Abstract
Our hypothesis was that cross-linked UHMWPE stabilized with vitamin-E would be wear and fatigue resistant. Acetabular liners were radiation cross-linked, doped with vitamin E and γ-sterilized. Hip simulator wear rate of vitamin E-stabilized UHMWPE was approximately 1 and 6 mg/million-cycles in clean serum and in serum with third-body bone cement particles, respectively; a four to ten-fold decrease from that of conventional UHMWPE. The ultimate strength, yield strength, elongation-at-break and fatigue resistance of vitamin E-stabilized UHMWPE were significantly higher than that of 100-kGy irradiated and melted UHMWPE and were unaffected by accelerated aging. Rim impingement testing with 3.7 mm-thick acetabular liners up to 2 million-cycles showed no significant damage of the cross-linked liners compared to conventional, gamma-sterilized in inert UHMWPE vitamin-E stabilized liners. The data indicate good wear properties and improved mechanical and fatigue properties for vitamin-E stabilized cross-linked UHMWPE.
Keywords: Total hip arthroplasty, highly cross-linked polyethylene, oxidation resistance, fatigue resistance, vitamin E, biomaterials
Introduction
One of the major factors limiting the long-term performance of total hips is the peri-prosthetic osteolysis secondary to the wear of the ultra-high molecular weight polyethylene (UHMWPE) in acetabular components. Radiation cross-linking and heat treatment of UHMWPE has proven to be an important advance, which resulted in the discovery of a number of highly cross-linked UHMWPEs with improved wear resistance [1-6]. Some of these improved UHMWPEs have been in clinical use since 1998 with early clinical studies showing markedly reduced wear rates with the new UHMWPEs in comparison with conventional UHMWPEs [7, 8]. These novel UHMWPE materials are showing promise of reducing the incidence of osteolysis, which remains a major long-term complication in total hips.
Cross-linking of UHMWPE is achieved through the use of ionizing radiation [9]. Ionizing radiation forms free radicals through the radiolytic cleavage of C-H and C-C bonds in polyethylene. These free radicals recombine with each other and form cross-links in the amorphous portion of the polymer. The free radicals generated during irradiation in the crystalline phase, however, become trapped [10] and adversely affect the long-term oxidative stability of the material.
The effects of residual free radicals causing embrittlement in the long-term have been well documented for UHMWPE components that had been gamma sterilized in air [11, 12]. Oxidative embrittlement of UHMWPE is initiated when the residual free radicals react with oxygen. A complex cascade of events leads to the formation of peroxy free radicals, hydroperoxides, and ultimately carbonyl species, mainly ketones, esters, and acids, the formation of which can be accompanied by chain scission, reducing the molecular weight of the polymer. This eventually leads to recrystallization, increase in stiffness, and embrittlement of the UHMWPE component.
In highly cross-linked UHMWPEs currently in clinical use, a thermal treatment step follows the radiation cross-linking of UHMWPE to decrease the concentration of the residual free radicals and to minimize or eliminate the adverse effects of the residual free radicals on the properties of UHMWPE [1, 2, 6]. The most effective method of stabilization is to melt the irradiated UHMWPE, which reduces the concentration of the residual free radicals to undetectable levels. The method of irradiation and melting improves the wear resistance and does not compromise the oxidation resistance of UHMWPE. However, the post-irradiation melting step further reduces the mechanical properties and fatigue strength of irradiated UHMWPE [13], presumably due to a decrease in crystallinity that accompanies post-irradiation melting.
A clinical concern caused by the loss of fatigue strength of irradiated and melted UHMWPEs in the hip is the fatigue damage and fracture of mal-positioned liners under adverse loading conditions [14]. Since highly cross-linked UHMWPEs have enabled the use of larger femoral heads with thinner acetabular liners, these liners are exposed to higher stresses. An instance of rim fracture has been reported with irradiated and melted liners loaded to extremely high stresses with the shell placed in a vertical, mal-aligned position. It may be highly desirable to improve the mechanical properties of highly cross-linked polyethylenes.
We propose to avoid post-irradiation melting by stabilizing the residual free radicals by diffusing a-tocopherol (vitamin-E), an antioxidant, into irradiated UHMWPE. a-Tocopherol is one of the isomers of vitamin E, which is the most abundant and effective chain breaking antioxidant in the body [15]. The major physiological role of Vitamin E is to react with free radicals in cell membranes and protect polyunsaturated fatty acids from degradation due to oxidation [16]. We previously showed that irradiated UHMWPE can be protected against oxidation by diffusion of vitamin E into it [13]. Our hypotheses are that by eliminating melting after irradiation, this novel highly cross-linked UHMWPE will avoid loss of mechanical properties and provide improved fatigue strength than irradiated and melted highly cross-linked UHMWPE as well as improved wear and oxidation resistance in comparison with conventional UHMWPE.
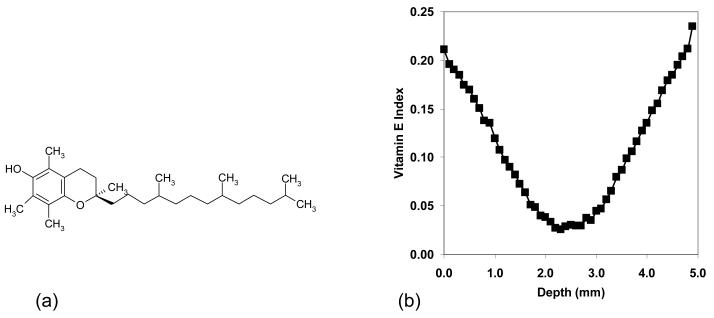
Cell membranes in the body typically contain about one a-tocopherol molecule per 2000 phospholipids to prevent against oxidative damage [16]. Oxidation of polyunsaturated fatty acids in cell membranes results in active free radicals (LOO•, LO•), which are stabilized by a-tocopherol. The antioxidant activity of a-tocopherol is due to hydrogen abstraction from the OH group on the chroman ring by a peroxyl free radical (Fig 1a). Hydrogen abstraction results in a tocopheryl free radical, which can combine with another peroxy free radical. Therefore, tocopherol can theoretically prevent two peroxy free radicals from attacking other fatty acid chains and producing more free radicals [17-19].
