Abstract
Chronic hypoxia during pregnancy has profound effects on uterine artery (UA) contractility and attenuates uterine blood flow. The present study tested the hypothesis that chronic hypoxia inhibits the pregnancy-induced reduction in pressure-dependent myogenic tone of resistance-sized UAs. UAs were isolated from nonpregnant ewes (NPUAs) and near-term pregnant ewes (PUAs) that had been maintained at sea level (∼300 m) or at high altitude (3,801 m) for 110 days. In normoxic animals, the pressure-dependent myogenic response was significantly attenuated in PUAs compared with NPUAs. Hypoxia significantly increased myogenic tone in PUAs and abolished its difference between PUAs and NPUAs. Consistently, there was a significant increase in PKC-mediated baseline Ca2+ sensitivity of PUAs in hypoxic animals. Hypoxia significantly increased phorbol 12,13-dibutyrate (PDBu)-induced contractions in PUAs but not in NPUAs. Whereas the inhibition of ERK1/2 by PD-98059 potentiated PDBu-mediated contractions of PUAs in normoxic animals, it failed to do so in hypoxic animals. Hypoxia decreased ERK1/2 expression in PUAs. PDBu induced membrane translocation of PKC-α and PKC-ɛ. Whereas there were no significant differences in PKC-α translocation among all groups, the translocation of PKC-ɛ was significantly enhanced in NPUAs compared with PUAs in normoxic animals, and hypoxia significantly increased PKC-ɛ translocation in PUAs. In the presence of PD-98059, there were no significant differences in PDBu-induced PKC-ɛ translocation among all groups. Treatment of PUAs isolated from normoxic animals with 10.5% O2 for 48 h ex vivo significantly increased PDBu-induced contractions and eliminated its difference between PUAs and NPUAs. The results suggest that hypoxia upregulates pressure-dependent myogenic tone through its direct effect in suppressing ERK1/2 activity and increasing the PKC signal pathway, leading to an increase in the Ca2+ sensitivity of the myogenic mechanism in the UA during pregnancy.
Keywords: hypoxia, protein kinase C
during pregnancy, the development of uteroplacental circulation with decreased vascular tone accommodates a >30-fold increase in uterine blood flow in term pregnant sheep and humans, which helps to ensure normal fetal development. Chronic hypoxia during pregnancy has profound effects on uterine artery (UA) contractility and attenuates the pregnancy-induced increase in uterine blood flow, which is associated with an increased risk of preeclampsia and fetal intrauterine growth restriction (16, 19, 33, 46, 54, 55). The adaptation of UA contraction and relaxation mechanisms to pregnancy is complex and poorly understood. In addition to growth and remodeling of the uterine vasculature, the decreased UA resistance is accomplished by both increased endothelial nitric oxide synthesis/release and decreased vascular contractility and myogenic response. Our recent study (47) in sheep has demonstrated that the pressure-induced myogenic response is significantly less and the distensibility of the UA is greater in pregnant ewes. Similar findings have been demonstrated in pregnant mice and rats (10, 28, 29, 41). The physiological importance of the myogenic response in the regulation of uterine blood flow in human pregnancy has been demonstrated in myometrial arteries in term pregnant women (20, 21). Given that pressure-dependent myogenic contraction is an important physiological mechanism that regulates basal vascular tone and is a major contributor to the modulation of organ blood flow, the decreased myogenic tone of the UA is likely to contribute significantly to the adaptation of uterine vascular hemodynamics in pregnancy.
Myogenic activity is an intrinsic property of vascular smooth muscle in response to pressure or stretch. It is modulated by paracrine or endocrine substances but does not require the nerves or endothelium to occur. Among other mechanisms, numerous studies (5, 22, 30, 32) have demonstrated an important role of PKC in the regulation of the arterial myogenic response. We (47, 48, 51, 53) have recently demonstrated that PKC plays a key role in the regulation of myogenic tone of resistance-sized UAs, and the reduced myogenic tone in the pregnant UA is primarily mediated by a decrease in the PKC signaling pathway. Consistent with our findings, it has been shown that pregnancy is associated with attenuated arterial PKC activity (6, 7, 17, 18, 25). In addition, we (47, 49, 50, 51, 53) have demonstrated that ERK1/2 functions as an upstream signal in the suppression of PKC activity in pregnant UAs. The activation of ERK1/2 depends on the dual phosphorylation on Tyr185 and Thr187 by MEK (1). In ovine UAs, the ERK1/2 inhibitor PD-98059 inhibited the phosphorylation and activation of ERK1/2 (50, 51, 53). The inhibition of ERK1/2 increased PKC-mediated contractions and myogenic tone in pregnant UAs (47, 53), suggesting a physiological mechanism of ERK1/2 in the increased uterine blood flow by suppressing the basal vascular tone during pregnancy.
It is unknown whether and to what extent chronic hypoxia modulates the adaptation of myogenic mechanisms of the UA during pregnancy. In the present study, we investigated the effects of chronic hypoxia on pressure-dependent myogenic tone of resistance-sized UAs obtained from nonpregnant and near-term pregnant ewes with normoxic control or high-altitude (3,801 m) hypoxic (arterial Po2: 60 mmHg) treatment for 110 days. We hypothesized that chronic hypoxia inhibited the pregnancy-mediated attenuation of the myogenic response in UAs, which was due to a suppression in ERK1/2 activity and an increase in the PKC signal pathway, leading to an increase in the Ca2+ sensitivity of the myogenic mechanism. To address whether chronic hypoxia has direct effects on the ERK/PKC pathway in the regulation of the UA myogenic response and its adaptation to pregnancy, experiments were also performed in isolated UAs treated ex vivo with prolonged hypoxia.
METHODS
Tissue preparation.
As previously described (52), nonpregnant and time-dated pregnant sheep were obtained from the Nebeker Ranch in Lancaster, CA (altitude: ∼300 m, arterial Po2: 102 ± 2 mmHg). UAs were obtained from nonpregnant and near-term (∼140 days of gestation) pregnant sheep. For chronic hypoxic treatment, nonpregnant and pregnant (30 days of gestation) animals were transported to the Barcroft Laboratory, White Mountain Research Station, in Bishop, CA (altitude, 3,801 m, maternal arterial Po2: 60 ± 2 mmHg) and maintained there for ∼110 days. Animals were transported to the laboratory immediately before the experiments. Ewes were anesthetized with thiamylal (10 mg/kg) administered via the external left jugular vein. Animals were then intubated, and anesthesia was maintained on 1.5–2.0% halothane in oxygen throughout surgery. An incision in the abdomen was made, and the uterus was exposed. The UAs were isolated, removed without being stretched, and placed into cold physiological salt solution (PSS) containing (in mM) 130 NaCl, 10.0 HEPES, 6.0 glucose, 4.0 KCl, 4.0 NaHCO3, 1.80 CaCl2, 1.2 MgSO4, 1.18 KH2PO4, and 0.025 EDTA (pH 7.4). After removal of the tissues, animals were killed with T-61 (euthanasia solution, Hoechst-Roussel, Somerville, NJ). All procedures and protocols used in the present study were approved by the Animal Research Committee of Loma Linda University and followed guidelines put forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Measurement of myogenic tone.
