Abstract
Understanding the genetic influence on ECG time intervals and heart rate (HR) is important for identifying the genes underlying susceptibility to cardiac arrhythmias. The objective of this study was to determine the genetic influence on ECG parameters and their age-related changes in mice. ECGs were recorded in lead I on 8 males and 8 females from each of 28 inbred strains at the ages of 6, 12, and 18 mo. Significant interstrain differences in the P-R interval, QRS complex duration, and HR were found. Age-related changes in the P-R interval, QRS complex duration, and HR differed among strains. The P-R interval increased with age in 129S1/SvlmJ females. The QRS complex duration decreased with age in C57BR/J males and DBA2/J females but increased in NON/ShiLtJ females. HR decreased in C57L/J females and SM/J and P/J males but increased in BALB/cByJ males. Differences between males and females were found for HR in SJL/J mice and in the P-R interval in 129S1/SvlmJ mice. Broad-sense heritability estimates of ECG time intervals and HR ranged from 0.31 for the QRS complex duration to 0.52 for the P-R interval. Heritability estimates decreased with age for the P-R interval. Our study revealed that genetic factors play a significant role on cardiac conduction activity and age-related changes in ECG time intervals and HR.
Keywords: cardiac arrhythmia, broad-sense heritability
the cardiac conduction system initiates electrical impulses and controls the route of the impulses through the myocardium. Abnormalities in the system may cause cardiac arrhythmias, the major causes of mortality and morbidity in developed countries. More than 2.2 million Americans, particularly the elderly, suffer from atrial fibrillation (AF), the most common type of cardiac arrhythmia (33). As the percentage of elderly in the population is rising, so is the incidence of AF (17). About 479,700 Americans died of cardiac arrhythmias in 2002–more than died from stroke, lung cancer, breast cancer, and acquired immunodefiency syndrome combined (36). Of these, 185,000 died of sudden arrhythmic death, the most dangerous and difficult to prevent form of arrhythmia (7).
If new therapeutic targets for cardiac arrhythmias are to be developed, the genetic influences on cardiac electrophysiology must be identified and understood (2). Yet, the mechanisms of arrythmogenesis are complex and largely unknown (24). Although several rare inherited lethal cardiac arrhythmias are caused by mutations in genes encoding ion channels or other membrane components (11, 26), very few studies have sought to identify the genetic influences on cardiac conduction, cardiac arrhythmia, and especially aging-related cardiac electrophysiological changes in the general population. Studies using inbred mice may reveal these influences, as inbred mice have been used to dissect the genetic factors underlying many human diseases (31).
The ECG is a diagnostic and research tool that measures and records the electrical activity of the cardiac conduction system. Various ECG measurements, such as the P-R interval, QRS complex duration, QT, and QTc interval, have been used to diagnose and predict some cardiac arrhythmias and other cardiac diseases. Forty years ago, Goldbarg et al. (18) noted ECG differences between anesthetized mice of two strains. Since then, ECG characteristics of different mouse strains have been reported, but the inconsistent techniques used make it impossible to compare them. To conduct meaningful research on the cardiac conduction system, accurate and comparable ECG data from a large number of inbred strains, using conscious mice, are needed. Modern ECG technology has made collecting this data possible (6). To that end, we characterized ECG time intervals in 6-, 12-, and 18-mo-old mice from 28 inbred mouse strains. The resulting data will greatly facilitate discovering the genetic factors that regulate the cardiac conduction system and susceptibility to cardiac arrhythmias.
MATERIALS AND METHODS
Mice.
