Abstract
Chikungunya virus (CHIKV) is an arbovirus (genus Alphavirus, family Togaviridae) that has recently caused disease outbreaks in the Indian Ocean basin and southern Europe. These outbreaks could be associated with a possible shift in primary vector from Aedes aegypti to Ae. albopictus. To evaluate vector competence differences in possible CHIKV vectors, we evaluated the dose-dependant susceptibility of Florida strains of Ae. albopictus and Ae. aegypti for infection with a La Réunion island strain of CHIKV. Pledget and water-jacketed membrane feeding systems were also evaluated. We show that both Aedes spp. were susceptible to the highest CHIKV doses, whereas only Ae. albopictus developed disseminated infections after exposure to the two lowest doses. Infection rates for both mosquito species were significantly affected by the bloodmeal delivery method used. This information is important in assessing risk of an outbreak of imported CHIKV in the United States, in determining differences in vectorial capacity of these two vector species, and in evaluating arbovirus delivery methods in the laboratory.
Keywords: arbovirus, viremia, vector competence, vectorial capacity, blood feeding
Chikungunya virus (CHIKV) (genus Alphavirus, family Togaviridae) is a mosquito-borne virus initially isolated in Tanzania in 1953 (Ross 1956). Although CHIKV is rarely fatal, symptoms of infection include sudden onset of fever, rash, headache, photophobia, vomiting, and severe arthralgia that can result in chronic joint problems persisting for several years (McGill 1995). Historically, epidemics of CHIKV have occurred in Africa, Southeast Asia, and India (Ross 1956, Jupp and McIntosh 1990). A large-scale epidemic of CHIKV is underway that began in Kenya in 2004 and subsequently spread to the Indian Ocean islands of La Réunion, Mayotte, Comoros, Mauritius, the Seychelles, and Madagascar in 2005–2006 (Chretien et al. 2007). The epidemic continues to spread through India, where 1.3 million human cases were reported between 2005 and 2006 (Arankalle et al. 2007). Recently, human cases have been reported in Indonesia, Sri Lanka, and Singapore (PROMED 2008) and, in a historic range expansion, CHIKV was reported in Italy in 2007 (Rezza et al. 2007).
In tropical Africa, CHIKV has a sylvatic transmission cycle between nonhuman primates and forest gallery Aedes mosquitoes, and there is likely continuous transmission to immune human populations, resulting in sporadic cases (Jupp and McIntosh 1990, Diallo et al. 1999). Outside of Africa, CHIKV is primarily transmitted by Aedes aegypti and Aedes albopictus and can cause large-scale human epidemics (Laras et al. 2005, Pialoux et al. 2007). Recent outbreaks of CHIKV have been characterized by greater morbidity and mortality than previous outbreaks (Powers and Logue 2007). In the Indian Ocean outbreak, a novel strain of CHIKV emerged that contains a point mutation, which changes the amino acid at position 226 in the envelope protein from an alanine to a valine (Schuffenecker et al. 2006). This minor genetic change seems to increase the vector competence for CHIKV in Ae. albopictus but not Ae. aegypti (Tsetsarkin et al. 2007, Vazeille et al. 2007). The enhanced transmission of this Indian Ocean strain of CHIKV by Ae. albopictus is one of several hypotheses for the recent intensified outbreaks and may help to explain the expansion of CHIKV transmission into new regions, such as Italy, where only Ae. albopictus is present.
In prior CHIKV outbreaks, Ae. aegypti has been considered the primary epidemic vector of CHIKV and Ae. albopictus a secondary vector. These mosquito species inhabit artificial containers in their immature stages, live near and within human dwellings, have been spread throughout much of the world through global trade, and have overlapping distributions (Knudsen 1995, Benedict et al. 2007). The worldwide distribution of epidemic vectors and travel and global trade in endemic regions have increased the risk for spread of CHIKV into previously unaffected areas (Parola et al. 2006). Previous research has shown that North American strains of Ae. aegypti and Ae. albopictus are capable of transmitting a Thai strain of CHIKV from 1962, with Ae. albopictus showing higher infection and dissemination rates (Turell et al. 1992). Florida strains of Ae. aegypti and Ae. albopictus are susceptible to a high dose of a La Réunion strain of CHIKV (titers ranging between log10 5.9 and log10 6.5 PFU/ml), with Ae. albopictus exhibiting higher dissemination rates than Ae. aegypti (Reiskind et al. 2008). The impact of the differential vector competence of Ae. aegypti and Ae. albopictus for CHIKV on disease transmission cycles remains unexplored.
