2005). A landmark study by Maguire et al (Maguire et al., 2000) showing that the hippocampus of London taxi drivers, performing memory-demanding navigation, was larger than those of controls, was among the first to suggest that MRI-measurable changes in the brain could occur with learning. Other studies followed. These include one by Draganski et al (Draganski et al., 2004) demonstrating that those who learned how to juggle obtained a higher concentration of gray matter bilaterally in MT. This same group also investigated changes associated with learning abstract information rather than a skill (Draganski et al., 2006). Images were obtained at three different time points while medical students studied for their medical examination. During the learning period, the gray matter increased significantly in the posterior and lateral parietal cortex bilaterally and did not change significantly toward the third scan 3 months after the exam. The posterior hippocampus showed a different pattern over time: the initial increase in gray matter during the learning period was even more pronounced toward the third time point.
Lastly, recently published was perhaps the first true longitudinal study in which the same group was imaged with MRI and fMRI both before and after subjects became proficient in mirror reading (Ilg et al., 2008). Mirror reading activated dorsolateral occipital cortex, medial occipital cortex, superior parietal cortex, medial and dorsolateral prefrontal cortex, as well as anterior insula and cerebellum. Daily practice of 15 min for 2 weeks was carried out. Practice-related decrease of activation at the right superior parietal cortex and increase of activation at the right dorsal occipital cortex was found. These results are shown in Figure 13. The longitudinal VBM analysis showed an increase of gray matter in the right dorsolateral occipital cortex that corresponded to the peak of mirror-reading-specific activation. This area, while overlapping with the peak of activation, in some atlases, curiously appears outside the brain. Nevertheless, this finding is the first of its kind and is exactly the right type of research to be doing to explore these gray matter changes. The mechanism that the authors proposed for the increase in gray matter concentration was synaptic remodeling within specific processing areas. It's clear that many more exciting studies of this kind will be coming out in the very near future.
Figure 13.
Obtained from (Ilg et al., 2008). This shows practice related changes in activation and in gray matter density related to performing the task of mirror reading. The green areas indicate the overall activation. The blue area is where the activation induced signal change increased with practice. The red area is where the activation induced signal change decreased with practice. Lastly, the white area is where the change in gray matter density was found.
VBM is in an extremely exciting stage of development at this point. Precise timings and mechanisms for gray matter changes are still not known. New comparisons are coming out almost daily even though the sources of variability are not fully characterized. The field also may go in the direction of making comparisons in very small regions in very high resolution scans of the brain rather than in low resolution images entire brain. Another direction that seems relatively unexplored is that of pulse sequence choice for VBM. Perhaps sequences that are weighted towards blood volume, perfusion, or susceptibility effects may be more sensitive to changes and may help towards elucidating mechanisms of change. An study by O'Gorman et al. (O'Gorman et al., 2008) demonstrates the use of VBM with arterial spin labeling based resting state perfusion data rather than anatomical data in the assessment of subjects with ADHD. Clear increases in perfusion for ADHD subjects were shown in the left caudate nucleus, and frontal and parietal regions. Psychomotor stimulation normalized perfusion within the frontal and caudate regions and decreased perfusion below normal levels in parietal and parahippocampal regions. This last study indicates not only a new direction for VBM, but a new direction and potentially enhanced utility of non-invasive quantitative baseline perfusion data.
b. Diffusion Tensor Imaging
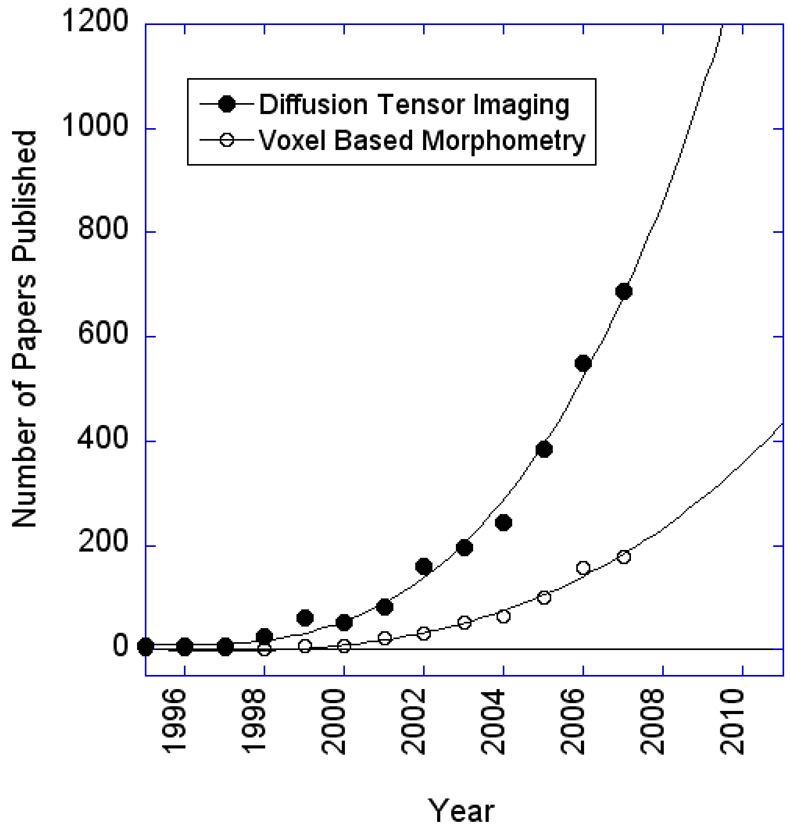
Voxel based morphometry can be generally thought of as the characterization of any voxel-wise changes in anatomical images. Most studies involving VBM, however, focus on characterizing changes in gray matter density. Diffusion tensor imaging (DTI) mostly involves the characterization of white matter, and specifically, the directionality of white matter tracts. DTI generated maps may be used in the context of VBM but do not have to be, as a considerable amount of unique information resides in the maps themselves for individual subjects. DTI involves the use of diffusion gradients sensitized to diffusion in multiple directions to determine the preferential directionality of diffusing spins (P. J. Basser & Jones, 2002). With the understanding that spins diffuse more rapidly along white matter tracts as opposed to perpendicularly to them, this method can create voxel-wise maps of fractional anisotropy (FA) as well as computed maps of white matter tracts. This avenue of neuroimaging has picked up considerable momentum as methods become more sophisticated and robust. Figure 14 shows the exponential growth of the number of papers published per year for both DTI and VBM.
Figure 14.
Results of a literature search using “Scopus” of all diffusion tensor imaging and voxel based morphometry imaging studies published in the past 12 years. A rapid growth in both is seen after 2002.
Dr. Peter J. Basser, arguably the creator of diffusion tensor imaging, having published the first several papers in the field on the topic (P. J. Basser, 1995; P. J. Basser, Mattiello, & Lebihan, 1994a, 1994b; Peter J. Basser & Pierpaoli, 1995), received, in 2008. the International Society for Magnetic Resonance in Medicine Gold Medal for his contribution to the field. This is the highest honor of the society and certainly well deserved.
