Abstract
Heart failure carries a poor prognosis with few treatment options. While myocardial stem cell therapeutic trials have traditionally relied on intracoronary infusion or intramyocardial injection routes, these cell delivery methods are invasive and can introduce harmful scar tissue, arrhythmia, calcification, or microinfarction in the heart. Given that patients with heart failure are at an increased surgical risk, the development of a noninvasive stem cell therapeutic approach is logistically appealing. Taking advantage of the trophic effects of bone marrow mesenchymal stem cells (MSCs) and using a hamster heart failure model, the present study demonstrates a novel noninvasive therapeutic regimen via the direct delivery of MSCs into the skeletal muscle bed. Intramuscularly injected MSCs and MSC-conditioned medium each significantly improved ventricular function 1 mo after MSC administration. MSCs at 4 million cells/animal increased fractional shortening by ∼40%, enhanced capillary and myocyte nuclear density by ∼30% and ∼80%, attenuated apoptosis by ∼60%, and reduced fibrosis by ∼50%. Myocyte regeneration was evidenced by an approximately twofold increase in the expression of cell cycle markers (Ki67 and phosphohistone H3) and an ∼13% reduction in mean myocyte diameter. Increased circulating levels of hepatocyte growth factor (HGF), leukemia inhibitory factor, and macrophage colony-stimulating factor were associated with the mobilization of c-Kit-positive, CD31-positive, and CD133-positive progenitor cells and a subsequent increase in myocardial c-Kit-positive cells. Trophic effects of MSCs further activated the expression of HGF, IGF-II, and VEGF in the myocardium. The work highlights a cardiac repair mechanism mediated by trophic cross-talks among the injected MSCs, bone marrow, and heart that can be explored for noninvasive stem cell therapy.
Keywords: trophic factors
advances in patient management and treatment have lowered death rates from heart disease over the last 30 years but have led to an increasing patient population living with heart failure. Unfortunately, the only therapy available to reverse the decline in cardiac function is heart transplantation. However, this option is available to very few patients due to a shortage of donor hearts. Late sequela of immunosuppression and rejection further limit the efficacy of this approach (4). Recent interests in stem cell therapeutics have prompted preclinical and clinical studies on the feasibility and safety of stem cells for the treatment of heart disease (6, 46). Although mixed results have been documented without a clear consensus on the best cell for cardiac regeneration, the ease of large-scale cell expansion and immunoprivileged status of bone marrow mesenchymal stem cells (MSCs) are attractive features of adult stem cells (13, 48, 57).
Myocardial stem cell therapy often uses invasive cell delivery approaches such as intramyocardial injection or intracoronary infusion. Given that patients with heart failure are at an increased surgical risk, the development of a noninvasive cell delivery regimen is logistically appealing. A salient feature of MSCs is their ability to produce a plethora of trophic factors (7, 15), which may be harnessed for noninvasive stem cell therapy for heart failure. Indeed, documented cardiovascular beneficial effects of MSCs have largely been attributed to their paracrine actions independent of their differentiation potentials (14, 52, 56, 58). This recognition stems from the findings that efficiencies of myocardial recruitment and engraftment after local or systemic stem cell administration are typically too low to account for functional improvement. Our recent cell tracking study (32) estimated that only 1–2% of intracoronary-infused MSCs engrafted in the pig heart with no evidence of MSC differentiation into cardiomyocytes. Furthermore, since diseased tissue environments often exhibit pathological levels of ischemia, inflammation, and fibrosis, which can impair cell survival, the therapeutic delivery of stem cells to areas away from the damaged heart offers a novel concept.
The multiple trophic factors produced by MSCs are capable of attenuating tissue injury, inhibiting fibrotic remodeling, promoting angiogenesis, stimulating the recruitment and proliferation of tissue stem cells, or reducing inflammatory oxidative stress (7, 15, 34, 48). We hypothesize that MSCs, via secretion of these functionally synergistic trophic factors, are able to rescue the failing heart even when delivered away from the myocardium. Delivery of MSCs by intramuscular injection offers a feasible noninvasive strategy as skeletal muscle, being the most abundant tissue in the body, is amenable to repeated injection of large numbers of stem cells. Along this line, we have shown by PCR analysis that intramuscularly injected MSCs are trapped in the muscular bed with no detectable migration to other tissues (48). This cell injection regimen is used here to provide the ultimate proof that the trophic actions of MSCs underlie their cardiovascular therapeutic effects. Using a hamster heart failure model characterized by us and others (12, 16, 39, 45), we demonstrate, for the first time, that the noninvasive administration of MSCs or MSC-derived trophic factors via intramuscular injection effectively rescues the failing heart through intricate tissue cross-talk mechanisms. This noninvasive stem cell administration regimen, if validated clinically, is expected to facilitate future stem cell therapy for heart failure.
MATERIALS AND METHODS
Animals.
F1B (normal) and TO2 (cardiomyopathic) male hamsters were obtained from Bio Breeders (Watertown, MA). All procedures and protocols conformed to institutional guidelines for the care and use of animals in research.
Echocardiography.
Echocardiographic measurements were performed in a blind-folded fashion and were as described in our recent work (39).
MSC culture and intramuscular implantation.
Porcine bone marrow MSCs were isolated as previously described (34, 59). To produce MSC-conditioned serum-free medium, MSCs were plated on a fibronectin-coated surface and grown to subconfluency. Cells were then washed thoroughly with HBSS and maintained in serum- and phenol red-free MEM for 24 h. The conditioned medium was harvested and filtered before use. For intramuscular implantation, MSCs (0.25, 1, or 4 million cells/animal) were resuspended in 0.8 ml HBSS and injected in equally divided doses into the left and right hamstrings of 4-mo-old TO2 hamsters. Control TO2 hamsters received the same volume of HBSS. Animals received a second intramuscular MSC implantation 2 wk later since preliminary cell injection trials indicated more prominent therapeutic effects with a repeated cell injection. For medium injection, TO2 hamsters received three weekly injections each of 0.8 ml of the conditioned medium for 4 wk.
ELISA assay.
