Abstract
Following pulmonary artery banding (PAB), the contractile function of right ventricle diminishes over time. Subsequently, the right atrium (RA) has to contract against a higher afterload, but it is unknown to what extent ventricular dysfunction has an effect on the atrial contractility. We hypothesized that right ventricular pressure overload may have an affect on atrial contractility and Ca2+ transport protein expression. Therefore, we induced pressure overload of the right ventricle by PAB for 10 wk in rabbits and examined the changes in the expression of Ca2+ transport proteins in the atrium. We demonstrate that PAB significantly decreased the expression of sarco(endo)plasmic reticulum Ca2+-ATPase (Serca) 2a while expression of Na+/Ca2+ exchanger-1 was significantly upregulated in the RA but not in the left atria of rabbit hearts, indicating that pressure is the major trigger. A decrease in Serca2a expression was concomitant with a significant decrease in sarcolipin (SLN), possibly indicating a compensatory role of SLN. The decreased expression of SLN was unable to completely restore sarcoplasmic reticulum Ca2+ uptake function of Serca2a. Functional contractile assessments in isolated trabeculae showed no difference between PAB- and sham-operated rabbits at 1 Hz but displayed an enhanced force development at higher frequencies and in the presence of isoproterenol, while twitch timing was unaffected. Our results indicate that right ventricular mechanical overload due to PAB affects the expression of the Ca2+-handling proteins in the RA in rabbits.
Keywords: calcium ion transport proteins, pressure overload, sarcoplasmic reticulum
cardiac hypertrophy is an adaptive response of the heart that occurs in response to a requirement for increased contractile power and pressure-volume work (12, 39). Most common among these is pressure overload hypertrophy wherein the ventricular wall is grossly thickened in the absence of chamber enlargement. It is associated with an increase in myocyte cross-sectional area relative to cell length, with parallel deposition of sarcomeres (30). It involves changes at the level of gene transcription, stimulation of the rate of protein synthesis, and increased assembly of myofibrils.
The sarcoplasmic reticulum (SR) is an important determinant of cardiac contractile and relaxation function in hypertrophied hearts by virtue of its ability to regulate intracellular free Ca2+ concentrations (1, 22, 24). The SR serves as a source for Ca2+ by releasing Ca2+ through a Ca2+ release channel, the ryanodine receptor (25). It also serves as a sink during muscle relaxation, which is brought about by SR Ca2+-ATPase (SERCA) at the cost of ATP (31). The majority of Ca2+ transported in the SR lumen is bound to the calsequestrin (CASQ), a high-capacity, moderate-affinity, Ca2+-binding protein. The heart muscle expresses primarily the cardiac/slow-twitch muscle isoform of SR Ca2+-ATPase (Serca2a). Serca2a activity is under inhibitory control of phospholamban (PLB), and phosphorylation of PLB relieves this inhibition (13, 29). Recent studies have shown that, in addition to PLB, sarcolipin (SLN) could also play an important role in the regulation of Serca pump activity (2–4). SLN has been shown to be expressed more abundantly in the atria compared with the ventricle (5). During muscle relaxation, some Ca2+ is also removed to extracellular milieu by the Na+/Ca2+ exchanger (NCX) to maintain cellular Ca2+ homeostasis (8). Therefore, it is evident that a precise control of Ca2+ cycling plays a key role to preserve normal contractile beat-to-beat activity of the cardiac myocyte; abnormal Ca2+ handling may significantly contribute to the contractile dysfunction.
Given that Ca2+-handling proteins play a major role in controlling intracellular Ca2+ levels, we hypothesized that the expression of these proteins may be altered in the right atrium (RA) following right ventricular pressure overload. We studied the rabbit because of their similarity to humans in cycling roughly 70% of the Ca2+ transient through the SR, and the rest via transmembrane processes (mainly L-type Ca2+ channel and NCX; see Ref. 8). Also, myofilament isoforms, such as the myosin heavy chain, are similar in human and rabbit, and at the molecular level, the rabbit atria and ventricle closely parallels that of the human heart (32). Results from the study demonstrate that expression of Serca2a was significantly downregulated while NCX-1 was significantly upregulated in the RA of rabbits subjected to pulmonary artery banding (PAB). The expression of SLN was significantly downregulated in the RA of PAB rabbits while expression of PLB and phosphorylation status of PLB both at Ser16 and Thr17 was unchanged. The decreased expression of Serca2a was concomitant with a significant decrease in the Ca2+ uptake function in the RA. Interestingly, despite these protein level changes, overall, no loss of contractile function was observed, and the PAB atrial muscles displayed an enhancement of function at high frequency and in the presence of isoproterenol.
MATERIALS AND METHODS
Pressure overload by PAB.