We recorded ECG characteristics of the following 28 inbred mouse strains: 129S1/SvImJ (129S1), A/J, AKR/J (AKR), BALB/cByJ (BALB), BTBR-T+tf/J, BUB/BnJ (BUB), C3H/HeJ (C3H), C57BL/10J, C57BL/6J (B6), C57BLKS/J (BLKS), C57BR/cdJ (C57BR), C57L/J (C57L), CBA/J, DBA/2J (D2), FVB/NJ (FVB), KK/HIJ, LP/J, MRL/MpJ, NOD.B10Sn-H2/J (NOD), NON/ShiLtJ (NON), NZO/H1LtJ (NZO), NZW/LacJ, P/J, PL/J, RIIIS/J (R3), SJL/J (SJL), SM/J (SM), and SWR/J. Mice were born, raised, and maintained at The Jackson Laboratory. At the ages of 6–8 wk, they were transferred from breeding rooms to a specific pathogen-free room, where they remained until ECGs were recorded. Same-sex mice were housed as 4 mice/pen in duplex polycarbonate cages (31 × 31 × 214 cm) equipped with pressurized individually ventilated mouse racks (Thoren Caging Systems) with a high efficiency particulate air-filtered supply and exhaust. Water and food pellets containing 6% fat (Lab Diet 5K52, PMI Nutritional, Bentwood, MO) were provided ad libitum. Mouse rooms were maintained at an ambient temperature of 21–23°C and a 12:12-h light-dark cycle. Colonies were regularly monitored for 15 viruses, 17 bacterial species (including Helicobacter spp., Pasteurella pneumotropica, and two Mycoplasma spp.), ecto- and endoparasites, and the microsporidium Encephalitozoon cuniculi. All animal protocols were approved by The Jackson Laboratory Animal Care and Use Committee. Mouse handling and care complied with Public Health Service animal welfare policies.
ECG recording.
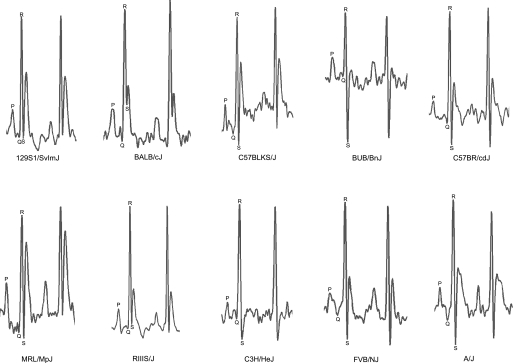
ECGs (1,244 total) of 8 males and 8 females from each of the 28 inbred strains were recorded at 6, 12, and 18 mo of age. One hundred mice died before they reached the age for ECG analysis. To eliminate circadian influences, ECGs were recorded between 8:30 and 11:00 AM. Mice were gently removed from their cages and carefully positioned on the ECG recording platform (Mouse Specifics, Boston, MA). The size and arrangement of the electrodes were configured to contact three paws, providing an ECG signal equivalent to Eithenoven lead I. To minimize stress, mice were accustomed to the platform by placing them on it for 10 min before ECGs were recorded. ECG signals were digitized at a sampling rate of 2,500 samples/s (Fig. 1). Only data from continuous recordings of 20–30 signals were used in the analysis. Each signal was analyzed using e-MOUSE (Mouse Specifics) (6). The QRS duration, P-R interval, and heart rate (HR) were measured and reported automatically. One of the authors (S. Xing) visually examined each trace for clear P, Q, R, and S peaks before accepting the automatic calculations. All raw data have been deposited in the Mouse Phenome Database (www.jax.org/phenome) and is available for download.
Fig. 1.
ECG (lead I) from 10 strains representing the variety of ECGs in the survey.
Statistical analysis.
ECGs of 1,244 mice were evaluated, and data from 1,194 mice with a regular sinus rhythm were analyzed. Data from 50 mice were excluded because of irregular rhythms and/or bad signals. Data are presented as means ± SD. One-way ANOVA was performed to test the main effect of strain and sex, and the interaction effect of strain by sex on the ECG parameters was measured at 6, 12, and 18 mo of age. If the test of interaction was significant (P < 0.001), then a t-test was applied to identify the sex effect within strain, and the post hoc pairwise multiple-comparison procedure of Tukey-Krammer honestly significant difference (HSD) test was used to analyze the difference between strain means. To test the overall effect of age on the ECG parameters measured across three time points, we conducted multivariate regression analysis. If the interaction effect of strain by age was revealed, the Tukey-Krammer HSD test was used to analyze the difference of means of each strain between groups. P < 0.05 was considered as statistically significant. All statistical analyses were performed using JMP statistical analysis software (SAS Institute).