Circulating cardiac troponin I (cTnI) was assayed with a rat cTnI ELISA kit (Life Diagnostics) using plasma samples collected 1 mo after MSC administration. Assays of circulating leukemia inhibitory factor (LIF) and granulocyte/macrophage-colony stimulating factor (G/M-CSF) were performed using the MAP program (Rules-Based Medicine). Hepatocyte growth factor (HGF), VEGF, and IGF-II were analyzed by the following ELISA kits from R&D Systems: mouse HGF DuoSet (no. DY2207), rat VEGF DuoSet (no. DY564), and mouse IGF-II DuoSet (no. DY792). Heart tissues were homogenized in ice-cold lysis solution containing 0.1% Triton X-100 and 2 mM EDTA. Lysates were clarified, diluted to 1 mg protein/ml, and used for ELISA per the manufacturer's instructions.
Flow cytometry.
Peripheral blood mononuclear cells were isolated 3 days after MSC administration, and red blood cells were removed with lysis buffer (150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA). Cells were washed and resuspended in normal saline. After being blocked with an Fc receptor blocker for 30 min, cells were labeled with phycoerythrin (PE)-conjugated CD133 (no. AC133, Miltenyi Biotec), PE-conjugated CD31 (no. 12-0311, eBioscience), and PE-conjugated c-Kit (no. 12-1171, eBioscience) antibodies. Flow cytometry was performed on ∼25,000 cells, and data were analyzed using FCS Express (De Novo Software). Proper isotype-matched IgGs were used as controls. Dead cells were excluded by 7-amino-actinomycin D counterstaining.
Quantification of capillary and cardiomyocyte nuclear density.
Freshly excised tissues were immersed in OCT, frozen in liquid nitrogen, and stored at −80°C until use. Ventricular cross sections of 5 μm thick were obtained using a cryostat and fixed in an acetone-ethanol mixture (3:1 ratio) for 5 min. Sections were blocked with Serum-Free Protein Block (Dako) for 30 min. FITC-labeled Griffonia simplicifolia isolectin B4 (diluted 1:100) was incubated with the tissue sections overnight at 4°C. Cardiomyocytes were stained with a rabbit TnI antibody (no. sc15368, Santa Cruz Biotechnology) the next day for 3 h. The TnI antibody reacts with both cTnI and skeletal TnI of rodent and human origin. Sections were then incubated with a Texas red-conjugated anti-rabbit secondary antibody for 1 h and then mounted using Vectashield's Mounting Medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Images were taken in 15–25 random fields using Zeiss's Axioimager fluorescence microscope at ×200 magnification. Numbers of capillaries (FITC channel) and total nuclei (DAPI channel) were quantified by ImageJ software using the analyze particle feature. Noncardiomyocte nuclei quantified from the merged images by their lack of TnI staining were subtracted from the total nuclei count to determine cardiomyocyte nuclear density. Black areas from images were subtracted using Photoshop-aided quantification of black pixels to calculate the total tissue area. Capillary and cardiomyocyte nuclear density were normalized to the total tissue area (in mm2).
Quantification of apoptosis.
Analysis of apoptosis was performed on frozen sections prepared as described above using the ApopTag kit (Millipore) per the manufacturer's instructions. TnI antibody was used to identify apoptotic myocytes, and analysis was performed similarly as described above. All apoptotic nuclei in each section were counted and normalized to total myocytes and nonmyocytes.
Quantification of fibrosis and cardiomyocyte diameter.
Masson trichrome-stained sections were used for fibrosis analysis and cardiomyocyte diameters. Fibrosis was performed by Photoshop-aided quantification of image pixels. The blue color range was selected to represent fibrotic areas. At least 15 random fields at ×200 magnification were assessed for each slide by 3 independent examiners with 1 examiner being blinded. Artifactual spaces (white clear areas) from images were subtracted using Photoshop-aided quantification of white pixels to calculate the total tissue area. The ratio of fibrotic areas to total tissue areas was calculated as a percentage of the fibrotic areas. For the quantification of cardiomyocyte diameters, at least 350 random cardiomyocytes were measured for each animal using AxioVision LE software's measurement tool (Carl Zeiss).
Quantification of c-Kit-positive, Ki67-positive, and phospho-histone H3-positive cells.
Paraformaldehyde-fixed, paraffin-embedded heart sections of 5 μm thick were used for c-Kit, Ki-67, and phospho-histone H3 (p-HH3) staining. Antigen retrieval was done by steaming in 10 mM citrate (pH 6) for 30 min followed by permeabilization in 1% Triton X-100 for 20 min. Sections were blocked with normal saline solution supplemented with 0.025% TW-20 and 2% nonfat milk powder for 30 min and incubated with diluted primary antibody overnight. The primary antibodies used were as follows: c-kit antibody (no. A4502, Dako), Ki67 antibody (no. RM-9106, Thermo Scientific), and p-HH3 antibody (no. 07-145, Millipore). Myocytes were stained with mouse cardiac troponin T (cTnT) antibody (no. MS-295, Thermo Scientific) the next day for 1 h. The cTnT antibody reacts with cTnT of multiple species. Sections were then incubated with Alexa 647-conjugated anti-rabbit and Alexa 488-conjugated anti-mouse secondary antibodies for 30 min and then mounted in Vectashield's Mounting Medium with DAPI. Sections were analyzed as described above using Zeiss's Axioimager fluorescence microscope at ×200 magnification.
Real-time quantitative RT-PCR.
RNA isolation and quantitative RT-PCR protocols were performed as previously described (34). β2-Microglobulin was used as the reference gene for calculations. Injected MSCs were quantified by pig-specific 16S rRNA primers. A standard curve was created by generating a serial dilution curve by plotting the threshold cycles of the 16S rRNA gene against a known number of MSCs. The primer sequences are shown in Table 1.
Table 1.