To help determine the importance of Ae. aegypti and Ae. albopictus for the potential transmission of CHIKV in the United States, and especially in Florida where both species of mosquito are present and abundant, the CHIKV bloodmeal titer necessary to infect local strains of Ae. aegypti and Ae. albopictus was evaluated. To determine minimum infectious titer for each species, we exposed Florida Ae. albopictus and Ae aegypti mosquitoes to serially diluted viral doses of a La Réunion strain of CHIKV and tested two artificial feeding techniques for exposing mosquitoes to virus.
Materials and Methods
Mosquitoes
The mosquitoes used for these experiments were F1 Ae. aegypti and Ae. albopictus from field collections made in Palm Beach County from April to June 2007. Larvae (F1) were hatched in tap water and put in groups of 50–100 in 1 liter of tap water with 0.3 g 1:1 yeast:lactalbumin added as a food source. Larvae were reared in trays at 28°C with a 14 L:10 D cycle.
Virus
We used CHIKV strain LR2006-OPY1, isolated from a febrile patient in France who had been infected in La Réunion (Parola et al. 2006) and passaged once in African green monkey kidney (Vero) cells. Virus stocks were prepared by inoculating Vero cell cultures maintained in M199 media supplemented with 10% fetal bovine serum, antibiotics, and antimycotics (Invitrogen, Carlsbad, CA). The virus stock titer was determined (log10 = 7.8 pfu/ml) using aplaque assay as described previously (Gargan et al. 1983), except that a second overlay, containing neutral red, was added 24 h after the first overlay. Virus stocks were stored at −80°C. To produce fresh virus for mosquito infection, a 275-ml tissue culture flask with a confluent monolayer of Vero cells in 10 ml of cell culture media was inoculated with 250 µl of stock virus and allowed to incubate with 5% CO2 at 35°C for 24 h. Visual inspection of cells after this period showed noticeable cytopathology, and titers of greater than log10 7.0 CHIKV PFU/ml in the supernatant of the tissue culture flasks were achieved with this method.
Oral Infection of Mosquitoes
Seven- to 10-d-old mosquitoes were placed in cylindrical, waxed cardboard (14 cm high by 11 cm diameter) containers (Dade Paper, Miami, FL) in groups of ≈50 females, and cages were put into an incubator (14 L10 D, with an automated dusk/dawn period of 1 h, at 28°C, ≈95% rh) within a certified Biosafety Level 3 containment facility. Mosquitoes were sucrose-starved 72 h before being offered an infectious blood meal. Mosquitoes were either exposed to cotton pledgets or water-jacketed glass membrane feeders (Rutledge et al. 1964) with Edicoll collagen film (Devor, Sandy Run, SC). Blood meals contained citrated bovine blood (Hemostat Laboratories, Dixon, CA) mixed with 10-fold dilutions of supernatant from a CHIKV-inoculated 275-ml tissue culture flask diluted in BA-1 media (EMEM, 1% bovine serum albumin, 0.6% Tris, pH 7.6, 7.5% sodium bicarbonate, 10 ml penicillin-streptomycin, 4 ml fungizone, and water to 1 liter, with HCl added for a final pH of 7.4). Adenosine triphosphate was added as a phagostimulant to blood meals at a final concentration of 5 mM. Two pledgets containing 3 ml of infectious blood meal each were heated in a 35°C incubator for 20 min, and 0.25 ml 20% sugar water was added, to better induce mosquitoes to feed. Blood meals delivered through water-jacketed glass membrane feeders were constantly maintained at 35°C during feeding.
After 1 h of feeding, mosquitoes were anesthetized with cold, and fully engorged mosquitoes were placed in new containers and returned to the incubators as described above. We delivered blood meals with four infectious doses: log10 6.1, log10 5.2, log10 4.4, and log10 3.6 PFU CHIKV/ml, determined by plaque assay. Immediately after blood feeding, five whole mosquitoes from each respective virus dilution were tested for virus titer: log10 2.9, log10 2.2, log10 1.3, and log10 0.9 PFU CHIKV/whole body, determined by Taqman quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). Six days after exposure, surviving mosquitoes were aspirated from cages and killed by freezing. Legs and bodies were triturated separately in 900 µl of BA-1 containing two 4.5-mm zincplated beads (BB-caliber air gun shot) and stored at −80°C until further processing.
