Abstract
Chronic Kidney Disease-Mineral Bone Disorder (CKD-MBD) is a newly defined disorder that describes the interacting triad of (1) biochemical abnormalities of calcium, phosphorus, and PTH, (2) vascular calcification, and (3) abnormal bone in patients with CKD. We describe a novel animal model of slowly progressive CKD that spontaneously develops all three components of CKD-MBD while fed a normal phosphorus diet. The advantage of this model is the natural progression of the disease, allowing manipulation early in the course of CKD to better understand the pathophysiology of CKD-MBD. We further demonstrate that different sources of dietary protein, despite having similar total phosphorus contents, can have profound effects on the progression of CKD-MBD, likely due to differences in intestinal bioavailability of these phosphorus sources. Animals with early, but established, CKD fed a casein-based protein source, compared to grain-based protein source, had no differences in serum phosphorus. However, the casein protein-fed animals had increased urinary phosphorus excretion and elevated serum FGF23. Thus, this animal model will allow us to examine early changes in the course of CKD that may lead to CKD-MBD.
Vascular calcification is common in patients with chronic kidney disease (CKD), appearing in 30-65% of patients with Stage 3-5 CKD, 50-80% of patients with Stage 5D CKD and is associated with increased morbidity and mortality (1-3). In the coronary arteries, this calcification is typically intimal, within atherosclerotic plaques or as circumferential intimal lesions, whereas in the aorta, calcification occurs in both the intimal and medial layers of the vessel wall (atherosclerosis and Mönkeberg's medial calcific sclerosis). The pathogenesis of vascular calcification is complex, but in vitro studies support the concept of an initial transformation of vascular smooth muscle cells to chondrocyte/osteoblast-like cells in response to hyperphosphatemia, uremia, inflammation, and elevated glucose levels (4). These transformed cells then lay down a matrix of collagen and non-collagenous proteins and produce matrix vesicles that serve as the initial nidus for calcification, similar to the process of normal bone mineralization. This process is accelerated in the clinical setting of CKD, possibly due to hyperphosphatemia and hyperparathyroidism, the use of high dose calcium salts as phosphate binders that increase the overall calcium load, abnormal bone remodeling, and relative deficiencies of circulating and locally produced inhibitors of calcification. This interrelationship of vascular calcification with abnormal serum biochemistries and abnormal bone remodeling is the basis for the recently named syndrome, Chronic Kidney Disease -Mineral Bone Disorder (CKD-MBD) (5). The complexity of this interrelationship makes studies in humans difficult, as one cannot easily control one variable without impacting another. Thus, there is a clear need for an appropriate animal model in which to understand the progressive pathophysiology of CKD-MBD, as well as to test interventions.
Several animal models of vascular calcification exist, including the adenine nephrotoxic model (6, 7) and the 5/6th nephrectomy models in rats (8, 9). In both models, animals develop severe hyperphosphatemia and hyperparathyroidism due to an acute kidney injury, which is followed by CKD. These models have provided useful information on the pathophysiology of vascular calcification. However, they represent advanced CKD, due to the severity of acute injury, with creatinine clearances that approximate late 4 or 5 stage human CKD. In both disease states, diet is an important factor. The 5/6th nephrectomy rat model is generally fed a high phosphorus diet to induce hyperphosphatemia (8, 9). The adenine model has a more rapid onset and severe course of kidney disease as well as hyperparathyroidism and arterial calcification than the 5/6th nephrectomy model. Curiously, the adenine model develops more consistent arterial calcification on a low protein diet (7). The type of calcium deposition may also differ in these models (10). In mice, spontaneous calcification in the setting of surgically induced acute kidney injury followed by CKD, is not found unless there is a concomitant genetic abnormality that is pro-atherogenic, such as ablation of the LDL receptor or the ApoE genes (11, 12). While useful, none of these rodent models provide the opportunity to study a slowly progressive CKD, nor earlier stages of CKD-MBD. This is important because studies demonstrate that patients in earlier stages of CKD also have coronary artery calcification (1, 13, 14) suggesting that the process begins prior to beginning dialysis. Therefore, there is a clear need for additional animal models is needed to provide the opportunity to study slowly progressive CKD to better understand the triad of CKD-MBD: 1) abnormal serum biochemistries, 2) abnormal bone remodeling, and 3) vascular calcification. In this report, we describe a novel model of progressive CKD-MBD, the Cy/+ rat, and demonstrate its usefulness for evaluating early pathogenesis and the impact of different dietary regimens on the course of CKD-MBD.
Results
Main Study Assessment of CKD
To assess the magnitude of uremia, we compared BUN, creatinine, weight, and hematocrit in the 38 week Cy/+ CKD animals each treated for 18 weeks with the casein protein based diet with either 0.7% (normal) or 0.2% phosphorus (low), comparing these CKD animals to normal littermates. The 38 week animals with CKD had elevated BUN at 20 weeks, which persisted (Figure 1A). The animals in the CKD groups also had elevated serum creatinine values (Normal- 0.62 ± 0.01mg/dl; CKD/0.7% Pi- 3.05 ± 0.27mg/dl; CKD/0.2 % Pi- 1.93 ± 0.25mg/dl, p < 0.01). The low phosphorus diet attenuated the progressive CKD as assessed by BUN (Figure 1A). In the 38 week end point groups, the CKD animals in both treatment groups weighed less than normal littermate animals (at week 38: Normal= 588 ± 9 g; CKD/0.7% Pi- 471 ± 17 g; CKD/0.2% Pi-537 ± 8 g, p < 0.01 between normal and both CKD groups). Lastly, animals in the two CKD treatment groups were anemic by 38 weeks, with more severe anemia occurring in the animals on the normal (0.7%) phosphorus diet: (hematocrit in week 38 end point animals: Normal, 0.50 ± 0.00%; CKD/0.7% Pi, 0.29 ± 0.01%; CKD/0.2% Pi, 0.36 ± 0.02%; p < 0.01, between each of the groups).
