SUMMARY
Cancer immunotherapy seeks to mobilize a patient's immune system for therapeutic benefit. It can be passive, i.e., transfer of immune effector cells (T cells) or proteins (antibodies); or active, i.e., vaccination. Early clinical trials testing vaccination with ex-vivo generated dendritic cells (DCs) pulsed with tumor antigens provide a proof-of-principle that therapeutic immunity can be elicited. Yet, the clinical benefit measured by regression of established tumors in patients with stage IV cancer has been observed in a fraction of patients only. The next generation of DC vaccines is expected to generate large numbers of high avidity effector CD8+ T cells and to overcome regulatory T cells and suppressive environment established by tumors, a major obstacle in metastatic disease. Therapeutic vaccination protocols will combine improved DC vaccines with chemotherapy to exploit immunogenic chemotherapy regimens. We foresee adjuvant vaccination in patients with resected tumors but at high risk of relapse to be based on in vivo targeting of DCs with fusion proteins containing anti-DCs antibodies, antigens from tumor stem/propagating cells and DC activators.
PRINCIPLES OF DENDRITIC CELL BIOLOGY
The system of dendritic cells
Lymphocytes (T cells, B cells, NK and NK T cells) and their products are controlled by DCs (1-3). DCs represent a complex system of cells with 1) different anatomical localization; 2) distinct subsets; and 3) distinct functions. The anatomical localization is often linked with specific function and/or distinct subset. DCs are present in the blood, in peripheral tissues and in lymphoid tissues. Blood contains at least two subsets of DC precursors, i.e., CD14+CD11c+ monocytes, which contain precursors of myeloid DCs (mDCs), and LINnegCD11c−IL-3Rα+ plasmacytoid DCs (pDCs). pDC release upon pathogen recognition high amounts of type I IFN thereby limiting the spread of infection. They also cross endothelial barriers and yield DCs in the tissue, particularly upon inflammation. The role of blood mDCs is less well established; it is likely that they represent human counterpart of patrolling DCs recently described in the mouse, i.e. Langerin+ dermal DCs which migrate from blood to dermis and then to draining lymph nodes (4). Skin contains at least two subsets of mDCs: epidermal Langerhans cells (LCs) and dermal (interstitial) DC. These tissue-residing immature DC possess high endocytic and phagocytic capacity and are posed to capture antigens and process them for presentation to lymphocytes in secondary lymphoid tissues. It is thought that antigen-loaded DCs migrate from tissues into the draining lymph nodes where they present processed antigens to T cells via both classical (MHC class I and class II) and non-classical (CD1 family) antigen presenting molecules. However, the role of lymph node resident DCs in the human in the presentation of antigens from peripheral tissues cannot be excluded and remains to be established. In the classical view, immature (non-activated) antigen-loaded DCs present antigens to T cells, which leads to tolerance as opposed to mature DCs, which are geared towards the launching of antigen-specific immunity. Yet, recent studies demonstrate that not all DC maturation signals are equal and under certain circumstances mature DCs can expand regulatory/suppressor T cells (5). Furthermore, the determination of tolerance might be related to the threshold of DC activation (6) and/or action of a unique set of inhibitory molecules, such as signalling through CTLA-4 or PD-1 or Immunoglobulin-like transcript 3 (ILT3) and ILT4 (7). Accordingly, DCs are now considered essential in both central, i.e. thymic (8), and peripheral tolerance (9).
Regulation of T cell differentiation
DCs control lymphocyte priming and the type of induced T cell immunity. This is important, because the type of immune response can be a matter of life and death as for example in leprosy. There, the tuberculoid form of the disease is characterized by a “type 1” response and low morbidity, but the lepromatous form which is characterized by a “type 2” response, often kills the host. Type 1 response is associated with IFN-γ secretion by T cells (10, 11) while type 2 response is classically associated with secretion of IL-4, IL-5 and IL-13 (12). Recent years witnessed the considerable expansion of functional T cell phenotypes controlled by DCs including i) T cells secreting IL-17 (13-15), and ii) revival of regulatory/suppressor T cells that might protect us from autoimmunity (16) but also represent a major obstacle to successful vaccination in cancer (17-19). Th17 cells have been shown to be important in immune responses to infectious agents, as well as in autoimmune diseases (20). While their role in cancer remains to be established, early studies show their presence in human tumors (21) and potential role in tumor rejection in murine models of cancer (22). Two broad subsets of CD4+ T cells with regulatory function have been characterized (23). Naturally occurring CD4+CD25+T cells (17, 18, 24) are produced in the thymus and mediate their suppressive effects in a cell contact-dependent, antigen-independent manner, without the requirement of suppressive cytokines such as IL-10 or TGF-β. These cells are naturally “anergic” and require stimulation via their TCR for optimal suppressive function. Mature DCs allow their expansion which is partially dependent upon the production of IL-2 by the T cell and B7 co-stimulation by the DCs (25). The induced T regulatory (TR) cells are derived from CD4+25− T cells and mediate their effects through the production of IL-10 and TGF-β (26). Two types have been described: TR1 cells that produce large amounts of IL-10 and low to moderate levels of TGF-β (27) and Th3 cells that produce preferentially TGF-β and provide help for IgA production (28). Immature DCs induce the differentiation of naïve T cells into TR cells (27). Injection of immature DCs pulsed with influenza-derived peptide into healthy adults leads to antigen-specific silencing of effector T-cell function (29). Therefore, strategies need to be developed that will limit either T regs generation and/or their suppressive function while enhancing the development of potent helper CD4+ T cells as we will discuss hereunder.
Mature DCs
Mature DC, present within secondary lymphoid organs, express high levels of costimulatory molecules and are remarkably efficient in antigen presentation (2). Lymphoid organs contain such a variety of DCs as splenic marginal DC and T zone interdigitating cells, germinal center DC, and thymic DCs, including those in Hassall's corpuscles which drive differentiation of CD4+CD25+ regulatory T cells (8). The DC maturation is associated with several coordinated events including i) loss of endocytic/phagocytic receptors and diminished antigen capture (30); ii) change in morphology permitting high cellular motility; iii) translocation of MHC class II antigen presenting molecules to cell surface (30); iv) upregulation of costimulatory molecules such as CD80, CD86 and several members of TNF/TNF receptor family (for example 4-1BB-L, and OX40-L) (31); and v) secretion of cytokines including IL-12 and IL-23, which are important for generation of IFN-γ or IL-17 secreting T cells, respectively (32).
DCs can receive maturation signals through several pathways including: i) microbes which act on DCs via Toll receptors (TLRs), C type lectins (33) as well as and intracytoplasmic NOD-like receptors (NLRs) (reviewed in (34-37)); ii) cells including T cells, NK cells, NKT cells and γ/δ T cells (reviewed in (38)); iii) cell products such as CD40 ligand and proinflammatory cytokines including IL-1β, TNF, IL-6 and PGE2; and iv) products of dying cells (39). These endogenous activating molecules, called damage-associated molecular pattern molecules (DAMPs) (40), include: heat shock proteins (HSP) (41), high-mobility group box 1 protein (HMGB1) (42), beta-defensin (43), and uric acid (44). Most likely at any given time point the DCs will be exposed to a combination of these signals which will influence the net result of T cell activation. Interestingly, DC subsets display distinct repertoire of TLRs. Thus, Langerhans cells (LCs) isolated from epidermis express TLRs 1, 2, 3, 6 and 10, but lack TLR4 and TLR5 expression. The expression of TLR7/8 remains unclear (45, 46). In contrast to LCs, dermal interstitial DCs (IntDCs) express TLRs recognizing bacterial PAMPs, such as TLR2, 4, and 5 (46). Importantly, in the human only plasmacytoid DCs express TLR9 (47, 48), as opposed to mouse where both myeloid and plasmacytoid DCs express TLR9 (49, 50). Similarly, different DC subsets express unique lectins, which may confer different maturation signals yielding distinct type of immune responses (49).