RNA Extraction
Samples were homogenized at 25 Hz for 3 min using a Tissuelyser tissue homogenizer (Qiagen, Valencia, CA) and clarified by centrifugation (3,148g for 4 min). Viral RNA was extracted using the MagNA Pure LC Instrument (Roche Diagnostics, Mannheim, Germany) and the MagNA Pure LC Total Nucleic Acid Isolation kit (Roche Diagnostics) following the manufacturer's instructions.
Quantitative RT-PCR
One-step qRT-PCR was used to determine infection status and body titer of samples. Final reaction volumes of 20 µl containing 5 µl of nucleic acid, 10 µl of 2 × SuperScript reaction mix buffer (Invitrogen), 4 pmol of the TaqMan probe, 10 pmol of each primer, 40 ng of bovine serum albumin (New England Biolabs, Ipswich, MA), and 0.4 µl of SuperScript III Platinum Taq mix (Invitrogen) were run on a LightCycler 480 (Roche Diagnostics) under the following conditions: 48°C for 20 min (reverse transcription), 95°C for 2 min (initial denaturation), and 40 cycles of 60°C for 15 s, 95°C for 10 s, followed by cool down at 50°C for 30 s. Primers were designed from the E1 gene and had the following sequences: forward, 5′-ACC CGG TAA GAG CGA TGA ACT-3′; reverse, 5′-AGG CCG CAT CCG GTA TGT-3′. The probe sequence was 5′-/5Cy5/CCG TAG GGA ACA TGC CCA TCT CCA/3BHQ 2/-3′ (IDT DNA, Coralville, IA). Virus titers were calculated by comparing crossing point values to standard curves based on data acquired from 10-fold serial dilutions of virus stocks (Reiskind et al. 2008).
Statistical Analysis
Standard curves and calculation of body titers by qRT-PCR were carried out with LC 480 software (Roche Diagnostics). A mosquito with virus found in the body but not in the legs was considered a nondisseminated infection limited to the midgut, and when virus was found in both the body and legs, the mosquito was determined to have a disseminated infection. Proportion infected was calculated by dividing the number of infected bodies by the total number of mosquitoes tested. Proportion with disseminated infection was calculated as the number of mosquitoes with positive legs divided by the total number of infected mosquitoes. Effects of titer and blood delivery system on proportion infected and disseminated were determined by categorical analysis of variance (ANOVA) with maximum likelihood estimates compared with χ2 distribution (PROC CATMOD, SAS version 9.1; SAS Institute, Cary, NC). Post hoc pairwise comparisons with the χ2 distribution were used to detect differences in proportions of infection and dissemination between groups, with α adjusted for multiple comparisons (SAS Institute 2002).
Results
Effect of Titer on Infection and Dissemination
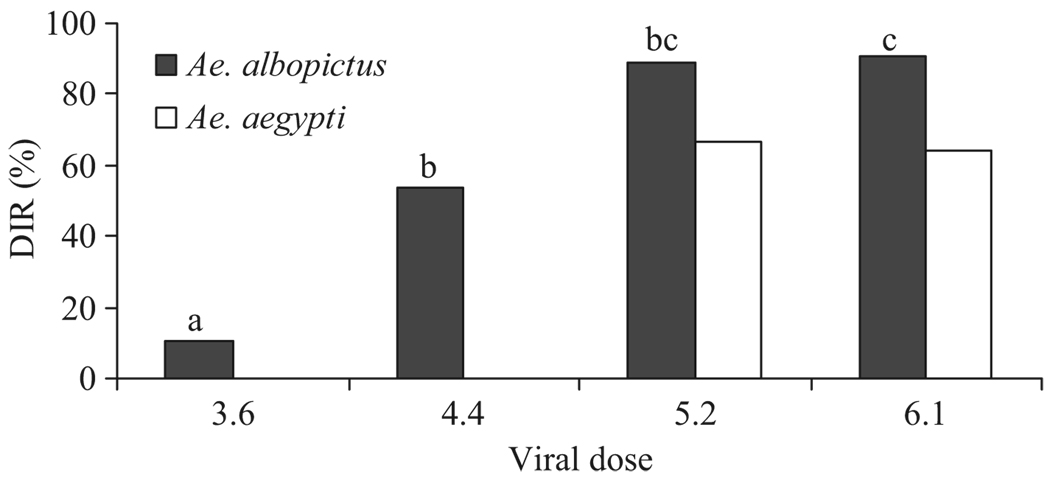
After an extrinsic incubation period of 6 d, Ae. albopictus was significantly more susceptible to CHIKV infection than Ae. aegypti, with infection rates of up to 100% when feeding on water-jacketed membranes and high viral doses (species: χ21 = 141.09, P < 0.0001). Viral doses produced different infection rates for both mosquito species (Table 1; bloodmeal titer for infection: χ23 = 87.73, P < 0.0001) and disseminated infection rates for Ae. albopictus (Fig. 1; bloodmeal titer for dissemination: χ23 = 23.84, P < 0.0001). Ae. aegypti had lower infection and disseminated infection rates than Ae. albopictus at each dose and was not infected when exposed to the lowest dose on either pledgets or membranes (Table 1).