Heritability of ECG phenotypes was calculated by estimating broad-sense heritability. Interclass correlations (r1) and coefficients of genetic determination (g2), both measures of broad-sense heritability, were calculated using methods as outlined by Festing (13). r1 was defined as the proportion of the total variation that is accounted for by differences between strains and was estimated by the following formula:
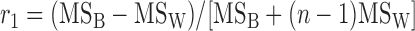 |
where MSB is the mean square of the between-strain comparison, MSW is the mean square of the within-strain comparison, and n is number of mice tested per strain with appropriate corrections for differences in mouse numbers per strain; n = 16 for our calculation. A modification of this formula gives g2, which takes into account the doubling of the additive genetic variance with inbreeding. It was calculated as follows:
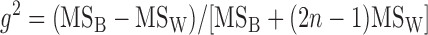 |
Whereas MSW was determined separately for each strain at each age, MSB was determined from ANOVA between stains at different ages for the estimation of broad-sense heritability.
RESULTS
Strain differences in ECG characteristics.
We calculated strain differences in ECG characteristics of 6-, 12-, and 18-mo-old males and females. We collected data as part of another larger study that included three wild-derived strains: PWD/PhJ, WSB/EiJ, and CAST/EiJ. Due to their nervous behaviors, we could not record the ECGs of conscious mice from these strains. Hence, we excluded them from our study. ECG characteristics measured included HR, the P-R interval, and the QRS complex duration. Because HR is a reciprocal of the R-R interval, and more commonly used than the R-R interval, we reported HR instead of the R-R interval. Before ECGs were recorded, all mice were weighed. No correlations between body weight and any ECG parameters were found (data not shown).
Data from 6-mo-old mice are shown in Table 1 for females and Table 2 for males. Data from 12- and 18-mo-old mice are shown in Supplemental Tables 1 and 2.1
Table 1.
HRs and ECG time intervals in females for 28 inbred mouse strains at 6 mo of age
| Strain | HR, beats/min |
P-R Interval, ms | QRS Complex Duration, ms | |||
|---|---|---|---|---|---|---|
| n | Means ± SD | n | Means ± SD | n | Means ± SD | |
| 129S1/SvImJ | 8 | 710±44b,c,d,e | 8 | 26.5±4.3a,b,c,d,e | 8 | 10.8±1.1a,b,c |
| A/J | 8 | 723±73a,b,c,d,e | 7 | 29.6±1.3a,b,c | 8 | 10.0±1.3b,c |
| AKR/J | 8 | 782±15a,b,c | 8 | 22.3±5.1d,e,f | 8 | 10.7±0.9a,b,c |
| BALB/cByJ | 7 | 697±24c,d,e | 7 | 27.7±1.6a,b,c,d,e | 7 | 12.2±1.5a |
| BTBR-T+tf/J | 6 | 722±29a,b,c,d,e | 5 | 31.5±1.8a | 6 | 9.5±0.3c |