Fig 1.
The structure of a-tocopherol (a). The a-tocopherol concentration profile in the 4.9 mm acetabular liners that were used in the present study (b): the profile was determined by infrared microscopy scanning of a thin film and determining the relative absorbance of a-tocopherol with respect to polyethylene.
Two potential methods of incorporating a-tocopherol into UHMWPE are pre-consolidation blending or post-irradiation diffusion. In the former, UHMWPE is consolidated with and irradiated in the presence of a-tocopherol, which is known to reduce the cross-linking efficiency of UHMWPE [20]. A decrease in the cross-linking efficiency also results in the decrease of wear resistance in the a-tocopherol blended and irradiated UHMWPE compared to a virgin, irradiated UHMWPE [21]. Therefore it may be more beneficial to incorporate a-tocopherol into consolidated UHMWPE after irradiation. This can be achieved by using diffusion, a method that was recently reported [13].
In that recent study [13], the incorporation of a-tocopherol (vitamin-E) into an irradiated UHMWPE resulted in high wear resistance and did not compromise the oxidation resistance of UHMWPE. 100-kGy irradiated and a-tocopherol diffused UHMWPE had at least 32% higher fatigue crack propagation resistance than that of 100 kGy irradiated and melted UHMWPE [13]. The hypothesis of that study was that, when present, a-tocopherol would stabilize the residual free radicals in irradiated UHMWPE and that this presence would not adversely affect the wear rate of irradiated UHMWPE. a-Tocopherol penetrated approximately less than 0.5 mm from the surface, where the material was oxidation resistant; while the bulk did not contain any a-tocopherol and was prone to oxidation.
By our current techniques, we fully penetrated a-tocopherol into finished acetabular liners (Fig 1b). One hypothesis of this study was that irradiated UHMWPE with full penetration of a-tocopherol would not compromise the wear resistance of highly cross-linked UHMWPE and another was that the fatigue strength would be improved compared to highly cross-linked and melted UHMWPE. We further hypothesized that the presence of a-tocopherol throughout the thickness of the samples would prevent deterioration of mechanical properties upon exposure to an accelerated aging environment.
In order to test these hypotheses, we performed hip simulator wear testing on 85-kGy irradiated, vitamin E-stabilized and finally gamma-sterilized acetabular liners. The final gamma-sterilization was performed to ensure the sterility of the vitamin E in the liners. To test mechanical and fatigue strength, we performed tensile testing and fatigue crack propagation testing on an MTS machine with samples having the same vitamin E concentration profile as that of the acetabular liners. In a more advanced device fatigue test, we performed a rim impingement study on the hip simulator with a worst-case acetabular liner dimension, namely 3.7 mm in thickness.
Materials and Methods
Preparation of a-tocopherol-doped, irradiated UHMWPE and conventional UHMWPE Test Samples for hip simulator, tensile and fatigue testing
All specimens were manufactured from isostatically molded UHMWPE bar stock (Biomet Inc., Warsaw, IN). The UHMWPE resin utilized was GUR1050 (Ticona, Bishop TX).
Two different groups of test samples, an a-tocopherol doped, irradiated UHMWPE and conventional UHMWPE were compared. Both groups of test samples were terminally gamma sterilized in argon gas before testing. Conventional UHMWPE samples were machined from a GUR1050 UHMWPE stock, packaged in argon gas, and gamma sterilized.
The a-tocopherol doped, irradiated UHMWPE samples were machined into their respective shapes from annealed GUR1050 UHMWPE stock below the melting point of UHMWPE. This annealing of bar stock materials was to reduce the thermal stresses in the UHMWPE stock resulting in greater dimensional stability of machined parts at elevated temperatures. These samples were packaged under argon gas with an oxygen scavenger (Fresh Pax™, Multisorb Technologies Inc., Buffalo, NY), and the packages were gamma-irradiated to 85 kGy. Since gamma-irradiation is a lengthy process (several days for 85-100 kGy), the probability of samples coming into contact with oxygen even though they are in inert packaging is high. The purpose of the scavenger was to provide an extra measure to eliminate any oxygen present in the environment during irradiation. The irradiated samples were then doped with D, L-a-tocopherol (vitamin E, >98%, Fisher Scientific, Houston, TX) by immersion into a-tocopherol at 120 °C and subsequently annealed at 120 °C under argon flow for homogenization. The samples were then packaged in argon gas with an oxygen scavenger and gamma sterilized. Immersion in a-tocopherol and the subsequent homogenization durations were adjusted to obtain similar amounts of a-tocopherol in samples with different thicknesses (Table 1).
Table 1.
Doping and homogenization durations for samples of different thickness
| Sample (thickness) | Doping duration (h) | Homogenization duration (h) |
|---|---|---|
| Thin sections for tensile testing (3.2 mm) | 0.33 | 9 |
| Acetabular liners (3.7 mm) | 1.5 | 24 |
| Acetabular liners (4.9 mm) | 2 | 24 |
| Compact tension samples for fatigue testing (8.2 mm) | 2.5 | 40 |
In the following studies, we used hip simulator testing in clean serum and in serum with third-body bone cement particulate to determine adhesive and third-body abrasive wear resistance, differential scanning calorimetry (DSC) to determine crystalline content, tensile testing to determine mechanical properties such as ultimate tensile strength (UTS), yield strength (YS) and elongation at break (EAB), and fatigue crack propagation testing to determine fatigue resistance. Tensile testing samples were tested before and after accelerated aging at 70°C for 2 weeks at 5 atm. of oxygen.
Hip Simulator Testing
We used hip simulator wear testing to determine if the addition of a-tocopherol in highly cross-linked acetabular liners would adversely affect the adhesive and third body abrasive wear resistance of cross-linked UHMWPE.
We chose a geometry that would result in high contact stresses by using a liner thickness of 4.9 mm for both groups of liners. The conventional UHMWPE liners had an inner diameter of 28 mm. We tested the a-tocopherol doped, irradiated UHMWPE liners with both an inner diameter of 28 mm and 36 mm. The 28mm liners allowed comparison of the two groups of liners. Since larger femoral heads result in higher wear, we also tested the a-tocopherol doped, irradiated UHMWPE liners with a 36 mm femoral head.