DCs are composed of subsets
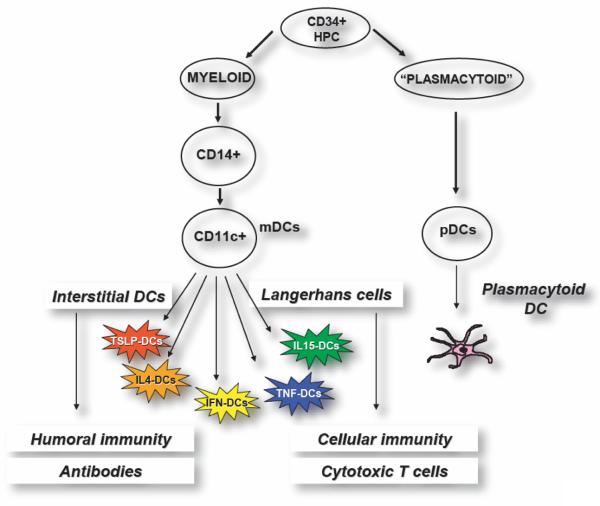
Classically, two main DC differentiation pathways from CD34+ hematopoietic progenitor cells (HPCs) are recognized. One pathway generates plasmacytoid DCs (pDCs) (51), which secrete large amounts of IFN-α/β after viral infection (52). A myeloid pathway generates LCs, which are found in stratified epithelia such as the skin, and intDCs, which are found in all other tissues (53).
Distinct DC subsets are endowed with distinct functional properties
Each of these DC subsets has common as well as unique biological functions, determined by a unique combination of cell-surface molecules and cytokines. Thus, in vitro LCs and interstitial DCs generated from CD34+ HPCs differ in their capacity to activate lymphocytes: interstitial DCs induce the differentiation of naïve B cells into immunoglobulin-secreting plasma cells (53), whereas LCs seem to be particularly efficient activators of cytotoxic CD8+ T cells (Fig. 1) (54). They also differ in the cytokines that they secrete, i.e., only interstitial DCs produce IL-10, and in their enzymatic activity (53). Such different enzymatic activity may be fundamental for antigen processing and selection of peptides that will be presented to T cells. Thus, distinct DC subsets could be possibly leading to distinct antigen-specific T-cell repertoires. This concept was proven most recently in mice in vivo (55) thanks to development of a new strategy to exploit DCs, i.e., their in vivo targeting with specific antibodies. There, CD8+DEC-205+ DC subset is specialized in MHC class I presentation, whereas the CD8−DCIR2+ DC subset is specialized in MHC class II presentation (56).
Figure 1. Subsets of human dendritic cells.
Blood DCs, mobilized by FLT3 ligand, contain both CD11c+ myeloid DCs (mDCs) and plasmacytoid DCs (pDCs). mDCs can be generated by culturing CD34+ HPCs with GM-CSF and TNF. In this way, two DC subsets can be obtained: Langerhans cells (LCs) that are specialized in the induction of cellular immunity including generation of cytotoxic T cells, and interstitial DCs that are specialized in launching humoral immunity and antibody response. Most studies of DCs so far have been carried out with DCs made by culturing monocytes with GM-CSF and IL-4, a simple procedure that yields a homogenous population of DCs. These preparations contain cells that resemble interstitial DCs and are devoid of LCs due to transcriptional regulation of Langerin expression by IL-4. These DCs are immature and require exogenous factors for maturation. Replacement of IL-4 with other cytokines leads to generation of a “Rainbow” of mDCs whose composition and function is determined by cytokine to which monocytes are exposed. Thus, in combination with GM-CSF, thymic stroma lymphopoietin (TSLP) generates mDCs promoting type 2 immunity while TNF and IL-15 generate mDCs containing LCs. The role of pDCs in vaccination against cancer remains to be determined. However, in the human these cells are likely to be the target of CpG since they uniquely express TLR9. Their capacity to rapidly secrete high amounts of type I IFN might contribute to anti-tumor activity of CpG.
Monocytes yield distinct DCs in vitro
Different cytokines skew the in vitro differentiation of monocytes into DCs with different phenotypes and function. Thus, when activated (for example by GM-CSF) monocytes encounter IL-4 they will yield IL4-DCs (57). By contrast, after encounter with IFN-α/β, TSLP, TNF or IL-15, activated monocytes will differentiate into IFN-DCs (58), TSLP-DCs (59), TNF-DCs or IL15-DCs (60), respectively. This spectrum of DCs represents immunostimulatory DCs. However, there exists a whole repertoire of DCs that exhibit immunoregulatory/tolerogenic functions, for example DCs generated by culturing monocytes with IL-10 in the presence of inflammatory cytokines such as GM-CSF or IFN-α (61). DCs derived in the presence of IL-10 are highly efficient in generation of anergic T cells and expansion of suppressor T cells (62-64). Thus, distinct DCs will induce distinct types of T cell immunity. The challenge for years to come will be to link these distinct DC phenotypes in vitro with a specific type of immune response and immune pathology in vivo as exemplified by TNF and IFN-α in autoimmunity or by TSLP in allergic inflammation (59). Furthermore, more studies are needed to determine the value of distinct DC subsets in the induction of therapeutic immunity in vivo as discussed hereunder.
DENDRITIC CELLS IN VACCINATION AGAINST CANCER
Vaccines act through DCs and even the classical vaccination via skin relies upon random interaction of the vaccine with epidermal or dermal DCs. A lack of encounter of vaccine antigen with DCs might result in the absence of an immune response. An inappropriate encounter, for example with non-activated DCs or with the wrong subset of DCs, may lead to silencing of immune response. Both of these scenarios may explain some of the shortcomings in the development of effective cancer vaccines (65). However, the use of ex vivo generated autologous DCs that are loaded with tumor antigen under controlled conditions might permit to establish the parameters for optimal vaccination against cancer. The discovery of methods to generate large numbers of autologous DCs facilitated such clinical studies (57, 66). Ex vivo-generated and antigen-loaded DCs have now been used as vaccines to improve immunity in patients with cancer (67) and chronic HIV infection (68), thus providing a “proof-of-principle” that DC vaccines can work. Yet, the clinical benefit measured by regression of established tumors in patients with stage IV disease has been observed in a fraction of patients. These early studies have been discussed in details earlier (69). They are invaluable as they permitted us to establish the parameters of DC vaccination that require a better understanding to allow us to design better vaccines that induce effective immunity against cancer (70). Here we will highlight a few key points that need to be incorporated in design of future clinical trials to improve immunity and subsequent clinical outcomes (3, 54, 70, 71). We define an improved immunity as i) the induction of broad T cell immunity with specificity against several tumor antigens; ii) the ability to rapidly yield effector cells that are multifunctional i.e., secrete multiple cytokines and kill tumors; iii) generation of tumor antigen-specific immune memory; and iv) decrease in tumor antigen-specific regulatory T cell expansion and/or function.