| BUB/BnJ | 7 | 791±25a,b | 7 | 16.0±3.1f | 7 | 11.1±1.5a,b,c |
| C3H/HeJ | 8 | 744±72a,b,c,d,e | 8 | 20.7±3.8e,f | 8 | 9.6±0.9c |
| C57BL/10J | 7 | 711±63a,b,c,d,e | 7 | 30.1±4.5a,b | 7 | 11.3±1.7a,b,c |
| C57BL/6J | 8 | 735±11a,b,c,d,e | 8 | 29.4±4.5a,b,c,d | 8 | 10.3±0.7a,b,c |
| C57BLKS/J | 8 | 678±47e | 8 | 30.2±2.3a | 8 | 11.2±1.0a,b,c |
| C57BR/cdJ | 7 | 729±23a,b,c,d,e | 7 | 31.9±1.3a | 7 | 11.2±0.4a,b,c |
| C57L/J | 8 | 739±21a,b,c,d,e | 8 | 30.7±2.3a | 7 | 10.4±0.2a,b,c |
| CBA/J | 7 | 745±44a,b,c,d,e | 7 | 26.0±5.0a,b,c,d,e | 7 | 10.3±1.5a,b,c |
| DBA/2J | 8 | 712±16a,b,c,d,e | 8 | 25.9±7.1a,b,c,d,e | 8 | 11.2±1.7a,b,c |
| FVB/NJ | 8 | 771±33a,b,c,d | 8 | 23.5±3.5b,c,d,e | 7 | 10.1±0.3a,b,c |
| KK/HIJ | 8 | 728±17a,b,c,d,e | 8 | 29.5±1.9a,b,c | 8 | 11.3±1.6a,b,c |
| LP/J | 6 | 726±51a,b,c,d,e | 5 | 29.4±1.6a,b,c,d | 6 | 10.5±0.9a,b,c |
| MRL/MpJ | 6 | 726±27a,b,c,d,e | 6 | 32.0±1.0a | 6 | 10.9±0.6a,b,c |
| NOD.B10Sn-H2<b>/J | 6 | 801±33a | 5 | 29.5±1.0a,b,c,d | 6 | 10.6±0.9a,b,c |
| NON/ShiLtJ | 8 | 714±19a,b,c,d,e | 8 | 23.2±4.7b,c,d,e | 8 | 10.1±1.2a,b,c |
| NZO/H1LtJ | 8 | 737±42a,b,c,d,e | 8 | 32.2±1.4a | 8 | 11.9±0.9a,b |
| NZW/LacJ | 8 | 688±72d,e | 8 | 31.6±2.2a | 8 | 11.1±1.0a,b,c |
| P/J | 5 | 751±19a,b,c,d,e | 5 | 20.3±2.9e,f | 5 | 10.1±0.8a,b,c |
| PL/J | 10 | 744±37a,b,c,d,e | 0 | 28.5±4.5a,b,c,d | 0 | 11.8±0.9a,b |
| RIIIS/J | 8 | 672±98e | 8 | 28.8±5.3a,b,c,d | 8 | 10.5±1.7a,b,c |
| SJL/J | 8 | 699±44c,d,e | 8 | 28.4±1.4a,b,c,d | 8 | 11.7±0.7a,b |
| SM/J | 8 | 744±27a,b,c,d,e | 8 | 23.2±3.7c,d,e | 8 | 9.9±0.7b,c |
| SWR/J | 8 | 743±40a,b,c,d,e | 7 | 29.9±1.2a,b,c | 8 | 10.9±0.6a,b,c |
n, no. of mice/group. HR, heart rate. One-way ANOVA analysis was significantly different for ECG parameters among strains (P < 0.001). The Tukey-Krammer honestly significant difference (HSD) post hoc test was used for all pair comparison.
Parameter levels not connected by the same letter are significantly different (P < 0.05).
Table 2.
HRs and ECG time intervals in males for 27 inbred mouse strains at 6 mo of age
| Strain | HR, beats/min |
P-R Interval, ms | QRS Complex Duration, ms | |||
|---|---|---|---|---|---|---|
| n | Means ± SD | n | Means ± SD | n | Means ± SD | |
| 129S1/SvImJ | 7 | 753±54a,b,c,d | 7 | 28.4±5.7a,b,c | 7 | 10.2±1.1b,c |
| A/J | 8 | 703±86d,e,f | 8 | 28.0±4.0a,b,c | 8 | 10.9±1.6a,b |
| AKR/J | 7 | 766±20a,b,c,d | 7 | 21.5±4.4c,d,e,f | 7 | 11.3±1.1a,b |
| BALB/cByJ | 8 | 673±21e,f | 8 | 25.9±1.8a,b,c,d,e | 8 | 12.9±1.2a |
| BUB/BnJ | 7 | 787±20a,b | 7 | 18.1±6.1e,f | 7 | 10.5±1.3b,c |
| C3H/HeJ | 7 | 808±26a | 7 | 16.9±1.1f | 7 | 8.7±0.6c |