All liners were coupled with the corresponding cobalt-chrome femoral head sizes for testing. Each group included 4 liners that were subjected to both motion and load and 2 load-soak liners that were only subjected to load without motion. The lubricant used for the study was 100% bovine serum, stabilized with 10.7 millimoles of ethylenediamine tetraacetate (EDTA, Fisher Scientific, Pittsburgh, PA) and 33 ml of penicillin-streptomycin solution (Sigma-Aldrich, St. Louis, MO) per 500 ml of serum.
All testing was performed on the AMTI 12-Station Hip Simulator (Watertown, MA). Testing was carried out at 2Hz for a total of 5 million-cycles (MC) in clean serum or at 1 Hz for a total of 3 MC for testing in serum with third-body bone cement particles. The kinematics used was a standard walking gait cycle with the peak load of 3000 N. All stations were temperature controlled at 37 °C with circulating bovine serum. The simulator was interrupted at approximately 500,000 cycle intervals for gravimetric assessment of wear.
For gravimetric measurements, the liners were cleaned by sonication in a soap solution for 10 minutes, followed by sonication in distilled water for 10 minutes, followed by an ethanol bath for 10 minutes and finally by drying in air for 10 minutes before weighing. The liners were weighed three times and then averaged using an A-250 balance (Denver Instrument Co., Arvada, CO). The articular surfaces were photographed at the dome and at 4 quadrants at about 3 to 4 mm from the dome using an Olympus SZX12 optical microscope and an Olympus DP11 camera at every gravimetric measurement.
Weight loss of each liner was used to calculate a wear rate before and after correction for fluid absorption. The correction for fluid absorption was done by subtracting the average weight gain of the load soak components from the weight change of the motion components. The actual wear rate was calculated by applying a linear regression. The slope of the linear regression is reported as the wear rate in milligrams per million cycles (mg/MC).
Surface Analysis by Scanning Electron Microscopy and Oxidative Stability Testing after Hip Simulator Testing
At the completion of the 5 million cycles, the motion liners of all three groups; conventional UHMWPE with 28 mm inner diameter, a-tocopherol-doped, irradiated UHMWPE with 28 mm inner diameter and a-tocopherol-doped, irradiated UHMWPE with 36 mm inner diameter, were cut into sections. One of the sections was gold-coated and analyzed with an environmental scanning electron microscope equipped with a field emission gun (SEM) (Phillips/FEI, XL30, Hillsboro, OR) to qualitatively evaluate changes in the articular surface morphology after simulated gait wear testing.
Determination of Percent Crystallinity by Differential Scanning Calorimetry (DSC)
We determined the crystallinity of a-tocopherol-doped, irradiated UHMWPE before and after accelerated aging and compared it to the crystallinity of conventional gamma-sterilized UHMWPE.
3.2 mm-thick sections were prepared for the determination of crystallinity. DSC specimens were prepared by cutting small slivers (5-10 milligrams) for testing. The DSC specimens were weighed with a Sartorius CP 225D balance to a resolution of 0.01 milligrams and placed in aluminum sample pans. The pan was crimped with an aluminum cover and placed in a Q-1000 Differential Scanning Calorimeter (TA Instruments, Newark, DE). The sample and the reference were then heated at a heating rate of 10°C/min from −20°C to 180°C, cooled to −20°C at −10°C/min and subjected to another heating cycle from −20°C to 180°C at 10°C/min. Heat flow as a function of time and temperature was recorded and the cycles are referred to as 1st heat, 1st cool and 2nd heat, respectively.
Crystallinity of unaged and aged conventional and a-tocopherol-doped, irradiated UHMWPEs (n=5 each) was determined by integrating the enthalpy peak from 20°C to 160°C, and normalizing it with the enthalpy of melting of 100% crystalline polyethylene, namely 291J/g.
Mechanical testing
Tensile specimens (Type V) were stamped out of 3.2 mm-thick sections in accordance with ASTM D-638. These dogbone-shaped specimens were tested on an MTS machine (Eden Prairie, MN) at a cross head speed of 10 mm/min. The axial displacement and force were sampled at a rate of 100 Hz.
The test was also recorded on videotape to visually determine elongation-at-break (EAB). For this purpose, a gauge length of approximately 7 mm was marked on the specimens as per ASTM D638. The thickness of the specimens and the width of the specimens in the gauge region were measured using calipers before deformation. The separation of the gauge marks just prior to failure was measured from the recorded videos and the true elongation-at-break (EAB) was computed as the ratio of the change in gauge length at fracture and the initial gauge length.
The engineering stress was computed based on the nominal cross sectional area (before any deformation) of the specimens as a function of engineering strain. The yield strength (YS) in MPa and the ultimate tensile strength (UTS) in MPa were calculated per ASTM D638.
Five samples each of unaged and aged conventional and a-tocopherol-doped, irradiated UHMWPE were tested.
Fatigue Crack Propagation Testing
Fatigue crack propagation testing was performed on compact tension (CT) specimens on a MiniBionix 858 (MTS, Eden Prairie MN) following ASTM E 647-00, the standard method for measurement of fatigue crack growth rates.
CT specimens were pre-cracked at the notch using a razor blade. They were pre-soaked for approximately 10 minutes in an aqueous bath at 40°C (also the testing environment) prior to loading. Testing was conducted at a sinusoidal load cycle frequency of 5 Hz and stress ratio of 0.1 in tension. Crack length was monitored by optical microscopy every 20,000 cycles. The average of the crack length on both sides of the CT specimen was used as the representative crack length for the computation of crack growth rates.
Stress intensity factor ranges at crack inception (ΔKincep) were reported at a threshold crack growth rate of 10−6 mm/cycle. All testing was done at 40°C to simulate the physiologic temperature of the joint.
At least three specimens were tested for unaged conventional UHMWPE and a-tocopherol-doped, irradiated UHMWPE.
Preparation of a-tocopherol-doped, irradiated UHMWPE and conventional UHMWPE test samples for rim impingement testing
All specimens were manufactured from isostatically molded UHMWPE bar stock (Biomet, Inc., Warsaw, IN). The UHMWPE resin utilized was GUR1050 (Ticona, Bishop TX).