Resistance-sized UA segments (∼150 μm in diameter) were dissected and cannulated in an organ chamber (Living Systems, Burlington, VT) followed by placement on the stage of an inverted microscope. The proximal cannula was connected to a pressure transducer and reservoir of PSS, and the intraluminal pressure was controlled by a servo system to set transmural pressures. The distal cannula was connected to a luer-lock valve that was opened to flush the lumen during the initial cannulation. After cannulation, the valve was closed, and all measurements were conducted under no-flow conditions. Arterial diameter was recorded using the SoftEdge Acquisition Subsystem (IonOptix, Milton, MA), as previously described (47). After being mounted, vessels were equilibrated in PSS for 10 min at an intraluminal pressure of 20 mmHg followed by a pressure increase from 20 to 70 mmHg and a return to 20 mmHg immediately. Vessels were then allowed to equilibrate at 20 mmHg in the presence of the nitric oxide synthase inhibitor NG-nitro-l-arginine (l-NNA; 100 μM) for 30 min. After the equilibration period, the pressure was increased in a stepwise manner from 20 to 100 mmHg in 20-mmHg increments, and each pressure was maintained for 3 min to allow the vessel diameter to stabilize before measurements. The passive pressure-diameter relationship was conducted in Ca2+-free PSS containing 3 mM EGTA to determine the maximum passive diameter. The following formula was used to calculate the percent myogenic tone at each pressure step: percent myogenic tone = (D1 − D2)/D1 × 100, where D1 is the passive diameter in Ca2+-free PSS (0 mM Ca2+ with 3 mM EGTA) and D2 is the active diameter with normal PSS in the presence of extracellular Ca2+.
Contraction experiments.
The fourth branches of main UAs were separated from the surrounding tissue and cut into 2-mm ring segments. Isometric tension was measured in PSS in a tissue bath at 37°C, as previously described (53). After 60 min of equilibration, each ring was stretched to the optimal resting tension as determined by the tension developed in response to 120 mM KCl added at each stretch level. Tissues were pretreated with either the ERK1/2 inhibitor PD-98059 (30 μM, Sigma, St. Louis, MO) or vehicle (DMSO) for 30 min followed by stimulations with the PKC activator phorbol 12,13-dibutyrate (PDBu; Sigma) in the presence of l-NNA. Concentration-response curves of PDBu were obtained by the cumulative addition of the agonist in approximate one-half log increments. To investigate the direct effect of hypoxia, some arterial rings obtained from normoxic nonpregnant and pregnant ewes were incubated in a given culture dish with 5 ml of complete DMEM (Mediatech Cellgro) containing 1% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Tissues were incubated at 37°C in a humidified incubator with either 21% or 10.5% O2 for 48 h before they were subjected to contraction experiments. EC50 values for the agonist in each experiment were taken as the molar concentration at which the contraction-response curve intersected 50% of the maximum response and were expressed as pD2 (−log EC50) values. Responses were normalized to the maximal KCl contraction.
Measurement of baseline Ca2+ sensitivity.
Two main Ca2+ buffer solutions were used in the present study, as previously described (48). One solution was zero Ca2+ relaxing solution, which contained (in mM) 110 K-acetate, 5 EGTA, 5 ATP, 6 Mg-acetate, 1 DTT, 0.01 leupeptin, 20 imidazole, and 20 HEPES at pH 6.8 (titrated with KOH). The other solution contained 1 mM Ca2+ in addition to the same components in the zero Ca2+ buffer. As described in a previous study (48), Ca2+ buffer solutions were prepared by solving the multiequilibrium equations for interactions among the different ions, and solutions containing intermediate free Ca2+ concentrations were prepared by mixing the appropriate amounts of zero Ca2+ relaxing solution and maximum Ca2+ buffer solution and then titrated to pH 7.0 with 1 M KOH. Arterial rings were attached to isometric force transducers and bathed in PSS at 37°C. After 60 min of equilibration in the tissue bath, each ring was stretched to the optimal resting tension, as determined by the tension developed in response to 120 mM KCl added at each stretch level. Arterial rings were then placed into the zero Ca2+ relaxing solution and permeabilized as previously described (48). Briefly, chemical permeabilization was achieved by adding 40 μM β-escin to the relaxing solution and allowing the arteries to incubate for 20 min at 25°C. The permeabilized solution was then replaced with the relaxing solution containing 1 μM A-23187 to deplete Ca2+ from the sarcoplasmic reticulum. Concentration-response curves to Ca2+ were obtained by cumulative increases of Ca2+ concentrations in approximate one-half log increments in β-escin-permeabilized arterial rings in the presence or absence of the PKC activator PDBu.
Immunoblot analysis.
The protein abundance of ERK1/2 and phospho-ERK1/2 was determined as previously described (53). Briefly, tissues were homogenized in lysis buffer containing 150 mM NaCl, 50 mM Tris·HCl, 10 mM EDTA, 0.1% Tween 20, 0.1% β-mercaptoethanol, 0.1 mM PMSF, 5 μg/ml leupeptin, and 5 μg/ml aprotinin (pH 7.4). Sample homogenates were then centrifuged at 4°C for 5 min at 6,000 g, and the supernatants were collected. Samples with equal protein were loaded onto a 10% polyacrylamide gel with 0.1% SDS and separated by electrophoresis. Proteins were then transferred onto Immobilon P membranes and incubated with ERK1/2 and phospho-ERK1/2 antibodies (New England Biolabs, Beverly, MA). After an incubation with horseradish peroxidase-conjugated secondary antibody (Amersham, Arlington Heights, IL), immunoreactive bands were visualized by enhanced chemiluminescence. Blots were exposed to Hyperfilm and analyzed with Kodak 1D image analysis software. Data were normalized by an internal standard loaded in the same membrane within each group.
Measurement of PKC isozyme translocation.
To determine PDBu-induced translocation of PKC isozymes from the cytosolic and particulate fractions in UAs, arterial rings were equilibrated in the tissue bath, and the optimal tension was obtained as described above. Tissues were then incubated for 20 min with PD-98059 (30 μM) or vehicle alone in the organ bath at 37°C. After incubation, they were stimulated with 3 μM PDBu for 10 min. At the end of treatment, tissues were snap frozen in liquid N2, and the cytosolic and particulate fractions were prepared as previously described (56). Briefly, tissues were homogenized in ice-cold homogenization buffer A containing 20 mM Tris·HCl, 250 mM sucrose, 5 mM EDTA, 5 mM EGTA, 10 mM β-mercaptoethanol, 1 mM benzamidine, 1 mM PMSF, 50 μM leupetin, 1 mM DTT, and 2 μg/ml aprotinin (pH 7.5). Homogenates were centrifuged at 100,000 g for 20 min at 4°C, and supernatants were collected and used as the cytosolic fraction. Pellets were then resuspended in homogenization buffer A containing 1% Triton X-100 by stirring overnight at 4°C, diluted with buffer A to a final concentration of 0.2% Triton X-100, and then centrifuged at 100,000 g for 20 min at 4°C. Supernatants were then collected and referred to as the particulate fraction. Protein concentrations were determined with a protein assay kit (Bio-Rad). Protein samples (5 μg) of particulate fractions were subjected to electrophoresis on a 7.5% SDS-polyacrylamide gel and then transferred electrophoretically to nitrocellulose membranes. Membranes were incubated at room temperature for 1 h in Tris-buffered saline solution containing 5% dried milk and 0.5% Tween 20 followed by an incubation with primary antibodies for PKC-α and PKC-ɛ (Santa Cruz Biotechnology, Santa Cruz, CA), respectively, overnight at 4°C and secondary antibody for 1 h at room temperature. To confirm the phosphorylation of PKC-α and PKC-ɛ in PDBu-induced membrane translocation, some membranes were incubated with phospho-PKC-α and phospho-PKC-ɛ antibodies (Upstate, Lake Placid, NY). Bands were detected with enhanced chemiluminsecence, visualized on Hyperfilm, and analyzed with Kodak 1D image analysis software. To normalize the loading variation of each sample, the corresponding actin level presented in each sample was determined using monoclonal anti-actin as the primary antibody (Santa Cruz Biotechnology).
Data analysis.