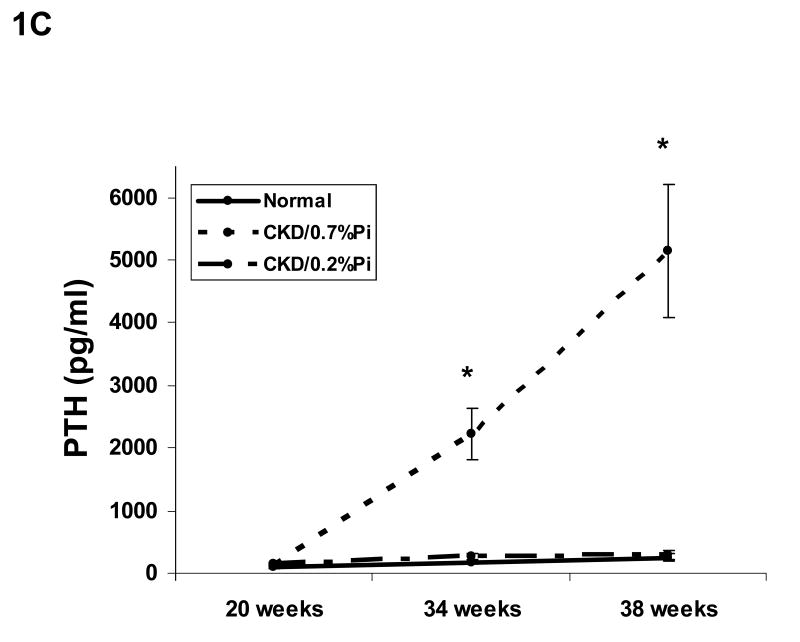
Figure 1. Biochemical changes over time.
Cy/+ rats were placed on the study diet at 20 weeks of age. The 38 week old animals had an intermediate blood draw at 34 weeks for phosphorus, parathyroid hormone, and calcium for a total of three time points, whereas BUN was measured at 20 weeks and 38 weeks. The graphs show changes in plasma levels over time for BUN (A), phosphorus (B), intact parathyroid hormone (C), and calcium (D). The data demonstrate that CKD animals fed a 0.7% phosphorus diet had progression of CKD as assessed by BUN, hyperphosphatemia, and hyperparathyroidism compared to the normal littermates. Feeding a low phosphorus diet (0.2%) ameliorated these changes. As detailed in the text, these changes over time were significant (p < 0.001), even when adjusted for baseline values and final BUN. At 38 weeks, the calcium levels were greater in the CKD/0.2% Pi animals than in the CKD/0.7% Pi fed animals (p = 0.04). n = 15-17 per group. * = p < 0.05 compared to normal animals, + = p < 0.05 0.2% phosphorus treated CKD animals vs. 0.7% phosphorus treated CKD animals.
The CKD animals in the 38 week end point groups were sicklier, particularly those with severe hyperparathyroidism/hyperphosphatemia in Group 2(CKD/0.7% phosphorus diet). Eighteen out of 43 animals in the CKD/0.7% phosphorus diet group died (n = 11) or became morbidly ill requiring euthanasia (n = 7), whereas only 1 of 31 normal animals died, and 3 of 29 animals in the CKD/0.2% Pi group died. Thus, by all indices (BUN, creatinine, body weight, hematocrit), the Cy/+ animals on both the normal (0.7% phosphorus) and low (0.2% phosphorus) diets had worse kidney disease when compared to the normal littermates, but the low phosphorus diet attenuated these abnormalities and prevented early morbidity and mortality.
Main Study Assessment of CKD-MBD
a) Biochemical parameters
To assess the biochemical parameters of CKD-MBD, plasma PTH, phosphorus, and calcium were assessed at the start of treatment (20 weeks), at 34 weeks, and again at 38 weeks (sacrifice). The CKD animals fed the normal 0.7% phosphorus diet developed progressive hyperphosphatemia and hyperparathyroidism. Figure 1 B-D demonstrates the change over time for these measures in the 38 week animals. These differences in end point measurements for phosphorus (p=0.03) and PTH (p=0.006) remained significant even if adjusted for baseline values of phosphorus or PTH (respectively), baseline BUN, end point BUN, or change in BUN using a mixed model, suggesting that the observed differences were not solely due to changes in kidney function and that the premature death did not alter our findings. In fact, the excessive animal deaths in the normal phosphorus diet group would have increased the observed differences. In contrast, the serum calcium was slightly increased only in the 38 week CKD/0.2% phosphorus treated animals compared to the CKD/0.7% Pi treated animals (p = 0.04 when adjusted for baseline calcium and BUN; Figure 1D). In the second group of animals, sacrificed at 34 weeks, biochemical assessments were made at baseline (week 20), mid point (week 29) and end point (week 34). Similar to the 38 week treatment group, the 34 week animals had progressive hyperphosphatemia and hyperparathyroidism (Table 1).
Table 1. Biochemical findings in 34 week animals.
| N = 12-17 | Group | Baseline (wk 20) | Week 29 | Week 34 |
|---|---|---|---|---|
| BUN (mg/dl) | Normal | 20.9 ± 0.5 | ND | 15.8 ± 1.2 |
| CKD/0.7% Pi | 47.0 ± 1.1 | ND | 106.7 ± 13.5* | |
| CKD/0.2% Pi | 47.8 ± 1.3 | ND | 52.7 ± 3.9 | |
| Phosphorus (mg/dl) | Normal | 6.9 ± 0.1 | 6.5 ± 0.2 | 6.1 ± 0.4 |
| CKD/0.7% Pi | 7.4 ± 0.2 | 8.5 ± 0.3 | 11.6 ± 1.0* | |
| CKD/0.2% Pi | 7.5 ± 0.1 | 6.4 ± 0.2 | 8.0 ± 0.9 | |
| Calcium (mg/dl) | Normal | 10.9 ± 0.3 | 11.9 ± 0.4 | 11.0 ± 0.6 |
| CKD/0.7% Pi | 11.1 ± 0.4 | 11.6 ± 0.5 | 9.8 + 0.3 | |
| CKD/0.2% Pi | 11.9 ± 0.2 | 10.5 ± 0.3 | 11.8 ± 1.0 | |
| PTH (pg/ml) | Normal | 91 ± 14 | 98 ± 10 | 177 ± 52 |
| CKD/0.7% Pi | 218 ± 73 | 1351 ± 246* | 3543 ± 648* | |
| CKD/0.2% Pi | 114 ± 18 | 110 ± 14 | 560 ± 163* | |
| Weight (g) | Normal | 487 ± 7 | 560 ± 10 | 585 ± 12* |
| CKD/0.7% Pi | 481 ± 5 | 535 ± 6 | 535 ± 11* | |
| CKD/0.2% Pi | 483 ± 6 | 510 ± 9 | 519 ± 10* |
Mean ± SEM,
p < 0.05 for difference from baseline.