All surgical procedures were completed in accordance with the Institutional Animal Care and Use Committee and National Institutes of Health guidelines. Right ventricular hypertrophy was induced by PAB in the rabbit. This model has previously been used by several groups (9, 37) and has been established in the laboratory. In line with previous reports, we also find that pressure overload by PAB induces right ventricular hypertrophy in these rabbits (K. D. Varian, A. Kijtawornrat, S. C. Gupta, C. A. A. Torres, M. M. Monasky, N. Hiranandani, D. A. Delfin, J. A. Rafael-Fortney, M. Perisamy, R. L. Hamlin, and P. M. L. Janssen, unpublished observations). To establish PAB, male New Zealand White rabbits (2–3 mo old, ∼2 kg wt) were premedicated with acepromazine (1.25 mg/kg) administered subcutaneously ∼30 min before anesthetizing with isoflurane (rate of 2.5–5.0%) as needed. Animals received 100% oxygen (rate of 400–600 ml/min) through a loose-fitting mask. Rabbits were placed in dorsal recumbency, and surgical anesthesia was confirmed by the absence of the pedal reflex. Chloramphenicol (30 mg/kg) was administered subcutaneously before surgery. After opening of the thorax, the pulmonary artery was constricted to 3.2 mm (outer diameter) using a sterile tube as a gauge at the origin of the vessel. Ligatures were performed using monofilament polypropylene suture. The muscle layers and the skin were sutured closed, and, after recovery, rabbits were given a postoperative dose of buprenorphine (0.01 mg/kg) intramuscularly. Chloramphenicol (30 mg/kg) was given subcutaneously 12 h from the first preoperative dose and again the following morning. The weights and body temperature of each rabbit were monitored and recorded 7 days postoperatively. Sham-operated rabbits were treated and handled identically, with the sole omission of placing the band around the pulmonary artery. The rabbits were kept 10 wk postsurgery (no rabbits died postoperatively) and killed, the hearts were harvested, and tissue was frozen immediately for protein expression analysis.
Western blots analysis.
The atrial tissues from both sham-operated and PAB rabbits were dissected in a Krebs-Henseleit solution and quickly frozen in liquid nitrogen. The total tissue homogenate was prepared, and Western blot analysis was carried out as described earlier (5). Briefly, tissue homogenates containing equal amount of proteins were resolved by performing SDS-PAGE on different gel concentrations [10% for Seca2a and CASQ, 9% for NCX-1, 15% for atrial natriuretic peptide (ANP), 12% for troponin I (TnI), 14% for PLB, Ser16 phospho-PLB and Thr17 phospho-PLB] and transferred to a nitrocellulose membrane. To detect SLN expression, tissue homogenates were electrophoretically separated on a 16% tricine gel and transferred to a nitrocellulose membrane. Membranes were probed with the following primary antibodies: Serca2a, SLN, and PLB (Zymed), CASQ and NCX-1 (Affinity Bioreagents), ANP (Santa Cruz Biotechnology), TnI (Fitzgerald Industries International), Ser16PPLB and Thr17PPLB (Badriella) followed by horseradish peroxidase-conjugated secondary antibody. Signals were detected by Super Signal West Dura substrate (Pierce) and quantified by densitometry.
Ca2+ uptake assay.
The RA from both PAB and sham-operated rabbits were used for Ca2+ uptake assays using Millipore filtration technique as described previously (16, 20). The tissues were homogenized in 8 vol of protein extraction buffer containing (in mM) 10 imidazole (pH 7.0), 300 sucrose, 10 NaF, and 1 EDTA. The tissue homogenates (150 μg) from atria were incubated at 37°C in a 1.5-ml Ca2+ uptake medium containing (in mM) 40 imidazole (pH 7.0), 100 KCl, 5 MgCl2, 5 NaN3, 5 potassium oxalate, 0.5 EGTA, and various concentrations of CaCl2 (1 μCi/μmol 45Ca2+) to yield 0.03–3 μM free Ca2+ as determined by the computer program described earlier (20). To obtain the maximal stimulation of SR Ca2+ uptake by inhibiting the SR Ca2+ release channel, 1 μM ruthenium red was added immediately before the addition of the substrates to begin the Ca2+ uptake. The reaction was initiated by the addition of 5 mM ATP and terminated after 1 min by filtration. The rate of Ca2+ uptake and the Ca2+ concentration required for half-maximal velocity of Ca2+ uptake (pCa50) were determined by nonlinear curve-fitting analysis using Graph Pad PRISM 4.0 software.
Muscle contractility.
After the heart was rapidly excised, ultra thin trabeculas (dimensions: 120–150 μm wide, 70–90 μm thick, and 2/3.5 mm long) were dissected from the RA of both PAB and sham-operated rabbits. Muscles of this size were chosen to avoid core hypoxia that will be present in muscles greater than ∼150 μm thick (34). The muscles were dissected in a Krebs-Henseleit solution containing (in mM) 137 NaCl, 5 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 20 NaHCO3, 10 glucose, and 0.25 CaCl2. Additionally, 20 mM 2,3-butanedione monoxime (BDM) was added to this solution to minimize cutting damage and to arrest the heart. Exposure to BDM for a short time has been shown to be reversible (28). Muscles were mounted in the setup as previously described (44, 45) and stimulated at 1 Hz, which is slightly below their physiological resting rates, while perfused with an oxygenated Krebs-Henseleit solution, now without BDM and containing 2 mM Ca2+. The muscles were stretched until an increase in passive (diastolic) force was no longer accompanied by a substantial increase in developed force. The muscles were allowed time to equilibrate at 37°C (∼20 min, or until twitches were stable). Previous studies have shown this length (i.e., optimal length) to correspond to a sarcomere length of ∼2.2 μm, which approximates the end-diastolic sarcomere length in the in vivo beating heart (35). Twitch amplitude, time-to-peak tension, and relaxation times were recorded under steady-state conditions. In addition to baseline contractility, we assessed the effect of different frequencies of stimulation, from 1 to 4 Hz by stimulating the muscle at these frequencies until forces had stabilized. Furthermore, we assessed the contractile response to β-adrenergic stimulation by performing a dose-response curve using isoproterenol (1 nM-1 μM).