Concentration-response curves were analyzed by computer-assisted nonlinear regression to fit the data using GraphPad Prism (GraphPad Software, San Diego, CA). Results are expressed as means ± SE obtained from the number of experimental animals given (n). Differences were evaluated for statistical significance (P < 0.05) by two-way ANOVA followed by the Newman-Keuls post hoc test.
RESULTS
Effect of long-term high-altitude hypoxia on myogenic tone in pressurized UAs.
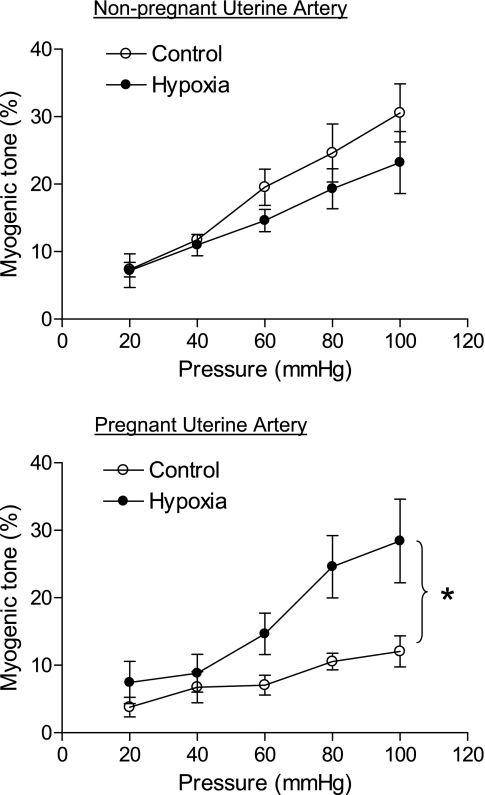
In the presence of Ca2+-containing PSS, UAs from all four groups of sheep (normoxic nonpregnant UAs, normoxic pregnant UAs, hypoxic nonpregnant UAs, and hypoxic pregnant UAs) developed myogenic tone in response to stepwise increases of intraluminal pressure (Fig. 1). In normoxic animals, pressure-dependent myogenic tone was significantly less in pregnant compared with nonpregnant UAs. Long-term high-altitude hypoxia resulted in a significant increase in the myogenic response in the UA of pregnant ewes but not in the UA of nonpregnant ewes (Fig. 1). Unlike normoxic animals, there were no significant differences in the myogenic response of UAs between nonpregnant and pregnant ewes in long-term high-altitude hypoxic animals.
Fig. 1.
Effect of high-altitude hypoxia on pressure-dependent myogenic tone in uterine arteries (UAs). Pressure-dependent myogenic tone was determined in UAs obtained from nonpregnant and pregnant ewes with normoxia (control) and long-term high-altitude hypoxia treatment. Myogenic tone was calculated as the percent difference between passive and active diameter at given pressures, as described in methods. Data are expressed as means ± SE of tissues from 4–8 animals/group. *P < 0.05, significant difference between control and hypoxic groups, as determined by repeated-measures two-way ANOVA.
Effect of long-term high-altitude hypoxia on PKC-mediated contractions in UAs.
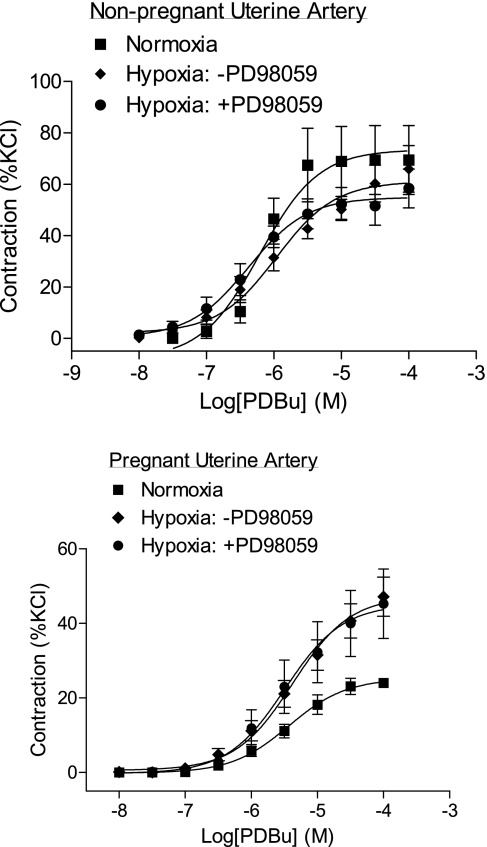
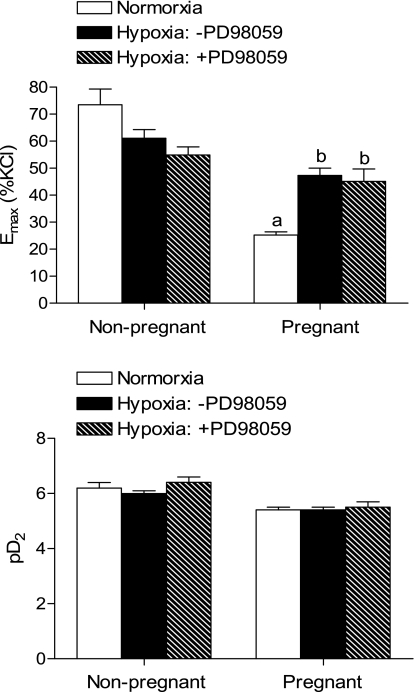
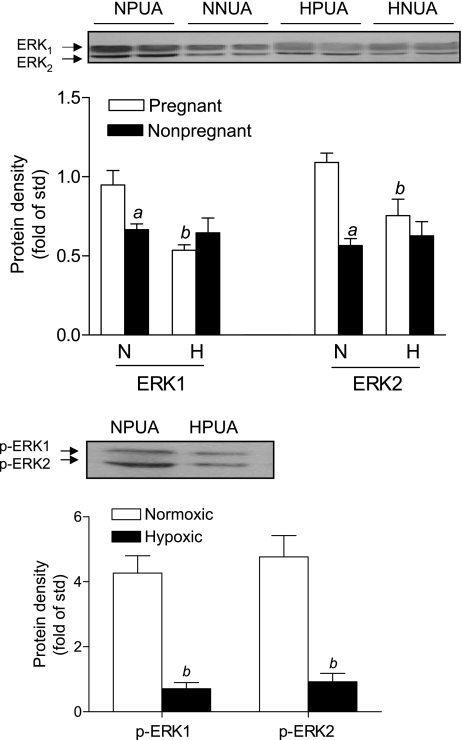
Given that PKC plays a key role in the regulation of the pressure-dependent myogenic response in UAs, and that reduced myogenic tone in pregnant UAs is primarily mediated by a decrease in the PKC signaling pathway (47), we determined the effect of hypoxia on PDBu-induced contractions. Consistent with our previous study (53), PDBu-induced contractions of UAs were significantly decreased in pregnant compared with nonpregnant ewes in normoxic animals (Figs. 2 and 3). Long-term high-altitude hypoxia did not alter pD2 values in either nonpregnant or pregnant UAs but significantly increased the maximal response of PDBu-induced contractions of UAs in pregnant but not nonpregnant ewes (Figs. 2 and 3). As we reported in a previous study (53) in normoxic animals, PD-98059 had no effect on PDBu-induced contractions in nonpregnant UAs but significantly increased contractions in pregnant UAs. In long-term high-altitude hypoxic animals, PD-98059 showed no significant effects on PDBu-induced contractions of UAs in either pregnant or nonpregnant ewes (Figs. 2 and 3). Furthermore, in hypoxic animals, there were no significant differences in PDBu-induced maximal responses in UAs between pregnant and nonpregnant ewes in the absence or presence of PD-98059 (Fig. 3). Consistent with these findings, chronic hypoxia caused a significant downregulation of ERK1/2 expression in UAs of pregnant but not nonpregnant ewes (Fig. 4, top). The decreased ERK1/2 expression in UAs of hypoxic pregnant animals was associated with a significant reduction in ERK1/2 activity and reduced phospho-ERK1/2 levels (Fig. 4, bottom).