DTI has gained popularity in the neuroimaging community because it provides a methodology for assessing unique structures in the brain which were previously unable to be imaged noninvasively. Functionally relevant anatomical information may be derived from white matter anisotropy as well as from tract-related connectivity measures. While publications using DTI are reaching about 1000 per year, the technique itself faces several major technical challenges. These include a high level of sensitivity to subject motion, eddy currents, susceptibility effects, gradient heating, scanner noise, and low resolution. Sensitivity to subject motion and brain pulsation can be reduced by single shot imaging techniques, which then increase the problems of susceptibility effects (longer TR, longer readout window) and also lower the image resolution. These problems are reduced by SENSE acquisition methods which, of course, require multi-channel acquisition. Eddy current effects cause image warping, and generally remain a problem. Pulse sequence based solutions as well as post processing based unwarping solutions have been proposed, and have helped reduce eddy currents. Gradient heating problems have been reduced by new gradient cooling methods involving water circulation through the gradient coil windings themselves. Scanner noise has also been reduced by mechanical stabilization/isolation approaches.
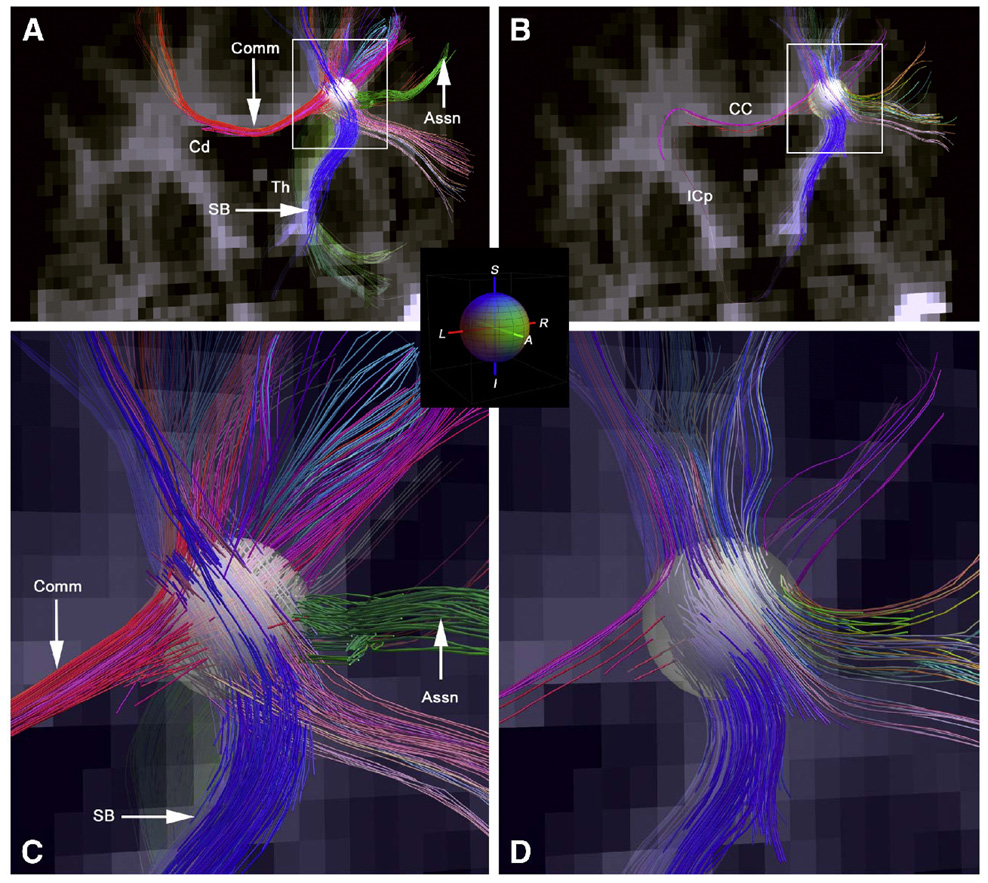
With regard to MRI tractography (Conturo et al., 1999; Mori & Van Zijl, 2002) the primary problem is having a sufficient resolution to separate crossing fibers from those that approach each other within a voxel and then go in parting directions. One solutions is to image at higher spatial resolution, although individual fibers cross at a much finer spatial scale than current techniques can resolve. Other solutions include application of diffusion gradients in more than just the three perpendicular directions, thus performing a more fine grained directional encoding. Wedeen et al (Wedeen et al., 2008) have taken DSI to dramatic new levels of detail in post mortem fixed macaque brain and in healthy human brain. A dramatic image of some of the results from that paper are shown in Figure 14.
Some developments and applications of DTI and fiber tracking in the past year include automated methods for fiber tracking (Zhang, Olivi, Hertig, van Zijl, & Mori, 2008), the use of DTI to predict clinical outcome to traumatic brain injury (Sidaros et al., 2008), the use of DTI to help explain synethesia as an increased structural connectivity (Rouw & Scholte, 2007), exploration of the limits of fMRI-guided DTI fiber tracking (Staempfli et al., 2008), and characterization of the microstructural maturation of the human brain from childhood to adulthood. (Lebel, Walker, Leemans, Phillips, & Beaulieu, 2008).
IV. EEG and MEG
Recently, both magnetoencphalography (MEG) and electroencephalography (EEG) have experienced new growth as those performing fMRI have sought more direct measures of brain activity to complement their studies. Both MEG and EEG have been useful and challenging in many regards. In terms of utility, MEG studies have uncovered specific transient dynamics associated with slower BOLD changes and fluctuations (Furey et al., 2001; K.D. Singh, Barnes, Hillebrand, Forde, & Williams, 2002; M. Singh, Kim, & Kim, 2003). BOLD changes have helped confine dipole source estimations, allowing forward solutions which delineate precise timing between regions (Dale & Halgren, 2001; Dale et al., 2000). EEG studies have the distinct advantage of being carried out simultaneously with fMRI studies (Herrmann & Debener, 2008; H. Laufs, Daunizeau, Carmichael, & Kleinschmidt, 2008), allowing the study of non-repeatable effects in the context of the study of epileptic patients (Jackson, 1994; Lemieux et al., 2008; Lemieux et al., 2001) and healthy volunteers (Horovitz et al., 2008; Horovitz, Rossion, Skudlarski, & Gore, 2004; Horovitz, Skudlarski, & Gore, 2002). Comparisons of dynamics of MEG and EEG with BOLD contrast have led to insights and additional puzzles regarding the parametric stimulus dependence of BOLD (Muthukumaraswamy & Singh, 2008), BOLD dynamics (Tuan, Birn, Bandettini, & Boynton, 2008) and fluctuations (R. Goldman, Stern, Engel, & Cohen, 2001; R. I. Goldman, Stern, Engel, & Cohen, 2002; Helmut Laufs, 2008; H. Laufs et al., 2007).
An ongoing question in MEG and EEG pertains to the functional significance of the various frequency bands that show relatively high levels of spatially specific spectral power. This is particularly relevant given a growing body of evidence that resting state oscillations in fMRI are functionally important to performance and perception - as mentioned above. The following four studies, published last year, stood out as cutting edge examples of how MEG and EEG oscillations are being utilized and probed.
The first study (Van Dijk, Schoffelen, Oostenveld, & Jensen, 2008) explored the functional role of alpha band (∼ 10 Hz) oscillations in MEG. Subjects performed a subtle visual detection task. Prestimulus alpha power was shown to be inversely correlated with performance. Source modeling found that the location of this performance related alpha modulation was in the parieto-occipital sulcus. The hypothesis is that this alpha power reflects inhibition of these areas by higher areas, therefore modulating the gain in visual stream. A lower gain effectively "gates" the information passing from occipital to dorsal parietal areas. Consequently, subtle details of a visual stimulus would less likely make their way to conscious perception during high alpha activity. Interestingly, reaction time was not affected, suggesting that alpha power modulations do not modulate vigilance (a potential confound in the study).