Statistics.
All the data shown are means ± SE. Comparison between the groups was performed by unpaired Student's t-test. A two-tailed value of P < 0.05 was considered statistically significant.
RESULTS
The increased pressure gradient in the ventricle ultimately resulted in atrial hypertrophy. We weighed the hearts and right atria and found that, in PAB heart, the right atrial weight was significantly greater than in sham rabbits (0.50 ± 0.03 vs. 0.35 ± 0.04 g, PAB, n = 15 vs. sham, n = 10, P < 0.01). Even when calculated as a fraction of total heart weight, this difference persisted; in PAB rabbits, the right atria accounted for 5.1 ± 0.2% of the total heart weight vs. only 3.9 ± 0.3% in the sham group (P < 0.01).
Expression of Ca2+-handling proteins in the atrial tissues of PAB- and sham-operated rabbits.
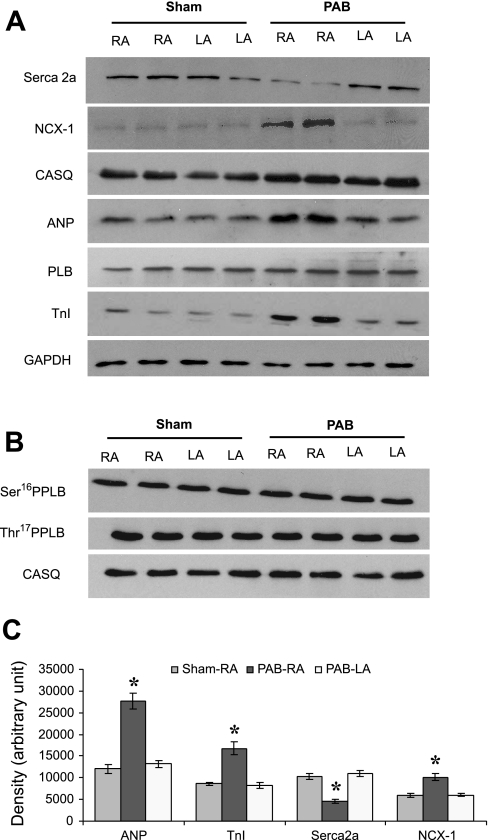
To determine the expression of Ca2+-handling proteins, total protein prepared from atrial tissues of both PAB- and sham-operated rabbits was analyzed by Western blot analysis. Because expression of ANP has been reported to be increased by pressure overload (46), we first choose to examine the expression of ANP. Expression of ANP was significantly induced in the RA but not in the left atria (LA) of PAB rabbits (Fig. 1, A and C). A significant upregulation of ANP protein was observed in the RA of PAB rabbits compared with RA of sham-operated rabbits (2.3-fold induction) (Fig. 1C). Like expression of ANP, TnI, a thin filament protein, was significantly induced in the RA of PAB rabbits compared with RA of sham-operated rabbits (Fig. 1, A and C).
Fig. 1.
Quantitation of sarcoplasmic reticulum (SR) Ca2+-handling proteins and Na+/Ca2+ exchanger (NCX)-1 in the atrial tissues of sham-operated and pulmonary artery banding (PAB) rabbits. A total of 12 (A) or 20 (B) μg of homogenate were loaded in each well. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and calsequestrin (CASQ) were used as loading controls. C: expression of sarco(endo)plasmic reticulum Ca2+-ATPase (Serca) 2a was significantly decreased, whereas expression of atrial natriuretic peptide (ANP), troponin I (TnI), and NCX-1 proteins was significantly increased in the right atrium of PAB rabbits (PAB-RA) compared with right atrium of sham-operated rabbits (Sham-RA). Values given are means ± SE from 4 independent rabbits. *Values significantly different compared with sham-RA. RA, right atrium; LA, left atrium; PLB, phospholamban.
We next examined the effect of PAB on the expression of Serca2a and NCX-1 proteins. The expression of Serca2a was significantly decreased (55% reduction) in the RA of PAB rabbits when compared with RA of sham-operated rabbits (Fig. 1, A and C). Serca2a levels remained unchanged in the LA. A comparison of expression of NCX-1 protein revealed a significant upregulation (1.7-fold) in the RA but remained unchanged in the LA of PAB rabbit hearts (Fig. 1, A and C).