Primer sequences for quantitative RT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β2-Microglobulin | 5′-TCTCTTGGCTCACAGGGAGT-3′ | 5′-ATGTCTCGTTCCCAGGTGAC |
| c-Kit | 5′-GCCACGTCTCAGCCATCTG-3′ | 5′-GTCGCCAGCTTCAACTATTAACT |
| Hepatocyte growth factor | 5′-AGAGGTCCCATGGATCACAC-3′ | 5′-AGCCCTTGTCGGGATATCTT |
| IGF-II | 5′-CAAGTCCGAGAGGGATGTGT-3′ | 5′-GGACTGTCTCCAGGTGTCGT |
| VEGF | 5′-TCACCAAAGCCAGCACATAG-3′ | 5′-AAATGCTTTCTCCGCTCTGA |
| Collagen type I-α1 | 5′-GAGCGGAGAGTACTGGATCG-3′ | 5′-GTTCGGGCTGATGTACCAGT |
| Collagen type I-α2 | 5′-CGAGACCCTTCTCACTCCTG-3′ | 5′-GCATCCTTGGTTAGGGTCAA |
| Collagen type II-α1 | 5′-GGGATCCAATGAGGGAGAAT-3′ | 5′-GGCCTTGCGTGTTTGATATT |
| MMP-13 | 5′-TTTATTGTTGCTGCCCATGA-3′ | 5′-AACTGGATTCCTTGCACGT |
| MMP-2 | 5′-TGGTTTCCCTAAGCTCATCG-3′ | 5′-TTGGTTCTCCAGCTTCAGGT |
| MMP-9 | 5′-GTCTTCCCCTTCGTCTTCCT-3′ | 5′-CACTTCTTGTCAGCGTCGAA |
| CD45 | 5′-GGCGTACAGGCACCTACATT-3′ | 5′-ATGTACTGGGCCTCCACTTG |
| TIMP-1 | 5′-CATGGAAAGCCTCTGTGGAT-3′ | 5′-CTCAGAGTACGCCAGGGAAC |
| TIMP-2 | 5′-GACTGGGTCACGGAGAAGAG-3′ | 5′-GGGTCCTCGATGTCAAGAAA |
| TIMP-3 | 5′-GCTGTGCAACTTTGTGGAG-3′ | 5′-GGTCACAAAGCAAGGCAAG |
MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
Statistical analysis.
Data are expressed as means ± SE. Comparisons were based on an unpaired Student's t-test. P values of <0.05 were considered significant.
RESULTS
The hamster heart failure model.
The TO2 hamster strain harbors a genetic defect in the δ-sarcoglycan gene that causes dilated cardiomyopathy leading to congestive heart failure clinically identical to that occurring in the general category of human heart failure (12, 16, 45). Functional deterioration of the TO2 heart is accompanied by prominent myocyte loss, inflammation, fibrosis, and calcified lesions (44, 45). We (39) have shown that the left ventricular (LV) ejection fraction (LVEF) and fractional shortening (FS) of the TO2 hamster heart decline by ∼25% and 35%, respectively, at 4 mo of age. The MSC therapeutic trials described here were performed using 4-mo-old TO2 hamsters.
Noninvasive MSC delivery for heart failure.
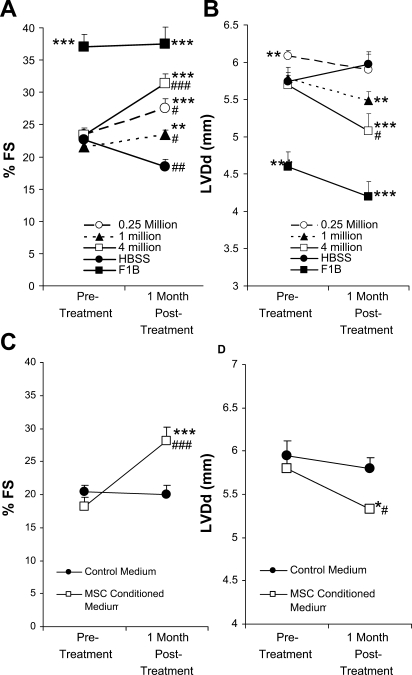
Preclinical and clinical studies of myocardial stem cell therapy often use invasive cell delivery approaches such as intramyocardial injection or intracoronary infusion. Given that patients with heart failure are at an increased surgical risk, it is logistically appealing to explore a noninvasive cell delivery approach. MSCs, via their ability of trophic factor production, may be harnessed toward achieving this goal. Using both histological and PCR detection methods, we (48) have recently shown that intramuscularly injected MSCs are largely trapped in the musculature with no detectable cell migration to other tissues including the heart. This noninvasive cell injection regimen takes advantage of the fact that skeletal muscle is the largest tissue of the body and is amenable to repeated injections, which is difficult to achieve with myocardial cell delivery routes. To address the feasibility of intramuscular injection of MSCs for heart failure, we first carried out cell dosage experiments comparing injections of 0.25, 1, and 4 million MSCs into the hamstring muscles of TO2 cardiomyopathic hamsters. Blinded echocardiography was performed 1 mo after cell injection and showed that all three MSC dosage groups significantly improved ventricular function and attenuated chamber dilation (Fig. 1, A and B). The 4 million cell dosage group clearly exhibited the most prominent functional improvement, as indicated by an ∼40% increase in FS and ∼10% decrease in LV diastolic diameter. The 4 million cell dosage group also caused showed an ∼80% increase in systolic wall thickening (data not shown).
Fig. 1.
Cardiac functional improvement by intramuscular injection of mesenchymal stem cells (MSCs) and MSC-conditioned medium. A and B: percent fractional shortening (%FS) and left ventricular diastolic diameter (LVDd) at preinjection and 1 mo postinjection (n = 5 animals/group) were obtained by blinded echocardiography. MSCs at the indicated cell dosages were injected into hamstring muscles of 4-mo-old TO2 hamsters. Control TO2 hamsters received HBSS injections. Normal F1B hamsters were not injected and are shown as a reference for A–D. C and D: %FS and LVDd before and 1 mo after the first intramuscular injection of MSC-conditioned medium versus control medium (n = 4 animals/group). The MSC-conditioned media used here were from the same batch. Note that the 0.25 million MSC dosage group exhibited a higher mean %FS at pretreatment than the 1 million MSC dosage group. MSC-mediated improvements in %FS between the two cell dosage groups were statistically similar after normalization to the pretreatment level. *P < 0.05 vs. control; **P < 0.01 vs. control; ***P < 0.001 vs. control; #P < 0.05 vs. pretreatment; ##P < 0.01 vs. pretreatment; ###P < 0.001 vs. pretreatment.