Two different groups of test samples, an a-tocopherol doped, irradiated UHMWPE and conventional UHMWPE were compared. Both groups of test samples were terminally gamma sterilized in argon gas before testing. 3.7 mm-thick conventional UHMWPE liners with 28 mm inner diameter were machined from a GUR1050 UHMWPE stock, packaged in argon gas, and gamma sterilized.
The a-tocopherol doped, irradiated UHMWPE samples were machined into 3.7 mm thick acetabular liners with 28 and 40 mm inner diameter from annealed GUR1050 UHMWPE stock below the melting point of UHMWPE. These samples were packaged under argon gas and irradiated to 100 kGy by electron-beam irradiation. Electron beam irradiation was utilized because it was faster than gamma irradiation and this also eliminated the need for an oxygen scavenger. The irradiated liners were then doped with D, L-a-tocopherol and homogenized at 120 °C under argon flow. The duration of doping and homogenization are shown in Table 1. The samples were then packaged in argon gas and gamma sterilized.
Rim impingement testing
The lip of four acetabular liners of each group (conventional-28 mm, Vitamin E-28 mm, Vitamin E-40 mm) were brought into contact with the femoral neck (Fig 2) and loaded for 2 million-cycles (MC) to a peak load of 125 lb at a rate of 0.5 Hz based on a torque of 100 in-lb, a moment necessary to cause dislocation of the hip joint [22]. The test was stopped at intervals of 500,000 cycles so that the components could be visually inspected for signs of fatigue failure. At the completion of the study, the liners were removed from their shells by shrinking them with exposure to liquid nitrogen in order to bypass the locking mechanism.
Fig 2.
Impingement of an acetabular liner by the femoral neck.
In the following studies, where n≥3, statistical analysis was performed using a Student's t-test for two-tailed distributions with unequal variance.
Results
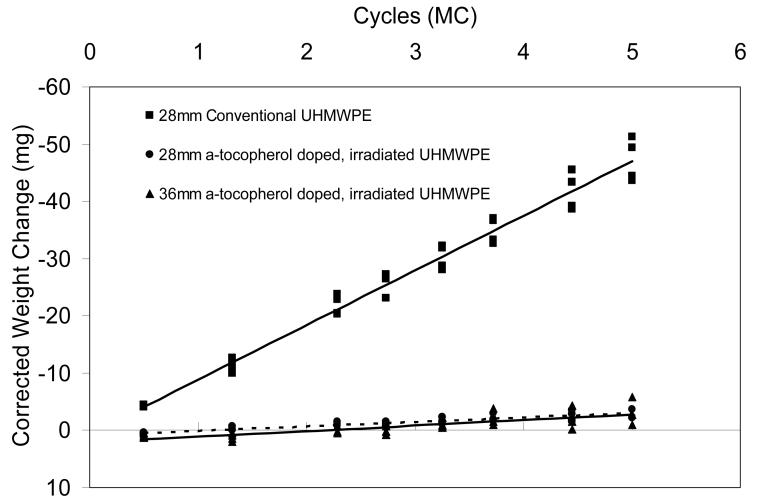
Average wear rate corrected for fluid absorption of the 28 mm conventional liners were 9.54±0.73 mg/million-cycles after 5 million cycles of simulated gait with clean serum. The average wear rates for the 28 mm and 36 mm inner diameter a-tocopherol doped, irradiated UHMWPE acetabular liners were 0.78±0.28 and 0.97±0.49 mg/million-cycles, a 10-fold decrease with respect to the conventional UHMWPE (Fig 3). The wear rate of the a-tocopherol doped, irradiated UHMWPE was not affected by the larger femoral head size. Optical microscopy of the articular surfaces of the tested liners showed the presence of original machining lines after 5 million-cycles of simulated gait (Fig 4); while the conventional liners exhibited no machining marks throughout the loaded region of the articular surfaces (Fig 5).
Fig 3.
Average total weight change of tested liners (n=4) tested in clean serum for 5 million-cycles corrected for fluid absorption shown with corresponding linear regression lines.
Fig 4.
Compilation of photos showing a 28mm a-tocopherol doped, irradiated UHMWPE liner after 5 million cycles of testing on the hip simulator. Machining marks are present in all four quadrants and also at the dome. All pictures were taken at the same magnification and the scale bar denotes 1 mm.
Fig 5.
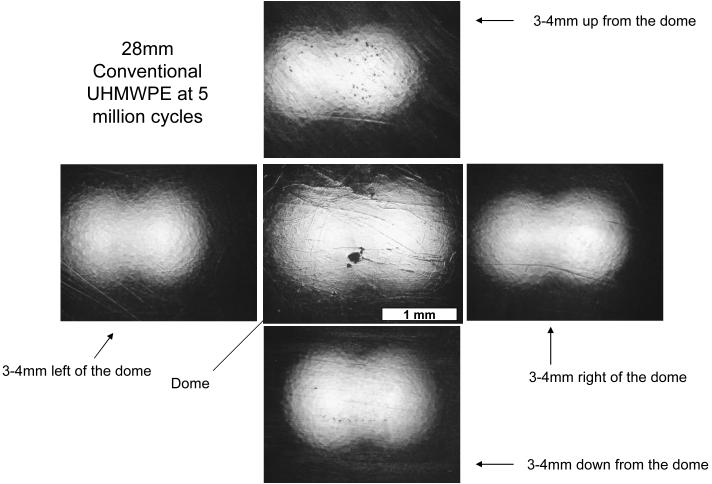
Compilation of photos showing a 28mm conventional UHMWPE liner after 5 million cycles of testing on the hip simulator. The machining marks have been polished away in all four quadrants and also at the dome. All pictures were taken at the same magnification and the scale bar denotes 1 mm.