Table 1.
Chikungunya virus percentage infection rates (n) in mosquitoes, processed 6 d after feeding on pledgets (p) or water jacketed membranes (m)
| Log virus dose |
Ae. albopictus (p) |
Ae. albopictus (m) |
Ae. aegypti (p) |
Ae. aegypti (m) |
|---|---|---|---|---|
| 6.1 | 92.3 (26) | 100 (22) | 18.8 (32) | 57.7 (26) |
| 5.2 | 83.8 (37) | 100 (9) | 4.5 (44) | 23.8 (42) |
| 4.4 | 48.9 (45) | 89.7 (29) | 0 (32) | 3.1 (32) |
| 3.6 | 19 (42) | 65.5 (29) | 0 (30) | 0 (16) |
Infection = % infection (n). Log virus dose = Log10 PFU CHIKV per ml. n = no. mosquitoes tested. Significantly different pairs within species and titer for membrane versus pledget at P < 0.05 by χ2 test are shown in bold.
Fig. 1.
Comparison of dissemination rates for Ae. aegypti and Ae. albopictus at different bloodmeal titers fed through water-jacketed membranes. DIR (disseminated infection rate) = % mosquitoes with positive legs/mosquitoes with positive bodies. Letters denote homogenous groups by posthoc pair wise comparisons at αadj. = 0.0083, comparing proportions to a χ2 distribution with one degree of freedom.
Effect of Bloodmeal Delivery Method on Infection Rates
In general, mosquitoes of both species had significantly lower infection rates when fed on pledgets compared with membranes (Table 1). Ae. aegypti had lower infection rates when fed on pledgets versus membranes when exposed to the higher two viral doses and had little or no infection after exposure to the two lower titers (membrane versus pledget at 6.1 log10 PFU CHIKV/ml: χ21 = 7.8, P < 0.01; membrane versus pledget at 5.2 log10 PFU CHIKV/ml: χ21 = 5.5, P < 0.05). For Ae. albopictus, pledget feeding resulted in significantly lower infection rates when exposed to the two lower viral doses but no significant difference in infection rates at the two higher viral doses (membrane versus pledget at 4.4 log10 PFU CHIKV/ml: χ21 = 12.9, P < 0.001; membrane versus pledget at 3.6 log10 PFU CHIKV/ml: χ21 = 15.7, P < 0.001).
Discussion
Our results showed that Ae. albopictus from Florida were more susceptible to infection with the La Réunion strain of CHIKV than sympatric Ae. aegypti, regardless of the bloodmeal delivery method we used. These results support a previous study using artificial membrane feeders (Reiskind et al. 2008). Ae. albopictus became infected at lower viral doses and developed disseminated infections at all viral doses tested, whereas Ae. aegypti was less susceptible, with no disseminated infections developing 6 d after exposure to the two lowest viral doses. These results were similar with both methods used to expose mosquitoes to infectious blood meals. However, the bloodmeal delivery method impacted infection rates, and mosquitoes of both species had significantly higher infection rates when exposed to water jacketed membranes compared with pledgets at the same viral titers.