ND = not done
b) Vascular calcification
To determine the extent and magnitude of arterial calcification, the calcium content of the thoracic aorta was examined by quantitative biochemistry and semi-quantitative histology. The thoracic aorta was more calcified in the CKD animals treated with the normal (0.7% phosphorus) diet than either the normal animals or the CKD animals treated with the low (0.2%) phosphorus diet (Figures 2 and 3A,B), but calcification was quantifiable at levels above normal animals only at 38 weeks. The magnitude of the calcification correlated with the phosphorus level (r = 0.67, p < 0.001), but not PTH or calcium. Histologic evaluation of the 38 week animals at identical levels of the upper thoracic aorta revealed that 60% (6 of 10) of the CKD/0.7% phosphorus treated animals had significant medial calcification (3 animals with 3+ calcification, 2 animals with 2+ calcification, and 1 animal with 1+ calcification). In contrast, none of the normal animals or CKD/0.2% phosphorus treated animals had calcification. No histologic evidence of calcification was observed at 34 weeks. Scanning electron microscopy with energy dispersive X-ray microanalysis demonstrated that the mineral deposits were composed of calcium, phosphorus, and oxygen indicative of brushite or hydroxyapatite (Figure 3 E).
Figure 2. Thoracic aorta calcification.
At the time of sacrifice, the thoracic aorta was removed and its calcium content determined biochemically. The results demonstrate that there is increased arterial calcification in the normal (0.7%) phosphorus treated CKD animals at 38 weeks, but not at 34 weeks. This implies that hyperphosphatemia and hyperparathyroidism precede arterial calcification. Interestingly, the low phosphorus diet (0.2%), despite producing modest hyperphosphatemia and hyperparathyroidism, did not increase calcification in CKD animals compared to the normal littermates. Solid black bars= normal littermates; open white bars = CKD (Cy/+) animals fed a 0.7% phosphorus casein-based diet; hatched bars = CKD animals fed a 0.2% phosphorus casein-based diet. Symbols: * different than normal littermates (p < 0.03) or low phosphorus diet, p = 0.06. (n = 8 per group)
Figure 3. Histologic evaluation of vascular calcification and bone.
A: A thoracic aorta from a normal (non-CKD) 40 week old animal stained with MacNeal's stain demonstrating no calcification. B thoracic aorta from a CKD animal fed with a normal (0.7%) phosphorus diet demonstrates calcification (in black) in the medial layer with a vascular smooth muscle cell visible within the calcified area. B: C and D. one from a CKD animal fed a normal (0.7%) phosphorus diet demonstrating osteitis fibrosa cystica with osteoclasts (arrowhead), active osteoblasts (arrows), and in D, peritrabecular fibrosis (arrow). E. Scanning electron microscopy with EDS X-ray microanalysis of the aortic calcifications. Backscatter imaging allowed the identification of the calcification (white region) in the aorta and energy dispersive spectrometry was used to assess the elemental composition. Compared with the non-calcified region (pink arrow and top spectrum), the calcified region (green arrow and bottom spectrum) had increased amounts of calcium, oxygen, and phosphorus (compared to carbon and sodium peaks) indicating a calcium phosphate material such as hydroxyapatite or brushite.
c) Bone abnormalities
Static histomorphometry was performed in the CKD animals treated with normal (0.7%) and low (0.2%) phosphorus diets in the 34 week and 38 week animals and compared to the normal animals in each time group. The bone changes in the CKD animals were indicative of secondary hyperparathyroidism (Table 2, Figure 3 C and D: increased fibrosis, osteoblasts, and osteoclasts) in the CKD animals treated with the 0.7% phosphorus diet, and a mineralization defect (increased osteoid volumes and osteoid surface) in the animals treated with 0.2% phosphorus diet. The bone volume and trabecular thickness were not different between the two groups, but the trabecular number was higher and the trabecular separation lower in the CKD animals treated with 0.7% phosphorus consistent with excessive and uncontrolled hyperparathyroidism (Table 1). The differences from 34 to 38 weeks reflected continued progression of severe secondary hyperparathyroidism in the Cy/+ animals treated with the 0.7 % phosphorus diet, and stabilization or slight improvement of the mineralization defect in the 0.2% phosphorus treated animals.
Table 2. Static bone histomorphometric parameters in normal and CKD rats.