Fig. 2.
Effect of high-altitude hypoxia on phorbol 12,13-dibutyrate (PDBu)-induced contractions in UAs. Cumulative concentration-response curves to PDBu were determined in UAs obtained from nonpregnant and pregnant ewes with normoxia and long-term high-altitude hypoxia treatment in the absence or presence of PD-98059 (PD; 30 μM, pretreatment for 20 min). Data are expressed as percentages of contraction generated by 120 mM KCl, and each point represents the mean ± SE of tissues from 4–10 animals.
Fig. 3.
Effect of high-altitude hypoxia on the maximal response (Emax) and potency (pD2) of PDBu-induced contractions in UAs. Emax and pD2 values were determined from the PDBu concentration-response curves shown in Fig. 2 by computer-assisted nonlinear regression to fit the data using GraphPad Prism. Data are expressed as means ± SE of tissues from 4–10 animals. aP < 0.05, pregnant vs. nonpregnant animals; bP < 0.05, hypoxia vs. normoxia.
Fig. 4.
Effect of high-altitude hypoxia on ERK1/2 and phosphorylated (p-)ERK1/2 protein abundance in UAs. ERK1/2 and p-ERK1/2 protein abundance were determined in UAs obtained from normoxic nonpregnant ewes (NNUAs), normoxic pregnant ewes (NPUAs), high-altitude hypoxic nonpregnant ewes (HNUAs), and high-altitude hypoxic pregnant ewes (HPUAs). N, normoxia; H, hypoxia. Data are means ± SE of tissues from 5–6 animals. aP < 0.05, nonpregnant vs. pregnant animals; bP < 0.05, hypoxic vs. normoxic.
Effect of long-term high-altitude hypoxia on PKC-mediated baseline Ca2+ sensitivity.
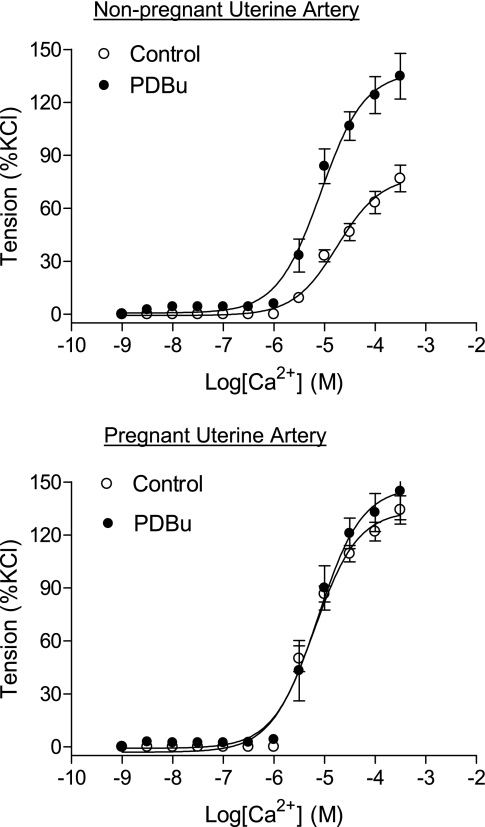
Our previous study (48) in normoxic animals demonstrated that pregnancy attenuated PKC-mediated baseline Ca2+ sensitivity in the UA. Figure 5 shows that cumulative increases of Ca2+ produced concentration-dependent contractions of permeabilized UAs from both nonpregnant and pregnant ewes in long-term high-altitude hypoxic animals. In the absence of PDBu, both pD2 values (4.7 ± 0.1 vs. 5.2 ± 0.1, P < 0.05) and maximal responses (78.2 ± 3.4 vs. 133.8 ± 3.2% of the KCl maximum, P < 0.05) of Ca2+-induced contractions were significantly increased in UAs from pregnant compared with nonpregnant ewes. In the presence of PDBu, there were significant increases in pD2 values (4.7 ± 0.1 vs. 5.0 ± 0.1, P < 0.05) and maximal responses (78.2 ± 3.4 vs. 137.3 ± 5.3% of the KCl maximum, P < 0.05) of Ca2+-induced contractions of UAs in nonpregnant ewes but not in pregnant ewes (pD2: 5.2 ± 0.1 vs. 5.1 ± 0.1, P > 0.05; maximal response: 133.8 ± 3.2 vs. 147.3 ± 6.4% of the KCl maximum, P > 0.05). PDBu abolished the difference in Ca2+-induced contractions of UAs between nonpregnant and pregnant ewes in long-term high-altitude hypoxic animals.
Fig. 5.
Effect of PKC activation on the Ca2+-force relation in β-escin-permeabilized UAs in high-altitude hypoxic animals. Concentration-response curves of Ca2+-induced contractions were determined in β-escin-permeabilized UAs obtained from nonpregnant and pregnant long-term high-altitude hypoxic animals in the absence (control) or presence of PDBu (3 μM, pretreatment for 20 min). Data are expressed as percentages of maximal KCl-induced contractions of the same tissue before permeabilization to normalize the tissue size and are means ± SE of tissues from 5–14 animals. pD2 and Emax values are presented in results.
Effect of long-term high-altitude hypoxia on PDBu-induced translocation of PKC isozymes.
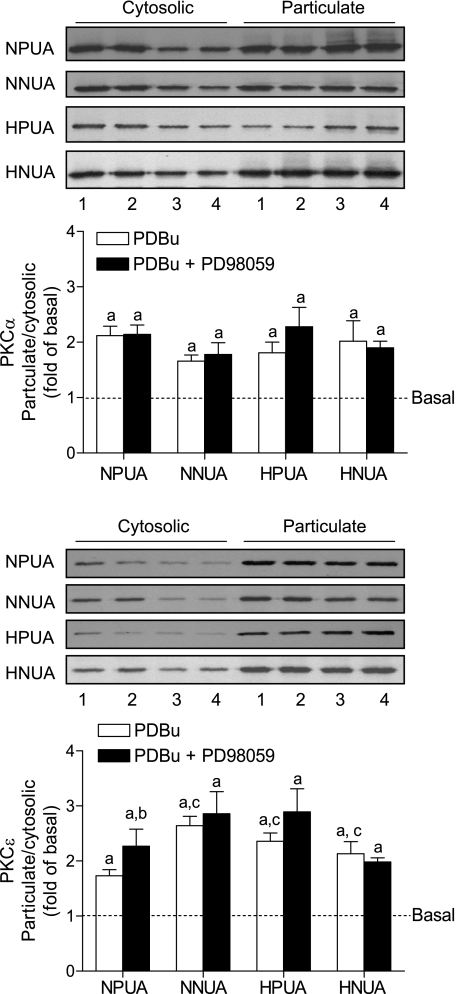
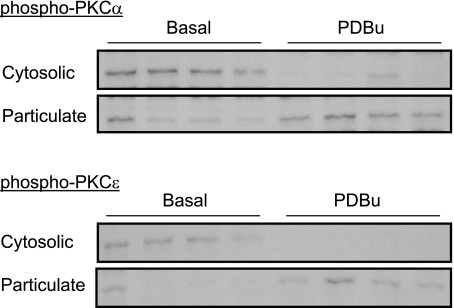
PDBu significantly increased the levels of PKC-α in membrane particulate fractions, suggesting that PDBu induced translocation and activation of PKC-α in UAs (Fig. 6). However, these PDBu-induced translocations of PKC-α in UAs were not significantly different among all four groups and were not significantly affected by PD-98059 (Fig. 6). Unlike PKC-α, in normoxic animals, PDBu-induced translocations of PKC-ɛ were significantly decreased in UAs of pregnant ewes compared with nonpregnant animals (Fig. 6). PD-98059 significantly increased PDBu-induced translocations of PKC-ɛ in pregnant but not nonpregnant UAs of normoxic ewes. In the presence of PD-98059, there were no significant differences in PDBu-induced translocations of PKC-ɛ between nonpregnant and pregnant UAs (Fig. 6). Long-term high-altitude hypoxia significantly increased PDBu-induced translocations of PKC-ɛ in UAs of pregnant ewes (Fig. 6). PD-98059 had no significant effect on PDBu-induced translocations of PKC-ɛ in either pregnant or nonpregnant UAs in hypoxic animals (Fig. 6). Whereas PKC translocation from cytosolic to membrane particulate fractions generally has been considered the hallmark of activation and frequently has been used as a surrogate measure of PKC isoform activation in cells (37), phosphorylation of PKC-α and PKC-ɛ was confirmed in the PDBu-induced translocation in UAs (Fig. 7).