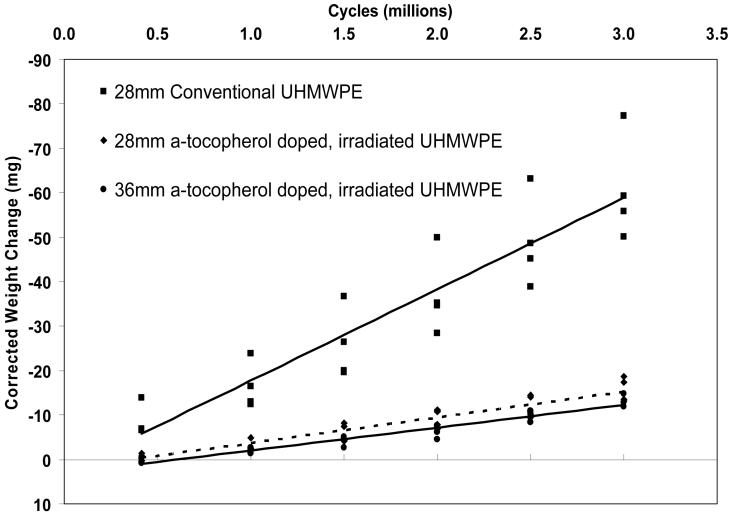
Average wear rates corrected for fluid absorption of the 28 mm conventional liners were 20.55±0.50 mg/million-cycles after 3 million cycles of simulated gait with third body bone cement particles. The average wear rates for the 28 mm and 36 mm inner diameter a-tocopherol-doped, irradiated UHMWPE acetabular liners were 5.76±0.82 and 5.13±0.34 mg/million cycles (Fig 6). These values were 72 and 75% lower than that for the conventional UHMWPE. Optical microscopy of the articular surfaces of the tested liners showed the presence numerous scratching on all tested liners.
Fig 6.
Average total weight change (corrected for fluid absorption) over 3 million cycles of the acetabular liners of each test group tested in serum with third-body bone cement particulates (n=4) shown with corresponding linear regression lines.
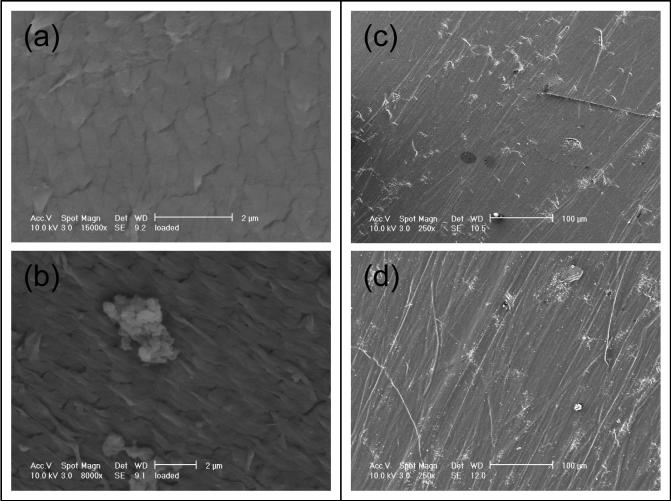
Scanning electron microscopy of the articular surfaces of the tested liners showed abundant formation of fibrils in the conventional liners that were tested in clean serum (Fig 7b); whereas the a-tocopherol-doped, irradiated UHMWPE liners showed reduced fibril formation (Fig 7a). All liners tested in serum with third-body bone cement particles exhibited extensive scratching of the articular surface (Fig 7c and 7d).
Fig 7.
Representative SEM micrographs of the articular surfaces of tested motion liners (a-Vitamin E, b-conventional) following 5 million cycles of simulated normal gait with clean serum and (c-Vitamin E, d-conventional) following 3 million cycles of simulated normal gait with abrasive third body bone cement particles added to the lubricating bovine serum.
The crystallinity of the a-tocopherol doped, irradiated UHMWPE was not significantly different (p>0.05) than that of the conventional UHMWPE. Following accelerated aging, the crystallinity of conventional UHMWPE increased (p<0.0001); whereas the crystallinity of a-tocopherol-doped, irradiated UHMWPE did not change upon accelerated aging (p=0.46, Table 2). The DSC thermogram and peak melting point of a-tocopherol doped, irradiated UHMWPE, indicative of crystalline lamellar thickness, was not different from that of conventional UHMWPE (Fig 8).
Table 2.
Crystallinity of unaged and accelerated aged conventional and a-tocopherol-doped, irradiated UHMWPE
| Group | Crystallinity (%) |
|---|---|
| Conventional UHMWPE | |
| Unaged | 68 ± 2 |
| Aged | 77 ± 2 |
| a-tocopherol Doped, Irradiated UHMWPE | |
| Unaged | 71 ± 2 |
| Aged | 71 ± 1 |
Fig 8.
Representative DSC thermograms of gamma-sterilized conventional and highly cross-linked, Vitamin E-doped UHMWPE.
The ultimate tensile strength (UTS) and yield strength (YS) of a-tocopherol doped, irradiated UHMWPE was comparable to those of conventional UHMWPE; the elongation at break (EAB) was lower (Table 3). Accelerated aging resulted in a significant (p<0.001) decrease in the UTS of the conventional UHMWPE, whereas it did not affect the UTS of the a-tocopherol doped, irradiated UHMWPE (p=0.22, Table 3). Similarly, accelerated aging resulted in a significant change in the YS and EAB of conventional UHMWPE (p<0.01 and p<0.05, respectively), whereas it did not significantly affect the YS and EAB of a-tocopherol-doped, irradiated UHMWPE (p=0.33, p=0.74).
Table 3.
Tensile properties for conventional and a-tocopherol doped, irradiated UHMWPE
| Sample | UTS (MPa) | YS (MPa) | EAB (%) |
|---|---|---|---|
| Unaged conventional UHMWPE | 52 ± 5 | 24 ± 1 | 347 ± 35 |
| Aged conventional UHMWPE | 33 ± 1 | 28 ± 1 | 434 ± 40 |
| Unaged a-tocopherol doped, irradiated UHMWPE | 46 ± 3 | 25 ± 1 | 230 ± 9 |
| Aged a-tocopherol doped, irradiated UHMWPE | 45 ± 2 | 25 ± 1 | 234 ± 21 |
The a-tocopherol doped, irradiated UHMWPE showed a significantly higher stress factor range at crack inception (ΔKincep), i.e. higher fatigue resistance, than 100-kGy irradiated and melted UHMWPE (p<0.001, Table 4). Also, both of the irradiated UHMWPEs showed lower fatigue resistance than conventional UHMWPE (p<0.00001).