DC subsets
Several groups have now used IL4-DCs as vaccines (67) following pioneering clinical studies in patients with metastatic melanoma by Nestle et al. (72) (using tumor-lysate-loaded DCs) and by Schuler and colleagues (73) (using melanoma-peptide-loaded DCs). However, recent discoveries point to some alternatives to the classical way of generating DCs. For example, melanoma-peptide-pulsed IL15-DCs are much more efficient than IL4-DCs for the induction of antigen-specific CTL differentiation in vitro, whereas their ability to stimulate CD4+ T cells is comparable (60, 74). Also, IFN-α-DCs generated in three-day cultures are efficient for the induction of specific immunity. Thus, the immunogenicity of these distinct DC vaccines needs to be tested in vivo in clinical studies.
Monocytes are not the only source of DC precursors/progenitors that have been used in clinical studies. Blood DCs loaded have been used as vaccines in patients with i) follicular B-cell lymphoma upon loading with specific idiotype protein (75), and immune and clinical responses were observed; and ii) prostate cancer after loading with a recombinant fusion protein consisting of prostatic acid phosphatase (PAP) linked to granulocyte-macrophage colony-stimulating factor (76). Furthermore, Fong et al. used FLT3 ligand mobilized blood DCs (77). We have vaccinated patients with metastatic melanoma with antigen-loaded DCs derived from CD34+ hematopoietic progenitor cells (CD34-DCs). Vaccination elicited melanoma-specific immunity and patients who survived longer were those who mounted immunity to > 2 melanoma antigens (78). These results justify the design of larger follow-up studies with a range of different DC vaccines to assess their immunological and clinical efficacy.
Loading DC vaccines with antigen
Loading MHC class I and MHC class II molecules on DCs with peptides derived from defined antigens is the most commonly used strategy for DC vaccination (79). Although this technique is important for “proof of concept” studies, the use of peptides has limitations: restriction to a given HLA type; the limited number of well characterized tumor-associated antigens (TAA); the relatively rapid turnover of exogenous peptide-MHC complexes resulting in comparatively low antigen presentation at the time the DC arrive into draining lymph node after injection; and, the induction of a restricted repertoire of T-cell clones, thus limiting the ability of the immune system to control tumor antigen variation. Thus, loading DCs with total antigen preparations and allowing “natural” processing and epitope selection is expected to improve efficacy as well as to allow the generation of a diverse immune response involving many clones of CD4+ T cells and CTLs. These strategies include loading DCs with recombinant proteins, exosomes, viral vectors, plasmid DNA, RNA transfection, immune complexes and antibodies specific for DC surface molecules (reviewed in (80)). Coating tumor cells with antibodies that permit targeting of activating Fcγ receptors on DCs results in substantial improvement of anti-tumor T cell immunity as shown for example in myeloma (81) and glioma (82). Another technique involves exploiting the capacity of DCs to cross-prime (83, 84). This approach can be applied to load DC vaccines to elicit immunity against multiple antigens irrespectively of patients HLA type. Indeed, DCs cultured with killed allogeneic melanoma, prostate and breast cancer cell lines prime naïve CD8+ T cells against tumor antigens in vitro (84). We have vaccinated 20 patients with metastatic melanoma with autologous monocyte-derived DCs loaded with killed allogeneic melanoma cell line (8 vaccines on a monthly basis). Vaccination has proved to be safe (no autoimmunity or other adverse events) and has led to the induction of melanoma-specific T cell immunity. In two patients, who failed prior therapy for stage IV melanoma, this has resulted in long-lasting tumor regression (85). These results warrant larger clinical studies. Recent studies demonstrate that immunogenicity of killed tumor cells can be further enhanced through several means including i) heat treatment of tumor cells that leads to enhanced transcription of melanoma antigens and broadens the repertoire of elicited CD8+T cells (86); ii) opsonization of tumor cells (81); and iii) the mode of tumor cell death (87).
An important issue to consider is also the nature of antigen used for immunization. Classical tumor antigens include: i) unique (mutated) antigens; and ii) shared self-antigens including cancer/testis antigens and tissue differentiation antigens (79, 88-90). The choice between these types of antigens for vaccination could be viewed as choice between inducing immunity (mutated antigens) or breaking tolerance and inducing autoimmunity (self antigens). The potential of mutated antigens is opposed to i) relatively weak immunogenicity of shared antigens; and ii) the existence of T regs with specificity for shared antigens (88). Actually, we should aim at inducing an immune response to both antigen types, which might happen using either the autologous tumor to load DCs (91) or through epitope spreading (92). An important shift in the selection of antigen targets is brought about by the identification of cancer stem cells (93, 94). While a majority of studies have focused on eliminating mature cancer cells with limited proliferation capacity, it seems more efficient to target the self-renewing cancer stem cells. The importance of stem cell associated antigens in malignancy can be best illustrated by the presence of SOX-2-specific immunity in patients with monoclonal gammopathy (95). This immunity is lost in patients who developed multiple myeloma suggesting differential antigenic targets at pre-malignant and malignant stages.
Finally, tumors express altered lipids and sugars that can be bound by CD1 molecules on APCs and presented to NKT cells as well as T cells (96-98). These antigens can also be harnessed for improved vaccination.
DC migration
Monitoring the in vivo migration of labeled DCs in patients showed that only a small fraction (<1%) of intradermally injected DCs migrated rapidly to the regional lymph nodes (99, 100). A mouse study showed improved DC migration by conditioning the injection site with TNF (101). Intranodal injection may result in DCs getting trapped in the fat tissue surrounding the lymph node (102). Furthermore, it remains to be established whether overloading the lymph node with large numbers of injected DCs will indeed improve generation of immune and clinical responses in humans (103). Novel strategies to improve the access of injected DCs into secondary lymphoid organs involve overexpression of L-selectin in ex-vivo generated DCs by mRNA electroporation (104).
Furthermore, distinct maturation/activation signals, for example PGE2 (105), may induce the preferential expression of CCR7 by DCs, hence increasing the capacity of the DCs to respond to appropriate ligands such as CCL19 and CCL21 expressed in lymphatic vessels and secondary lymphoid organs. Yet, PGE2 might skew the T cell response towards type 2, hence illustrating challenges in the selection of maturation factors. The issue of DC migration will likely have to be revisited in view of recent studies in mouse demonstrating that DCs might arrive in the lymph node as late as three days after antigen inoculation (106).