The second study concerns gamma (> 30 Hz) oscillations. Gamma oscillations have been recently implicated in perceptual binding of multiple inputs within sensory systems and might also reflect functional coupling within distributed cortical networks underlying perceptual or cognitive processes. The spatial and parametric relationship between gamma oscillations and BOLD has been shown to be mostly similar but with some dramatic differences as discussed above (Muthukumaraswamy & Singh, 2008). This study used MEG to demonstrate gamma oscillations in primary motor cortex during self -paced movement, peaking at about 100 to 250 ms after movement onset (Cheyne, Bells, Ferrari, Gaetz, & Bostan, 2008). The hypothesis is that these gamma oscillations are within cortico -subcortical networks, and are involved with feedback control of discrete movements.
A third study involves MEG and EEG processing that is along a similar avenue as the growing area of fMRI decoding (Waldert et al., 2008). This type of work is significant in the context of building robust brain computer interfaces. Because of the much higher temporal resolution of these techniques, these techniques have a greater potential for real time interaction of brain activity with neuro-prosthetic devices or with feedback based on brain activity. In this study, subjects performed hand movements which caused power modulation of MEG activity in three frequency bands. They observed an increase in power for <7Hz (low frequency or theta) and for 62–87 Hz (high gamma) and a decrease for 10–30 Hz (beta). Interestingly, only the power modulations of low frequency activity were informative with regard to decoding direction of movement from the MEG data. In addition, the informative decoding time period began just before movement onset.
The last study involves exploring the significance of theta band frequencies with regard to speech discrimination performance (Luo & Poeppel, 2007). The key to this study is that the focus was not on spectral power but on theta band phase. MEG signal was recorded from subjects listening to spoken sentences of varying intelligibility. It was found that theta band phase pattern predominantly in the right hemisphere reliably discriminated spoken sentence signals and that this tracking was correlated with sentence intelligibility. These results suggest that continuous speech is processed through a temporal window of about 200 ms - which is the period of the theta band.
These studies are only a small sampling of the very large and growing areas of MEG and EEG as they relate to BOLD contrast and as they relate to questions regarding the fundamental mechanisms of cortical processing. It is clear that we are just at the very beginning of making sense of the biological significance and the utility of these oscillations. As more robust, sensitive, and perhaps standardized processing methods come about for exploring this highly multi-dimensional data (Dalal et al., 2008), insights will certainly come more rapidly.
V. Optical Imaging
Optical imaging is an extremely wide field, extending well beyond neuroscience and medicine. Within neuroscience, the techniques range from invasive approaches in animal models with probes directly over brain tissue to noninvasive approaches with probes passing light directly through the skull. The technique is generally based on the observation that activation related hemodynamic changes and, in a small but growing number of studies, nerve cell changes have been shown to cause localized detectable changes in light absorption. Time courses and maps of deoxygenated, oxygenated, and total hemoglobin can be routinely created. The array of optical imaging techniques have been extremely helpful in elucidating fMRI based contrast (Huppert, Hoge, Dale, Franceschini, & Boas, 2006; Huppert, Hoge, Diamond, Franceschini, & Boas, 2006; Malonek et al., 1997) as well as fundamental brain organization (Grinvald, Slovin, & Vanzetta, 2000). Recently, practical, clinical applications have been increasing. Specifically, applications in the field of pediatric assessment of development and brain function are a high growth area in optical imaging (Franceschini et al., 2007). Children are more optimal for optical imaging due to their thinner skull. The technique is advantageous over fMRI because of its portability, and relative ease of use.
Optical imaging is also a technique that is still rapidly evolving technically. Three cutting edge papers, published in the past year, highlight areas of impressive technical advancement in the field of optical imaging, and provide a glimpse of some of the directions that the field may be going.
The first is that of Zeff et al (Zeff, White, Dehghani, Schlaggar, & Culver, 2007). In this study, a high density diffuse optical imaging (DOT) system is shown to be capable of performing retinotopic mapping - something that was a major achievement in the mid 90's for fMRI. These maps are shown in Figure 16. The technique is reported to resolve functional responses of 1.7 cm and subtle spatial shifts in responses of less than 1 cm. This dramatic increase in resolution may pave the way to increased usage clinically.
Figure 16.
Obtained from (Zeff et al., 2007). This shows polar angle retinotopic mapping with diffuse optical tomography.
The second study(Liebert et al., 2006) is a bit more radical. Even though it was published in 2006, it is worth mentioning because of its innovative approach. The study is the first demonstration that fluorescence of exogenous dyes, introduced intravenously to healthy human volunteers, inside the brain can be excited and detected noninvasively. A bolus of indocyanine green was introduced, and the passage of the dye was monitored by detection of the flourescence signal following a pulsed laser excitation at 780 nm. This technique may evolve into a portable and noninvasive alternative to present day MRI based techniques involving intravascular susceptibility-based contrast agents to assess blood volume and vascular patency.
The last study is not non-invasive and is not demonstrated on humans or even on brains. The study is a demonstration, on stimulated lobster leg nerves, of a novel technique for imaging action potentials at high temporal resolution using near-infrared video microscopy and polarized light(Schei, McCluskey, Foust, Yao, & Rector, 2008). Biological tissue can alter light polarization through birefringence, light scattering polarization changes, and absorption of light with specific polar angles. Sodium channel activation causes reorientation of peptide bonds, altering the polarization of scattered light. Nerve shape changes and swelling may also cause changes in polarized light. Action potentials were detected both in transmission and reflection mode. Different parts of the nerve showed different effects in transmission and reflection mode. Movies of action potentials were created, demonstrating a complex interaction of the larger, faster axons and slower, smaller axons. In general, polarization methods appear to be more hopeful than scattering detection methods since polarization provides more specificity to activated neurons. It will be challenging, yet hypothetically possible, to use this technique in intact brains having random nerve cell orientation.
VI. PET
Positron emission tomography (PET), while perhaps taking a back seat to functional MRI with regard to brain mapping, remains a highly influential neuroimaging technique due to its continuing demonstration of advancement in the fields of molecular imaging, and more specifically, neuroreceptor mapping. Currently, fMRI and NMR spectroscopy lack the sensitivity of PET with regard to imaging specific tracers (or biomarkers) in vivo. A well attended neuroreceptor mapping meeting with over 200 abstracts presented, took place in July, 2008. The proceedings of this meeting are published in NeuroImage, Volume 41, Supplement 2, 2008.
One technical advance of PET has generated excitement is that of a machine that performs simultaneous PET and fMRI (Judenhofer et al., 2008). This paper outlines a 7T animal MRI-PET system. The primary challenge that was overcome in creating this system was the fact that conventional PET scanners incorporate photomultiplier tubes which are very sensitive to magnetic fields. One solution is the use of optical fibers that lead scintillation light outside the magnetic field. For this system, an alternative solution was used. Here a novel PET detector based on lutetium oxyorthosilicate scintillation crystals and avalanche photodiodes was used in the MRI bore. This manuscript demonstrates that these work just as well as conventional detectors.
In contrast to the recently introduced PET-CT scanners, PET-MRI has more potential than biomarker tracing and anatomical imaging. It can synergistically combine fMRI, anatomical MRI, spectroscopy, and PET, opening up new avenues in research involving studies that are extremely difficult to identically reproduce across separate systems.