Since MSC migration to the heart was undetectable after the intramuscular injection (48), the observed functional improvement must have been mediated by trophic factors. To provide the evidence, MSC-conditioned medium was used to determine whether the administration of cell-free medium would similarly rescue the failing heart. The medium was administered by multiple injections into the hamstring muscle, and echocardiography was performed after 1 mo. This experiment showed that MSC-conditioned medium was again effective in improving ventricular function and decreasing dilation compared with the control medium (Fig. 1, C and D). These functional experiments thus demonstrated the feasibility of noninvasive MSC therapy for heart failure using the convenient intramuscular injection route.
Active regeneration of the failing heart.
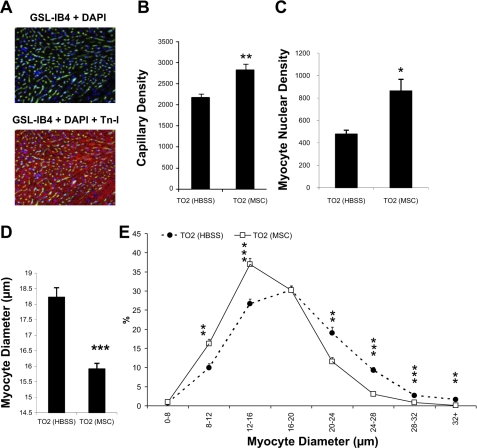
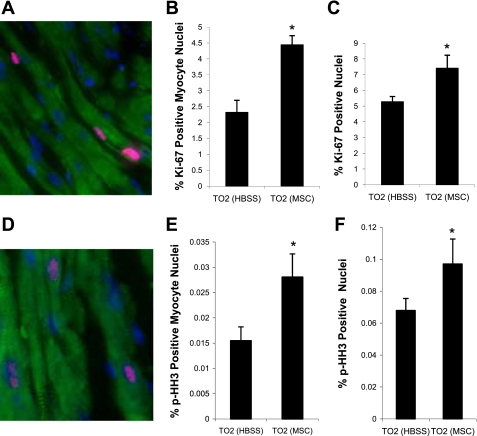
Histological experiments were further performed to ascertain that the functional improvement caused by intramuscularly injected MSCs was associated with myocardial tissue regeneration. We found that capillary and myocyte nuclear density in the MSC treatment group was ∼30% and ∼80% higher than the control injection group, respectively (Fig. 2, A–C). The higher myocyte nuclear density can be contributed by newly regenerated myocytes, which are typically smaller (37, 48). Figure 2D indeed shows that the mean cross-sectional myocyte diameter was smaller in the MSC-treated group (15.8 ± 0.9 μm) than in the control group (18.2 ± 0.3 μm). The frequency histogram demonstrated a shift toward smaller myocyte diameters in the MSC group, suggesting the prominent presence of newly regenerated myocytes (Fig. 2E). To provide additional evidence along this line, we quantified two specific markers associated with cell cycle activity: Ki67 and p-HH3 (18, 33). Quantitative analyses showed that the expression of Ki67 (Fig. 3, A–C) and p-HH3 (Fig. 3, D–F) were increased by approximately twofold in the MSC group and that increased expression of the cell cycle markers could be detected in both myocyte and nonmyocyte populations. Thus, cardiac functional improvement after intramuscular injection of MSCs is mediated by active myocardial regeneration.
Fig. 2.
Increased capillary and myocyte nuclear densities after MSC administration. A: representative images of capillary and myocyte staining using FITC-labeled Griffonia simplicifolia isolectin B4 (GSL-IB4; green) and troponin I (TnI) antibody (red), respectively. 4′,6-Diamidino-2-phenylindole (DAPI; blue) was used for nuclear staining. B and C: computer analysis of capillary and myocyte nuclear densities (expressed as numbers/mm2). D: computer analysis of myocyte cross-sectional diameters. At least 15 fields of ×200 magnification and >350 myocytes were evaluated in each hamster. E: frequency histogram of diameters showing a greater number of smaller myocytes in the MSC-treated group (n = 4 animals/group). *P < 0.05 vs. control; **P < 0.01 vs. control; ***P < 0.001 vs. control.
Fig. 3.
MSC administration augments cell cycle activities in the myocardium. A: representative image of Ki67-positive cells (pink nuclei). Myocytes were stained by a troponin T (TnT) antibody (green). Nuclei were stained by DAPI (blue). B and C: percentages of Ki67-positive myocytes and total Ki67-positive nuclei (n = 4 animals/group). D: representative image of phospho-histone H3 (p-HH3)-positive cells (pink nuclei). E and F: percentages of p-HH3-positive myocytes and total p-HH3-positive nuclei (n = 4 animals/group). *P < 0.05 vs. control.
Downregulation of apoptosis and tissue injury.
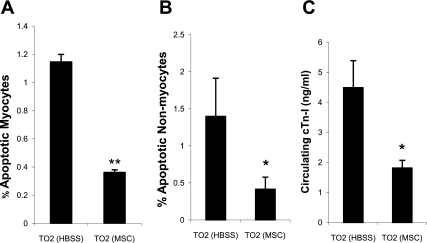
Since many trophic factors produced by MSCs possess antiapoptotic function (7, 15), the observed myocardial tissue regeneration could also be contributed by reduced apoptosis. Histological analysis of myocardial tissue sections revealed that MSCs decreased apoptosis of myocytes and nonmyocytes each by ∼60% (Fig. 4, A and B), suggesting that trophic factors promoted cell survival in the myocardium. Consistent with this finding, circulating cTnI levels were decreased by ∼60% (MSC group vs. saline control group: 1.8 ± 0.25 vs. 4.49 ± 0.9 ng/ml; Fig. 4C), reflecting a significant attenuation of myocardial tissue injury.