| C57BL/10J | 8 | 760±18a,b,c,d | 8 | 26.3±3.5a,b,c,d | 8 | 10.9±0.5a,b |
| C57BL/6J | 8 | 754±16a,b,c,d | 8 | 30.0±2.6a,b | 8 | 10.3±0.4b,c |
| C57BLKS/J | 7 | 720±36b,c,d,e,f | 7 | 29.3±3.1a,b,c | 7 | 11.0±1.0a,b |
| C57BR/cdJ | 7 | 746±23a,b,c,d | 6 | 31.8±0.8a | 6 | 11.0±0.2a,b |
| C57L/J | 8 | 734±27b,c,d,e | 8 | 31.3±1.1a | 8 | 10.3±0.3b,c |
| CBA/J | 8 | 768±19a,b,c,d | 8 | 26.6±5.1a,b,c | 8 | 10.2±1.0b,c |
| DBA/2J | 8 | 733±35b,c,d,e | 8 | 25.2±8.3a,b,c,d,e | 8 | 10.4±1.6b,c |
| FVB/NJ | 7 | 781±21a,b,c | 7 | 27.1±1.2a,b,c | 7 | 10.4±0.5b,c |
| KK/HIJ | 8 | 747±28a,b,c,d | 8 | 27.8±2.4a,b,c | 8 | 10.5±1.1b,c |
| LP/J | 8 | 777±49a,b,c | 7 | 29.4±0.9a,b | 8 | 10.4±0.6b,c |
| MRL/MpJ | 8 | 757±26a,b,c,d | 8 | 28.2±5.4a,b,c | 8 | 10.8±0.7b,c |
| NOD.B10Sn-H2<b>/J | 4 | 785±25a,b,c | 4 | 27.8±1.8a,b,c | 4 | 10.3±0.9b,c |
| NON/ShiLtJ | 8 | 707±30d,e,f | 8 | 25.7±6.5a,b,c,d,e | 8 | 11.3±2.0a,b |
| NZO/H1LtJ | 7 | 761±22a,b,c,d | 7 | 30.2±2.0a,b | 7 | 11.7±0.8a,b |
| NZW/LacJ | 7 | 709±78c,d,e,f | 6 | 32.1±1.2a | 7 | 11.4±1.7a,b |
| P/J | 7 | 770±17a,b,c,d | 7 | 19.1±2.0d,e,f | 7 | 10.9±1.3a,b |
| PL/J | 5 | 742±37a,b,c,d,e | 5 | 80.8±2.5a,b | 5 | 10.9±1.4a,b |
| RIIIS/J | 7 | 657±60f | 7 | 29.3±4.4a,b,c | 7 | 10.0±1.3b,c |
| SJL/J | 8 | 778±17a,b,c | 7 | 28.1±0.9a,b,c | 7 | 11.2±0.4a,b |
| SM/J | 8 | 764±13a,b,c,d | 8 | 23.8±5.7b,c,d,e,f | 8 | 10.4±0.9b,c |
| SWR/J | 8 | 722±51b,c,d,e,f | 7 | 32.4±1.2a | 8 | 11.3±1.1a,b |
n, no. of mice/group. One-way ANOVA analysis was significantly different for ECG parameters among strains (P < 0.001). The Tukey-Krammer HSD post hoc test was used for all pair comparison.
Parameter levels not connected by the same letter are significantly different (P < 0.05).
Sex differences in ECG time intervals and HR.
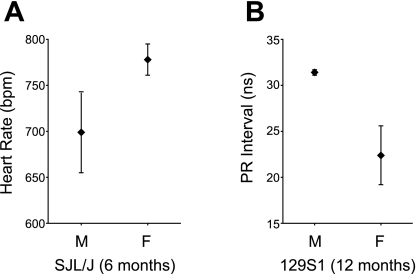
Because sex plays an important role in the development of cardiovascular disease, we analyzed its influence on cardiac conduction function. Overall, males had higher HRs than females. However, when tested for individual strains at the different time points, only SJL males at 6 mo of age had significantly higher HRs than females (778 ± 17 vs. 699 ± 44 beats/min, nominal P = 0.001; Fig. 2A). For the intervals, we only found a difference for the P-R interval at 12 mo in one strain: 129S1 females had longer P-R intervals than males (31.4 ± 0.3 vs. 22.4 ± 3.2 ms, nominal P < 0.001; Fig. 2B).
Fig. 2.
Sex differences in heart rates [in beats/min (bpm)] between 6-mo-old male (M) and female (F) SJL/J mice (A) and in P-R intervals between 12-mo-old male and female 129S1/SvImJ (129S1) mice (B). Graphs show means ± SD.
Age-related changes in HR and ECG time intervals.