VII. Conclusions
This review has is an attempt at a very narrow snapshot of the cutting edge of the rapidly moving - accelerating - discipline of neuroimaging methods. The areas of fMRI, DTI, VBM, MEG, EEG, Optical Imaging, and PET were covered - but, admittedly, with considerable more emphasis on fMRI methods and contrast mechanisms. The hope is that some of the most innovative work and most interesting controversies were highlighted in such a way that the reader can come away not only with a bit more information about specific papers but a general perspective of where the field is currently at and where it's going, from a methodological perspective. It's also hoped that a clear sense of excitement is also conveyed as these methods show no indication of stabilizing any time soon. Methods are continually advanced as new technology comes about, as we understand more of the biophysics and neurophysiology of the signal being measured, and as we come up with ever more clever ways of pulling subtle information from our data. From these advancements, high impact applications inevitably follow. It's clear that many methods described are still in their infancy, and a considerable amount of collaborative work between experts of all disciplines remains to be carried out towards developing better methods not only to better understand the human brain, but to use this ever growing understanding in the most effective way possible.
Figure 15.
Obtained from (Wedeen et al., 2008). These panels show crossing fibers within the centrum semiovale of in vivo human brain using DSI (A, C - magnification of A) and DTI (B, D -magnification of B). Assn, long association fibers; CC corpus callosum; Cd caudate nucleus; Comm, commissural fibers; ICp, posterior limb of internal capsule; SB subcortical bundle projection fibers; Th, thalamus. DSI shows superior fiber tracking resolution over DTI.
Acknowledgements
I very appreciate the helpful input of David Boas, Jerzy Bodurka, Jeff Duyn, Dan Handwerker, Alan Koretsky, Niko Kriegeskorte, Hellmut Merkle, Marieke Mur, Lalith Talagala, Carlo Pierpaoli, David Poeppel, Adam Thomas, and Arno Villringer. I also appreciate the administrative assistance of Kay Kuhns and Dorian Van Tassell with regard to obtaining permission from various publishers to use most of the figures in this article.
References
- Aguirre GK, Detre JA, Zarahn E, Alsop DC. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage. 2002;15(3):488–500. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- Ances BM, Leontiev O, Perthen JE, Liang C, Lansing AE, Buxton RB. Regional differences in the coupling of cerebral blood flow and oxygen metabolism changes in response to activation: Implications for BOLD-fMRI. NeuroImage. 2008;39(4):1510–1521. doi: 10.1016/j.neuroimage.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. Journal of Neuroscience. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry-The methods. Neuroimage. 2000;11(6 I):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: Chronic pain hurts the brain, disrupting the default-mode network dynamics. Journal of Neuroscience. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Petridou N, Bodurka J. Direct detection of neuronal activity with MRI: Fantasy, possibility, or reality? Applied Magnetic Resonance. 2005;29(1):65–88. [Google Scholar]
- Bandettini PA, Wong EC. A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. Nmrin Biomedicine. 1997;10(4–5):197–203. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<197::aid-nbm466>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. Nmrin Biomedicine. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: Theory, experimental design and data analysis-technical review. Nmrin Biomedicine. 2002;15(7–8):456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Lebihan D. Estimation of the Effective Self-Diffusion Tensor from the NMR Spin Echo. Journal of Magnetic Resonance, Series B. 1994a;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994b;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Diffusion tensor MRI: a new tool for elucidating tissue microstructure and organization; Paper presented at the American Society of Mechanical Engineers, Bioengineering Division (Publication) BED; 1995. [Google Scholar]
- Birn RM, Murphy K, Bandettini PA. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Human Brain Mapping. 2008;29(7):740–750. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: The temporal dynamics of fMRI signal fluctuations related to changes in respiration. NeuroImage. 2008;40(2):644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar Mri. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boas DA, Jones SR, Devor A, Huppert TJ, Dale AM. A vascular anatomical network model of the spatio-temporal response to brain activation. Neuroimage. 2008;40(3):1116–1129. doi: 10.1016/j.neuroimage.2007.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: A voxel-based morphometry MRI study. Neuroimage. 2004;23(1):364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Bodurka J, Bandettini PA. Toward direct mapping of neuronal activity: MRI detection of ultraweak, transient magnetic field changes. Magnetic Resonance in Medicine. 2002;47(6):1052–1058. doi: 10.1002/mrm.10159. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(29):12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Phillips C, Balteau E, Schnakers C, Degueldre C, Moonen G, et al. Consciousness and cerebral baseline activity fluctuations. Human Brain Mapping. 2008;29(7):868–874. doi: 10.1002/hbm.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biological Psychiatry. 2004;55(1):77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Buracas GT, Liu TT, Buxton RB, Frank LR, Wong EC. Imaging periodic currents using alternating balanced steady-state free precession. Magnetic Resonance in Medicine. 2008;59(1):140–148. doi: 10.1002/mrm.21457. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT. Cerebral atrophy in Parkinson's disease with and without dementia: A comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127(4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23:S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human Brain Mapping. 2008;29(7):828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson TA, Schrater P, He S. Patterns of activity in the categorical representations of objects. Journal of Cognitive Neuroscience. 2003;15(5):704–717. doi: 10.1162/089892903322307429. [DOI] [PubMed] [Google Scholar]
- Cassara AM, Hagberg GE, Bianciardi M, Migliore M, Maraviglia B. Realistic simulations of neuronal activity: A contribution to the debate on direct detection of neuronal currents by MRI. Neuroimage. 2008;39(1):87–106. doi: 10.1016/j.neuroimage.2007.08.048. [DOI] [PubMed] [Google Scholar]
- Cassara AM, Maraviglia B. Microscopic investigation of the resonant mechanism for the implementation of nc-MRI at ultra-low field MRI. Neuroimage. 2008;41(4):1228–1241. doi: 10.1016/j.neuroimage.2008.03.051. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-Precuneus Interactions: A New Locus of Dysfunction in Adult Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, De la Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13(15):1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage. 2008;42(1):332–342. doi: 10.1016/j.neuroimage.2008.04.178. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA, Bulte DP, Wise R, Gallichan D, Jezzard P. A calibration method for quantitative BOLD fMRI based on hyperoxia. NeuroImage. 2007;37(3):808–820. doi: 10.1016/j.neuroimage.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Chuang KH, van Gelderen P, Merkle H, Bodurka J, Ikonomidou VN, Koretsky AP, et al. Mapping resting-state functional connectivity using perfusion MRI. Neuroimage. 2008;40(4):1595–1605. doi: 10.1016/j.neuroimage.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NUF, Miezin FM, Dierker D, Van Essen DC, et al. Defining functional areas in individual human brains using resting functional connectivity MRI. NeuroImage. 2008;41(1):45–57. doi: 10.1016/j.neuroimage.2008.