Antigen-loaded DCs may prime T-cell responses regardless of the route of injection, but the quality of responses might be affected. Thus, recent studies in mice show that the induction of tissue-specific immunity is related to the tissue origin of DCs (107). Both intravenous and subcutaneous immunization with peptide-pulsed DCs in a mouse model of melanoma induced peptide-specific memory T cells in spleen and permitted the control of lung metastasis. However, whereas subcutaneous immunization also induced memory T cells in the lymph nodes allowing subsequent protection against subcutaneously growing tumors, intravenous immunization failed to do so (107). Thus, DCs can prime T cells with different homing capacities. The consequence could be that, for example, intravenous administration of a DC vaccine for melanoma would be unlikely to induce skin-homing effector T cells.
DC maturation
Key factor in the quality of vaccination is DC activation status and most particularly the repertoire of co-stimulatory and co-inhibitory molecules which they express. DCs express at different activation (maturation) stages different molecules from the B7 family: CD80 (B7-1), CD86 (B7-2), ICOS-ligand, PD-L1 (B7-H1), PD-L2 (B7-DC), B7-H3, and B7-H4 (108). B7 family includes members which can stimulate immune response and others that can inhibit them (109, 110). For instance, CD80 and CD86 bind to both CD28 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) (111). While CD28 delivers signals for T cells to become functional effector cells, CTLA-4 delivers inhibitory signals that suppress their functions (112). Furthermore, through its mode of action one molecule might promote both the effector and the regulatory response as exemplified by ICOS ligand. Indeed, ICOS:ICOS ligand interaction helps generation of T regs (113) but also appears important in the stimulation of effector T cells and T cell-dependent B cell responses (114-116). Another member, OX40L shuts down the generation of IL-10-producing CD4+ type 1 regulatory T (Tr1) cells (117, 118) by DCs but induces the differentiation of pro-inflammatory Th2 cells secreting TNF and IL-13 (119), which might actually have a cancer promoting effect (120).
In the context of cancer, PD-1/PD-L interaction represents an important pathway to control (121) for several reasons. Thus, PD-1 ligands include PD-L1 and PD-L2 both of which bind to PD-1, and deliver potent inhibitory signals (122). While PD-L2 expression is limited to DCs and macrophages, PD-L1 is broadly expressed on hematopoietic and non-hematopoietic tissues and is considered as a molecular shield protecting from autoreactive attack (123). T cells in patients with chronic diseases such as cancer and HIV (124) express PD-1, a phenotype of chronically activated “exhausted” T cells. Indeed, in HIV patients PD-1 expression on antigen-specific CD8+ T cells correlates with their impaired function and with the predictors of disease progression: positively with viral load in the plasma and inversely with CD4+ T cell count (125, 126). The blockade of PD-1 reversed T cell dysfunction (125, 126). Emerging evidence suggests the role of PD-L molecules on DCs not only as ligands for negative T cell regulation but also as receptors involved in DC regulation (121, 127). Thus, engaging DCs with soluble PD-1 decreased the expression of maturation markers and increased IL-10 production (128). We should therefore aim at developing vaccines that do not express inhibitory molecules (PD-L1 or ICOS ligand) but do express high levels of molecules enhancing CTL priming (CD80 and 4-1BBL).
Vaccination frequency
We have found in 18 patients with metastatic melanoma that four vaccinations over 6 weeks with melanoma peptide-loaded CD34-DCs result in an increase in the number of melanoma-specific CD8+ T cells in the blood as documented by IFN-□ ELISPOT (129) and CTL assay (130). However, the melanoma-specific CD8+ T-cell immunity in the blood was short-lived: all analyzed patients lost specific T cells detectable by direct ELISPOT and 4/9 patients lost all recall responses by two months after the last vaccination. Several explanations might be considered. T cells might migrate from the blood to peripheral tissue (tumor site). Alternatively, the four bi-weekly vaccinations might have provided too frequent antigen stimulation for optimal T cell differentiation. Mouse and human studies of vaccination against infectious agents (131) indicate that priming should be followed by a boost 4-6 weeks later for an optimal response. However, these rules may not apply to a chronic disease such as cancer. By analogy, chronic viral infections are associated with exhausted T cells owing to chronic antigen presentation (132), and their reactivation through vaccination is likely to require different schedules. Accordingly, recent studies demonstrate that DC vaccination stimulates a pathway of accelerated generation of memory T cells that undergo vigorous secondary expansion in response to a variety of booster immunizations, leading to elevated numbers of effector and memory T cells and enhanced protective immunity (133).
Regulatory/suppressor T cells
A major obstacle to the success of cancer vaccines might be the presence of regulatory/suppressor T cells. Indeed, a large body of experimental evidence shows that these T cells suppress anti-tumor immunity and that their removal allows tumor eradication (134). An increased frequency of CD4+CD25+ T cells has been observed in the blood and tissues of patients with cancer (135). Our studies in metastatic melanoma patients recently revealed the presence in of circulating IL-10 producing CD4+ T cells specific for a broad range of melanoma antigens including NY-ESO1, survivin, TRP-1 and gp-100 (136). These melanoma antigen-specific IL-10-secreting CD4+ T cells show suppressive activity in vitro though it appears to be IL-10 independent but contact dependent. It is conceivable that distinct DC subsets and/or distinct DC maturation stimuli will have different capacities to induce regulatory T cells. This aspect needs to be explored further. Naturally occurring CD4+CD25+ suppressor T cells may be controlled by pre-treatment of patients with drugs that can eliminate and/or control these cells, meaning that DC vaccination may be more effective when combined with other therapies. Indeed, studies in the late 1970s and early 1980s showed that cytostatic drugs (for example, cyclophosphamide) facilitate adoptive immunotherapy for tumors in animal models (137). The proposed mechanism was the elimination of suppressor T cells (137). Recent data showing improved outcomes of vaccination with DCs in myeloablated animals (138) reinforce this concept and indicate that controlled “immune ablation” may improve the clinical efficacy of DC vaccination trials in cancer. Besides the elimination of suppressor T cells, the mechanism may also involve the elimination of pre-existing memory T cells, which might not be of the most effective phenotype (for example, Th2). Thus, pre-treatment of patients with metastatic cancer with Cyclophosphamide prior to vaccination with DCs is the best example of combination therapy and might significantly improve DC vaccine efficacy. Indeed, in the late 1980's, clinical trials in patients with melanoma using whole tumor cells as vaccines showed improved immunity when vaccination was combined with Cyclophosphamide (139). The efficacy of Cytoxan to control T regs in humans remains however to be established and several trials testing this drug in combination with vaccines are currently ongoing. In alternative approach, treatment of patients with metastatic renal cancer with recombinant IL-2 diphtheria toxin conjugate (ONTAK) reduced number of T regs in the blood and enhanced tumor-specific T cell responses to vaccines (140, 141). T regs depletion upon treatment with ONTAK has also been observed in patients with metastatic melanoma (142).
Combining DC vaccination with other therapies
Other therapies that offset the suppressive environment created by the tumor (143-145) involve several drugs that target different pathways (146). The drugs can be grouped as: i) antibodies; ii) cytokines; iii) chemotherapeutic agents; and iv) other drugs, for example anti-inflammatory COX-2 inhibitors (147). The therapeutic targets are: i) tumor cells and their products; ii) effector and regulatory lymphocytes; and iii) DCs themselves.