Expression of PLB was unaffected in the RA of both sham-operated and PAB rabbits (Fig. 1A). Western blotting with phoshospecific antibodies showed that the basal phosphorylation of PLB at Ser16 and Thr17 was comparable between PAB-RA and sham-operated RA (Fig. 1B). The expression of CASQ was not much different between sham-operated and PAB RA samples (Fig. 1A).
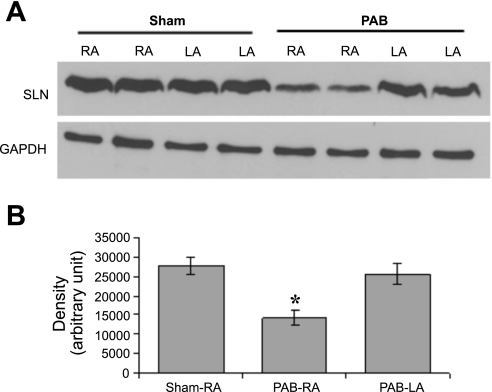
We next choose to examine the expression of SLN in the atrial tissues of both PAB- and sham-operated rabbits using a rabbit polyclonal antibody (SLN-CTAb) (5). An abundance of SLN was observed in the rabbit atrial tissues that was significantly downregulated in the RA of PAB (32% reduction) compared with RA of sham-operated rabbits (Fig. 2, A and B).
Fig. 2.
A: representative Western blot of sarcolipin (SLN) protein in the atrial tissues of sham-operated and PAB rabbits. B: densitometric analysis of SLN protein between the right atrial tissues of sham-operated and PAB rabbits. The expression of SLN protein was significantly decreased in the RA of PAB rabbits compared with RA of sham-operated rabbits. Values given are means ± SE from 4 independent rabbits. *Values significantly different compared with sham-RA.
PAB decreases the rate of Ca2+ uptake in the RA of rabbit heart.
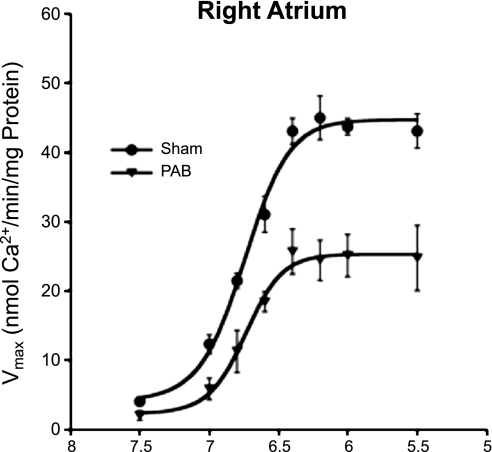
To determine whether PAB affected the SR Ca2+ transport function, we measured rates of SR Ca2+ uptake in total homogenates from RA of PAB- and sham-operated rabbits. The Ca2+ uptake was significantly decreased in the RA of PAB rabbits compared with RA of sham-operated rabbits (Fig. 3). The maximum velocity was significantly decreased in the RA of PAB rabbits compared with RA of sham-operated rabbits (sham = 41.1 ± 1.6 nmol Ca2+·min−1·mg protein−1; PAB = 24.0 ± 2.4 nmol Ca2+·min−1·mg protein−1; P < 0.05). On the other hand, there was a nonsignificant change (P > 0.05) in the pCa50 value (−log [Ca2+]) in the RA of PAB rabbits compared with that observed in RA of sham-operated rabbits (sham = 6.75 ± 0.02; PAB = 6.74 ± 0.09).
Fig. 3.
Ca2+ uptake in the right atrial tissues of sham-operated and PAB rabbits. Ca2+ uptake assays were performed using total atrial homogenates from 3 independent rabbits.
Contractile performance of isolated trabeculas.
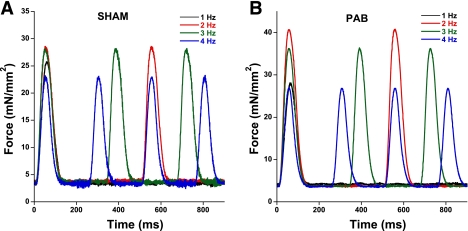
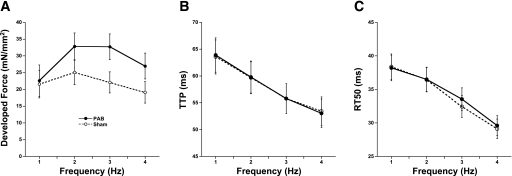
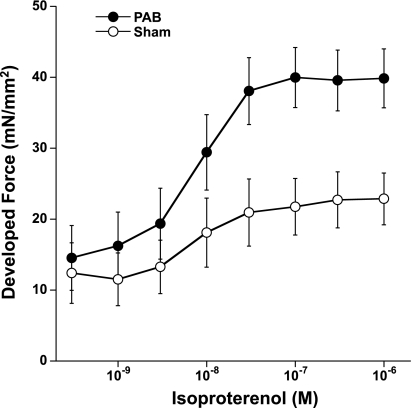
Trabeculas isolated from the RA of both sham-operated and PAB rabbits showed that, per cross-sectional area of muscle tissue, the developed forces at a stimulation frequency of 1 Hz at body temperature are similar (22.6 ± 4.7 vs. 21.5 ± 4.1 mN/mm2, PAB vs. sham, n = 9/group). However, the PAB muscles showed a somewhat enhanced frequency-dependent activation of contraction and were significant at 3 and 4 Hz (Fig. 4). Although average force development at different frequencies was somewhat enhanced in PAB muscles (Fig. 5A), twitch kinetics, assessed as time from stimulation to peak tension and time from peak tension to 50% force decline, were not different between PAB and sham muscles (Fig. 5, B and C). The response to isoproterenol was enhanced in PAB atrial muscles, whereas the EC50 for isoproterenol was unchanged (Fig. 6).