Fig. 6.
Effect of high-altitude hypoxia on PDBu-induced membrane translocation of PKC isozymes in UAs. PDBu-induced membrane translocations of PKC-α and PKC-ɛ were determined in NNUAs, NPUAs, HNUAs, and HPUAs in the absence or presence of PD-98059 (30 μM, pretreatment for 20 min). PBDu-induced increases in the ratio of the particulate to cytosolic distribution of PKC-α and PKC-ɛ are expressed as fold changes of basal levels of each isozyme blotted in the same membrane. Lane 1, basal; lane 2, PD-98059; lane 3, PDBu; lane 4, PDBu + PD-98059. Data are means ± SE of tissues from 5–6 animals. aP < 0.05, PDBu vs. basal; bP < 0.05, PDBu + PD98059 vs. PDBu alone; cP < 0.05, all groups vs. NPUAs.
Fig. 7.
PDBu-induced membrane translocation of p-PKC-α and p-PKC-ɛ in UAs. PDBu-induced membrane translocations of p-PKC-α and p-PKC-ɛ were determined in UAs obtained from four pregnant ewes.
Direct effect of chronic hypoxia on PKC-mediated contractions in UAs.
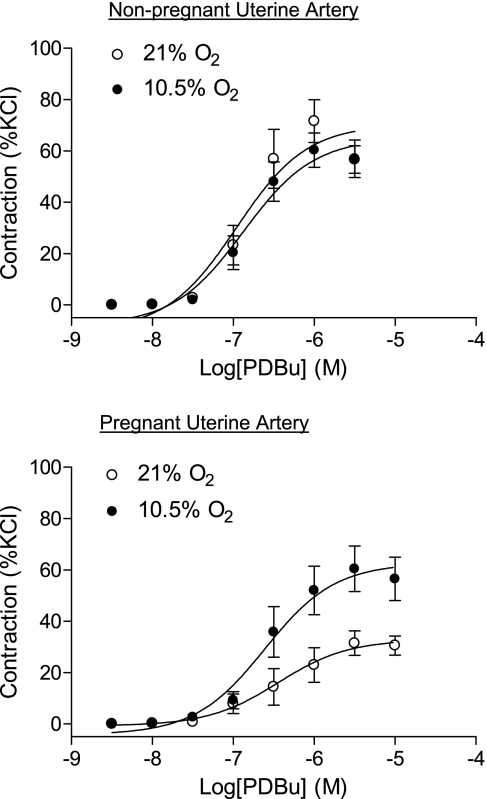
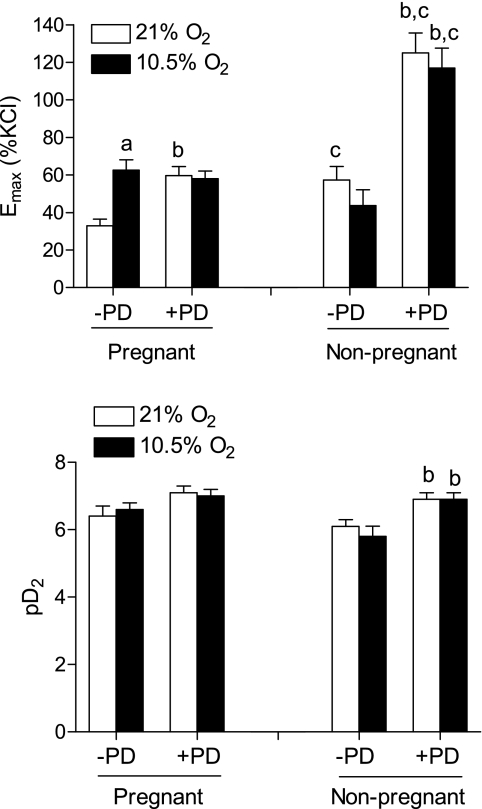
To determine the direct effect of hypoxia on contractility, UAs isolated from nonpregnant and pregnant ewes of normoxic animals were treated ex vivo with either 21% or 10.5% O2 for 48 h before they were subjected to contraction experiments. The hypoxic treatment of 10.5% O2 for 48 h had no significant effect on KCl-induced contractions in either nonpregnant (10.4 ± 0.9 vs. 11.0 ± 0.8 g/mm2, P > 0.05) or pregnant (10.7 ± 0.7 vs. 11.4 ± 0.6 g/mm2, P > 0.05) UAs. In contrast, PDBu-induced contractions were significantly increased by the hypoxic treatment in UAs of pregnant ewes (Figs. 8 and 9). PD-98059 significantly increased PDBu-induced contractions of pregnant UAs (Fig. 9). In the presence of PD-98059, hypoxia had no further effect on the PDBu-mediated contraction of UAs of pregnant ewes, which was not significantly different from that of the hypoxic treatment in the absence of PD-98059 (Fig. 9). Whereas PDBu-mediated contractions were significantly increased in UAs of nonpregnant ewes compared with those of pregnant animals, hypoxia did not affect PDBu-mediated contractions in nonpregnant UAs in the absence or presence of PD-98059 (Figs. 8 and 9).
Fig. 8.
Effect of ex vivo hypoxia on PDBu-induced contractions in UAs. UAs isolated from normoxic nonpregnant and pregnant ewes were incubated at 37°C in a humidified incubator with either 21% or 10.5% O2 for 48 h before they were subjected to contractions induced by increased concentrations of PDBu. Data are expressed as percentages of contraction generated by 120 mM KCl, and each point represents the mean ± SE of tissues from 7–12 animals.
Fig. 9.
Effect of ex vivo hypoxia on the Emax and pD2 of PDBu-induced contractions in UAs. UAs isolated from normoxic nonpregnant and pregnant ewes were incubated at 37°C in a humidified incubator with either 21% or 10.5% O2 for 48 h before they were subjected to contractions induced by increased concentrations of PDBu in the absence or presence of PD-98059 (30 μM, pretreatment for 20 min). Emax and pD2 values were determined from PDBu concentration-response curves by computer-assisted nonlinear regression to fit the data using GraphPad Prism. Data are expressed as means ± SE of tissues from 7–12 animals. aP < 0.05, 10.5% vs. 21% O2; bP < 0.05, with (+) PD-98059 vs. without (−) PD-98059; cP < 0.05, nonpregnant vs. pregnant animals.