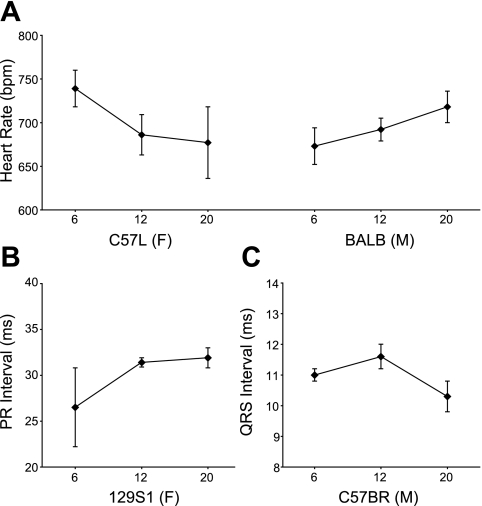
To analyze the effect of age on cardiac conduction function, we compared ECG time intervals and HRs among 6-, 12-, and 18-mo-old mice (except for AKR and SJL mice, which died before 18 mo of age). HRs significantly decreased with age in female C57L (Fig. 3A) and male SM and P/J mice but increased in male BALB mice (Fig. 3A). NZO and R3 mice showed, respectively, a decrease and an increase at 12 mo compared with 6 mo but then were at similar levels again at 18 mo of age. The P-R interval only significantly increased with age in 129S1 female mice (Fig. 3B). The QRS complex duration significantly decreased over time in C57BR males (Fig. 3C) and D2 females but increased in NON females.
Fig. 3.
Examples of age differences in heart rates for C57L/J (C57L) females and BALB/cByJ (BALB) males (A), differences in P-R intervals in 129S1 females (B), and differences in QRS intervals in C57BR/cdJ (C57BR) males (C). Graphs show means ± SD.
Heritability estimates.
r1 is defined as the proportion of the total variation accounted for by interstrain differences. g2 is a modification of r1 that controls the additive genetic variance occurring with inbreeding. Because g2 results in more conservative heritability estimates, it has been considered as a better indicator of broad-sense heritability (13). However, because it is rarely reported, we report both estimates for ECG time intervals and HRs (Table 3). Heritability estimates were different for different time intervals and HRs. Regardless of sex, P-R interval broad-sense heritability decreased significantly from 0.52 [95% confidence interval (CI): 0.48–0.60] at 6 mo to 0.22 (95% CI: 0.17–0.27) at 18 mo for r1 and from 0.37 (95% CI: 0.29–0.45) at 6 mo to 0.13 (95% CI: 0.09–0.17) at 18 mo for g2 (Table 3). Broad-sense heritability estimates were stable for the QRS complex duration (0.20 for g2 and 0.3 for r1) and HR (0.25 for g2 and 0.40 for r1) in different age groups, indicating that age did not influence heritability (variance data for these estimates and sex-specific heritability estimates are shown in Supplemental Tables 3, 4, and 5).
Table 3.
Estimates of broad-sense heritability
| g2 (95% CI) | r1 (95% CI) | |
|---|---|---|
| HR, beats/min | ||
| 6 mo | 0.27 (0.20–0.34) | 0.40 (0.31–0.49) |
| 12 mo | 0.21 (0.16–0.26) | 0.34 (0.27–0.41) |
| 18 mo | 0.24 (0.19–0.29) | 0.37 (0.30–0.44) |
| P–R interval, ms | ||
| 6 mo | 0.37 (0.29–0.45) | 0.52 (0.48–0.60) |
| 12 mo | 0.33 (0.26–0.40) | 0.47 (0.40–0.54) |
| 18 mo | 0.13 (0.09–0.17) | 0.22 (0.17–0.27) |
| QRS complex duration, ms | ||
| 6 mo | 0.20 (0.13–0.27) | 0.31 (0.23–0.39) |
| 12 mo | 0.18 (0.12–0.24) | 0.29 (0.22–0.36) |
| 18 mo | 0.16 (0.11–0.21) | 0.27 (0.21–0.33) |
g2, coefficient of genetic determination; r1, interclass correlation; CI, confidence interval.
DISCUSSION
Both genetic and environmental factors influence HR, which may consequently affect ECG measurements (35). To obtain comparable and reliable data, we controlled possible extraneous variables by the following strategies: 1) the environment (light, temperature, noise, etc.) was carefully controlled; 2) ECGs of obviously sick mice were not used; 3) to eliminate circadian influences, ECGs were measured at fixed times; and 4) ECGs were recorded in conscious mice acclimated to the instrument.