Fig. 4.
Raw recordings of a single sham trabecula (A) and a single trabecula from a PAB heart (B). In both groups, as can be seen from the representative traces, diastolic force remained completely unaltered, even at the highest frequency, indicating no relaxation disorders.
Fig. 5.
Contractile performance of atrial trabeculas from PAB and sham rabbits. A: active developed force at 1–4 Hz shows near-equal forces at 1 Hz and a significantly higher force at 3 and 4 Hz. B: time-to-peak tension (TTP) was abbreviated with frequency equally in PAB and sham trabeculas. C: relaxation, assessed as time from peak tension to 50% relaxation (RT50) became faster with frequency; no differences were observed between the two groups.
Fig. 6.
Isoproterenol response was significantly enhanced in PAB trabeculas, since maximal developed force was significantly higher at high concentrations of isoproterenol. No differences were found in the EC50.
DISCUSSION
The present study demonstrated that pressure overload due to PAB has an adverse effect on the expression of Ca2+-handling proteins in the RA of the rabbit hearts. To our knowledge, this is the first report showing that the PAB affects the expression of Ca2+-handling proteins in the RA of rabbits. The overall results observed in the present study may be summarized as 1) a significant downregulation of Serca2a protein, 2) a significant upregulation of NCX-1, TnI, and ANP proteins, 3) a significant downregulation of SLN, 4) a significant decrease in the Ca2+ uptake function in the RA of PAB rabbits, but 5) no apparent loss of contractile function, and unchanged twitch kinetics.
Previous studies have shown that the level of expression of Serca mRNA and the rate of SR Ca2+ uptake (26) are diminished in pressure overload hearts. Conversely, there are reports showing that these parameters were enhanced (11) or unchanged with pressure overload (47). Importantly, these studies were focused on the molecular and functional changes in ventricular chamber, and knowledge about changes in the atria following pressure overload is lacking. Therefore, it is important to examine whether the observed changes are seen only in the affected cardiac chamber or uniformly throughout the heart. The major finding of this study is that changes in protein expression are chamber specific, since PAB selectively affected the expression of Ca2+-handling proteins only in the RA and not in the LA. This finding may have important clinical relevance, since right ventricular pressure overload over time may affect right atrial gene expression and can contribute to muscle dysfunction. Our finding also suggests that changes in protein expression are directly related to mechanical stress because such changes were not observed in the LV (K. D. Varian et al., unpublished observation). Interestingly, our data suggest that changes in protein expression can be seen even before the onset of severe muscle dysfunction can be observed.
We found that the expression of Serca2a was decreased in the RA of PAB rabbits. Previous studies have demonstrated that mRNA levels of Serca2a in the atria are decreased by mechanical stress (33, 38), which is consistent with data from many studies investigating the change in Secra2a transcripts in the pressure or volume-overloaded ventricles (10, 23, 26, 42). Also, Bauer et al. (7) demonstrated that, in the pig ventricle, in a similar model, mRNA levels of Serca decrease and mRNA levels of atrial natriuretic factor (ANF) increase. Our findings add to these observations that the pressure overload on the ventricle causes a stress on the atrium, resulting in similar molecular-level changes. In addition, we assessed the changes at the protein level, rather than the mRNA level, where changes often do not reflect the quantitative alterations in actual protein levels. The role of SLN expression in PAB animals is less understood. Recently, Shimura et al. (41) showed that SLN mRNA was downregulated in the atrial myocardium of mice due to pressure overload. Currently, there are no protein data available to validate the importance of SLN in PAB rabbits. Our observations now indicate a downregulation of SLN protein in the RA of PAB rabbits. Both SLN and its homolog PLB are regulatory proteins that inhibit SR Ca2+-ATPase, resulting in decreased cardiac relaxation (15). Dephosphorylated PLB is an inhibitor of Serca activity, and phosphorylation relieves this inhibition (43). This inhibition has been suggested to involve direct protein-protein interaction followed by conformational changes in the SR Ca2+-ATPase, resulting in a decrease in the affinity of the pump for Ca2+ (21). Because SLN is abundant in the atria, Serca2a/SLN stoichiometry may be an important determinant of atrial functions. The significant downregulation in the expression of SLN as observed in the atria may be a compensatory response to a decrease in Serca2a levels. However, the depressed SLN did not completely restore Ca2+ uptake by Serca2a, since a significant decrease in the maximum velocity of Ca2+ uptake was still observed in the PAB rabbits. Because PLB is the major component of ventricle, an insignificant change observed in the level of PLB and phospho-PLB in the atria of PAB rabbit is not unlikely. SLN has a conserved threonine (Thr5) residue at the NH2-terminus that can be phosphorylated by serine/threonine kinases such as calmodulin kinase II (6). Because of nonavailability of a specific antibody, we could not detect the level of phospho-SLN in PAB rabbit. Future studies may be directed to assess the changes in the SLN phosphorylation, if altered by pressure overload.