DISCUSSION
The following several key observations were made in the present study: 1) long-term high-altitude hypoxia during pregnancy increases the pressure-dependent myogenic tone of resistance-sized UAs and eliminates the difference in the myogenic response between nonpregnant and pregnant UAs, 2) high-altitude hypoxia potentiates PKC-mediated contractions of pregnant UAs and abolishes the difference in PKC-mediated contractions between nonpregnant and pregnant UAs in the absence or presence of ERK1/2 inhibition with PD-98059, 3) chronic hypoxia caused a decrease in ERK1/2 and phospho-ERK1/2 protein abundance in pregnant UAs, 4) high-altitude hypoxia results in an increase in PKC-mediated baseline Ca2+ sensitivity in UAs of pregnant ewes, 5) high-altitude hypoxia inhibits ERK1/2-mediated downregulation of PKC-ɛ in UAs of pregnant ewes, and 6) chronic hypoxia has a direct effect in the upregulation of PKC-induced contractions in pregnant UAs by removing the ERK1/2-mediated inhibition.
Our previous study (47) in sheep has demonstrated that pregnancy is associated with a significant decrease in pressure-induced myogenic tone in the UA. Similar findings of a pregnancy-mediated decrease in the vascular myogenic response have been obtained in mice and rats (10, 28, 29, 41). In the present study, we found that long-term high-altitude hypoxia during pregnancy increased the pressure-dependent myogenic response in UAs of pregnant ewes and abolished the differences in myogenic tone in UAs between pregnant and nonpregnant animals. To our knowledge, this is the first study showing that chronic hypoxia increases myogenic contractions in a systemic vessel. This finding is somewhat surprising given previous findings showing that hypoxia suppressed vasoconstrictions of systemic vessels but increased contractions of pulmonary arteries. Indeed, our previous studies (13, 14) have demonstrated that long-term high-altitude hypoxia decreased α1-adrenoceptor-mediaded contractions of UAs in pregnant ewes by suppressing the agonist-mediated inositol 1,4,5-trisphosphate pathway. These findings suggest a complex effect of chronic hypoxia in differentiating an agonist-mediated response and myogenic contractions in UAs. Consistent with the present finding of increased pressure-dependent myogenic tone of UAs, a previous study (4) has demonstrated that chronic hypoxia significantly increases the myogenic response in pulmonary arteries. Additionally, maternal diet restriction has been shown to increase the myogenic tone of the UA in pregnant rats (40). In contrast, a study (27) in guinea pigs showed an absence of the pressure-dependent myogenic response in the UA, which was not affected by either pregnancy or chronic hypoxia. The lack of a pressure-dependent myogenic response in this latter study was probably due to the use of a relatively large conduit vessel of the main UA with an inner diameter of 434–684 μm (27). We and others (4) have shown that pressure-dependent myogenicity is not observed in larger vessels (>300 μm inner diameter). Interestingly, a previous study (9) has demonstrated that pregnancy-induced changes in myogenic tone of the UA are size dependent, with increased tone in the proximal portion but a reduction in the more distal portion.
In the present study, we demonstrated that high-altitude hypoxia significantly increased PKC-induced contractions of the UA in pregnant ewes and abolished the differences in PKC-mediated UA contractions between nonpregnant and pregnant animals. PKC plays an important role in the regulation of the pressure-dependent myogenic response of arteries, including the UA (5, 11, 22, 30, 32, 47). We (47, 48, 51, 53) have demonstrated that the reduced myogenic tone of the UA in pregnancy is primarily mediated by a decrease in the PKC signaling pathway. Consistent with our findings, it has been shown that pregnancy is associated with attenuated arterial PKC activity (6, 7, 17, 18, 25). Taken together, these findings indicate that long-term high-altitude hypoxia inhibits the pregnancy-induced downregulation of the myogenic response in the UA by upregulating the PKC signaling pathway. This is most likely due to the direct effect of chronic hypoxia because of the finding in the present study that ex vivo prolonged hypoxic treatment of isolated UAs from normoxic ewes produced similar effects on PKC-induced contractions as those found in UAs from high-altitude hypoxic animals. In agreement with the present finding, several previous studies (3, 24, 31, 34, 39, 44) have demonstrated that the activation of PKC played a central role in modulating hypoxic pulmonary vasoconstriction and hypertension.
The lack of a direct effect of prolonged hypoxia on KCl-induced contractions of the UA is consistent with the previous finding in long-term high-altitude hypoxic animals (57) and reinforces the notion that it is unlikely that the hypoxia-mediated increase in UA contractility is attributed to increased L-type Ca2+ channels and influx of extracellular Ca2+. It has been demonstrated that the PKC-dependent pathway modulates primarily the Ca2+ sensitivity of myogenic mechanisms (2, 8, 23, 26, 32). In the UA, the activation of PKC by PDBu decreased the diameter of pressurized vessels without increasing intracellular Ca2+ concentrations, indicating a key role of Ca2+ sensitization in PKC-mediated changes in vascular tone in the UA (47). In the present study, we determined the effect of chronic hypoxia on baseline myofilament Ca2+ sensitivity in the UA by examining Ca2+-induced contractions of β-escin-permeabilized arterial preparations, a method that has been widely used to study baseline Ca2+ sensitivity (48). In contrast to our previous findings in normoxic animals showing that pregnancy decreased baseline Ca2+ sensitivity in the UA (48), Ca2+ sensitivity was significantly increased in pregnant UAs in high-altitude hypoxic animals. Interestingly, PDBu-mediated PKC activation increased the maximum Ca2+-induced contraction in nonpregnant UAs but had no effect on pregnant UAs. One possible explanation accounting for this observation is that chronic hypoxia activates PKC in pregnant but not nonpregnant UAs. This is consistent with the findings of PDBu-induced contractions, which were increased by chronic hypoxia in pregnant but not nonpregnant UAs. These findings support the notion that chronic hypoxia may promote PKC signaling in pregnant UAs, resulting in increased baseline Ca2+ sensitivity and the myogenic response. Unlike UAs, hypoxia-mediated augmentation of Ca2+ sensitivity in pulmonary arteries appeared through the activation of RhoA/Rho kinase but not PKC signaling (15), suggesting heterogeneity in the vascular response to chronic hypoxia.
Both PKC-α and PKC-ɛ have been implicated in contractions of vascular smooth muscle through increasing Ca2+ sensitivity (12, 42, 43). The present finding that PDBu-induced activation of PKC-α was similar in UAs from all four groups of animals in the absence or presence of PD-98059 suggests that PKC-α is not involved in pregnancy- and/or hypoxia-mediated changes in PKC-induced contractions of UAs. In contrast, the finding of a significant decrease in PDBu-induced PKC-ɛ activation in UAs of pregnant ewes indicates a key role of PKC-ɛ in the pregnancy-mediated decrease in PKC-mediated contractions of UAs. Chronic hypoxia significantly increased the activation of PKC-ɛ in pregnant UAs and abolished the difference in PKC-ɛ activity between nonpregnant and pregnant UAs. This suggests that chronic hypoxia inhibits the pregnancy-mediated downregulation of PKC-ɛ activity, resulting in increased PKC-mediated contractions in UAs. Consistent with the present finding, a previous study (24) in a PKC-ɛ knockout mouse in which PKC-ɛ was absent, and expression of all other isozymes was unaffected, demonstrated a direct involvement of PKC-ɛ in pulmonary pressor responses to hypoxia. The effect of PKC-ɛ ablation appeared to be specific to hypoxia-mediated vasoconstriction in perfused lungs, because no differences in the pressure response to infused angiotensin II or cumulative KCl were detected in PKC-ɛ−/− mice compared with PKCɛ+/+ mice (24).