Table 4.
Stress factor range at fatigue crack inception for conventional UHMWPE and a-tocopherol-doped, irradiated UHMWPE
| Sample | ΔKincep (MPa m1/2) |
|---|---|
| Conventional UHMWPE | 1.19 ± 0.02 |
| a-Tocopherol-doped, irradiated UHMWPE | 0.77 ± 0.02 |
| 100-kGy irradiated and melted UHMWPE [13] | 0.56 ± 0.02 |
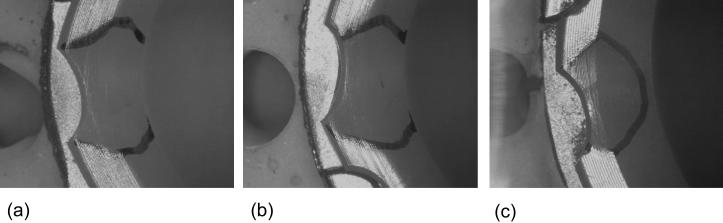
After 2 million cycles of simulated rim impingement, all three groups of liners showed a slight depression on the rim (Fig 9). There was no evidence of cracking, pitting or gross surface damage in any of the liners.
Fig 9.
Representative optical micrographs of the rim of (a) conventional-28 mm, (b) irradiated, Vitamin E-doped-28 mm and (c) irradiated, Vitamin E-doped-40 mm liners following 2 million cycles of simulated rim impingement. The depressed area is marked.
Discussion
The first hypothesis of this study; that a-tocopherol would not detrimentally affect the wear resistance of highly cross-linked UHMWPE was supported by the hip simulator data. The hip simulator wear rates of a-tocopherol doped, irradiated UHMWPE reported here are comparable to those reported for the highly cross-linked, irradiated and melted UHMWPE [2, 4].
Hip simulator testing is a well-accepted method of determining the wear behavior of acetabular liners. The testing commonly replicates the in vivo attributes such as component positioning, temperature, loading, and kinematics of normal gait [23-27], all which are known to affect the wear rate of UHMWPE acetabular liners. One common limitation of hip simulator testing is the choice of kinematics. Most tests utilize simulated normal gait kinematics. Yet the implants are subjected to more complex kinematics including many other activities of daily living. Another limitation is the choice of bovine serum as the lubricant, which is the analogue for synovial fluid. Some laboratories use undiluted bovine serum while others use dilute the bovine serum by adding deionized water. Wear rate of conventional polyethylene has been shown to increase with dilution of bovine serum [28]. However, at extreme dilutions, non-physiological wear mechanisms have been shown to dominate the wear process [29]. In the present study we used 100% bovine serum diluted only by a chelating agent and penicillin. This bovine serum preparation has been shown to produce clinically relevant wear rates and wear mechanisms for metal-on-polyethylene articulations in hip simulators [30, 31].
Surface analysis of conventional UHMWPE liners showed polishing and wearing away of the machining marks following the simulated gait wear test; in contrast, the a-tocopherol doped, irradiated UHMWPE liners showed the persistence of original machining marks throughout the five million cycles of testing, corroborating the improved wear rate determined gravimetrically. The acetabular shells in which the acetabular liners were mounted for hip simulator testing had a screw-hole at the dome. As a result, the acetabular liners were minimally loaded at the dome region because of the lacking support on the backside. During the initial stages of hip simulator testing, the dorsal aspects of the acetabular liners showed the persistence of machining marks in both the conventional UHMWPE liners as well as the a-tocopherol doped, irradiated UHMWPE liners. In the case of the conventional UHMWPE liners, wear was high enough to ultimately result in a higher load contact with the dorsal aspect of the liners and polish away the machining marks in the dorsal aspect (Fig 5). The a-tocopherol doped, irradiated UHMWPE liners maintained the machining marks at the dorsal aspect even after the five million cycles of testing (Fig 4).
The fibrils observed on conventional polyethylene liners under the scanning electron microscope following wear testing (Fig 7) have been proposed as precursors for particulate wear debris [32, 33]. The propensity of conventional UHMWPE to form fibrils due to its high plasticity is also reflected in its high elongation at break (Table 3). The fact that these fibrils were rare on the articular surfaces of a-tocopherol doped, irradiated UHMWPE liners indicated that the ability of this polymer to undergo large-scale deformation was reduced by cross-linking. This manifested itself as a decrease in the elongation at break of a-tocopherol doped, irradiated UHMWPE.
We used a two-week accelerated aging protocol under oxygen pressure at high temperature (70°C) to determine the relative oxidative resistance of a-tocopherol-doped, irradiated and conventional UHMWPE. We determined the mechanical properties of a-tocopherol, irradiated and conventional UHMWPEs before and after accelerated aging to compare the resistance of these properties to oxidative stress. The accelerated aging method that was used was not intended to simulate shelf aging or oxidation in vivo. Accelerated aging tests are useful in determining if a material will or will not oxidize in the long-term when exposed to oxygen. The extent and rate of oxidation in real-time cannot be predicted from accelerated aging methods.
The mechanical and fatigue strength of UHMWPE is a function of crystallinity and cross-link density. There is a decrease in mechanical and fatigue strength of irradiated and melted UHMWPEs, caused both by cross-linking and the loss of crystallinity during melting, which quenches the residual free radicals [5, 34]. We expected to avoid the loss of crystallinity by doping irradiated UHMWPE with a-tocopherol instead of melting it after irradiation. Crystallinity of 100-kGy irradiated and melted UHMWPE is typically lower than that of conventional UHMWPE [1]; the crystallinity of a-tocopherol doped, irradiated UHMWPE was not significantly different than that of conventional UHMWPE (p<0.001). Accelerated aging of conventional UHMWPE caused a significant increase in crystallinity (Table 2) consistent with oxidation. Because the residual free radicals caused by gamma sterilization had not been eliminated, this material would oxidize leading to chain scission and re-crystallization of shorter chains. The effects of oxidative degradation can be clearly seen in the decrease in the ultimate tensile strength (Table 3) of conventional UHMWPE following accelerated aging. The crystallinity of a-tocopherol-doped, irradiated UHMWPE was not significantly affected by accelerated aging (Table 2) because the residual free radicals in this irradiated UHMWPE had been stabilized by a-tocopherol, preventing oxidation. As a result, the mechanical properties of a-tocopherol-doped, irradiated UHMWPE were not adversely affected by accelerated aging.