Despite the lower levels of Serca, time-to-peak tension and time to 50% relaxation were not significantly changed. The upregulated levels of NCX may thus potentially be sufficient to ensure adequate Ca2+ removal from the cytosol. This is in line with a finding by Hasenfuss et al. (18), who observed that, in failing human hearts where NCX was upregulated, diastolic function was better preserved. Little is known regarding the impact of Serca expression levels on the force-frequency relationship in atrial tissue. An elegant study by Schotten et al. (40) showed that, in patients with atrial fibrillation, the positive force-frequency relationship was preserved. Although they did not observe a decrease in Serca levels, in close agreement with our work, they show a robust upregulation of NCX, resulting in an increase in the NCX-to-Serca ratio. Thus, both in patients [Schotten et al. (40)] and in rabbits in diseased states, the atria (unlike the ventricles) are able to maintain a positive force-frequency relationship.
Despite the changes on the molecular level, no functional changes in developed force were observed in isolated trabeculae. It is, however, known that, particularly at resting levels, contractile function is compensated to maintain normal levels of contractile developed force, and this observation is in agreement with several human isolated muscle studies at resting rates (0.5 or 1 Hz), in which twitch developed force under conditions close to those used in our studies (1 Hz and 37°C) are not significantly depressed (17, 19, 36), and have similar time-to-peak tension and half-relaxation in failing vs. nonfailing myocardium. More surprisingly was that, at faster pacing rates, PAB muscles continued to outperform sham muscles, a finding in significant contrast to ventricular failing tissue (17, 19, 36). Both at higher pacing rates (but still within the physiological range of the animal) and in the presence of isoproterenol, force production was enhanced in PAB atrial muscles. Thus the compensation that is present in ventricular tissue at low rates, at least in this stage of the atrial myocardium, seems to be likewise preserved at higher pacing rates, and the enhanced contractility may stem from this compensatory mechanism. Two recent studies show that, even when the Serca is completely blocked in isolated muscles, the rabbit continues to display a positive force frequency behavior (27) and that complete pharmacological knock out of Serca in working rabbit hearts preserves ventricular contractile function for the largest part (14). Thus a change in Serca expression may not necessarily lead in a large alteration of contractile behavior in the rabbit. If SR load, and subsequent release, is decreased, it could be that the inactivation of the L-type Ca2+ channel is less effective, leading to a greater influx of Ca2+, potentially aiding in contraction. The upregulated levels of NCX observed may, in this atrial tissue at this stage of compensatory hypertrophy, be sufficient to maintain diastolic function. Furthermore, the higher levels of NCX may promote increased reverse mode during high pacing, potentially contributing to the contractile effects observed. In ventricular myocardium, this compensatory mechanism is incompletely understood as well, and future studies aimed in this direction are needed to further address this observation, as well as what modulated the increase isoproterenol effect in this stage of compensatory hypertrophy in the atria.
In conclusion, the present study demonstrates that PAB has the ability to alter the expression of Ca2+-handling proteins of rabbit atrium. Because pressure overload may be a milestone in the pathophysiological progression of heart failure, knowledge of the levels of proteins involved in SR function is important to understand the pathophysiology of the failing heart and to develop new therapeutic strategies for the treatment of heart failure. The findings showing that SLN expression is diminished in the RA of PAB rabbits suggest that SLN may be a regulator of the Serca2a. However, future studies are needed to further identify the regulatory mechanisms that control expression of these molecules in PAB heart, as well as the compensatory mechanisms that preserve contractile function in light of the protein level changes observed at this stage of the model.
GRANTS
This research was partially supported by National Heart, Lung, and Blood Institute Grants HL-073816 and HL-083957 (to P. M. L. Janssen) and HL64140 and HL088555 (to M. Periasamy) and by Established Investigator Award 0740040N from the American Heart Association (P. M. L. Janssen).
Acknowledgments
We thank Anusak Kijtawornrat and Annemarie Hoffman for technical assistance.