Additionally, the present study suggests that hypoxia-mediated upregulation of PKC activation is likely due to an inhibition of ERK1/2 activity. Our previous studies (47, 50, 53) have demonstrated that pregnancy-increased ERK1/2 acted as an upstream signal in suppressing PKC-mediated contractions and pressure-dependent myogenic tone in UAs. In the present study, we demonstrated that inhibition of ERK1/2 by PD-98059 increased PDBu-induced PKC-ɛ activation in pregnant UAs, and, in the presence of PD-98059, there were no significant differences in PKC-ɛ activity between nonpregnant and pregnant UAs. This supports the previous findings and indicates a selective cross talk between PKC-ɛ and ERK1/2 pathways in the adaptation of the UA myogenic response to pregnancy. The finding of the lack of effect of PD-98059 on PDBu-induced PKC-ɛ activation in UAs of pregnant ewes of high-altitude animals suggests that chronic hypoxia during pregnancy inhibits ERK1/2 activity, resulting in increased PKC activity in UAs. This is further supported by the findings that chronic hypoxia abolished the effect of PD-98059 and increased PDBu-induced contractions in pregnant UAs in the absence or presence of PD-98059. Additionally, we demonstrated in the present study that long-term high-altitude hypoxia resulted in a significant decrease in ERK1/2 protein expression in UAs of pregnant but not nonpregnant ewes, which was associated with a reduction in ERK1/2 activity. Although many previous studies have suggested that hypoxia increased ERK activity, most if not all of these studies were conducted in cell cultures with short-term hypoxia treatment within 24 h. Few studies have examined the effect of long-term hypoxia in animals on ERK1/2 expression and activity in tissue. A study (45) of male rats treated with hypoxia for 14 days showed an increase in ERK activity in pulmonary but not in aortic fibroblasts. This tissue-specific response of ERK to long-term hypoxia was also demonstrated in the heart, in which 25-day hypoxia treatment of rats resulted in a partial upregulation of ERK2 in the right ventricle, but expression of ERK1/2 in the left ventricle as well as their activities in both ventricles were not affected by chronic hypoxia (38). In the present study, given that in normoxic animals there was a significant increase in ERK1/2 in pregnant compared with nonpregnant UAs and this difference was abolished in hypoxic animals, the results suggest that chronic hypoxia during pregnancy inhibits the upregulation of ERK1/2 in the UA that normally occurs in pregnancy. This is supported by previous studies (35, 36) demonstrating that long-term high-altitude hypoxia diminished the proliferative response of UA vascular smooth muscle cells in pregnant guinea pigs, although ERK1/2 was not measured. The similar findings obtained in UAs of long-term high-altitude hypoxic animals and those of prolonged ex vivo hypoxic treatment strongly suggest a direct effect of chronic hypoxia on the cross talk between ERK1/2 and PKC pathways in the adaptation of the UA myogenic response to pregnancy.
In summary, we have shown in the ovine UA that pregnancy-induced downregulation of pressure-dependent myogenic tone is inhibited by chronic hypoxia in pregnancy. The increased myogenic response by hypoxia is mediated in part by a decrease in the inhibitory effect of ERK1/2, resulting in an increase in the PKC signal pathway, which leads to an increase in baseline Ca2+ sensitivity of the myogenic mechanism in the UA during pregnancy. It should be noted that it is unlikely that ERK1/2 is the only possible mechanism in hypoxia-induced changes in UA contractility because hypoxia is known to activate many contractile signaling pathways in vascular smooth muscle. For instance, inhibition of K+ channels may also be important in the hypoxic effect. Given that pressure-dependent myogenic contraction is an important physiological mechanism that regulates basal vascular tone and is a major contributor to the modulation of organ blood flow, the increased myogenic response of the UA in pregnancy with chronic hypoxia is likely to contribute significantly to the dysregulation of uterine vascular hemodynamics and results in reduced uterine blood flow, which may have deleterious consequences on fetal development during pregnancy. Indeed, it has been shown that long-term high-altitude hypoxia attenuates the pregnancy-induced increase in uterine blood flow, which is associated with an increased risk of preeclampsia and fetal intrauterine growth restriction (46, 54, 55). The potential mechanisms of sex steroid hormones in the regulation of the PKC/ERK1/2 pathway and the myogenic response of UAs in the adaptation to pregnancy and chronic hypoxia present an intriguing area for future investigation.
GRANTS
This work was supported in part by National Institutes of Health Grants HD-31226 and HL-89012 and by Loma Linda University School of Medicine.
REFERENCES
- 1.Anderson NG, Maller JL, Tonks NK, Sturgill TW. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature 343: 651–653, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Bakker EN, Kerkhof CJ, Sipkema P. Signal transduction in spontaneous myogenic tone in isolated arterioles from rat skeletal muscle. Cardiovasc Res 41: 229–236, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Bialecki RA, Fisher CS, Murdoch WW, Barthlow HG. Chronic hypoxia increases staurosporine sensitivity of pulmonary artery smooth muscle to endothelin-1. Pulm Pharmacol Ther 11: 159–163, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 294: L797–L806, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Farley DB, Ford SP. Evidence for declining extracellular calcium uptake and protein kinase C activity in uterine arterial smooth muscle during gestation in gilts. Biol Reprod 46: 315–321, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Ford SP Control of blood flow to the gravid uterus of domestic livestock species. J Anim Sci 73: 1852–1860, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Gokina NI, Knot HJ, Nelson, Osol G. Increased Ca2+ sensitivity as a key mechanism of PKC-induced constriction in pressurized cerebral arteries. Am J Physiol Heart Circ Physiol 277: H1178–H1188, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Gokina NI, Mandala M, Osol G. Induction of localized differences in rat uterine radial artery behavior and structure during gestation. Am J Obstet Gynecol 189: 1489–1493, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Grandley RE, Conrad KP, McLaughlin MK. Endothelin and nitric oxide mediate reduced myogenic reactivity of small renal arteries from pregnant rats. Am J Physiol Regul Integr Comp Physiol 280: R1–R7, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Hill MA, Falcone JC, Meininger GA. Evidence for protein kinase C involvement in arteriolar myogenic reactivity. Am J Physiol Heart Circ Physiol 259: H1586–H1594, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz A, Clement-Chomienne O, Walsh MP, Morgan KG. ɛ-Isoenzyme of protein kinase C induces a Ca2+-independent contraction in vascular smooth muscle. Am J Physiol Cell Physiol 271: C589–C594, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Hu XQ, Longo LD, Gilbert RD, Zhang L. Effects of long-term high altitude hypoxemia on α1-adrenergic receptors in the ovine uterine artery. Am J Physiol Heart Circ Physiol 270: H1001–H1007, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Hu XQ, Yang S, Pearce WJ, Longo LD, Zhang L. Effect of chronic hypoxia on α1-adrenoceptor-mediated inositol 1,4,5-trisphosphate signaling in ovine uterine artery. J Pharmacol Exp Ther 288: 977–983, 1999. [PubMed] [Google Scholar]