| Parameter N=5-8 per group | Group 1: Normal | Group 2: CKD Cy/+ 0.7% Pi diet | Group 3: CKD Cy/+ 0.2% Pi diet | |||
|---|---|---|---|---|---|---|
| 34 wks | 38 wks | 34 wks | 38 wks | 34 wks | 38 wks | |
| Turnover: | ||||||
| Fibrosis (%) | None* | None* | 8.5 ± 2.7 | 4.9 ± 1.8 | None* | None* |
| Ob.S/BS (%) | 1.8 ± 0.5* | 2.5 ± 0.6* | 18.5 ± 3.4 | 14.9 ± 3.7 | 10.2 ± 1.5*+ | 4.9 ± 1.4* |
| N.Ob/B.Pm (n/mm) | 1.4 ±0.3* | 1.7 ± 0.4* | 12.4 ± 2.3 | 10.1 ± 2.6 | 7.1 ± 1.0*+ | 3.5 ± 0.9*+ |
| Oc.S/BS (%) | 7.6 ±0.7* | 10.3±0.8* | 16.5 ± 1.7 | 22.1 ± 4.5* | 23.8 ± 2.3*+ | 10.2 ± 1.5* |
| N.Oc/B.Pm (n/mm) | 1.5 ± 0.1* | 2.3 ± 0.1* | 3.2 ± 0.3 | 4.1 ± 0.6 | 4.3 ± 0.3*+ | 2.2 ± 0.2* |
| Mineralization | ||||||
| OV/BV (%) | 0.2 ± 0.1 | 0.29±0.1* | 0.9 ± 0.3 | 1.0 ± 0.3 | 1.8 ± 0.4+ | 1.2 ± 0.6 |
| OS/BS (%) | 6.3 ± 3.0 | 4.2 ± 0.8 | 6.3 ± 3.0 | 6.4 ± 1.3 | 14.9 ± 1.8+ | 9.8 ± 2.4 |
| Volume | ||||||
| BV/TV (%) | 11.5 ±1.4 | 8.4 ± 0.9 | 10.8 ± 1.6 | 10.6±1.3 | 4.8 ± 1.0 | 6.9 ± 1.5 |
| Tb.Th (µm) | 51.4± 2.3* | 48.9 ± 2.8 | 48 ± 3.1* | 41.8 ± 2.8 | 39.0 ± 2.6*+ | 40.8 ± 2.7 |
| Tb.Sp (µm) | 427 ± 50* | 571 ± 44 | 433 ± 55 | 405 ± 61 | 1027 ± 211* | 689 ± 107* |
| Tb.N (n/mm) | 2.2 ± 0.2 | 1.6 ± 0.1* | 2.2 + 0.2 | 2.5 ± 0.2 | 1.2 ± 0.2 | 1.6 ± 0.2* |
| Cross-sectional area (mm2) | 6.8 ± 0.1 | 6.6 ± 0.1 | 8.1 ± 0.9* | 6.7 ± 0.1 | 6.1 + 0.1 | 6.1 ± 0.2* |
Mean + SEM
Group 1 or 3 different from Group 2 at same time point, p < 0.05
Group 3 different from Group 1 at same time point, p < 0.05
Static parameters: trabecular bone volume (BV/TV), intertrabecular fibrosis (Fb.V/TV), osteoblast and osteoclast surface (Ob.S/BS and Oc.S/BS), osteoblast and osteoclast number per millimeter bone perimeter (N. Ob/B.Pm and N. Oc/B.Pm), osteoid volume (OV/BV), osteoid surface (OS/BS), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N).
Thus, the Cy/+ animal model spontaneously reflects all three components of CKD-MBD: biochemical abnormalities, vascular calcification, and bone abnormalities. The biochemical and bone abnormalities show a progressive change over time, whereas the vascular calcification was only apparent in the older animals, perhaps due to more severe hyperphosphatemia and hyperparathyroidism.
Diet Study
We previously noted (unpublished observations) that when our male Cy/+ CKD animals were treated with standard rat chow, in which protein content was not casein-based, the magnitude of hyperparathyroidism was not as severe as with a casein based diet. Therefore, we treated another group of animals on a grain based diet. This was a commercially available diet (Purina 5002) that paralleled the protein and phosphorus content of the casein diet. Comparing the results from Cy/+ animals on the two diets (Table 3) demonstrated a marked difference in magnitude of hyperphosphatemia, hyperparathyroidism, and severity of CKD compared to the casein based, 0.7% phosphorus diet fed animals (whose biochemical data is also shown in Figure 1).
Table 3. Effect of dietary protein source in Cy/+ male animals with CKD.
| Casein Protein Source | Grain Protein Source | |||
|---|---|---|---|---|
| 34 weeks | 38 weeks | 34 weeks | 38 weeks | |
| BUN (mg/dl) | 107 ± 13.4 | 140 ± 16.2 | 62 ± 3.0 | 86 ± 6.9 |
| Phosphorus (mg/dl) | 11.6 ± 1.0 | 14.9 ±1.5 | 5.12 ± 0.3 | 7.2 ± 0.6 |
| Calcium (mg/dl) | 9.9 ± 0.3 | 9.8 ± 0.6 | 8.86 ± 0.3 | 8.9 ± 0.3 |
| Intact PTH (pg/ml) | 3,543 ± 648 | 5,151 ± 1067 | 303 ± 116 | 421 ± 97 |
Mean ± SEM, casein different than grain in all categories and all time points, p < 0.01.
Metabolic Cage Study
To better understand how the protein source could cause such a marked difference, we conducted one week long metabolic studies in 20 week old male Cy/+ CKD rats. At baseline, before the diet intervention, the BUN levels were 38.0 mg/dl ± 1.4 in the animals on the casein diet, and 42.1 mg/dl ± 1.8 in the animals on the grain diet, which represents an approximate 50-60% reduction in GFR. After one week of diet intervention, there were no differences in serum phosphorus, calcium, or PTH values between the two groups, but the FGF23 levels were higher in the CKD animals fed a casein diet versus grain diet (p < 0.001) (Table 4). There was also increased urinary phosphate excretion in the casein compared to the grain diet, and an increase in the urine calcium/creatinine ratio (Table 4). Grain diets typically contain much of the phosphate source as phytate, which is poorly absorbed from the gastrointestinal tract. We thus assessed the diets for phytate content, demonstrating that 60% of the phosphate in the grain based diet was in the non-bioavailable phytate form, whereas the casein diet was devoid of phytate so all of the phosphate was bioavailable.