Fig. 4.
MSC administration reduces myocardial apoptosis and tissue damage. A and B: percentages of myocyte and nonmyocyte apoptosis. At least 25 fields of ×200 magnification and >8,000 cells were evaluated from each hamster. C: decreased circulating levels of cardiac TnI (cTnI) after MSC administration (n = 3 animals/group). *P < 0.05 vs. control; **P < 0.01 vs. control.
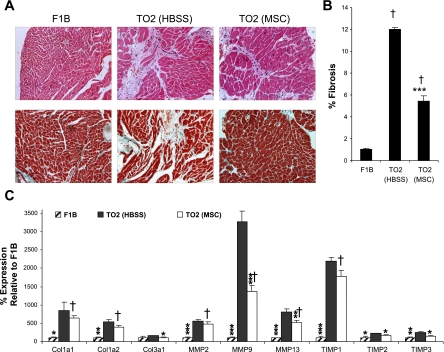
Attenuation of pathological fibrosis.
The TO2 cardiomyopathic hamster heart exhibits progressive fibrosis marked by the elevated expression of collagens, matrix metalloproteinases (MMPs), and tissue inhibitor of metalloproteinases (TIMPs) (11, 44, 45). Examinations of histological sections revealed greatly diminished LV fibrosis and leukocyte infiltration after MSC treatment (Fig. 5A). Fibrotic areas in the TO2 saline control group were ∼12%, in contrast to the ∼1% fibrotic areas in the normal F1B hamster heart. MSC injection caused an ∼50% decrease in fibrosis in the TO2 heart (Fig. 5B). Since the balance between collagen synthesis and degradation is mediated by MMPs and TIMPs and is of crucial relevance in maintaining myocardial structural integrity (49), we further used quantitative RT-PCR to assess the expression of these molecular players. Figure 5C shows that the control TO2 heart exhibited an elevated expression of collagens, MMPs, and TIMPs, as shown previously (11), and that MSC administration reversed the abnormal expression profiles of collagens, MMPs, and TIMPs. Thus, the noninvasive delivery of MSCs, through trophic activities, rescued the failing heart by improving ventricular function, promoting myocardial tissue regeneration and survival, and attenuating pathological fibrosis.
Fig. 5.
Attenuation of myocardial fibrosis by MSCs. A: hematoxylin-esosin-stained (top) and Masson trichrome-stained (bottom) heart sections. B: quantification of fibrotic areas (n = 5 animals/group). C: quantitative RT-PCR analysis of expression of genes involved in extracellular tissue remodeling. Col1a1, collagen type I-α1; Col1a2, collagen type I-α1; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase. Results are representative of 2 independent experiments. *P < 0.05 vs. control; **P < 0.01 vs. control; ***P < 0.001 vs. control; †P < 0.001 vs. F1B.
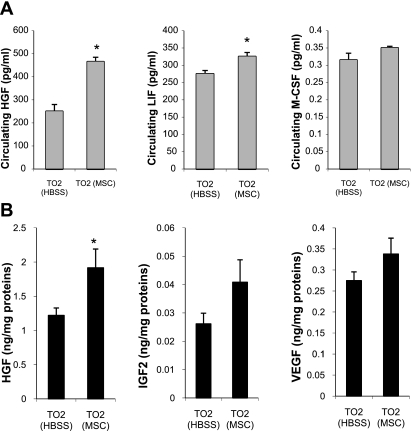
Increased circulating and myocardial trophic factors.
We next sought to identify the therapeutic mechanisms mediated by the intramuscularly injected MSCs. The multiple trophic factors produced by MSCs are known to possess functionally synergistic and redundant activities that are beneficial to the heart (7, 15, 34). These trophic factors may be initially released from intramuscularly injected MSCs and subsequently affect the expression of growth factors in the myocardium. Several major growth factors and cytokines present in the plasma and heart tissue homogenates were analyzed by ELISA 1 mo after MSC administration. These immunoassays revealed increased circulating levels of HGF, LIF, and G/M-CSF in the MSC treatment group (Fig. 6A). Elevated expressions of HGF, IGF-II, and VEGF in the myocardium were further identified by both ELISA (Fig. 6B) and quantitative RT-PCR (data not shown), indicating that the trophic activities of MSCs could amplify the expression of host growth factor genes in the myocardium.
Fig. 6.
Increased circulating and myocardial trophic factors. A: circulating (plasma) levels of hepatocyte growth factor (HGF), leukemia inhibitory factor (LIF), and granulocyte/macrophage colony-stimulating factor (G/M-CSF) 1 mo after MSC administration (n = 3 animals/group). B: heart tissues were collected 1 mo after MSC administration and homogenized. Clarified lysates were assayed by HGF, IGF-II, and VEGF ELISA kits (n = 4 animals/group). *P < 0.05 vs. the HBSS control.
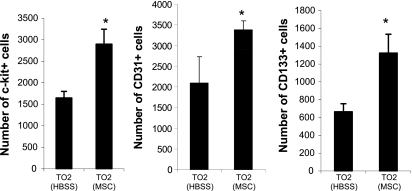
Mobilization of bone marrow progenitor cells.
Mobilization of bone marrow progenitor cells plays an important role in tissue repair (30). Among the multiple MSC trophic factors, HGF, VEGF, G/M-CSF, stem cell factor (SCF), IGF, and stromal-derived factor (SDF)-1 are known to be able to mobilize bone marrow progenitor cells (19, 23, 24, 28, 29, 31). Along this line, we (34, 59) have previously demonstrated that the MSCs used in the present study express IGF-2, LIF, G/M-CSF, SDF-1, and VEGF. We therefore investigated whether bone marrow progenitor cells might be mobilized in response to injected MSCs. Flow cytometric analysis of peripheral blood indeed showed that MSCs significantly increased circulating progenitor cells expressing c-Kit, CD31, or CD133 surface markers (Fig. 7), which have been shown to originate from the bone marrow compartment and contribute to tissue repair (21). A contribution by injected MSCs to these circulating progenitor cells can be ruled out because we and others have shown that intramuscularly injected MSCs are trapped in the musculature with no detectable migration (10, 48) and that MSCs do not express c-Kit (CD117), CD31, and CD133 markers (1, 59).