REFERENCES
- 1.Arai M, Matsui H, Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res 74: 555–564, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Asahi M, Kurzydlowski K, Tada M, MacLennan DH. Sarcolipin inhibits polymerization of phospholamban to induce superinhibition of sarco(endo)plasmic reticulum Ca2+-ATPases (SERCAs). J Biol Chem 277: 26725–26728, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Asahi M, Otsu K, Nakayama H, Hikoso S, Takeda T, Gramolini AO, Trivieri MG, Oudit GY, Morita T, Kusakari Y, Hirano S, Hongo K, Hirotani S, Yamaguchi O, Peterson A, Backx PH, Kurihara S, Hori M, MacLennan DH. Cardiac-specific overexpression of sarcolipin inhibits sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2a) activity and impairs cardiac function in mice. Proc Natl Acad Sci USA 101: 9199–9204, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babu GJ, Zheng Z, Natarajan P, Wheeler D, Janssen PM, Periasamy M. Overexpression of sarcolipin decreases myocyte contractility and calcium transient. Cardiovas Res 65: 177–186, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol 43: 215–222, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, Periasamy M. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci USA 104: 17867–17872, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer EP, Kuki S, Zimmermann R, Schaper W. Upregulated and downregulated transcription of myocardial genes after pulmonary artery banding in pigs. Ann Thorac Surg 66: 527–531, 1998. [DOI] [PubMed] [Google Scholar]
- 8.Bers DM Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Bishop JE, Rhodes S, Laurent GJ, Low RB, Stirewalt WS. Increased collagen synthesis and decreased collagen degradation in right ventricular hypertrophy induced by pressure overload. Cardiovasc Res 28: 1581–1585, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Calderone A, Takahashi N, Izzo NJ Jr, Thaik CM, Colucci WS. Pressure- and volume-induced left ventricular hypertrophies are associated with distinct myocyte phenotypes and differential induction of peptide growth factor mRNAs. Circulation 92: 2385–2390, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho BM, Bassani RA, Franchini KG, Bassani JW. Enhanced calcium mobilization in rat ventricular myocytes during the onset of pressure overload-induced hypertrophy. Am J Physiol Heart Circ Physiol 291: H1803–H1813, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Chien KR, Zhu H, Knowlton KU, Miller-Hance W, van Bilsen M, O'Brien TX, Evans SM. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol 55: 77–95, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Edes I, Kranias EG. Regulation of cardiac sarcoplasmic reticulum function by phospholamban. Membr Biochem 7: 175–192, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Elliott EBA, Kelly A, Rankin A, Smith GL, Loughrey CM. A quantitative assessment of selective pharmacological inhibition of Serca in isolated rabbit working hearts. Biophys J 96: 515a, 2009. [Google Scholar]
- 15.Gramolini AO, Kislinger T, Asahi M, Li W, Emili A, MacLennan DH. Sarcolipin retention in the endoplasmic reticulum depends on its C-terminal RSYQY sequence and its interaction with sarco(endo)plasmic Ca2+-ATPases. Proc Natl Acad Sci USA 101: 16807–16812, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene AL, Lalli MJ, Ji Y, Babu GJ, Grupp I, Sussman M, Periasamy M. Overexpression of SERCA2b in the heart leads to an increase in sarcoplasmic reticulum calcium transport function and increased cardiac contractility. J Biol Chem 275: 24722–24727, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res 75: 434–442, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+-Ca2+-exchanger protein levels and diastolic function of failing human myocardium. Circulation 99: 641–648, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Holubarsch C, Lüdemann J, Wiessner S, Ruf T, Schulte-Baukloh H, Schmidt-Schweda S, Pieske B, Posival H, Just H. Shortening versus isometric contractions in isolated human failing and non-failing left ventricular myocardium: dependency of external work and force on muscle length, heart rate and inotropic stimulation. Cardiovasc Res 37: 46–57, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Ji Y, Loukianov E, Periasamy M. Analysis of sarcoplasmic reticulum Ca2+ transport and Ca2+-ATPase enzymatic properties using mouse cardiac tissue homogenates. Anal Biochem 269: 236–244, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Kim HW, Steenaart NAE, Ferguson DG, Kranias EG. Functional reconstitution of the cardiac sarcoplasmic reticulum Ca2+-ATPase with phospholamban in phospholipid vesicles. J Biol Chem 265: 1702–1709, 1990. [PubMed] [Google Scholar]
- 22.Laver DR Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Clin Exp Pharmacol Physiol 34: 889–896, 2007. [DOI] [PubMed] [Google Scholar]