We hypothesize that the differences we observed in infection rates between blood delivery methods were produced by a smaller initial dose ingested through pledgets, because of a phenomenon known as “discontinuous feeding,” which results in fluids being diverted to the diverticula (Christophers 1960) and could create the appearance of distension by blood meal when in fact distension was caused by air. The actual ingested blood meal would have been split between the diverticula and the midgut, resulting in lower viral doses and thus lower infection rates, although freshly fed mosquitoes had similar viral titers. The significantly different results produced by these two feeding methods underscore the importance of taking into account feeding method when comparing studies. Although pledget feeding resulted in lower infection rates than water jacketed membrane feeding, we observed the same pattern of infection regardless of feeding apparatus (i.e., Ae. albopictus had higher infection and disseminated infection rates than Ae. aegypti). The information obtained with this feeding method can still be useful, as long as it is not compared directly with vector competence information gained from other artificial feeding methods or live animal studies. We also observed a higher feeding rate on the water jacketed membranes (>50% of Ae. albopictus exposed, whereas <20% of Ae. albopictus fed on pledgets), which allows for larger initial sample sizes and enables easier experimental setup.
One hypothesis for the severity of the recent CHIKV outbreak in La Réunion and other areas concerns a mutation in the envelope protein gene that has been associated with strains in the outbreak areas and could enhance the vector competence of Ae. albopictus (Schuffenecker et al. 2006). Recent research has shown that Ae. albopictus mosquitoes from La Réunion became infected and developed disseminated infections with the emerging virus strain at twice the rate as with a previous strain (Vazeille et al. 2007). Other research has shown that Ae. albopictus (from highly colonized strains) develops disseminated infections after a much lower infectious dose of the emergent CHIKV strain compared with other strains and compared with altered strains that have been engineered to take away the hypothesized susceptibility conferring alteration (Tsetsarkin et al. 2007). Our research agrees with these previous studies and shows that a Florida strain of Ae. albopictus is susceptible at lower doses of the La Réunion strain of CHIKV, relative to Florida Ae. aegypti.
Even at the lowest viral dose tested, >50% of Ae. albopictus became infected after feeding on water-jacketed membranes. This low threshold for infection may heighten the risk of an imported infection spreading to resident mosquitoes in areas without endemic CHIKV, such as Florida. From April to December 2006, CHIKV antibodies were detected in 35 travelers returning to the United States from Asia and Africa, and 8 of these were circulating virus at titers from 3.9 to 6.8 log10 PFU CHIKV/ml (Lanciotti et al. 2007). Taken together with our findings, at the high end, these titers could readily infect local Florida Ae. albopictus or Ae. aegypti and likely infect Ae. albopictus at the low end.
The importance of Ae. albopictus, relative to Ae. aegypti, in CHIKV epidemiology remains an open question, but the stark differences in susceptibility may explain the prominence of Ae. albopictus in recent outbreaks, particularly the epidemic in Italy in 2007. The theoretical importance of multiple vectors for vector-borne infectious disease epidemiology has not been well examined. Although variation in vector susceptibility to infection has not been modeled, host diversity and differences in host viremia have been modeled previously and may represent an analogous situation to variation in vector susceptibility (Lord et al. 2006). The high susceptibility of a vector to low host viremia, as our findings suggest for Ae. albopictus to CHIKV, would effectively extend the period of host infectiousness, another important parameter in vector-borne disease transmission models (Dye 1986). The wide range of epidemiological outcomes caused by differences in vector susceptibility to CHIKV infection is additionally dependent on other aspects of vectorial capacity. However, because these systems are complicated, there is a need for empirical studies comparing vectors to evaluate the potential effects of vector diversity on the dynamics of disease transmission. Future studies should compare strains of Ae. albopictus and Ae. aegypti from Florida to strains from the epidemic areas of the Indian Ocean basin and also contrast the vector competence of Ae. albopictus from the southern United States to strains derived from the northern United States.
The autochthonous transmission of CHIKV in Europe, abundance of both of these mosquito species in urban areas nearby international airports in Florida (Rey et al. 2006), and the numerous imported CHIKV viremic cases to the United States demonstrate the potential importance of this emerging arbovirus for the southern United States.
Acknowledgments
We thank S. Richards and C. Smartt for critical reviews of earlier versions of this manuscript. This work was funded under NIH Grant R01AI-044793 to L.P.L. and Civilian Research and Development Foundation Grant DTRA04-059 to C.N.M.