Another aim of the present study was to determine if a-tocopherol doped, irradiated UHMWPE would have better strength than irradiated and melted UHMWPE, as hypothesized. The literature reported value for the ultimate tensile strength of 100 kGy irradiated and melted UHMWPE is approximately 35MPa [1]; the ultimate tensile strength of a-tocopherol doped, irradiated UHMWPE was measured to be 46 MPa. We attribute the improved strength of the latter to the retention of the crystallinity of the base material.
The fatigue strength of surgical grade UHMWPE is typically quantified by determining the resistance to fatigue crack propagation by cyclically loading a specimen designed to concentrate stresses at a crack tip. The stress range that needs to be applied to propagate the crack at a rate of 10−6 mm/cycle is reported as a measure of the resistance to crack propagation. The values for unirradiated or gamma-sterilized UHMWPE, which has high fatigue resistance, have been reported as 1.4-2.0 [13, 35]. In contrast, irradiated and melted highly cross-linked UHMWPEs had values of 0.55-0.69 [13, 35]. The ranges of these values were a result of testing environment (air or liquid), testing temperature and testing frequency. We compared the a-tocopherol doped, irradiated UHMWPE to irradiated and melted UHMWPE tested on our system in water at 40°C and the former showed significantly higher fatigue crack propagation resistance (Table 4), presumably due to its higher crystallinity.
A more clinically relevant device fatigue test involved the impingement of the rim of thin polyethylene liners by the neck of the femoral components (Fig 2), simulating the adverse case of vertical alignment of the shell and mal-positioning of the implant. As the increased wear-resistance of irradiated UHMWPEs has enabled the use of larger femoral heads, we tested the a-tocopherol-doped, irradiated liners with a 40-mm femoral head in comparison with 28-mm inner diameter group of conventional liners. Our hypothesis of improved fatigue strength was supported and our findings from the fatigue crack propagation test were corroborated because there were no fractures in any of the tested liners and no apparent differences between sterilized UHMWPE and vitamin E-stabilized, highly cross-linked UHMWPE.
In conclusion, irradiated, a-tocopherol-doped and terminally gamma sterilized highly cross-linked UHMWPE has high wear resistance and improved mechanical properties and fatigue crack propagation resistance than that of irradiated and melted first-generation highly cross-linked UHMWPE.
Acknowledgements
This work was funded in part by NIH RO1 AR051142 and by a research grant from Biomet Inc. We acknowledge with thanks the assistance of Dave Schroeder and Jordan Freedman in sample preparation and lengthy discussions.
This work was funded by NIH RO1 AR051142 and a research grant from Biomet, Inc..
References
- 1.Muratoglu OK, Bragdon CR, O'Connor DO, Jasty M, Harris WH, Gul R, McGarry F. Unified Wear Model for Highly Crosslinked Ultra-high Molecular Weight Polyethylenes (UHMWPE) Biomaterials. 1999;20(16):1463–1470. doi: 10.1016/s0142-9612(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 2.Muratoglu OK, Bragdon CR, O'Connor DO, Jasty M, Harris WH. 1999 HAP Paul Award. A novel method of crosslinking UHMWPE to improve wear, reduce oxidation and retain mechanical properties. J Arthroplasty. 2001;16(2):149–160. doi: 10.1054/arth.2001.20540. [DOI] [PubMed] [Google Scholar]
- 3.Muratoglu OK, O'Connor DO, Bragdon CR, Delaney J, Jasty M, Harris WH, Merrill EW, Venugopalan P. Gradient crosslinking of UHMWPE using irradiation in molten state for total joint arthroplasty. Biomaterials. 2001;23:717–724. doi: 10.1016/s0142-9612(01)00176-4. [DOI] [PubMed] [Google Scholar]
- 4.Muratoglu OK, Bragdon CR, O'Connor DO, Perinchief RS, Estok DM, Jasty M, Harris WH. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: A new concept. J Arthroplasty. 2001;16(8 suppl):24–30. doi: 10.1054/arth.2001.28376. [DOI] [PubMed] [Google Scholar]
- 5.Muratoglu OK, Merrill EW, Bragdon CR, O'Connor DO, Hoeffel D, Burroughs B, Jasty M, Harris WH. Effect of Radiation, Heat, and Aging on In Vitro Wear Resistance of Polyethylene. Clinical Orthopaedics & Related Research. 2003;417:253–262. doi: 10.1097/01.blo.0000093004.90435.d1. [DOI] [PubMed] [Google Scholar]
- 6.McKellop H, Shen F-W, Lu B, Campbell P, Salovey R. Development of an extremely wear resistant ultra-high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157–167. doi: 10.1002/jor.1100170203. [DOI] [PubMed] [Google Scholar]
- 7.Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop. 2004;(429):16. [PubMed] [Google Scholar]
- 8.Martell JM, Verner JJ, Incavo SJ. Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J Arthroplasty. 2003;18(7 Suppl 1):9. doi: 10.1016/s0883-5403(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 9.Charlesby A. Cross-linking of polythene by pile radiation. Proc. Roy. Soc. Lond. 1952;A215:187–215. [Google Scholar]
- 10.Bhateja S, Duerst R, Aus E, Andrews E. Free radicals trapped in polyethylene crystals. Journal of Macromolecular Science-Physics. 1995;B34(3):263–272. [Google Scholar]
- 11.Sutula L, Collier J, Saum K, Currier B, Currier J, Sanford W, Mayor M, Wooding R, Sperling D, Williams I, et al. The Otto Aufranc Award. Impact of gamma sterilization on clinical performance of polyethylene in the hip. Clin Orthop. 1995;(319):28–40. [PubMed] [Google Scholar]