Table 4. Comparison of impact of diets in 20 week animals.
| Cy/+ CKD Animals | P value | ||
|---|---|---|---|
| Casein (n = 15) | Grain (n = 9) | ||
| Serum: | |||
| Calcium (mg/dl) | 9.6 ± 0.4 | 8.8 ± 0.3 | NS |
| Phosphorus (mg/dl) | 5.4 ± 0.2 | 5.4 ± 0.3 | NS |
| Intact PTH (pg/ml)
(Median 25/75%) |
469 ± 87 | 347 ± 56 | NS |
| FGF23 (pg/ml)
(Median 25/75%) |
1254 (1183-1411) | 869 (799-903) | < 0.001 |
| Urine: | |||
| FE phosphorus (%)
(Median 25/75%) |
80.1 (67.2-116.7 | 24.2 (21.0-49.3) | < 0.001 |
| Phosphorus (mg/24 hr)
(Median 25/75%) |
76.3 (58.3-87.8) | 44.3 (39.4-49.0) | < 0.001 |
| Calcium/creatinine
Ratio (mg/mg) |
0.21 ± 0.02 | 0.12 ± 0.02 | 0.013 |
| Calcium (mg/24 hrs) | 3.2 ± 1.1 | 2.9 ± 1.2 | NS |
| Stool: | |||
| Calcium (mg/24 hrs) | 49.7 (38.9-44.8) | 74.0 (54.3-78.7) | NS |
| Phosphate (mg/24 hrs) | 33.1 (18.4-44.8) | 30.6 (12.4-44.3) | NS |
FE = fractional excretion; Mean ± SEM unless otherwise indicated
Discussion
In the present study, we describe a novel animal model of spontaneous CKD-MBD. In previous rodent models of kidney disease, vascular calcification developed only on a high phosphorus diet greater than 1% (6, 8, 15). The advantage of the Cy/+ rat includes the ability to induce all three components of CKD-MBD with normal (0.7%) dietary phosphorus concentration. The biochemical and bone abnormalities demonstrate a progressive course, whereas the calcification was only observed late in the disease course. The aorta vascular calcification was spontaneous and medial in location, paralleling findings in patients with CKD. Studies in humans demonstrate that abnormal biochemical changes (16), begin when GFR is around 40-50 ml/min (CKD 3-4), whereas bone (17) and vascular calcification disorders (1, 13, 14) are more prevalent with CKD stages 4-5D. Thus, any effort to prevent CKD-MBD must first treat the biochemical abnormalities at this early stage. This Cy/+ rat model develops vascular calcification at 38 weeks, suggesting the presence of very severe and/or protracted hyperphosphatemia and hyperparathyroidism are required to induce arterial calcification. CKD-MBD with arterial calcification in rodents is a rare finding. Taken together, this model allows a characterization of the progressive onset and course of CKD-MBD. This model also allows us to test interventions early and chronically to ensure that prevention of CKD-MBD can be done safely. To our knowledge, this represents the first animal model of CKD-MBD that occurs spontaneously (no surgery or drug) on a normal phosphorus diet.
Our study also demonstrates the importance of diet on CKD-MBD. Specifically, a low phosphorus diet can slow or ameliorate CKD-MBD progression. We also found that dietary protein source was an important factor. Casein-based (synthetic) protein sources are often recommended by laboratory feed manufacturers for experiments because they can be more easily manipulated to achieve final desired protein, phosphorus, and calcium concentrations, compared to grain-based diets. The latter can also be seasonally variable depending on the available grains. However, grain-based diets contain most of the phosphate as phytate, which lowers the bioavailability of this mineral compared to casein sources. Previous investigators analyzing the effects of various protein diets found that soy-based diets, compared to casein-based diets, slowed the progression of cystic kidney disease and reduced interstitial fibrosis in the Cy/+ rat (18-21). These investigators hypothesized that this could be due to differences in IGF-1 or fatty acids, but they did not investigate the potential role of phosphorus or parathyroid hormone. Our metabolic studies suggest differences in bioavailable phosphorus and its impact on the development of CKD-MBD. However, it is unknown whether the differences in phosphorus could also account for the observed changes in kidney disease progression identified by these other authors (18-21). These soy, compared to casein-based diets, have also been found in humans to reduce albuminuria in non-diabetic nephropathy (22), and would likely have an impact on overall control of hyperphosphatemia in patients with CKD. However, our diet studies were specifically designed to test two diets in which we found different outcomes in older animals. The diets were not perfectly matched, but were very comparable for protein, phosphorus, calcium and fat content. However, more research is needed to specifically identify the critical element or elements in a diet that contributes to the progression of CKD-MBD, and this model should be useful for these evaluations.
Another advantage of this animal model is the ability to study early changes in CKD-MBD. Our metabolic studies, where younger (20 week old) Cy/+ animals were exposed to different protein sources demonstrated, that despite normal phosphorus levels, there was an increase in serum fibroblast growth factor 23 (FGF23) concentrations with the casein based diet. FGF23 is secreted by osteocytes in response to hyperphosphatemia (23). We saw an elevation in this factor in response to an increased phosphorus load, even without overt hyperphosphatemia. However, it is possible that we missed a rise in serum phosphorus due to the timing of our blood draws. Interestingly, we did not see a difference in PTH in animals fed these two diets. The classic trade off hypothesis (24) suggests that PTH increases early in the course of CKD due to an inability of the kidney to excrete a phosphorus load. PTH, in turn, further increases the urinary phosphorus excretion but at the expense of secondary hyperparathyroidism. Research in dogs and rats with more advanced CKD clearly show the importance of PTH (25), but our results suggest that FGF23 may also be important in early CKD. We may have not detected an increase in PTH because we studied animals at a relatively earlier stage of CKD (generally equivalent to human CKD stage 3), or our blood draw timing failed to detect an acute rise. However, the urine calcium-to-creatinine ratio was higher in the casein versus grain treated animals, the opposite of what one would expect with increased PTH. Both PTH and FGF23 are phosphaturic hormones, but they have opposing effects on calcitriol synthesis. Unfortunately, we did not assess the vitamin D axis in our study. However, analyzing the interplay of PTH, FGF23, and vitamin D metabolism during the course of progressive CKD in future rat and human studies may provide insight into both normal and abnormal physiology.
In conclusion, we describe a novel rat model of progressive CKD-MBD that manifests all three components of CKD-MBD in a manner similar to humans with progressive CKD. We further demonstrate that the progression of CKD-MBD and kidney dysfunction itself, is affected by both total phosphorus content and dietary protein type and we therefore recommend that protein source be clarified in all reports describing experimental animal models. Lastly, our metabolic studies imply that FGF23 may have a role in the pathophysiology of CKD-MBD early in the course of CKD.