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, et al. Tracking neuronal fiber pathways in the living human brain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(18):10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Lyoo IK, Sung SM, Ahn KH, Kim MJ, Hwang J, et al. Reduced cortical gray matter density in human MDMA (Ecstasy) users: A voxel-based morphometry study. Drug and Alcohol Dependence. 2003;72(3):225–235. doi: 10.1016/j.drugalcdep.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Cox DD, Savoy RL. Functional magnetic resonance imaging (fMRI) “brain reading”: detecting and classifying distributed patterns of fMRI activity in human visual cortex. Neuroimage. 2003;19(2):261–270. doi: 10.1016/s1053-8119(03)00049-1. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Guggisberg AG, Edwards E, Sekihara K, Findlay AM, Canolty RT, et al. Five-dimensional neuroimaging: Localization of the time-frequency dynamics of cortical activity. Neuroimage. 2008;40(4):1686–1700. doi: 10.1016/j.neuroimage.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Halgren E. Spatiotemporal mapping of brain activity by integration of multiple imaging modalities. Current Opinion in Neurobiology. 2001;11(2):202–208. doi: 10.1016/s0959-4388(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, et al. Dynamic statistical parametric mapping: Combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26(1):55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C, Ruparel K, Fan Y, Shen DG, Acharyya M, Loughead JW, et al. Classifying spatial patterns of brain activity with machine learning methods: Application to lie detection. NeuroImage. 2005;28(3):663–668. doi: 10.1016/j.neuroimage.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: Mapping the dynamics of oxidative metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(4):1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Munck JC, Gonçalves SI, Faes TJC, Kuijer JPA, Pouwels PJW, Heethaar RM, et al. A study of the brain's resting state based on alpha band power, heart rate and fMRI. Neuroimage. 2008;42(1):112–121. doi: 10.1016/j.neuroimage.2008.04.244. [DOI] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion Imaging. Magnetic Resonance in Medicine. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EMC, Narayanan SN, et al. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. Journal of Neuroscience. 2007;27(16):4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136(3):407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Changes in grey, matter induced by training. Nature. 2004;427(6972):311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Büchel C, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(23):6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioural Brain Research. 2008;192(1):137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Duff EP, Johnston LA, Xiong J, Fox PT, Mareels I, Egan GF. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Human Brain Mapping. 2008;29(7):778–790. doi: 10.1002/hbm.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyn JH, Van Gelderen P, Li TQ, De Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(28):11796–11801. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger E, Ashburner J, Haynes JD, Dolan RJ, Rees G. fMRI activity patterns in human LOC carry information about object exemplars within category. Journal of Cognitive Neuroscience. 2008;20(2):356–370. doi: 10.1162/jocn.2008.20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Aragri A, Pesaresi I, Cirillo S, Tedeschi G, Marciano E, et al. Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI. Magnetic Resonance Imaging. 2008 doi: 10.1016/j.mri.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic Fluctuations within Cortical Systems Account for Intertrial Variability in Human Behavior. Neuron. 2007;56(1):171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Frahm J, Baudewig J, Kallenberg K, Kastrup A, Merboldt KD, Dechent P. The post-stimulation undershoot in BOLD fMRI of human brain is not caused by elevated cerebral blood volume. Neuroimage. 2008;40(2):473–481. doi: 10.1016/j.neuroimage.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Franceschini MA, Thaker S, Themelis G, Krishnamoorthy KK, Bortfeld H, Diamond SG, et al. Assessment of infant brain development with frequency-domain near-infrared spectroscopy. Pediatric Research. 2007;61(5 PART 1):546–551. doi: 10.1203/pdr.0b013e318045be99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biological Psychiatry. 2002;51(2):134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Friston K, Chu C, Mourão-Miranda J, Hulme O, Rees G, Penny W, et al. Bayesian decoding of brain images. Neuroimage. 2008;39(1):181–205. doi: 10.1016/j.neuroimage.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Furey ML, Tanskanen T, Beauchamp MB, Avikainen S, Haxby JV, Hari R. Temporal characteristics of selective attention to faces and houses: A MEG study. Neuroimage. 2001;13(6):S316–S316. [Google Scholar]
- Gitelman DR, Ashburner J, Friston KJ, Tyler LK, Price CJ. Voxel-based morphometry of herpes simplex encephalitis. Neuroimage. 2001;13(4):623–631. doi: 10.1006/nimg.2000.0734. [DOI] [PubMed] [Google Scholar]
- Goldman R, Stern J, Engel J, Cohen M. Tomographic mapping of alpha rhythm using simultaneous EEG/fMRI. Neuroimage. 2001;13(6):S1291–S1291. [Google Scholar]
- Goldman RI, Stern JM, Engel J, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13(18):2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001a;14(3):685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001b;14(1I):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Greicius M, Kiviniemi V, Tervonen O, Vainionpää V, Alahuhta S, Reiss A, et al. Persistent default-mode network connectivity during light sedation. Human Brain Mapping, current issue. 2008 doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biological Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A, Slovin H, Vanzetta I. Non-invasive visualization of cortical columns by fMRI. Nature Neuroscience. 2000;3(2):105–107. doi: 10.1038/72045. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. Neuroimage. 2004;23(1):425–433. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Hamandi K, Laufs H, Nöth U, Carmichael DW, Duncan JS, Lemieux L. BOLD and perfusion changes during epileptic generalised spike wave activity. NeuroImage. 2008;39(2):608–618. doi: 10.1016/j.neuroimage.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Hanson SJ, Matsuka T, Haxby JV. Combinatorial codes in ventral temporal lobe for object recognition: Haxby (2001) revisited: is there a “face” area? Neuroimage. 2004;23(1):156–166. doi: 10.1016/j.neuroimage.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Hasson U, Yang E, Vallines I, Heeger DJ, Rubin N. A hierarchy of temporal receptive windows in human cortex. Journal of Neuroscience. 2008;28(10):2539–2550. doi: 10.1523/JNEUROSCI.5487-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293(5539):2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Rees G. Predicting the orientation of invisible stimuli from activity in human primary visual cortex. Nature Neuroscience. 2005a;8(5):686–691. doi: 10.1038/nn1445. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Rees G. Predicting the stream of consciousness from activity in human visual cortex. Current Biology. 2005b;15(14):1301–1307. doi: 10.1016/j.cub.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nature Reviews Neuroscience. 2006;7(7):523–534. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- Haynes JD, Sakai K, Rees G, Gilbert S, Frith C, Passingham RE. Reading Hidden Intentions in the Human Brain. Current Biology. 2007;17(4):323–328. doi: 10.1016/j.cub.2006.11.072. [DOI] [PubMed] [Google Scholar]