Antibodies, or other soluble antagonists, can be exploited for the blockade of suppressive cytokines such as IL-10 (148), IL-13 (149), TGF-β (150) and VEGF (151, 152). They can also be used to block inhibitory ligand:receptor interactions (153) by acting on antigen presenting cells such as tumor or DCs (for example anti-PD-L1) or on lymphocytes as illustrated by anti-CTLA-4 (111) or anti-PD1 (125). In contrast, agonistic antibodies (151, 152) might further promote co-stimulation of effector T cells as for example with anti-CD137 (31), a ligand for 4-1BB (154).
Cytokines can be exploited to improve vaccination outcomes by acting on both the DCs and lymphocytes. Thus, concomitant administration of cytokines or cytokine triggers (such as TLRs) could improve the performance of the DC vaccine by promoting its survival, migration, and possibly protecting it from tumor-derived inhibitory factors such as VEGF or IL-10 (151). Cytokines either produced by DCs or delivered as a part of combination therapy can also help T cells, for example IL-7 and IL-15 (155, 156). IL-7 might be of particular importance given its capacity to mobilize T cells in vivo in cancer patients (157). This might allow an increase of the pool of naïve T cells which decreases with age when the risk of cancer increases (158).
Traditional antitumor chemotherapeutic agents might be combined with DC vaccines to harness their different features:
i) Immunomodulatory, as already discussed in the context of Tregs.
ii) Ablative, to create a niche for autologous transplant (depleted of Tregs) and expansion of new naïve T cells, which in turn can be primed by DC vaccines. Improved outcomes of vaccination with DCs in myeloablated animals (138, 159) reinforce this concept. Furthermore, recent studies demonstrated improved function of adoptively transferred CD8+ T cells as a result of gut-derived LPS (160). It is possible that this beneficial effect of LPS is mediated by activation of host DCs. In the human, adoptive transfer of antigen-specific T cells following lymphodepletion with high dose Cytoxan and Fludarabin can mediate considerable regression of metastatic melanoma in refractory patients (161).
iii) Therapeutic, aimed to destroy cancer cells in vivo by inducing “immunogenic” tumor cell death (162, 163) and providing substrate for DCs and immune responses, called chemo immunotherapy as for example appears to be the case with anthracyclins (164) or proteasome inhibitors (Bortezomib) (165). Recent studies from Zitvogel group unraveled the contribution of the innate and adaptive immune systems, and most particularly DCs, to the anti-tumor effects of conventional treatment modalities in cancer such as radiotherapy and chemotherapy (166). Thus, radiotherapy and some chemotherapeutic agents, in particular anthracyclines, can induce specific immune responses that result either in immunogenic cancer cell death or in immunostimulatory side effects. For example, tumor cell death triggered by chemotherapy or radiotherapy initiates an immunoadjuvant pathway that relies on the interaction of high mobility group box 1 protein (HMGB1) released from dying tumor cells with Toll-like receptor 4 (TLR4) on DCs (167). This pathway was required for the crosspresentation of tumor antigens and the promotion of tumor specific cytotoxic T-cell responses. Importantly, a direct link with outcomes of chemotherapy was found as breast cancer patients harboring the loss-of-function Asp299Gly polymorphism of TLR4 relapsed earlier after receiving anthracycline-based chemotherapy (167, 168). These data bring about a new view on the role of DC in cancer therapy that might be exploited for improved outcomes of both conventional cancer therapy as well as DC vaccination. For example, chemotherapy combined with intra-tumoral DC injection or with their in vivo mobilization with FLT3 ligand (169-171).
Finally, drugs such as COX-2 inhibitors (147) might permit to improve outcomes by blocking inflammation that allows tumor progression (172, 173). In this context, it is important to consider tumor microenvironment. There, antigen-specific T cells that effectively traffic to tumor sites are exposed to immunosuppressive mechanisms (174, 175) including: i) insufficient or inappropriate costimulation, for example inhibition by ligands such as programmed death ligand-1 (PDL-1) (122) which is expressed at high levels by tumor cells; ii) suppression by cells such as T regs (176) and myeloid suppressor cells (177-180); iii) suppression by cell products such as IL-10, TGF-β (181-183) or by IL-13 produced by Th2 (120) and/or NKT cells (149); iv) products of enzymatic activity of indoleamine-2,3-dioxygenase (IDO) (184) or arginase (185, 186); and v) subversion of effector cell function as for example by shedding NKG2D ligands (MICA) (187), which in membrane form act as T cell and NK cells activators but are inhibitory in soluble form (188). These might also disrupt the interaction of DCs and NK cells (189). Thus, the analysis of transcription and proteomics patterns in tumor biopsies might permit the identification of patient-specific suppressive pathways and therefore, tailoring combination therapy to overcome them (190). Just as different tumors are treated with different combinations of cytostatic drugs and targeted therapies, we foresee development of clinical protocols combining DC vaccines with individualized adjunct therapies.
Assessing immune efficacy
Detailed analysis of elicited T-cell and B-cell responses in the blood can provide important clues as to the efficacy of a given DC vaccine and is therefore fundamental for the development of improved vaccines.
The type of T cell immunity
Vaccine-specific T cell immunity has been classically measured by the quantity of tumor-antigen specific CD8+ T cells. The elicited tumor antigen-specific T cells should be capable of cytokine production, proliferation upon antigen re-exposure, migration to the tumor site, and CTL function. Markers indicative of T cell migratory capacity include differential expression of CCR7 and CD45 isoforms: CCR7+ CD45RO+ T cells (central memory) will most likely migrate to lymph nodes, whereas the shift towards a CCR7− phenotype (effector memory) should be associated with migration to the tissue. A global assessment of immune responses is needed to determine different types of induced immunity. With this in mind, we developed a technology called EPIMAX, which allows a global measurement of the immune repertoire (85, 136). EPIMAX measures simultaneously cell proliferation and secretion of multiple cytokines that distinguish Type 1, Type 2 cytokines and IL-10 secretion using the Multiplex Cytokine Analysis. This technology permitted us to measure directly in the blood melanoma antigen-specific IL-10 secreting T cells (136). Furthermore, this technology might permit us to measure the baseline status of tumor-specific immunity in patients and therefore identify patients with pre-existing immunity that might benefit the most from vaccination therapy.
Activation of other immune effectors
CD4+ T cells, NKT and NK cells, and B cells also need to be taken into account when analyzing vaccine specific immunity. In particular, CD4+ T cells seem to be fundamental for priming long-lived CD8+ T-cell memory (191). In fact, the lack of CD4+T-cell activation in peptide-vaccination strategies might explain their limited efficacy in patients with cancer. Although a large number of circulating effector CD8+ T cells might be elicited by such vaccines, in the absence of CD4+ T-cell help, their quality might be compromised and the establishment of specific CD8+ T-cell memory is unlikely (192). The induction of NKT cells, which kill a wide spectrum of tumor cells (193), or NK cells, which recognize MHC-class-I-deficient tumor cells (194), could be desirable, yet caution must be taken with regard to the cytokines that they produce. For example, IL-13-producing NKT cells may inhibit CTL-mediated tumor elimination and favor tumor progression (149).