Fig. 7.
Mobilization of bone marrow progenitor cells. Circulating c-Kit-positive, CD133-positive, and CD31-positive cells were quantified by flow cytometry 3 days after MSC injections. Cell numbers per 1 million peripheral blood mononuclear cells are shown (n = 3 animals/group). *P < 0.05 vs. control.
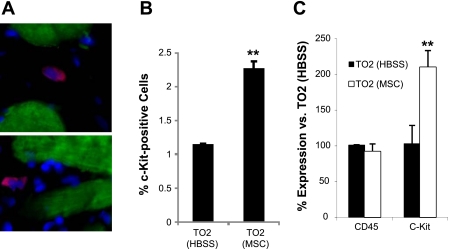
Myocardial c-Kit-positive progenitor cells.
Mobilized bone marrow progenitor cells are thought to participate in tissue repair through tissue homing mechanisms (19, 21, 30). Increased capillary and myocyte densities, as shown in Fig. 2, could be mediated by myocardial recruitment and the subsequent differentiation of circulating progenitor cells, some of which have been found to express the c-Kit marker (3). To explore this possibility, we further examined whether the myocardium might harbor elevated pools of c-Kit-positive progenitor cells after MSC administration. Immunostaining revealed an approximately twofold increase after MSC administration in ventricular c-Kit-positive cells (Fig. 8, A and B). This increase was further corroborated by quantitative RT-PCR analysis of myocardial c-Kit expression (Fig. 8C). Thus, MSC-mediated mobilization of bone marrow progenitor cells is coupled with increased myocardial c-Kit-positive progenitor cells. Taken together, the noninvasive cell therapeutic regimen for heart failure takes advantage of the powerful trophic activities of MSCs, resulting in functional improvement and myocardial regeneration.
Fig. 8.
Increased myocardial c-Kit-positive progenitor cells. A: representative images of interstitial c-Kit-positive cells (red). Myocytes were stained by a TnT antibody (green). Nuclei were stained by DAPI (blue). B: quantification of c-Kit-positive cells. C: quantitative RT-PCR confirmed the quantification data in B. Note that the expression of CD45 (leukocyte common antigen) was not affected by MSC administration. **P < 0.01 vs. the HBSS control.
DISCUSSION
The present study demonstrates a novel noninvasive MSC therapeutic regimen for heart failure based on an intramuscular delivery route. Intramuscularly injected MSCs or MSC-conditioned medium improved ventricular function, promoted myocardial regeneration, attenuated apoptosis and fibrotic remodeling, recruited bone marrow progenitor cells, and induced the myocardial expression of multiple growth factor genes. These findings highlight the critical cross-talks between injected MSCs and host tissues, culminating in effective cardiac repair for the failing hamster heart.
Advantages of intramuscular MSC delivery.
Patients with heart failure are at an increased surgical risk. While most stem cell trials have used intracoronary infusion or intramyocardial injection for cell delivery, these delivery methods are invasive, often clinically unsuitable, and can introduce harmful scar tissue, arrhythmia, calcification, or microinfarction in the heart (5, 60–62). Systemic delivery by intravenous infusion of MSCs has been found to cause entrapment of MSCs in the lungs (2). An important issue to consider is whether the engrafted stem cells may become electromechanically coupled with resident cardiomyocytes. Myoblasts, for instance, do not exhibit optimal electrophysiological integration upon myocardial engraftment, leading to postimplantation arrythmogenesis (38). Successful clinical applications may be more feasible with a noninvasive approach, delivering MSCs to areas away from the damaged heart. Given the powerful trophic effects of MSCs, delivery of MSCs via an intramuscular route may be a superior noninvasive strategy, allowing the repeated administration of large numbers of cells and circumventing entrapments and dilution by other tissue. In addition, since we and others (9, 35, 48) have shown that MSC treatment ameliorates muscular dystrophy, intramuscular injection of MSCs is expected to be well suited for treating muscular dystrophy patients with cardiomyopathies.
Cardiac repair mediated by trophic mechanisms.
While early preclinical studies have suggested therapeutic mechanisms mediated by stem cell transdifferentiation or fusion (42, 55), it has become apparent that these mechanisms do not occur in sufficiently high frequency to account for the observed functional improvement after stem cell administration (15). Our cell tracking study (32) estimated that only 1–2% of intracoronary-infused MSCs engrafted in the pig heart, and yet this low efficiency of cell engraftment was able to significantly improve function in the porcine hibernating myocardium (50). We note that although systemic delivery of MSCs by intravenous infusion caused cell entrapment in the lungs (2), this cell delivery strategy was found to improve cardiac function in rats with acute myocardial infarction (40). These findings are consistent with increasing evidence suggesting that the cardiovascular beneficial effects of stem cell therapy are largely due to the actions of trophic factors or paracrine mediators (14, 53, 56). In addition, studies have attributed trophic activities of myoblasts and endothelial progenitor cells as critical cardioprotective mechanisms (20, 43). Our demonstration here that intramuscularly injected MSCs and MSC-conditioned medium are both therapeutically effective for treating hamster heart failure provides the ultimate proof for the critical role of trophic factors in stem cell therapy. While various single growth factor therapeutic regimens have been attempted for FGF, HGF, IGF, and VEGF, with encouraging results (36, 41, 47, 51), the MSC therapy is unique in its engagement of functionally synergistic and redundant trophic factors (7, 15) that may be required for the activation of the endogenous stem cell repair mechanism and a more sustained therapeutic effect.
Molecular cross-talks between MSCs and host tissues.