- 23.LekanneDeprez RH, van den Hoff MJ, de Boer PA, Ruijter PM, Maas AA, Chamuleau RA, Lamers WH, Moorman AF. Changing patterns of gene expression in the pulmonary trunk-banded rat heart. J Mol Cell Cardiol 30: 1877–1888, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Lompre AM, Anger M, Levitsky D. Sarco(endo)plasmic reticulum calcium pumps in the cardiovascular system: Function and gene expression. J Mol Cell Cardiol 26: 1109–1121, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Lukyanenko V, Ziman A, Lukyanenko A, Salnikov V, Lederer WJ. Functional groups of ryanodine receptors in rat ventricular cells. J Physiol 583: 251–269, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui H, MacLennan DH, Alpert NR, Periasamy M. Sarcoplasmic reticulum gene expression in pressure overload-induced cardiac hypertrophy in rabbit. Am J Physiol Cell Physiol 268: C252–C258, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Monasky MM, Janssen PM. The positive force-frequency relationship is maintained in absence of sarcoplasmic reticulum function in rabbit, but not in rat myocardium. J Comp Physiol [B] 179: 469–477, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Mulieri LA, Hasenfuss G, Ittleman F, Blanchard EM, Alpert NR. Protection of human left ventricular myocardium from cutting injury with 2,3-butanedione monoxime. Circ Res 65: 1441–1449, 1989. [DOI] [PubMed] [Google Scholar]
- 29.Münch G, Bölck B, Karczewski P, Schwinger RH. Evidence for calcineurin-mediated regulation of SERCA 2a activity in human myocardium. J Mol Cell Cardiol 34: 321–334, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodeling. Lancet 367: 356–367, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Ottenheijm CA, Fong C, Vangheluwe P, Wuytack F, Babu GJ, Periasamy M, Witt CC, Labeit S, Granzier H. Sarcoplasmic reticulum calcium uptake and speed of relaxation are depressed in nebulin-free skeletal muscle. FASEB J 22: 2912–2919, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel R, Nagueh SF, Tsybouleva N, Abdellatif M, Lutucuta S, Kopelen HA, Quinones MA, Zoghbi WA, Entman ML, Roberts R, Marian AJ. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation 104: 317–324, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavlovic M, Schaller A, Pfammatter JP, Carrel T, Berdat P, Gallati S. Age-dependent suppression of SERCA2a mRNA in pediatric atrial myocardium. Biochem Biophys Res Commun 326: 344–348, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Raman S, Kelley MA, Janssen PML. Effect of muscle dimensions on trabecular contractile performance under physiological conditions. Eur J Physiol 451: 625–630, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez EK, Hunter WC, Royce MJ, Leppo MK, Douglas AS, Weisman HF. A method to reconstruct myocardial sarcomere lengths and orientations at transmural sites in beating canine hearts. Am J Physiol Heart Circ Physiol 263: H293–H306, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Rossman EI, Petre RE, Chaudhary KW, Piacentino 3rd V, Janssen PML, Gaughan JP, Houser SR, Margulies KB. Abnormal frequency-dependent responses represent the pathophysiologic signature of contractile failure in human myocardium. J Mol Cell Cardiol 36: 33–42, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Rouleau JL, Kapuku G, Pelletier S, Gosselin H, Adam A, Gagnon C, Lambert C, Meloche S. Cardioprotective effects of ramipril and losartan in right ventricular pressure overload in the rabbit: importance of kinins and influence on angiotensin II type 1 receptor signaling pathway. Circulation 104: 939–944, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Sadamatsu K, Urabe Y, Tsutsui H, Tagawa H, Maruoka F, Igarashi-Saito K, Takeda K, Kawachi Y, Yasui H, Takeshita A. Sarcoplasmic reticulum Ca2+ regulatory protein gene expression in human right atrium under hemodynamic overload. Heart Vessels 14: 208–215, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 446: 444–448, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Schotten U, Greiser M, Benke D, Buerkel K, Ehrenteidt B, Stellbrink C, Vazquez-Jimenez JF, Schoendube F, Hanrath P, Allessie M. Atrial fibrillation-induced atrial contractile dysfunction: a tachycardiomyopathy of a different sort. Cardiovasc Res 53: 192–201, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Shimura M, Minamisawa S, Yokoyama U, Umemura S, Ishikawa Y. Mechanical stress-dependent transcriptional regulation of sarcolipin gene in the rodent atrium. Biochem Biophys Res Comm 334: 861–866, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Takizawa T, Arai M, Yoguchi A, Tomaru K, Kurabayashi M, Nagai R. Transcription of the SERCA2 gene is decreased in pressure-overloaded hearts: a study using in vivo direct gene transfer into living myocardium. J Mol Cell Cardiol 31: 2167–2174, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Traaseth NJ, Ha KN, Verardi R, Shi L, Buffy JJ, Masterson LR, Veglia G. Structural and dynamic basis of phospholamban and sarcolipin inhibition of Ca2+-ATPase. Biochemistry 47: 3–13, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varian KD, Raman S, Janssen PML. Measurement of myofilament calcium sensitivity at physiological temperature in intact cardiac trabeculae. Am J Physiol Heart Circ Physiol 290: H2092–H2097, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Varian KD, Janssen PML. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol 292: H2212–H2219, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Witt H, Schubert C, Jaekel J, Fliegner D, Penkalla A, Tiemann K, Stypmann J, Roepcke S, Brokat S, Mahmoodzadeh S, Brozova E, Davidson MM, Ruiz Noppinger P, Grohé C, Regitz-Zagrosek V. Sex-specific pathways in early cardiac response to pressure overload in mice. J Mol Med 86: 1013–1024, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yue P, Long CS, Austin R, Chang KC, Simpson PC, Massie BM. Post-infarction heart failure in the rat is associated with distinct alterations in cardiac myocyte molecular phenotype. J Mol Cell Cardiol 30: 1615–1630, 1998. [DOI] [PubMed] [Google Scholar]