Clinical efficacy
The definition of clinical endpoints, and hence the measures that are used to assess vaccine efficacy, need to be revisited. Since cancer is a chronic disease, prolonged survival and good quality of life might be considered a therapeutic success and of benefit to the patient. Therefore, while critically assessing different therapies, we should be careful not to pre-maturely dismiss therapies that do not lead to a high rate of objective tumor regression (195). Furthermore, it might be considered unrealistic to expect even the most efficient immune responses to eliminate the total tumor burden in a patient with advanced cancer. However, true analysis of improved survival requires randomized studies and long-term follow-up, which creates another set of logistic/regulatory difficulties, particularly for academic centers (196). These issues have been most recently reviewed and a novel clinical development paradigm for cancer vaccines has been outlined in a milestone article by Hoos et al. (197). Indeed, the current assessment and development paradigm is based on the criteria used for testing cytotoxic drugs. The unique nature of therapeutic vaccination that builds on induction/activation of immune responses requires a specific strategy. It is also very important to consider that several of drugs targeting molecular pathways or chemotherapeutics have been concluded active based not on the rate of objective tumor rejections but rather based on the improved survival and/or time to disease progression in large scale phase III studies treating hundreds of patients. Thus far, only one phase III study comparing DC vaccination with standard chemotherapy (DTIC) was carried out in patients with melanoma. While no improvement in objective response rate was found in DC arm, post-hoc analysis demonstrated improved survival in patients with good performance status (Karnofsky 100) and a specific phenotype, i.e., HLA-A201+ HLA-B44− (198). Thus, DC vaccination therapy might need to be tailored to pre-identified cohorts of patients.
CONCLUDING REMARKS
DCs are the critical decision-making cells in the immune response. DCs are an attractive target for therapeutic manipulation of the immune system to enhance otherwise insufficient immune responses to tumor antigens. However, the complexity of the DC system requires their rational manipulation to achieve protective or therapeutic immunity. Thus, further research is needed to analyze the immune responses induced in patients by distinct ex vivo generated DC subsets activated via different pathways. DC-based vaccination will become an essential component in cancer management. The considerable progresses made in the knowledge of DC biology as well as effector/regulatory T cell biology clearly open the avenues for development of considerably improved clinical protocols (Fig. 2). These will include therapeutic vaccination of metastatic disease and preventive vaccination in patients with resected tumors (199).
Figure 2. Current obstacles to clinically effective DC vaccination protocols and strategy to overcome them.
First generation DC vaccines: Early clinical trials with first generation DC vaccines (left panel) showed the induction of immune responses to vaccine antigens. However, the clinical responses are still infrequent. Possible contributing factors can be grouped into: i) vaccine features, and ii) suppressive pathways established by tumors. Vaccine features include: i) insufficient CD4+ T cell help; ii) generation of low affinity CTLs; and iii) generation of T regs. Suppressive pathways involve: i) suppressor cells, both T regs as well as myeloid suppressor cells; ii) suppressor molecules expressed by tumors such as PD-L1; and iii) suppressive factors secreted by tumors, for example TGF-β or VEGF. Suppression can act at the level of the induction of immune response and at the effector function level including inhibiting the release of cytotoxic effector molecules Granzyme B and perforin.
Second generation DC vaccines: Improved next generation DC vaccines will harness Langerhans cells and microbial activation signals leading to: i) secretion of high amounts of cytokines such as IL-12, which will generate strong Th1 response and helper function for generation of memory T cells; and IL-15 which will help generation of high avidity CTLs that might be resistant to tumor microenvironment; and ii) strong costimulation mediated via at least three molecular pathways such as CD80, CD70 and 4-1BB. This in combination with therapies that will permit to eliminate T regs and block tumor microenvironment will results in the full activity of elicited CTLs and tumor rejection.
The ultimate ex vivo-generated therapeutic DC vaccine will be heterogeneous and composed of several subsets, each of which will target a specific immune effector. These ex vivo strategies should help to identify the parameters for DC targeting in vivo, which represents the next step in the development of DC-based vaccination, most particularly for preventive vaccination.
ACKNOWLEDGEMENTS
We thank our patients for volunteering to participate in our studies. We thank our colleagues and collaborators for their contribution to our progresses. We could only cite a fraction of an enormous amount of studies (over 30000 citations in PubMed as of June 2008), all of which contributed to our progresses. We are grateful to all former and current members of BIIR. We thank M. Ramsay and W. Duncan for support.
Supported by grants from Baylor Health Care Systems Foundation and the National Institutes of Health (PO1 CA84512, U19 AIO57234, CA78846, CA085540, and CA89440). JB holds the Caruth Chair for Transplantation Immunology Research. AKP holds the Michael A. Ramsay Chair for Cancer Immunology Research.
REFERENCES
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 4.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, Snoeck H, Randolph G, Merad M. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee D, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar K. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after DC injection of cytokine matured DCs in myeloma patients. Blood. 2006 doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999;189:611–614. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe N, Wang YH, Lee HK, Ito T, Cao W, Liu YJ. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 9.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 10.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 11.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 13.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 Family Cytokines and the Expanding Diversity of Effector T Cell Lineages. Annu Rev Immunol. 2007 doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 14.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 15.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 18.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 19.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 20.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 21.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, Demarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic Analysis of Prostate-Infiltrating Lymphocytes Reveals TH17 and Treg Skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann K, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008 doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 27.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 28.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 29.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 31.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 32.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 33.Cambi A, Figdor CG. Levels of complexity in pathogen recognition by C-type lectins. Curr Opin Immunol. 2005;17:345–351. doi: 10.1016/j.coi.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ting JP, Davis BK. CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu Rev Immunol. 2005;23:387–414. doi: 10.1146/annurev.immunol.23.021704.115616. [DOI] [PubMed] [Google Scholar]
- 35.Delbridge LM, O'Riordan M X. Innate recognition of intracellular bacteria. Curr Opin Immunol. 2006 doi: 10.1016/j.coi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 38.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 40.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 41.Srivastava PK, Maki RG. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991;167:109–123. doi: 10.1007/978-3-642-75875-1_7. [DOI] [PubMed] [Google Scholar]
- 42.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 43.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, Kwak LW. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 44.Rock KL, Hearn A, Chen CJ, Shi Y. Natural endogenous adjuvants. Springer Semin Immunopathol. 2005;26:231–246. doi: 10.1007/s00281-004-0173-3. [DOI] [PubMed] [Google Scholar]