Detailed Methods
Animal model
We utilized the Cy/+ rat, a Han:SPRD rat with autosomal dominant polycystic kidney disease (ADPKD) (26). The male Cy/+ rat develops a persistent azotemia starting at about 10 weeks of age which progresses to uremia by about 40 weeks. The renal pathology has been well characterized with initial cyst development in proximal tubules, followed by interstitial fibrosis (27). The progressive azotemia is accompanied by the usual manifestations of CKD, including anemia, hypertension, and secondary hyperparathyroidism (26). The spontaneous genetic mutation (Cy) that leads to cystic kidney disease and progressive CKD encodes for a protein of unknown function (28). This rat colony at the Indiana University School of Medicine has been maintained through successive breeding of heterozygous Cy/+ rats. This is an autosomal dominant condition, such that at birth, 1/4 of the animals are normal (+/+), 1/2 are heterozygotes (Cy/+), and 1/4 are homozygotes (Cy/Cy). Homozygotes (Cy/Cy) of either sex are easily identified after approximately 10 days of age by abdominal palpation of enlarged kidneys and elevation in blood urea nitrogen (BUN), a finding used to verify parental heterozygosity. Homozygous Cy/Cy rats develop massively enlarged kidneys and severe azotemia, and normally die by 4 weeks of age. Heterozygote male animals develop progressive chronic kidney disease (CKD) with a rise in blood urea nitrogen (BUN) by 10 weeks of age and become markedly uremic by 40-50 weeks. Heterozygote female animals (and castrated males) develop progressive CKD with a rise in BUN not detected until 20 weeks of age, followed by uremia at 80 weeks (18, 27, 29, 30). For the present study, male heterozygotes were utilized and all procedures reviewed and approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Main Study Experimental Design
Weaned rats were housed in open top, shoebox cages, and had free access to tap water and standard chow until they were 20 weeks old. At that time, animals were separated into 3 treatment groups:
Group 1. Normal (+/+) littermates fed a diet containing 18% casein-based protein, 0.7% phosphate, 0.7% calcium, 5% fat (Harlan Teklan TD.04539) (=normal)
Group 2. Cy/+ (cystic/CKD) rats fed the same diet as Group 1 (= CKD/0.7% Pi)
Group 3. Cy/+ (cystic/CKD) rats fed the same diet as Group 1, but containing 0.2% phosphate (=CKD/0.2% Pi)
Within each treatment group, there were 16-18 animals initially assigned to two different study durations: 34 week animals with blood draws at 20, 29, and 34 weeks, and 38 week animals with blood draws at 20, 34, and 38 weeks. At sacrifice, rats were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally.) and blood was collected by cardiac puncture. After initial studies, a high mortality rate was noted in the Group 2 animals and we added additional animals to ensure adequate sampling for the vascular and bone end points in each group. At the time of sacrifice, half of the animals from each study duration group were perfusion fixed with 4% paraformaldehyde in phosphate buffer to harvest tissue for bone and aorta histology and the remaining animals from each study duration group were saline perfused to harvest the thoracic aorta to determine calcium content. If animals in the 38 week endpoint required euthanasia or died after 25 weeks, they were replaced.
Plasma biochemistries: blood urea nitrogen (BUN), calcium, phosphorus, and creatinine were determined using colorimetric assays (Point Scientific or Sigma kits). Intact PTH was determined by ELISA (Alpacoa).
Bone histomorphometry: Tibiae were removed, cleaned of soft tissue, and cut into proximal and distal segments. Segments were fixed in 10% neutral buffered formalin (NBF), dehydrated in ethanol, and processed undecalcified into methyl methacrylate. Frontal sections (4 µm thick) of the proximal segments were cut using a Leica RM2165 microtome and stained with von Kossa-tetrachrome for measurement of static bone parameters at 250X magnification 1.0 mm distal to the chondro-osseous junction using a semiautomatic image analysis system (Osteomeasure Histomorphometry System, Osteometrics, Inc., Atlanta, GA) (31). Results are presented using standardized histomorphometry measures (32). To examine cortical bone geometry, cross-sections of the distal segments containing the tibial shaft immediately proximal to the tibiofibular junction were taken using a wire saw (Histo-saw, DDM P216) and mounted unstained. Total cross-sectional area was measured from these sections using a Bioquant image analysis system.
To quantify calcification, thoracic aortas from the saline perfused euthanized animals were snap frozen. At thawing, they were dried, weighed, and then incubated in 0.6N HCl for 48 hrs. The sample was homogenized, centrifuged, and the supernatant analyzed for calcium using the o-cresolphthalein complex one method (Calcium kit; Pointe Scientific). For histology, thoracic aorta from the paraformaldehyde perfused euthanized animals were placed in 4% paraformaldehyde, then embedded in paraffin, sectioned, and stained with MacNeal's tetrachrome stain (a combination of von kossa stain and toluidine blue) (33). The proximal thoracic aorta was used and 4-6 sections per animal analyzed. The sections were graded semi-quantitatively with 0 = no calcification, 1+ = small spots of calcification, 2+ = single area of calcification that spanned several cells and easily visualized by low power, 3+ multiple areas of calcification. In one animal, the aorta was processed for scanning electron microscopy. After the rat was perfusion fixed, a segment of aorta with calcifications was removed, rinsed, dehydrated in a graded series of ethanol and critical point dried. The tissue was attached to a carbon planchet with carbon dag and carbon coated in a vacuum evaporator. The specimen was analyzed using scanning electron microscopic backscattered imaging with a JEOL 8900 (JEOL USA Inc, MA) equipped with an energy-dispersive spectrometer (EDS). Adjacent calcified and noncalcified regions within the same specimen were analyzed for differences in elemental content of the calcified lesions.
Diet Study
After our initial results with the main study using the casein diet revealed that the magnitude of hyperphosphatemia and hyperparathyroidism was much more severe than our initial observations in animals fed a predominantly grain-based diet, we added a fourth treatment group of Cy/+ animals, which were fed a commercially available grain diet (Purina 5002) composed of 20.7 % protein, 0.6 % phosphorus, 0.8 % calcium to evaluate the effects of casein versus grain-based diets. These animals were similarly treated as detailed above in the main study. The phytate content of the grain and casein based diets were analyzed by the ferric precipitate method(34).