- Hennig J, Zhong K, Speck O. MR-Encephalography: Fast multi-channel monitoring of brain physiology with magnetic resonance. Neuroimage. 2007;34(1):212–219. doi: 10.1016/j.neuroimage.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Debener S. Simultaneous recording of EEG and BOLD responses: A historical perspective. International Journal of Psychophysiology. 2008;67(3):161–168. doi: 10.1016/j.ijpsycho.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: The deoxyhemoglobin dilution model. Magnetic Resonance in Medicine. 1999a;42(5):849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999b;96(16):9403–9408. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, De Zwart JA, Van Gelderen P, Fulton SC, Balkin TJ, et al. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Human Brain Mapping. 2008;29(6):671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Rossion B, Skudlarski P, Gore JC. Parametric design and correlational analyses help integrating fMRI and electrophysiological data during face processing. Neuroimage. 2004;22(4):1587–1595. doi: 10.1016/j.neuroimage.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Skudlarski P, Gore JC. Correlations and dissociations between BOLD signal and P300 amplitude in an auditory, oddball task: a parametric approach to combining fMRI and ERP. Magnetic Resonance Imaging. 2002;20(4):319–325. doi: 10.1016/s0730-725x(02)00496-4. [DOI] [PubMed] [Google Scholar]
- Huppert TJ, Hoge RD, Dale AM, Franceschini MA, Boas DA. Quantitative spatial comparison of diffuse optical imaging with blood oxygen level-dependent and arterial spin labeling-based functional magnetic resonance imaging. Journal of Biomedical Optics. 2006;11(6):064018. doi: 10.1117/1.2400910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage. 2006;29(2):368–382. doi: 10.1016/j.neuroimage.2005.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg R, Wohlschlager AM, Gaser C, Liebau Y, Dauner R, Woller A, et al. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(16):4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GD. New Techniques in Magnetic-Resonance and Epilepsy. Epilepsia. 1994;35:S2–S13. doi: 10.1111/j.1528-1157.1994.tb05985.x. [DOI] [PubMed] [Google Scholar]
- Jin T, Kim SG. Functional changes of apparent diffusion coefficient during visual stimulation investigated by diffusion-weighted gradient-echo fMRI. NeuroImage. 2008;41(3):801–812. doi: 10.1016/j.neuroimage.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H. Structural Plasticity: Rewiring the Brain. Current Biology. 2007;17(4):R141–R144. doi: 10.1016/j.cub.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129(6):1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, et al. Simultaneous PET-MRI: A new approach for functional and morphological imaging. Nature Medicine. 2008;14(4):459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nature Neuroscience. 2005;8(5):679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay KN, Naselaris T, Prenger RJ, Gallant JL. Identifying natural images from human brain activity. Nature. 2008;452(7185):352–355. doi: 10.1038/nature06713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cerebral Cortex. 2005;15(8):1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- Kida I, Rothman DL, Hyder F. Dynamics of changes in blood flow, volume, and oxygenation: Implications for dynamic functional magnetic resonance imaging calibration. Journal of Cerebral Blood Flow and Metabolism. 2007;27(4):690–696. doi: 10.1038/sj.jcbfm.9600409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konn D, Gowland P, Bowtell R. MRI detection of weak magnetic fields due to an extended current dipole in a conducting sphere: A model for direct detection of neuronal currents in the brain. Magnetic Resonance in Medicine. 2003;50(1):40–49. doi: 10.1002/mrm.10494. [DOI] [PubMed] [Google Scholar]
- Kraus RH, Jr, Volegov P, Matlachov A, Espy M. Toward direct neural current imaging by resonant mechanisms at ultra-low field. NeuroImage. 2008;39(1):310–317. doi: 10.1016/j.neuroimage.2007.07.058. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. NeuroImage. 2007a;38(4):649–662. doi: 10.1016/j.neuroimage.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Bandettini P. Combining the tools: Activation-and information-based fMRI analysis. NeuroImage. 2007b;38(4):666–668. doi: 10.1016/j.neuroimage.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(51):20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(10):3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaConte S, Strother S, Cherkassky V, Anderson J, Hu XP. Support vector machines for temporal classification of block design fMRI data. Neuroimage. 2005;26(2):317–329. doi: 10.1016/j.neuroimage.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Laufs H. Endogenous brain oscillations and related networks detected by surface EEG-combined fMRI. Human Brain Mapping, current volume. 2008 doi: 10.1002/hbm.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Daunizeau J, Carmichael DW, Kleinschmidt A. Recent advances in recording electrophysiological data simultaneously with magnetic resonance imaging. NeuroImage. 2008;40(2):515–528. doi: 10.1016/j.neuroimage.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Human Brain Mapping. 2007;28(10):1023–1032. doi: 10.1002/hbm.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Urayama SI, Aso T, Hanakawa T, Fukuyama H. Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(21):8263–8268. doi: 10.1073/pnas.0600644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lemieux L, Laufs H, Carmichael D, Paul JS, Walker MC, Duncan JS. Noncanonical spike-related BOLD responses in focal epilepsy. Human Brain Mapping. 2008;29(3):329–345. doi: 10.1002/hbm.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux L, Salek-Haddadi A, Josephs O, Allen P, Toms N, Scott C, et al. Event-related fMRI with simultaneous and continuous EEG: Description of the method and initial case report. Neuroimage. 2001;14(3):780–787. doi: 10.1006/nimg.2001.0853. [DOI] [PubMed] [Google Scholar]
- Liebert A, Wabnitz H, Obrig H, Erdmann R, Möller M, Macdonald R, et al. Non-invasive detection of fluorescence from exogenous chromophores in the adult human brain. Neuroimage. 2006;31(2):600–608. doi: 10.1016/j.neuroimage.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Lin FH, Witzel T, Mandeville JB, Polimeni JR, Zeffiro TA, Greve DN, et al. Event-related single-shot volumetric functional magnetic resonance inverse imaging of visual processing. Neuroimage. 2008;42(1):230–247. doi: 10.1016/j.neuroimage.2008.04.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA, Zhang CH, Glover G, Shepp L. Rapid three-dimensional functional magnetic resonance imaging of the initial negative BOLD response. Journal of Magnetic Resonance. 2008;191(1):100–111. doi: 10.1016/j.jmr.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Liu WC, Flax JF, Guise KG, Sukul V, Benasich AA. Functional connectivity of the sensorimotor area in naturally sleeping infants. Brain Research. 2008 doi: 10.1016/j.brainres.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131(4):945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang K, Yu C, He Y, Zhou Y, Liang M, et al. Regional homogeneity, functional connectivity and imaging markers of Alzheimer's disease: A review of resting-state fMRI studies. Neuropsychologia. 2008;46(6):1648–1656. doi: 10.1016/j.neuropsychologia.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Beall EB, Sakaie KE, Koenig KA, Stone L, Marrie RA, et al. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Human Brain Mapping. 2008;29(7):818–827. doi: 10.1002/hbm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Golay X, Pekar JJ, Van Zijl PCM. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magnetic Resonance in Medicine. 2003;50(2):263–274. doi: 10.1002/mrm.10519. [DOI] [PubMed] [Google Scholar]
- Lu HZ, Golay X, Pekar JJ, van Zijl PCM. Sustained poststimulus elevation in cerebral oxygen utilization after vascular recovery. Journal of Cerebral Blood Flow and Metabolism. 2004;24(7):764–770. doi: 10.1097/01.WCB.0000124322.60992.5C. [DOI] [PubMed] [Google Scholar]
- Lui S, Ouyang L, Chen Q, Huang X, Tang H, Chen H, et al. Differential interictal activity of the precuneus/posterior cingulate cortex revealed by resting state functional MRI at 3T in generalized vs. partial seizure. Journal of Magnetic Resonance Imaging. 2008;27(6):1214–1220. doi: 10.1002/jmri.21370. [DOI] [PubMed] [Google Scholar]