Mobilization of bone marrow progenitor cells plays an important role in tissue repair (30). The MSCs used here have been shown to express trophic factors such as HGF, LIF, G/M-CSF, SDF-1, and VEGF (34, 59), which are capable of mobilizing bone marrow progenitor cells. Along this line, the administration of G/M-CSF has been proposed as a potential new therapy for myocardial infarction (22), and intramuscular injection of LIF plasmid DNA has been found to be cardioprotective (63). We indeed detected elevated levels of circulating HGF, LIF, and G/M-CSF in MSC-treated animals, and, consistent with this finding, circulating c-Kit-positive, CD31-positive, and CD133-positive bone marrow progenitor cells were increased after MSC administration. Although unrestrained MSC secretions may cause an abnormally abundant mobilization of progenitor cells, this effect of MSCs is unlikely to be sustained because we observed a progressive loss of injected MSCs (48). The mobilized progenitor cells can repopulate the myocardium, as shown here by increased myocardial c-Kit-positive progenitor cells, and participate in endogenous cardiac repair mechanisms. Notably, we have obtained evidence that this cell mobilization mechanism becomes impaired in aged TO2 hamsters, which may explain at least in part why the MSC therapeutic regimen fails to rescue the aging heart (data not shown). This molecular cross-talk between injected MSCs and the bone marrow compartment thus illustrates the dynamic and functionally relevant signaling cascade involved in stem cell repair. The signaling cascade depicted here can further activate the myocardial expression of HGF, IGF, and VEGF genes, highlighting an additional cross-talk circuit between MSCs and the myocardium. Similar to this finding, Cho et al. (8) demonstrated that the myocardial expression of several growth factor genes, including HGF, IGF, and VEGF, was upregulated after intramyocardial stem cell implantation. Tateno et al. (54) found that implanted stem cells stimulated muscle cells to produce angiogenic factors that resulted in neovascularization. Our finding here is consistent with previous reports (27, 41) demonstrating that HGF administration could improve cardiac function in TO2 cardiomyopathic hamsters. Work is in progress to characterize the role and response of host tissues after MSC administration.
Exploring the immunomodulatory properties of MSCs.
MSCs are thought to possess unique immunomodulatory properties that can be explored for nonautologous or xenogeneic stem cell-based therapeutics (17, 57). The trophic action of MSCs can decrease the host production of inflammatory cytokines and induce T cell anergy. The immune phenotype of culture-expanded MSCs is widely described as major histocompatability complex (MHC) class I positive, MHC class II negative, CD40 negative, CD80 negative, and CD86 negative, which is regarded as nonimmunogenic, suggesting that MSCs are capable of trespassing species defense barriers. The use of MSCs in allogeneic and xenogeneic transplantation can reduce the incidence and severity of graft versus host disease (57). In this aspect, we (48) have recently demonstrated that intramuscularly injected human and porcine MSCs are well tolerated by TO2 dystrophic hamsters and that the MSC treatment leads to prominent skeletal muscle regeneration and attenuates oxidative stress without inflaming the host immune system. Given that stem cell function and potency can be impaired by aging and disease (25, 26), the use of nonautologous human MSCs isolated from healthy donors offers a major advantage since these adult stem cells can be routinely expanded in culture and thoroughly tested in advance for clinical applications.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-84590 and by the New York State Stem Cell Program.
Acknowledgments
The authors thank Dr. J. Canty for helpful discussions and Merced Leiker and Huey Lin for technical assistance.
REFERENCES
- 1.Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem 89: 1235–1249, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108: 863–868, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA 104: 14068–14073, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brann WM, Bennett LE, Keck BM, Hosenpud JD. Morbidity, functional status, and immunosuppressive therapy after heart transplantation: an analysis of the joint International Society for Heart and Lung Transplantation/United Network for Organ Sharing Thoracic Registry. J Heart Lung Transplant 17: 374–382, 1998. [PubMed] [Google Scholar]
- 5.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110: 1362–1369, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, Wen Y, Rapp JA, Kessler J. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA 299: 925–936, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 98: 1076–1084, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med 204: 3257–3269, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bari C, Dell'Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol 160: 909–918, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 309: 314–317, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Dixon IM, Ju H, Reid NL, Scammell-La Fleur T, Werner JP, Jasmin G. Cardiac collagen remodeling in the cardiomyopathic Syrian hamster and the effect of losartan. J Mol Cell Cardiol 29: 1837–1850, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Fiaccavento R, Carotenuto F, Minieri M, Fantini C, Forte G, Carbone A, Carosella L, Bei R, Masuelli L, Palumbo C, Modesti A, Prat M, Di Nardo P. Stem cell activation sustains hereditary hypertrophy in hamster cardiomyopathy. J Pathol 205: 397–407, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol 211: 27–35, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20: 661–669, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204–1219, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goineau S, Pape D, Guillo P, Ramee MP, Bellissant E. Hemodynamic and histomorphometric characteristics of dilated cardiomyopathy of Syrian hamsters (Bio TO-2 strain). Can J Physiol Pharmacol 79: 329–337, 2001. [PubMed] [Google Scholar]
- 17.Gotherstrom C Immunomodulation by multipotent mesenchymal stromal cells. Transplantation 84: S35–37, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M, Okawa K, Iwamatsu A, Okigaki T, Takahashi T, Inagaki M. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem 274: 25543–25549, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res 103: 1300–1308, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Muller S, Willhauck M, Spitzweg C, Gildehaus FJ, Munzing W, Hannappel E, Bock-Marquette I, DiMaio JM, Hatzopoulos AK, Boekstegers P, Kupatt C. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation 117: 2232–2240, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: isolation and characterization. Trends Cardiovasc Med 13: 201–206, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Ince H, Petzsch M, Kleine HD, Schmidt H, Rehders T, Korber T, Schumichen C, Freund M, Nienaber CA. Preservation from left ventricular remodeling by front-integrated revascularization and stem cell liberation in evolving acute myocardial infarction by use of granulocyte-colony-stimulating factor (FIRSTLINE-AMI). Circulation 112: 3097–3106, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Suzuki T, Mizuno S, Nakamura T, Sasaki H. Hepatocyte growth factor induces angiogenesis in injured lungs through mobilizing endothelial progenitor cells. Biochem Biophys Res Commun 324: 276–280, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM, Asahara T. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res 86: 1198–1202, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Keymel S, Kalka C, Rassaf T, Yeghiazarians Y, Kelm M, Heiss C. Impaired endothelial progenitor cell function predicts age-dependent carotid intimal thickening. Basic Res Cardiol 103: 582–586, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol 49: 2341–2349, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kondoh H, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Kitagawa-Sakakida S, Imanishi Y, Kawaguchi N, Matsuura N, Matsuda H. Combined strategy using myoblasts and hepatocyte growth factor in dilated cardiomyopathic hamsters. Ann Thorac Surg 84: 134–141, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Korbling M In vivo expansion of the circulating stem cell pool. Stem Cells 16, Suppl 1: 131–138, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Kucia M, Zhang YP, Reca R, Wysoczynski M, Machalinski B, Majka M, Ildstad ST, Ratajczak J, Shields CB, Ratajczak MZ. Cells enriched in markers of neural tissue-committed stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia 20: 18–28, 2006. [DOI] [PubMed] [Google Scholar]
- 30.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111: 589–601, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Lehrke S, Mazhari R, Durand DJ, Zheng M, Bedja D, Zimmet JM, Schuleri KH, Chi AS, Gabrielson KL, Hare JM. Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ Res 99: 553–560, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Leiker M, Suzuki G, Iyer VS, Canty JMJ, Lee T. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant 17: 911–922, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonardi E, Girlando S, Serio G, Mauri FA, Perrone G, Scampini S, Dalla Palma P, Barbareschi M. PCNA and Ki67 expression in breast carcinoma: correlations with clinical and biological variables. J Clin Pathol 45: 416–419, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin H, Shabbir A, Molnar M, Yang J, Marion S, Canty JMJ, Lee T. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulate bone marrow-derived mesenchymal stem cells. J Cell Physiol 216: 458–468, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Yan X, Sun Z, BC, QH, JL, CZR. Flk-1+ adipose-derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophy in mdx mice. Stem Cells Dev 16: 695–706, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 98: 2800–2804, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Lynch P, Lee TC, Fallavollita JA, Canty JM Jr, Suzuki G. Intracoronary administration of AdvFGF-5 (fibroblast growth factor-5) ameliorates left ventricular dysfunction and prevents myocyte loss in swine with developing collaterals and ischemic cardiomyopathy. Circulation 116: I71–76, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP, Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 41: 1078–1083, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Missihoun C, Zisa D, Shabbir A, Lin H, Lee T. Myocardial oxidative stress, osteogenic phenotype, and energy metabolism are differentially involved in the initiation and early progression of delta-sarcoglycan-null cardiomyopathy. Mol Cell Biochem 321: 45–52, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol 287: H2670–H2676, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Matsumoto K, Mizuno S, Sawa Y, Matsuda H, Nakamura T. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am J Physiol Heart Circ Physiol 288: H2131–H2139, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature 410: 701–705, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Ilzarbe M, Agbulut O, Pelacho B, Ciorba C, San Jose-Eneriz E, Desnos M, Hagege AA, Aranda P, Andreu EJ, Menasche P, Prosper F. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail 10: 1065–1072, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Ryoke T, Gu Y, Ikeda Y, Martone ME, Oh SS, Jeon ES, Knowlton KU, Ross J Jr. Apoptosis and oncosis in the early progression of left ventricular dysfunction in the cardiomyopathic hamster. Basic Res Cardiol 97: 65–75, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Sakamoto A, Ono K, Abe M, Jasmin G, Eki T, Murakami Y, Masaki T, Toyo-Oka T, Hanaoka F. Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, delta-sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc Natl Acad Sci USA 94: 13873–13878, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature 451: 937–942, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Serose A, Prudhon B, Salmon A, Doyennette MA, Fiszman MY, Fromes Y. Administration of insulin-like growth factor-1 (IGF-1) improves both structure and function of delta-sarcoglycan deficient cardiac muscle in the hamster. Basic Res Cardiol 100: 161–170, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T. Muscular dystrophy therapy by non-autologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation. In press. [DOI] [PMC free article] [PubMed]
- 49.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation 102: 1944–1949, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki G, Iyer V, Lee T, Canty JJ. Intracoronary injection of autologous mesenchymal stem cells (MSCs) increases the myocardial localization of CD133+ hematopoietic stem cells (HSCs) in swine with hibernating myocardium. Circulation 116, Suppl II: II–133, 2007.
- 51.Suzuki G, Lee TC, Fallavollita JA, Canty JM Jr. Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res 96: 767–775, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol 291: H886–H893, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips MI. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 80: 229–236, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Tateno K, Minamino T, Toko H, Akazawa H, Shimizu N, Takeda S, Kunieda T, Miyauchi H, Oyama T, Matsuura K, Nishi J, Kobayashi Y, Nagai T, Kuwabara Y, Iwakura Y, Nomura F, Saito Y, Komuro I. Critical roles of muscle-secreted angiogenic factors in therapeutic neovascularization. Circ Res 98: 1194–1202, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416: 542–545, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289: F31–F42, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Tyndall A, Walker UA, Cope A, Dazzi F, De Bari C, Fibbe W, Guiducci S, Jones S, Jorgensen C, Le Blanc K, Luyten F, McGonagle D, Martin I, Bocelli-Tyndall C, Pennesi G, Pistoia V, Pitzalis C, Uccelli A, Wulffraat N, Feldmann M. Immunomodulatory properties of mesenchymal stem cells: a review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res Ther 9: 301, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res 98: 1414–1421, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM Jr, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol 205: 194–201, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet 363: 783–784, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Widimsky P, Penicka M. Complications after intracoronary stem cell transplantation in idiopathic dilated cardiomyopathy. Int J Cardiol 111: 178–179, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation 109: 3154–3157, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Zou Y, Takano H, Mizukami M, Akazawa H, Qin Y, Toko H, Sakamoto M, Minamino T, Nagai T, Komuro I. Leukemia inhibitory factor enhances survival of cardiomyocytes and induces regeneration of myocardium after myocardial infarction. Circulation 108: 748–753, 2003. [DOI] [PubMed] [Google Scholar]