- 45.Flacher V, Bouschbacher M, Verronese E, Massacrier C, Sisirak V, Berthier-Vergnes O, de Saint-Vis B, Caux C, Dezutter-Dambuyant C, Lebecque S, Valladeau J. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- 46.van der Aar AM, Sylva-Steenland RM, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MB. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–1990. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 47.Kadowaki N, Ho S, Antonenko S, de Waal Malefyt R, Kastelein RA, Bazan F, Liu Y-J. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194:863–870. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 49.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O'Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J Exp Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 51.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 52.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood [In Process Citation] Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 53.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 54.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, Palucka AK, Banchereau J. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 55.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 56.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz V, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 57.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paquette RL, Hsu NC, Kiertscher SM, Park AN, Tran L, Roth MD, Glaspy JA. Interferon-alpha and granulocyte-macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J Leukoc Biol. 1998;64:358–367. doi: 10.1002/jlb.64.3.358. [DOI] [PubMed] [Google Scholar]
- 59.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 60.Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, Palucka AK, Banchereau J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, Suciu-Foca N. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 62.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 63.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 64.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 65.Finn O. Cancer Immunology. N Engl J Med. 2008;358:25. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 66.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 67.Davis ID, Jefford M, Parente P, Cebon J. Rational approaches to human cancer immunotherapy. J Leukoc Biol. 2003;73:3–29. doi: 10.1189/jlb.0502261. [DOI] [PubMed] [Google Scholar]
- 68.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 69.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 70.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 71.Koski GK, Cohen PA, Roses RE, Xu S, Czerniecki BJ. Reengineering dendritic cell-based anti-cancer vaccines. Immunol Rev. 2008;222:256–276. doi: 10.1111/j.1600-065X.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 72.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 73.Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Brocker EB, Steinman RM, Enk A, Kampgen E, Schuler G. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8(+) T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 75.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen- pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 76.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of Hormone-Refractory Prostate Cancer With Antigen-Loaded Dendritic Cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 77.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fay JW, Palucka AK, Paczesny S, Dhodapkar M, Johnston DA, Burkeholder S, Ueno H, Banchereau J. Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34(+) progenitor-derived dendritic cells. Cancer Immunol Immunother. 2005:1–10. doi: 10.1007/s00262-005-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 80.den Brok MH, Nierkens S, Figdor CG, Ruers TJ, Adema GJ. Dendritic cells: tools and targets for antitumor vaccination. Expert Rev Vaccines. 2005;4:699–710. doi: 10.1586/14760584.4.5.699. [DOI] [PubMed] [Google Scholar]
- 81.Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation ofcCellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med. 2002;195:125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Banerjee D, Matthews P, Matayeva E, Kaufman JL, Steinman RM, Dhodapkar KM. Enhanced T-cell responses to glioma cells coated with the anti-EGF receptor antibody and targeted to activating FcgammaRs on human dendritic cells. J Immunother. 2008;31:113–120. doi: 10.1097/CJI.0b013e31815a5892. [DOI] [PubMed] [Google Scholar]
- 83.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I- restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 84.Berard F, Blanco P, Davoust J, Neidhart-Berard EM, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini JC, Banchereau J, Palucka AK. Cross-Priming of Naive CD8 T Cells against Melanoma Antigens Using Dendritic Cells Loaded with Killed Allogeneic Melanoma Cells. J Exp Med. 2000;192:1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 86.Shi H, Cao T, Connolly JE, Monnet L, Bennett L, Chapel S, Bagnis C, Mannoni P, Davoust J, Palucka AK, Banchereau J. Hyperthermia enhances CTL cross-priming. J Immunol. 2006;176:2134–2141. doi: 10.4049/jimmunol.176.4.2134. [DOI] [PubMed] [Google Scholar]
- 87.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 88.Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol. 2007;178:1975–1979. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- 89.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 90.Finn OJ. Human tumor antigens, immunosurveillance, and cancer vaccines. Immunol Res. 2006;36:73–82. doi: 10.1385/IR:36:1:73. [DOI] [PubMed] [Google Scholar]
- 91.O'Rourke MG, Johnson M, Lanagan C, See J, Yang J, Bell JR, Slater GJ, Kerr BM, Crowe B, Purdie DM, Elliott SL, Ellem KA, Schmidt CW. Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol Immunother. 2003;52:387–395. doi: 10.1007/s00262-003-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Butterfield LH, Ribas A, Dissette VB, Amarnani SN, Vu HT, Oseguera D, Wang HJ, Elashoff RM, McBride WH, Mukherji B, Cochran AJ, Glaspy JA, Economou JS. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin. Cancer Res. 2003;9:998–1008. [PubMed] [Google Scholar]
- 93.Lobo NA, Shimono Y, Qian D, Clarke MF. The Biology of Cancer Stem Cells. Annu Rev Cell Dev Biol. 2007:23. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 94.Rossi DJ, Weissman IL. Pten, tumorigenesis, and stem cell self-renewal. Cell. 2006;125:229–231. doi: 10.1016/j.cell.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 95.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter G, Durie B, Crowley J, Shaughnessy JD, Jr., Scanlan MJ, Gure AO, Barlogie B, Dhodapkar MV. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–840. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hava DL, Brigl M, van den Elzen P, Zajonc DM, Wilson IA, Brenner MB. CD1 assembly and the formation of CD1-antigen complexes. Curr Opin Immunol. 2005;17:88–94. doi: 10.1016/j.coi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 97.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 98.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 99.Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK. Migration of human dendritic cells after injection in patients with metastatic malignancies [In Process Citation] Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- 100.de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, Bulte JW, Scheenen TW, Punt CJ, Heerschap A, Figdor CG. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23:1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 101.MartIn-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Verdijk P, Aarntzen EH, Punt CJ, de Vries IJ, Figdor CG. Maximizing dendritic cell migration in cancer immunotherapy. Expert Opin Biol Ther. 2008;8:865–874. doi: 10.1517/14712598.8.7.865. [DOI] [PubMed] [Google Scholar]
- 103.Lesimple T, Neidhard EM, Vignard V, Lefeuvre C, Adamski H, Labarriere N, Carsin A, Monnier D, Collet B, Clapisson G, Birebent B, Philip I, Toujas L, Chokri M, Quillien V. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res. 2006;12:7380–7388. doi: 10.1158/1078-0432.CCR-06-1879. [DOI] [PubMed] [Google Scholar]
- 104.Dorrie J, Schaft N, Muller I, Wellner V, Schunder T, Hanig J, Oostingh GJ, Schon MP, Robert C, Kampgen E, Schuler G. Introduction of functional chimeric E/L-selectin by RNA electroporation to target dendritic cells from blood to lymph nodes. Cancer Immunol Immunother. 2008;57:467–477. doi: 10.1007/s00262-007-0385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 106.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 107.Mullins DW, Sheasley SL, Ream RM, Bullock TNJ, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and efefctor T cells in lymphoid tissues and determines the pattern of regional tumor control. j exp med. 2003;198:1–13. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 109.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 110.Shin T, Yoshimura K, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J. Exp. Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006 doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 112.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 115.Gonzalo JA, Tian J, Delaney T, Corcoran J, Rottman JB, Lora J, Al-Garawi A, Kroczek R, Gutierrez-Ramos JC, Coyle AJ. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat. Immunol. 2001;2:597–604. doi: 10.1038/89739. [DOI] [PubMed] [Google Scholar]