- Luo H, Poeppel D. Phase Patterns of Neuronal Responses Reliably Discriminate Speech in Human Auditory Cortex. Neuron. 2007;54(6):1001–1010. doi: 10.1016/j.neuron.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biological Psychiatry. 2004;55(6):648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, et al. Brain morphology associated with obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine. 2002;166(10):1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: Relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14826–14831. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massana G, Serra-Grabulosa JM, Salgado-Pineda P, Gasto C, Junque C, Massana J, et al. Parahippocampal gray matter density in panic disorder: A voxel-based morphometric study. American Journal of Psychiatry. 2003;160(3):566–568. doi: 10.1176/appi.ajp.160.3.566. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence. 2005;77(1):23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- May A. A review of diagnostic and functional imagingin headache. Journal of Headache and Pain. 2006;7(4):174–184. doi: 10.1007/s10194-006-0307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Hajak G, Gänssbauer S, Steffens T, Langguth B, Kleinjung T, et al. Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cerebral Cortex. 2007;17(1):205–210. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- McKiernan K, D'Angelo B, Kucera-Thompson JK, Kaufman J, Binder J. Task-induced deactivation correlates with suspension of task-unrelated thoughts: An fMRI investigation. Journal of Cognitive Neuroscience. 2002:96–96. [Google Scholar]
- Miller G. Science. 5882. Vol. 320. New York, N.Y: 2008. Neuroimaging. Growing pains for fMRI; pp. 1412–1414. [DOI] [PubMed] [Google Scholar]
- Miller KL, Bulte DP, Devlin H, Robson MD, Wise RG, Woolrich MW, et al. Evidence for a vascular contribution to diffusion FMRI at high b value. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(52):20967–20972. doi: 10.1073/pnas.0707257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TM, Hutchinson R, Niculescu RS, Pereira F, Wang X, Just M, et al. Learning to decode cognitive states from brain images. Machine Learning. 2004;57(1–2 SPEC ISS):145–175. [Google Scholar]
- Mitchell TM, Shinkareva SV, Carlson A, Chang KM, Malave VL, Mason RA, et al. Predicting human brain activity associated with the meanings of nouns. Science. 2008;320(5880):1191–1195. doi: 10.1126/science.1152876. [DOI] [PubMed] [Google Scholar]
- Moore CI, Cao R. The hemo-neuro hypothesis: on the role of blood flow in information processing. J. Neurophysiology. in press doi: 10.1152/jn.01366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Van Zijl PCM. Fiber tracking: Principles and strategies-A technical review. Nmr in Biomedicine. 2002;15(7–8):468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Mourao-Miranda J, Bokde ALW, Born C, Hampel H, Stetter M. Classifying brain states and determining the discriminating activation patterns: Support Vector Machine on functional MRI data. Neuroimage. 2005;28(4):980–995. doi: 10.1016/j.neuroimage.2005.06.070. [DOI] [PubMed] [Google Scholar]
- Muhlau M, Rauschecker JP, Oestreicher E, Gaser C, Röttinger M, Wohlschlager AM, et al. Structural brain changes in tinnitus. Cerebral Cortex. 2006;16(9):1283–1288. doi: 10.1093/cercor/bhj070. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47(1):36–45. [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Singh KD. Spatiotemporal frequency tuning of BOLD and gamma band MEG responses compared in primary visual cortex. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.01.052. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nature Neuroscience. 2007;10(12):1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- O'Gorman RL, Mehta MA, Asherson P, Zelaya FO, Brookes KJ, Toone BK, et al. Increased cerebral perfusion in adult attention deficit hyperactivity disorder is normalised by stimulant treatment: A non-invasive MRI pilot study. Neuroimage. 2008;42(1):36–41. doi: 10.1016/j.neuroimage.2008.04.169. [DOI] [PubMed] [Google Scholar]
- O'Toole AJ, Jiang F, Abdi H, Haxby JV. Partially distributed representations of objects and faces in ventral temporal cortex. Journal of Cognitive Neuroscience. 2005;17(4):580–590. doi: 10.1162/0898929053467550. [DOI] [PubMed] [Google Scholar]
- Paley MNJ, Chow LS, Whitby EH, Cook GG. Modelling of axonal fields in the optic nerve for direct MR detection studies. Image and Vision Computing. 2008 [Google Scholar]
- Park TS, Lee SY, Park JH. Effect of nerve cell currents on MRI images in snail ganglia. Neuroreport. 2004;15(18):2783–2786. [PubMed] [Google Scholar]
- Pelletier D, Garrison K, Henry R. Measurement of whole-brain atrophy in multiple sclerosis. Journal of Neuroimaging. 2004;14 SUPPL. 3:11S–19S. doi: 10.1177/1051228404266264. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Padmala S. Quantitative prediction of perceptual decisions during near-threshold fear detection. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(15):5612–5617. doi: 10.1073/pnas.0500566102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S. Decoding near-threshold perception of fear from distributed single-trial brain activation. Cerebral Cortex. 2007;17(3):691–701. doi: 10.1093/cercor/bhk020. [DOI] [PubMed] [Google Scholar]
- Petridou N, Plenz D, Silva AC, Loew M, Bodurka J, Bandettini PA. Direct magnetic resonance detection of neuronal electrical activity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(43):16015–16020. doi: 10.1073/pnas.0603219103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE. Guidelines for reporting an fMRI study. Neuroimage. 2008;40(2):409–414. doi: 10.1016/j.neuroimage.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabrait C, Ciuciu P, Ribès A, Poupon C, Le Roux P, Dehaine-Lambertz G, et al. High temporal resolution functional MRI using parallel echo volumar imaging. Journal of Magnetic Resonance Imaging. 2008;27(4):744–753. doi: 10.1002/jmri.21329. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Kramer JH, Gorno-Tempini ML, Schuff N, Weiner M, Miller BL. Patterns of cerebral atrophy in primary progressive aphasia. American Journal of Geriatric Psychiatry. 2002;10(1):89–97. [PubMed] [Google Scholar]
- Rouw R, Scholte HS. Increased structural connectivity in grapheme-color synesthesia. Nature Neuroscience. 2007;10(6):792–797. doi: 10.1038/nn1906. [DOI] [PubMed] [Google Scholar]
- Rusch N, Tebartz Van Elst L, Ludaescher P, Wilke M, Huppertz HJ, Thiel T, et al. A voxel-based morphometric MRI study in female patients with borderline personality disorder. Neuroimage. 2003;20(1):385–392. doi: 10.1016/s1053-8119(03)00297-0. [DOI] [PubMed] [Google Scholar]
- Salmond CH, De Haan M, Friston KJ, Gadian DG, Vargha-Khadem F. Investigating individual differences in brain abnormalities in autism. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358(1430):405–413. doi: 10.1098/rstb.2002.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Martínez A, Pomarol-Clotet E, Gomar J, Vila F, Sarro S, et al. A simple view of the brain through a frequency-specific functional connectivity measure. NeuroImage. 2008;39(1):279–289. doi: 10.1016/j.neuroimage.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MCM, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. International Journal of Psychophysiology. 2008;67(3):242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Schei JL, McCluskey MD, Foust AJ, Yao XC, Rector DM. Action potential propagation imaged with high temporal resolution near-infrared video microscopy and polarized light. Neuroimage. 2008;40(3):1034–1043. doi: 10.1016/j.neuroimage.2007.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Shinkareva SV, Mason RA, Malave VL, Wang W, Mitchell TM, Just MA. Using fMRI brain activation to identify cognitive states associated with perception of tools and dwellings. PLoS ONE. 2008;3(1) doi: 10.1371/journal.pone.0001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Human Brain Mapping. 2008;29(7):751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Chaimow D, Logothetis NK, Ugurbil K. Spatio-temporal point-spread function of fMRI signal in human gray matter at 7 Tesla. Neuroimage. 2007;35(2):539–552. doi: 10.1016/j.neuroimage.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu XP, et al. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36(6):1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage. 2007;38(2):306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain. 2008;131(2):559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP, Duyn JH. Functional MRI impulse response for BOLD and CBV contrast in rat somatosensory cortex. Magnetic Resonance in Medicine. 2007;57(6):1110–1118. doi: 10.1002/mrm.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KD, Barnes GR, Hillebrand A, Forde EME, Williams AL. Task-related changes in cortical synchronization are spatially coincident with the hemodynamic response. Neuroimage. 2002;16(1):103–114. doi: 10.1006/nimg.2001.1050. [DOI] [PubMed] [Google Scholar]