- 15.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L515–L529, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J, Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol 295: R906–R915, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanashiro CA, Alexander BT, Granger JP, Khalil RA. Ca2+-insensitive vascular protein kinase C during pregnancy and NOS inhibition. Hypertension 34: 924–930, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Kanashiro CA, Cockrell KL, Alexander BT, Granger JP, Khalil RA. Pregnancy-associated reduction in vascular protein kinase C activity rebounds during inhibition of NO synthesis. Am J Physiol Regul Integr Comp Physiol 278: R295–R303, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res 54: 20–25, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Kublickiene KR, Cockell AP, Nisell H, Poston L. Role of nitric oxide in the regulation of vascular tone in pressurized and perfused resistance myometrial arteries from term pregnant women. Am J Obstet Gynecol 177: 1263–1269, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Kublickiene KR, Kublickas M, Lindblom B, Lunell NO, Nisell H. A comparison of myogenic and endothelial properties of myometerial and omental resistance vessels in late pregnancy. Am J Obstet Gynecol 176: 560–566, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Lagaud G, Gaudreault N, Moore ED, Van Breemen C, Laher I. Pressure-dependent myogenic constriction of cerebral arteries occurs independently of voltage-dependent activation. Am J Physiol Heart Circ Physiol 283: H2187–H2195, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Lagaud GJ, Lam E, Lui A, Van Breemen C, Laher I. Nonspecific inhibition of myogenic tone by PD98059, a MEK1 inhibitor, in rat middle cerebral arteries. Biochem Biophys Res Commun 257: 523–527, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Littler CM, Morris KG, Fagan KA, McMurtry IF, Messing RO, Dempsey EC. Protein kinase C-ɛ-null mice have decreased hypoxic pulmonary vasoconstriction. Am J Physiol Heart Circ Physiol 284: H1321–H1331, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Magness RR, Rosenfeld CR, Carr BR. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am J Physiol Endocrinol Metab 260: E464–E470, 1991. [DOI] [PubMed] [Google Scholar]
- 26.Massett MP, Ungvari Z, Csiszar A, Kaley G, Koller A. Different roles of PKC and MAP kinases in arteriolar constrictions to pressure and agonists. Am J Physiol Heart Circ Physiol 283: H2282–H2287, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Mateev SN, Mouser R, Young DA, Mecham RP, Moore LG. Chronic hypoxia augments uterine artery distensibility and alters the circumferential wall stress-strain relationship during pregnancy. J Appl Physiol 100: 1842–1850, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Meyer MC, Brayden JE, Mclaughlin MK. Characteristics of vascular smooth muscle in the maternal resistance circulation during pregnancy in the rat. Am J Obstet Gynecol 169: 1510–1516, 1993. [DOI] [PubMed] [Google Scholar]
- 29.Meyer MC, Osol G, Mclaughlin M. Flow decreases myogenic reactivity of mesenteric arteries from pregnant rats. J Soc Gynecol Investig 4: 293–297, 1997. [PubMed] [Google Scholar]
- 30.Nakayama K, Obara K, Tanabe Y, Saito M, Ishikawa T, Nishizawa S. Interactive role of tyrosine kinase, protein kinase C, and Rho/Rho kinase systems in the mechanotransduction of vascular smooth muscles. Biorheology 40: 307–314, 2003. [PubMed] [Google Scholar]
- 31.Orton EC, Raffestin B, McMurtry IF. Protein kinase C influences rat pulmonary vascular reactivity. Am Rev Respir Dis 141: 654–658, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res 68: 359–367, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol 180: 1161–1168, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Rathore R, Zheng YM, Li XQ, Wang QS, Liu QH, Ginnan R, Singer HA, Ho YS, Wang YX. Mitochondrial ROS-PKCɛ signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun 351: 784–790, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rockwell LC, Dempsey EC, Moore LG. Chronic hypoxia diminishes the proliferative response of guinea pig uterine artery vascular smooth muscle cells in vitro. High Alt Med Biol 7: 237–244, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Rockwell LC, Keyes LE, Moore LG. Chronic hypoxia diminishes pregnancy-associated DNA synthesis in guinea pig uteroplacental arteries. Placenta 21: 313–319, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg SF Structural basis of protein kinase C isoform function. Physiol Rev 88: 1341–1378, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strnisková M, Ravingerová T, Neckár J, Kolár F, Pastoreková S, Barancík M. Changes in the expression and/or activation of regulatory proteins in rat hearts adapted to chronic hypoxia. Gen Physiol Biophys 25: 25–41, 2006. [PubMed] [Google Scholar]
- 39.Tsai BM, Wang M, Pitcher JM, Meldrum KK, Meldrum DR. Hypoxic pulmonary vasoconstriction and pulmonary artery tissue cytokine expression are mediated by protein kinase C. Am J Physiol Lung Cell Mol Physiol 287: L1215–L1219, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Veerareddy S, Campbell ME, Williams SJ, Baker PN, Davidge ST. Myogenic reactivity is enhanced in rat radial uterine arteries in a model of maternal undernutrition. Am J Obstet Gynecol 191: 334–339, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Veerareddy S, Cooke CL, Baker PN, Davidge ST. Vascular adaptations to pregnancy in mice: effects on myogenic tone. Am J Physiol Heart Circ Physiol 283: H2226–H2233, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Walsh MP, Andrea JE, Allen BG, Clement-Chomienne O, Collins EM, Morgan KG. Smooth muscle protein kinase C. Can J Physiol Pharmacol 72: 1392–1399, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Walsh MP, Horowitz A, Clement-Chomienne O, Andrea JE, Allen BG, Morgan KG. Protein kinase C mediation of Ca2+-independent contractions of vascular smooth muscle. Biochem Cell Biol 74: 485–502, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Weissmann N, Voswinckel R, Hardebusch T, Rosseau S, Ghofrani HA, Schermuly R, Seeger W, Grimminger F. Evidence for a role of protein kinase C in hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol 276: L90–L95, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Welsh DJ, Peacock AJ, MacLean M, Harnett M. Chronic hypoxia induces constitutive p38 mitogen-activated protein kinase activity that correlates with enhanced cellular proliferation in fibroblasts from rat pulmonary but not systemic arteries. Am J Respir Crit Care Med 164: 282–289, 2001. [DOI] [PubMed] [Google Scholar]
- 46.White M, Zhang L. Effects of chronic hypoxia on maternal vascular changes during pregnancy. High Alt Med Biol 4: 157–169, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Xiao D, Buchholz JN, Zhang L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: role of PKC/ERK pathway. Am J Physiol Heart Circ Physiol 290: H2337–H2343, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Xiao D, Huang X, Longo LD, Pearce WJ, Zhang L. Regulation of baseline Ca2+ sensitivity in permeabilized uterine arteries: effect of pregnancy. Am J Physiol Heart Circ Physiol 291: H413–H420, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Xiao D, Longo LD, Zhang L. α1-Adrenoceptor-mediated phosphorylation of MYPT1 and CPI-17 in the uterine artery: role of ERK/PKC. Am J Physiol Heart Circ Physiol 288: H2828–H2835, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Xiao D, Pearce WJ, Longo LD, Zhang L. ERK-mediated uterine artery contraction: role of thick and thin filament regulatory pathways. Am J Physiol Heart Circ Physiol 286: H1615–H1622, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Xiao D, Zhang L. Adaptation of uterine artery thick- and thin-filament regulatory pathway to pregnancy. Am J Physiol Heart Circ Physiol 288: H142–H148, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Xiao D, Zhang L. Calcium homeostasis and contractions of the uterine artery: effect of pregnancy and chronic hypoxia. Biol Reprod 70: 1171–1177, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Xiao D, Zhang L. ERK MAP kinases regulate smooth muscle contraction in ovine uterine artery: effect of pregnancy. Am J Physiol Heart Circ Physiol 282: H292–H300, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol 79: 15–22, 1995. [DOI] [PubMed] [Google Scholar]
- 55.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effects of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol 79: 7–14, 1995. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Xiao DL, Longo L, Zhang L. Regulation of α1-adrenoceptor-mediated contractions of the uterine artery by PKC: effect of pregnancy. Am J Physiol Heart Circ Physiol 291: H2282–H2289, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Xiao DL. Effects of chronic hypoxia on Ca2+ mobilization and Ca2+ sensitivity of myofilaments in the uterine artery. Am J Physiol Heart Circ Physiol 274: H132–H138, 1998. [DOI] [PubMed] [Google Scholar]