- 116.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 117.Ito T, Wang YH, Duramad O, Hanabuchi S, Perng OA, Gilliet M, Qin FX, Liu YJ. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:13138–13143. doi: 10.1073/pnas.0603107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 122.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 126.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 127.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 128.Kuipers H, Muskens F, Willart M, Hijdra D, van Assema FB, Coyle AJ, Hoogsteden HC, Lambrecht BN. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. Eur J Immunol. 2006;36:2472–2482. doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- 129.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 130.Paczesny S, Banchereau J, Wittkowski KM, Saracino G, Fay J, Palucka AK. Expansion of Melanoma-specific Cytolytic CD8+ T Cell Precursors in Patients with Metastatic Melanoma Vaccinated with CD34+ Progenitor-derived Dendritic Cells. J Exp Med. 2004;199:1503–1511. doi: 10.1084/jem.20032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21:515–546. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]
- 132.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Badovinac VP, Messingham KA, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat Med. 2005;11:748–756. doi: 10.1038/nm1257. [DOI] [PubMed] [Google Scholar]
- 134.Steitz J, Bruck J, Lenz J, Knop J, Tuting T. Depletion of CD25(+) CD4(+) T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon alpha-induced, CD8(+) T-cell-dependent immune defense of B16 melanoma. Cancer Res. 2001;61:8643–8646. [PubMed] [Google Scholar]
- 135.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 136.Vence L, Palucka AK, Fay JW, Ito T, Liu YJ, Banchereau J, Ueno H. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.North RJ. The murine antitumor immune response and its therapeutic manipulation. Adv Immunol. 1984;35:89–155. doi: 10.1016/s0065-2776(08)60575-1. [DOI] [PubMed] [Google Scholar]
- 138.Ma J, Urba WJ, Si L, Wang Y, Fox BA, Hu HM. Anti-tumor T cell response and protective immunity in mice that received sublethal irradiation and immune reconstitution. Eur J Immunol. 2003;33:2123–2132. doi: 10.1002/eji.200324034. [DOI] [PubMed] [Google Scholar]
- 139.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor-inducer T-cells. Cancer Res. 1988;48:1671–1675. [PubMed] [Google Scholar]
- 140.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen specific immune responses to cancer vaccines. Blood. 2008 doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mahnke K, Schonfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, Bedke T, Johnson TS, Storn V, Schallenberg S, Enk AH. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723–2733. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 143.Pure E, Allison JP, Schreiber RD. Breaking down the barriers to cancer immunotherapy. Nat Immunol. 2005;6:1207–1210. doi: 10.1038/ni1205-1207. [DOI] [PubMed] [Google Scholar]
- 144.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 145.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 147.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 148.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 149.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 150.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming Growth Factor-beta Regulation of Immune Responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 151.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 152.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 154.Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, Riley JL, June CH. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat. Biotechnol. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 155.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 156.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother (1997) 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 159.Asavaroengchai W, Kotera Y, Mule JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci U S A. 2002;99:931–936. doi: 10.1073/pnas.022634999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8 T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dhodapkar MV, Dhodapkar KM, Palucka AK. Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell Death Differ. 2008;15:39–50. doi: 10.1038/sj.cdd.4402247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fonseca C, Dranoff G. Capitalizing on the immunogenicity of dying tumor cells. Clin Cancer Res. 2008;14:1603–1608. doi: 10.1158/1078-0432.CCR-07-2245. [DOI] [PubMed] [Google Scholar]
- 164.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68:4026–4030. doi: 10.1158/0008-5472.CAN-08-0427. [DOI] [PubMed] [Google Scholar]
- 167.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, Andre F, Tursz T, Kroemer G, Zitvogel L. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 168.Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, van Endert P, Ghiringhelli F, Apetoh L, Chaput N, Flament C, Ullrich E, de Botton S, Zitvogel L, Kroemer G. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 169.Candido K, Shimuzu K, McLaughlin JC, Kunkel R, Fuller JA, Redman BG, Thomas E, Nikoloff B, Mulé J. Local administration of dendritic cells inhibits established breast cancer growth: implications for apoptosis inducing agents. Cancer Res. 2001;61:228–231. [PubMed] [Google Scholar]
- 170.Tong Y, Song W, Crystal RG. Combined intratumoral injection of bone marrow-derived dendritic cells and systemic chemotherapy to treat pre-existing murine tumors. Cancer Res. 2001;61:7530–7535. [PubMed] [Google Scholar]
- 171.Song W, Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of naive dendritic cells. Cancer Res. 2005;65:5958–5964. doi: 10.1158/0008-5472.CAN-05-0406. [DOI] [PubMed] [Google Scholar]
- 172.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 173.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 174.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 175.Janicki CN, Jenkinson SR, Williams NA, Morgan DJ. Loss of CTL function among high-avidity tumor-specific CD8+ T cells following tumor infiltration. Cancer Res. 2008;68:2993–3000. doi: 10.1158/0008-5472.CAN-07-5008. [DOI] [PubMed] [Google Scholar]
- 176.Chaput N, Darrasse-Jeze G, Bergot AS, Cordier C, Ngo-Abdalla S, Klatzmann D, Azogui O. Regulatory T cells prevent CD8 T cell maturation by inhibiting CD4 Th cells at tumor sites. J Immunol. 2007;179:4969–4978. doi: 10.4049/jimmunol.179.8.4969. [DOI] [PubMed] [Google Scholar]
- 177.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8(+) T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 179.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 181.Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dercamp C, Chemin K, Caux C, Trinchieri G, Vicari AP. Distinct and overlapping roles of interleukin-10 and CD25+ regulatory T cells in the inhibition of antitumor CD8 T-cell responses. Cancer Res. 2005;65:8479–8486. doi: 10.1158/0008-5472.CAN-05-1319. [DOI] [PubMed] [Google Scholar]
- 183.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sinha P, Clements VK, Miller S, Ostrand-Rosenberg S. Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression. Cancer Immunol Immunother. 2005;54:1137–1142. doi: 10.1007/s00262-005-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 187.Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai Z, Mann HH, Strong RK, Groh V, Spies T. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447:482–486. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- 188.Gonzalez S, Groh V, Spies T. Immunobiology of human NKG2D and its ligands. Curr Top Microbiol Immunol. 2006;298:121–138. doi: 10.1007/3-540-27743-9_6. [DOI] [PubMed] [Google Scholar]
- 189.Kalinski P, Giermasz A, Nakamura Y, Basse P, Storkus WJ, Kirkwood JM, Mailliard RB. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol. 2005;42:535–539. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 190.Wang E, Panelli MC, Marincola FM. Gene profiling of immune responses against tumors. Curr Opin Immunol. 2005;17:423–427. doi: 10.1016/j.coi.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 191.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Northrop JK, Shen H. CD8+ T-cell memory: only the good ones last. Curr Opin Immunol. 2004;16:451–455. doi: 10.1016/j.coi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 193.Kronenberg M. Toward an Understanding of NKT Cell Biology: Progress and Paradoxes. Annu Rev Immunol. 2004 doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 194.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 195.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pardoll D, Allison J. Cancer immunotherapy: breaking the barriers to harvest the crop. Nat Med. 2004;10:887–892. doi: 10.1038/nm0904-887. [DOI] [PubMed] [Google Scholar]
- 197.Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U, Nichol G. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 198.Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Brocker EB, Grabbe S, Rittgen W, Edler L, Sucker A, Zimpfer-Rechner C, Berger T, Kamarashev J, Burg G, Jonuleit H, Tuttenberg A, Becker JC, Keikavoussi P, Kampgen E, Schuler G. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17:563–570. doi: 10.1093/annonc/mdj138. [DOI] [PubMed] [Google Scholar]
- 199.Santin AD, Bellone S, Palmieri M, Zanolini A, Ravaggi A, Siegel ER, Roman JJ, Pecorelli S, Cannon MJ. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. J Virol. 2008;82:1968–1979. doi: 10.1128/JVI.02343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]