- 12.Collier JP, Sperling DK, Currier JH, Sutula LC, Saum KA, Mayor MB. Impact of gamma sterilization on clinical performance of polyethylene in the knee. J. Arthroplasty. 1996;11(4):377–389. doi: 10.1016/s0883-5403(96)80026-x. [DOI] [PubMed] [Google Scholar]
- 13.Oral E, Wannomae KK, Hawkins NE, Harris WH, Muratoglu OK. α-Tocopherol Doped Irradiated UHMWPE for High Fatigue Resistance and Low Wear. Biomaterials. 2004;25(24):5515–5522. doi: 10.1016/j.biomaterials.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 14.Halley D, Glassman A, Crowninshield R. Recurrent dislocation after revision total hip replacement with a large prosthetic femoral head. J Bone and Joint Surg. 2004;86A(4):827–830. doi: 10.2106/00004623-200404000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53:1050S–1055S. doi: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 16.Packer L, Kagan VE. Vitamin E: The antioxidant harvesting center of membranes and lipoproteins. In: Packer L, Fuchs J, editors. Vitamin E in Health and Disease. Marcel Dekker, Inc.; New York: 1993. pp. 179–192. [Google Scholar]
- 17.Burton G, Ingold K. Autoxidation of Biological Molecules. 1. The Antioxidant Activity of Vitamin E and Related Chain-Breaking Phenolic Antioxidants in Vitro. J. Am. Chem. Soc. 1981;103:6472–6477. [Google Scholar]
- 18.Burton GW, Traber MG. Vitamin E: Antioxidant activity, biokinetics, and bioavailability. Annual Reviews in Nutrition. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- 19.Kamal-Eldin A, Appelqvist L. The Chemistry and Antioxidant Properties of Tocopherols and Tocotrienols. Lipids. 1996;31(7):671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 20.Parth M, Aust N, Lederer K. Studies on the effect of electron beam radiation on the molecular structure of ultra-high molecular weight polyethylene under the influence of alpha-tocopherol with respect to its application in medical implants. J Mater Sci-Mater Med. 2002;13(10):917–921. doi: 10.1023/a:1019892004830. [DOI] [PubMed] [Google Scholar]
- 21.Oral E, Greenbaum E, Malhi A, Muratoglu O. Characterization of blends of α-Tocopherol with UHMWPE. Biomaterials. 2005 doi: 10.1016/j.biomaterials.2005.04.026. (in print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scifert C, Brown T, Pedersen D, Callaghan J. A finite element analysis of factors influencing total hip dislocation. Clin Orthop Relat Res. 1998;355:152–162. doi: 10.1097/00003086-199810000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Paul JP. Forces transmitted by joints in the human body. Proc Instn Mech Engrs Pt 3F. 1966;8:181. [Google Scholar]
- 24.Johnston RC, Schmidt GL. Measurement of hip-joint motion during walking. J Bone Joint Surg(Am) 1969;51(A):1083. [PubMed] [Google Scholar]
- 25.Bergmann G, Graichen F, Rohlmann A. Hip Joint Loading During Walking and Running, Measured in Two Patients. J. Biomechanics. 1993;26(8):969–990. doi: 10.1016/0021-9290(93)90058-m. [DOI] [PubMed] [Google Scholar]
- 26.Ramamurti B, Bragdon C, O'Connor D, Lowenstein J, Jasty M, Estok D, Harris W. Loci of movement of selected points on the femoral head during normal gait. Three-dimensional computer simulation. J Arthroplasty. 1996;11:845–852. doi: 10.1016/s0883-5403(96)80185-9. [DOI] [PubMed] [Google Scholar]
- 27.Ramamurti BS, Estok DM, Jasty M, Harris WH. Analysis of the kinematics of different hip simulators used to study wear of candidate materials for the articulation of total hip arthroplasties. J Orthop Res. 1998;16(3):365–9. doi: 10.1002/jor.1100160313. [DOI] [PubMed] [Google Scholar]
- 28.Wang A, Essner A, Polineni V, Stark C, Dumbleton JH. Lubrication and wear of uhmwpe in total joint replacements. Tribol Int. 1998;31:17–33. [Google Scholar]
- 29.Liao Y-S, Benya P, McKellop H. Effect of Protein Lubrication on the Wear Properties of Materials for Prosthetic Joints. Journal of Biomedical Materials Research. 1999;48(no 4):465–473. doi: 10.1002/(sici)1097-4636(1999)48:4<465::aid-jbm10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 30.McKellop H, Clarke I, Markolf K, Amstutz H. Wear characteristics of UHMW polyethylene: a method for accurately measuring extremely low wear rates. J Biomed Mater Res. 1978;12:895–927. doi: 10.1002/jbm.820120611. [DOI] [PubMed] [Google Scholar]
- 31.Wang A. Comparison of the size and morphology of UHMWPE wear debris produced by a hip joint simulator under serum and water lubricated conditions. Biomaterials. 1996;17(9):865–871. doi: 10.1016/0142-9612(96)83281-9. [DOI] [PubMed] [Google Scholar]
- 32.Jasty MJ, Goetz DD, Lee KR, Hanson AE, Elder JR, Harris WH. Wear of polyethylene acetabular components in total hip arthroplasty. An analysis of 128 components retrieved at autopsy or revision operation. JBJS. 1997;79(A):349–358. [PubMed] [Google Scholar]
- 33.Edidin AA, Pruitt L, Jewett CW, Crane DJ, Roberts D, Kurtz SM. Plasticity-induced damage layer is a precursor to wear in radiation-cross-linked UHMWPE acetabular components for total hip replacement. Ultra-high-molecular-weight polyethylene. Journal of Arthroplasty. 1999;14(5):616–27. doi: 10.1016/s0883-5403(99)90086-4. [DOI] [PubMed] [Google Scholar]
- 34.Lyons B. Radiolytic unsaturation decay in polyethylene. Part II-the effect of irradaition temperature, thermal history and orientation. Radiation Physics and Chemistry. 2004;69(6):503–510. [Google Scholar]
- 35.Baker DA, Bellare A, Pruitt L. The effect of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res. 2003;66A:146–154. doi: 10.1002/jbm.a.10606. [DOI] [PubMed] [Google Scholar]