Metabolic Cage Study
To further examine the differences between phosphate homeostasis in animals fed a casein diet versus a grain diet, we performed additional metabolic studies. Twenty week old Cy/+ rats were fed either the grain (Purina 5002 diet: 20% protein, 0.6% phosphate, 0.8% calcium, 4.5% fat) or the casein (Harlan Teklan TD.04539 diet: 18% protein, 0.7% phosphorus, 0.7% calcium, 5% fat) based diets used in the main study. On day 6, animals were placed into metabolic cages and urine and feces collected for 24 hours. Animals were then sacrificed and blood was collected. Blood plasma was similarly analyzed for BUN, intact PTH, creatinine, calcium, and phosphorus as described above. In addition, blood was analyzed for FGF23 using an intact ELISA according to the manufacturer's protocol (Kainos Laboratories International; Tokyo, Japan). This assay uses monoclonal antibodies and has been shown to recognize full-length rodent FGF23 (35). Urine was analyzed for creatinine, calcium and phosphorus using the colorimetric methods described above. Feces were dried at 80° C for 48 hours, weighed, ground, and then incubated with HCl for 96 hrs and analyzed for calcium and phosphorus using the colorimetric methods described above.
Statistical analyses
As noted above, multiple animals in Group 2 died or were euthanized and additional animals added to ensure adequate numbers at end point. All of the available data from all animals were utilized in an ‘intention to treat’ analysis, such that there were more samples for the 20 week blood draw than the final blood draw. Thus, the actual sample size for each of the measures ranges from group-to-group and is provided in the Results section. If animals were euthanized prior to being profoundly moribund, the final blood draw was utilized and the actual time of the blood draw for all samples utilized in the mixed models. For the main study, comparisons between the different animal groups (Groups 1-3) were made by using regression models adjusting for potential confounding variables. For outcomes that were measured at three time points (PTH, calcium, phosphorus, weight), a mixed model repeated measures analysis was used to examine comparisons between the animal groups, adjusting for the baseline value of the outcome. For the outcomes that were measured twice (at baseline and end point= BUN) an ANOVA model adjusting for the baseline value of the outcome was used to predict the differences in the outcome between the animal groups. For the outcomes measured once (Hematocrit, bone histomorphometry, and aorta calcium content in the main study, and results for the metabolic studies) we used ANOVA with adjustment for multiple comparisons or Pearson Product to compare biochemical calcification to biochemical data. The results are presented as mean ± SEM (for normally distributed variables), or median with 25/75% (for non-normally distributed variables).
Acknowledgments
This work was supported by an unrestricted grant from Genzyme, Inc. Dr. Moe is a consultant and scientific advisor for Genzyme. The authors would also like to acknowledge NIH grant DK063934 (KEW) and the Indiana Genomics Initiative (INGEN) of Indiana University, supported in part by the Lilly Endowment, Inc. (KEW). The SEM-EDS evaluation of calcification was performed by the Indiana University Electron Microscopy Core. The phytate analyses of the diets were measured in the laboratory of Dr. Brad Joern at Purdue University. We are grateful to Faouzi Azzouz for his statistical expertise, and Michelle Murray for her assistance in preparation of the manuscript.
References
- 1.Block GA, Spiegel DM, Ehrlich J, Mehta R, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney international. 2005;68:1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 2.Mehrotra R, Budoff M, Hokanson JE, Ipp E, et al. Progression of coronary artery calcification in diabetics with and without chronic kidney disease. Kidney Int. 2005;68:1258–1266. doi: 10.1111/j.1523-1755.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- 3.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 4.Chen NX, O'Neill KD, Chen X, Duan D, et al. Fetuin-A uptake in bovine vascular smooth muscle cells is calcium dependent and mediated by annexins. Am J Physiol Renal Physiol. 2007;292:F599–606. doi: 10.1152/ajprenal.00303.2006. [DOI] [PubMed] [Google Scholar]
- 5.Moe SM, Drueke T, Lameire N, Eknoyan G. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14:3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Katsumata K, Kusano K, Hirata M, Tsunemi K, et al. Sevelamer hydrochloride prevents ectopic calcification and renal osteodystrophy in chronic renal failure rats. Kidney international. 2003;64:441–450. doi: 10.1046/j.1523-1755.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 7.Price PA, Roublick AM, Williamson MK. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int. 2006;70:1577–1583. doi: 10.1038/sj.ki.5001841. [DOI] [PubMed] [Google Scholar]
- 8.Cozzolino M, Dusso AS, Liapis H, Finch J, et al. The effects of sevelamer hydrochloride and calcium carbonate on kidney calcification in uremic rats. J Am Soc Nephrol. 2002;13:2299–2308. doi: 10.1097/01.asn.0000025782.24383.0d. [DOI] [PubMed] [Google Scholar]
- 9.Hirata M, Katsumata K, Endo K, Fukushima N, et al. In subtotally nephrectomized rats 22-oxacalcitriol suppresses parathyroid hormone with less risk of cardiovascular calcification or deterioration of residual renal function than 1,25(OH)2 vitamin D3. Nephrol Dial Transplant. 2003;18:1770–1776. doi: 10.1093/ndt/gfg296. [DOI] [PubMed] [Google Scholar]