- Singh M, Kim S, Kim TS. Correlation between BOLD-fMRI and EEG signal changes in response to visual stimulus frequency in humans. Magnetic Resonance in Medicine. 2003;49(1):108–114. doi: 10.1002/mrm.10335. [DOI] [PubMed] [Google Scholar]
- Sluming V, Barrick T, Howard M, Cezayirli E, Mayes A, Roberts N. Voxel-based morphometry reveals increased gray matter density in Broca's area in male symphony orchestra musicians. Neuroimage. 2002;17(3):1613–1622. doi: 10.1006/nimg.2002.1288. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Büchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360(9330):380–383. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Song AW, Harshbarger T, Li TL, Kim KH, Ugurbil K, Mori S, et al. Functional activation using apparent diffusion coefficient-dependent contrast allows better spatial localization to the neuronal activity: evidence using diffusion tensor imaging and fiber tracking. Neuroimage. 2003;20(2):955–961. doi: 10.1016/S1053-8119(03)00292-1. [DOI] [PubMed] [Google Scholar]
- Song AW, Woldorff MG, Gangstead S, Mangun GR, McCarthy G. Enhanced spatial localization of neuronal activation using simultaneous apparent-diffusion-coefficient and blood-oxygenation functional magnetic resonance Imaging. Neuroimage. 2002;17(2):742–750. [PubMed] [Google Scholar]
- Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, et al. Brain spontaneous functional connectivity and intelligence. NeuroImage. 2008;41(3):1168–1176. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- Staempfli P, Reischauer C, Jaermann T, Valavanis A, Kollias S, Boesiger P. Combining fMRI and DTI: A framework for exploring the limits of fMRI-guided DTI fiber tracking and for verifying DTI-based fiber tractography results. Neuroimage. 2008;39(1):119–126. doi: 10.1016/j.neuroimage.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Strothert SC, Anderson J, Hansen LK, Kjems U, Kustra R, Sidtis J, et al. The quantitative evaluation of functional neuroimaging experiments: The NPAIRS data analysis framework. Neuroimage. 2002;15(4):747–771. doi: 10.1006/nimg.2001.1034. [DOI] [PubMed] [Google Scholar]
- Sun P, Ueno K, Waggoner RA, Gardner JL, Tanaka K, Cheng K. A temporal frequency-dependent functional architecture in human V1 revealed by high-resolution fMRI. Nature Neuroscience. 2007;10(11):1404–1406. doi: 10.1038/nn1983. [DOI] [PubMed] [Google Scholar]
- Tang L, Avison MJ, Gatenby JC, Gore JC. Failure to direct detect magnetic field dephasing corresponding to ERP generation. Magnetic Resonance Imaging. 2008;26(4):484–489. doi: 10.1016/j.mri.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, et al. The distribution of structural neuropathology in pre-clinical Huntington's disease. Brain. 2002;125(8):1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Chang CE, Glover GH, Gabrieli JDE, Greicius MD, Gotlib IH. Default-mode function and task-induced deactivation have overlapping brain substrates in children. NeuroImage. 2008;41(4):1493–1503. doi: 10.1016/j.neuroimage.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Foland LC, Glover GH. Calibration of BOLD fMRI using breath holding reduces group variance during a cognitive task. Human Brain Mapping. 2007;28(1):59–68. doi: 10.1002/hbm.20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan AS, Birn RM, Bandettini PA, Boynton GM. Differential transient MEG and fMRI responses to visual stimulation onset rate. International Journal of Imaging Systems and Technology. 2008;18(1):17–28. [Google Scholar]
- van den Heuvel M, Mandl R, Hulshoff Pol H. Normalized cut group clustering of resting-state FMRI data. PLoS ONE. 2008;3(4) doi: 10.1371/journal.pone.0002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk H, Schoffelen JM, Oostenveld R, Jensen O. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. Journal of Neuroscience. 2008;28(8):1816–1823. doi: 10.1523/JNEUROSCI.1853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Waldert S, Preissl H, Demandt E, Braun C, Birbaumer N, Aertsen A, et al. Hand movement direction decoded from MEG and EEG. Journal of Neuroscience. 2008;28(4):1000–1008. doi: 10.1523/JNEUROSCI.5171-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Vargha-Khadem F, Ashburner J, Passingham RE, Connelly A, Friston KJ, et al. MRI analysis of an inherited speech and language disorder: Structural brain abnormalities. Brain. 2002;125(3):465–478. doi: 10.1093/brain/awf057. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WYI, Dai G, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41(4):1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kowatch RA, Delbello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: First results. Psychiatry Research-Neuroimaging. 2004;131(1):57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: Correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage. 2003;20(1):202–215. doi: 10.1016/s1053-8119(03)00199-x. [DOI] [PubMed] [Google Scholar]
- Williams MA, Dang S, Kanwisher NG. Only some spatial patterns of fMRI response are read out in task performance. Nature Neuroscience. 2007;10(6):685–686. doi: 10.1038/nn1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink AM, Bullmore E, Barnes A, Bernard F, Suckling J. Monofractal and multifractal dynamics of low frequency endogenous brain oscillations in functional MRI. Human Brain Mapping. 2008;29(7):791–801. doi: 10.1002/hbm.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999;122(11):2101–2107. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Poline JB, Friston KJ, Evans AC. Characterizing the response of PET and fMRI data using multivariate linear models. Neuroimage. 1997;6(4):305–319. doi: 10.1006/nimg.1997.0294. [DOI] [PubMed] [Google Scholar]
- Wright IC, McGuire PK, Poline JB, Travere JM, Murray RM, Frith CD, et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage. 1995;2(4):244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- Xiong J, Fox PT, Gao JH. Directly mapping magnetic field effects of neuronal activity by magnetic resonance imaging. Human Brain Mapping. 2003;20(1):41–49. doi: 10.1002/hbm.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonskiy DA, Ackerman JJH, Raichle ME. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(13):7603–7608. doi: 10.1073/pnas.97.13.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Ugurbil K. high-field fMRI unveils orientation columns in humans. Pro. Natl. Acad. Sci. USA. 2008;105(30):10607–10612. doi: 10.1073/pnas.0804110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Logothetis N, Uğurbil K. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. Neuroimage. 2007;37(4):1161–1177. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(15):9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeff BW, White BR, Dehghani H, Schlaggar BL, Culver JP. Retinotopic mapping of adult human visual cortex with high-density diffuse optical tomography. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(29):12169–12174. doi: 10.1073/pnas.0611266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Olivi A, Hertig SJ, van Zijl P, Mori S. Automated fiber tracking of human brain white matter using diffusion tensor imaging. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.04.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong K, Leupold J, von Elverfeldt D, Speck O. The molecular basis for gray and white matter contrast in phase imaging. Neuroimage. 2008;40(4):1561–1566. doi: 10.1016/j.neuroimage.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, et al. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophrenia Research. 2008;100(1–3):120–132. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Zhu CZ, Zang YF, Cao QJ, Yan CG, He Y, Jiang TZ, et al. Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. NeuroImage. 2008;40(1):110–120. doi: 10.1016/j.neuroimage.2007.11.029. [DOI] [PubMed] [Google Scholar]