References Cited
- Arankalle VA, Shrivastava S, Cherian S, Gunjikar RS, Walimbe AM, Jadhav SM, Sudeep AB, Mishra AC. Genetic divergence of chikungunya viruses in India (1963–2006) with special reference to the 2005–2006 explosive epidemic. J. Gen. Virol. 2007;88:1967–1976. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- Benedict MQ, Levine RS, Hawley WA, Lounibos LP. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector-borne Zoonotic Dis. 2007;7:76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien J-P, Anyamba A, Bedno SA, Breiman RF, Sang R, Sergon K, Powers AM, Onyango CO, Small J, Tucker CJ, Linthicum KJ. Drought-associated chikungunya emergence along coastal East Africa. Am. J. Trop. Med. Hyg. 2007;76:405–407. [PubMed] [Google Scholar]
- Christophers SR. Aedes aegypti: the yellow fever mosquito. United Kingdom: University Press, Cambridge; 1960. [Google Scholar]
- Diallo M, Thonnon J, Traore-Lamizana M, Fontenille D. Vectors of chikungunya virus in Senegal: current data and transmission cycles. Am. J. Trop. Med. Hyg. 1999;60:281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- Dye C. Vectoral capacity: must we measure all its components? Parasitol. Today. 1986;2:203–208. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- Gargan T, Bailey C, Higbee G, Gad A, El-Said S. The effect of laboratory colonization on the vector-pathogen interactions of Egyptian Culex pipiens and Rift Valley fever virus. Am. J. Trop. Med. Hyg. 1983;32:1154–1163. doi: 10.4269/ajtmh.1983.32.1154. [DOI] [PubMed] [Google Scholar]
- Jupp PG, McIntosh BM. Aedes furcifer and other mosquitoes as vectors of chikungunya virus at Mica, northeastern Transvaal, South Africa. J. Am. Mosq. Control Assoc. 1990;6:415–420. [PubMed] [Google Scholar]
- Knudsen AB. Global distribution and continuing spread of Aedes albopictus. Parassitologia. 1995;37:91–97. [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy O, Laven J, Panella A, Velez J, Lambert A, Campbell G. Chikungunya virus in US travelers returning from India, 2006. Emerg. Infect. Dis. 2007;13:764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laras K, Sukri NC, Larasati RP, Bangs MJ, Kosim, Djauzi R, Wandra T, Master J, Kosasih H, Hartati S. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 2005;99:128–141. doi: 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Lord CC, Rutledge CR, Tabachnick WJ. Relationships between host viremia and vector susceptibility for arboviruses. J. Med. Entomol. 2006;43:623–630. doi: 10.1603/0022-2585(2006)43[623:rbhvav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill PE. Viral infections: alpha-viral arthropathy. Bailliere's Clin. Rheumatol. 1995;9:145–150. doi: 10.1016/s0950-3579(05)80151-7. [DOI] [PubMed] [Google Scholar]
- Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, Minodier P, Brouqui P, Flahault A, Raoult D, Charrel RN. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg. Infect. Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect. Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J. Gen. Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- Reiskind MH, Pesko K, Westbrook CJ, Mores CN. Susceptibility of Florida mosquitoes to infection with chikungunya virus. Am. J. Trop. Med. Hyg. 2008;78:422–425. [PMC free article] [PubMed] [Google Scholar]
- Rey JR, Nishimura N, Wagner B, Braks MAH, O’Connell SM, Lounibos LP. Habitat segregation of mosquito arbovirus vectors in south Florida. J. Med. Entomol. 2006;43:1134–1141. doi: 10.1603/0022-2585(2006)43[1134:hsomav]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli A, Panning M, Cordioli P, Fortuna C, Boros S. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- Ross RW. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J. Hygiene. 1956;54:177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge LC, Ward RA, Gould DJ. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosq. News. 1964;24:407–419. [Google Scholar]
- SAS Institute. User’s guide for personal computers computer program, version by SAS Institute. Cary, NC: 2002. [Google Scholar]
- Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney M-C, Lavenir R, Pardigon N, Reynes J-M, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel M-P, Bréhin A-C, Cubito N, Desprès P, Kunst F, Rey FA, Zeller H, Brisse S. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathogens. 2007;3:1895–1906. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ, Beaman JR, Tammariello RF. Susceptibility of selected strains of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) to chikungunya virus. J. Med. Entomol. 1992;29:49–53. doi: 10.1093/jmedent/29.1.49. [DOI] [PubMed] [Google Scholar]
- Vazeille M, Moutailler S, Coudrier D, Rousseaux C, Khun H, Huerre M, Thiria J, Dehecq J-S, Fontenille D, Schuffenecker I, Despres P, Failloux A-B. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE. 2007;2:e1168. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]