- 10.Verberckmoes SC, Persy V, Behets GJ, Neven E, et al. Uremia-related vascular calcification: more than apatite deposition. Kidney Int. 2007;71:298–303. doi: 10.1038/sj.ki.5002028. [DOI] [PubMed] [Google Scholar]
- 11.Davies MR, Lund RJ, Hruska KA. BMP-7 Is an Efficacious Treatment of Vascular Calcification in a Murine Model of Atherosclerosis and Chronic Renal Failure. J Am Soc Nephrol. 2003;14:1559–1567. doi: 10.1097/01.asn.0000068404.57780.dd. [DOI] [PubMed] [Google Scholar]
- 12.Massy ZA, Ivanovski O, Nguyen-Khoa T, Angulo J, et al. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J Am Soc Nephrol. 2005;16:109–116. doi: 10.1681/ASN.2004060495. [DOI] [PubMed] [Google Scholar]
- 13.Mehrotra R, Adler S. Coronary artery calcification in nondialyzed patients with chronic kidney diseases. Am J Kidney Dis. 2005;45:963. doi: 10.1053/j.ajkd.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 14.Russo D, Miranda I, Ruocco C, Battaglia Y, et al. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72:1255–1261. doi: 10.1038/sj.ki.5002518. [DOI] [PubMed] [Google Scholar]
- 15.Hirata M, Makibayashi K, Katsumata K, Kusano K, et al. 22-Oxacalcitriol prevents progressive glomerulosclerosis without adversely affecting calcium and phosphorus metabolism in subtotally nephrectomized rats. Nephrol Dial Transplant. 2002;17:2132–2137. doi: 10.1093/ndt/17.12.2132. [DOI] [PubMed] [Google Scholar]
- 16.Levin A, Bakris GL, Molitch M, Smulders M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 17.Sprague SM. The role of the bone biopsy in the diagnosis of renal osteodystrophy. Seminars in dialysis. 2000;13:152–155. doi: 10.1046/j.1525-139x.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 18.Cowley BD, Jr, Grantham JJ, Muessel MJ, Kraybill AL, et al. Modification of disease progression in rats with inherited polycystic kidney disease. Am J Kidney Dis. 1996;27:865–879. doi: 10.1016/s0272-6386(96)90525-9. [DOI] [PubMed] [Google Scholar]
- 19.Aukema HM, Housini I. Dietary soy protein effects on disease and IGF-I in male and female Han:SPRD-cy rats. Kidney Int. 2001;59:52–61. doi: 10.1046/j.1523-1755.2001.00465.x. [DOI] [PubMed] [Google Scholar]
- 20.Fair DE, Ogborn MR, Weiler HA, Bankovic-Calic N, et al. Dietary soy protein attenuates renal disease progression after 1 and 3 weeks in Han:SPRD-cy weanling rats. J Nutr. 2004;134:1504–1507. doi: 10.1093/jn/134.6.1504. [DOI] [PubMed] [Google Scholar]
- 21.Ogborn MR, Nitschmann E, Weiler HA, Bankovic-Calic N. Modification of polycystic kidney disease and fatty acid status by soy protein diet. Kidney Int. 2000;57:159–166. doi: 10.1046/j.1523-1755.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- 22.Teixeira SR, Tappenden KA, Carson L, Jones R, et al. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J Nutr. 2004;134:1874–1880. doi: 10.1093/jn/134.8.1874. [DOI] [PubMed] [Google Scholar]
- 23.White KE, Larsson TM, Econs MJ. The Roles of Specific Genes Implicated as Circulating Factors Involved in Normal and Disordered Phosphate Homeostasis: Frp-4, MEPE, and FGF23. Endocr Rev. 2006 doi: 10.1210/er.2005-0019. [DOI] [PubMed] [Google Scholar]
- 24.Slatopolsky E, Bricker NS. The role of phosphorus restriction in the prevention of secondary hyperparathyroidism in chronic renal disease. Kidney international. 1973;4:141–145. doi: 10.1038/ki.1973.92. [DOI] [PubMed] [Google Scholar]
- 25.Slatopolsky E, Brown A, Dusso A. Role of phosphorus in the pathogenesis of secondary hyperparathyroidism. Am J Kidney Dis. 2001;37:S54–57. doi: 10.1053/ajkd.2001.20740. [DOI] [PubMed] [Google Scholar]
- 26.Kaspareit-Rittinghausen J, Deerberg F, Wcislo A. Hereditary polycystic kidney disease. Adult polycystic kidney disease associated with renal hypertension, renal osteodystrophy, and uremic enteritis in SPRD rats. Am J Pathol. 1991;139:693–696. [PMC free article] [PubMed] [Google Scholar]
- 27.Cowley BD, Jr, Gudapaty S, Kraybill AL, Barash BD, et al. Autosomal-dominant polycystic kidney disease in the rat. Kidney international. 1993;43:522–534. doi: 10.1038/ki.1993.79. [DOI] [PubMed] [Google Scholar]
- 28.Brown JH, Bihoreau MT, Hoffmann S, Kranzlin B, et al. Missense mutation in sterile alpha motif of novel protein SamCystin is associated with polycystic kidney disease in (cy/+) rat. J Am Soc Nephrol. 2005;16:3517–3526. doi: 10.1681/ASN.2005060601. [DOI] [PubMed] [Google Scholar]
- 29.Kaspareit-Rittinghausen J, Deerberg F, Rapp KG, Wcislo A. A new rat model for polycystic kidney disease of humans. Transplant Proc. 1990;22:2582–2583. [PubMed] [Google Scholar]
- 30.Cowley BD, Jr, Rupp JC, Muessel MJ, Gattone VH., 2nd Gender and the effect of gonadal hormones on the progression of inherited polycystic kidney disease in rats. Am J Kidney Dis. 1997;29:265–272. doi: 10.1016/s0272-6386(97)90039-1. [DOI] [PubMed] [Google Scholar]
- 31.Hjorth-Hansen H, Seifert MF, Borset M, Aarset H, et al. Marked osteoblastopenia and reduced bone formation in a model of multiple myeloma bone disease in severe combined immunodeficiency mice. J Bone Miner Res. 1999;14:256–263. doi: 10.1359/jbmr.1999.14.2.256. [DOI] [PubMed] [Google Scholar]
- 32.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. Journal of Bone & Mineral Research. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 33.Moe SM, O'Neill KD, Duan D, Ahmed S, et al. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney international. 2002;61:638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 34.Baxter CA, Joern BC, Ragland D, Sands JS, et al. Phytase, high-available-phosphorus corn, and storage effects on phosphorus levels in pig excreta. Journal of environmental quality. 2003;32:1481–1489. doi: 10.2134/jeq2003.1481. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]