HRs, P-R intervals, and QRS complex durations differed significantly among inbred strains (Tables 1 and 2), indicating that the genetic background influenced cardiac conduction. Our study showed that BLKS mice had lower HRs and NOD mice had higher HRs, which is similar to a report by Howden et al. (22). However, they reported a generally lower HR than ours. The difference of the mean value of HR may be due to the difference of age of tested mice or the difference in animal arousal levels. Chu et al. (6) reported that HR differs among three mouse strains: 129S1, B6, and FVB. Desai et al. (12) reported that HRs of anesthetized mice differ among six strains. The HRs reported in those strains are lower than those we recorded for the same six strains, a discrepancy that may be caused by the influence of anesthetic agents on HR. Goldbarg et al. (18) reported that HR is not significantly different among anesthetized mice from strains C57BL/10, SEC/J, and their F1 progeny. Anesthetic agents can significantly affect cardiovascular parameters and may have masked important physiological phenotypes in that study.
The P-R interval represents the time from the onset of atrial depolarization to the onset of ventricular depolarization. In 6-mo-old female mice, NZO mice had the longest P-R interval, double that of the shortest interval in BUB mice (32.2 vs. 16.0 ms). Humans and dogs display a wide range of P-R intervals (3, 20), although an optimal P-R interval has been demonstrated for both species (3). It has been suggested that the P-R interval increases linearly with heart length (28), a relationship that could have been responsible for the interstrain P-R interval differences we observed. Unfortunately, no data for mouse heart length were available for us to explore this relationship.
Our study revealed that the QRS complex duration differed significantly among strains. The QRS complex duration represents ventricle excitation time and marks the time required to depolarize the entire contractile myocardium. QRS durations ranged from 8.7 ms for C3H males to 12.9 ms for BALB males, a 48% difference. Another study (6) has reported interstrain differences in the QRS complex duration. Studies (23, 30) have suggested that the distribution pattern of the Purkinje fiber network in the myocardium and ventricular wall thickness play an important role in ventricle excitation time. Interstrain differences in the QRS complex duration may reflect interstrain differences in ventricular wall thickness.
Influence of sex on ECG time intervals and HR.
Overall, males had higher HRs than females. However, when strains were tested individually, only 6-mo-old SJL males had higher HRs. Twelve-month-old 129S1 females had a significantly higher P-R intervals than 129S1 males. Intersex differences in ECG time intervals were not found in other strains. These observations agree with a previous report by Mitchell et al. (29). However, Chu et al. (6) reported that 2-mo-old males in three strains have higher ECG time intervals than same-age females: P-R intervals are higher in 129S1 and FVB males and QRS durations are higher in 129S1 and B6 males. Chu et al.'s results may reflect the age of the mice studied: intersex HR differences are present in humans (37) and rats (5); in humans, it disappears with age (37).
Age-related changes of HR and ECG time intervals.
The mouse is currently the principal mammalian model for studying biological processes, particularly those related to cardiac pathophysiology (4). However, changes of cardiac electrical activity with age in different mouse strains were previously unknown. We found that the QRS complex duration increased with age in NON females and decreased in C57BR males and D2 females and that the P-R interval increased with age in 129S1 females. HR decreased with age in SM and P/J males and C57L females. These findings support that aging slows cardiac conduction. However, in BALB males, we found an increase of HR as they get older. Although the mechanisms are not understood, slowed conduction may be one of the strongest triggers for AF (1). In humans, increased P-R intervals with aging has been observed consistently (9, 15). QRS complex duration changes in humans have not been reported. Increased ECG time intervals with age might be explained by concomitant anatomic and electrophysiological changes. The main causes of slowed conductions are decreases in cell excitability (10), intercellular coupling (8, 32), and changes in the cellular architecture of cardiac tissue(16). In rats, electrophysiological and contractile properties of the heart change significantly with aging. For example, the action potential duration increases in senescent rat hearts (40). Increased ventricular action potential duration is an indication of the QRS duration. Furthermore, the number of sinoatrial nodal cells decreases dramatically with age (25), and interventricular septal and ventricular thickness due to cardiac myocyte enlargement with aging may slow cardiac conduction.
We observed age-related ECG changes in only a few strains, suggesting that the genetic background plays a crucial role in the cardiac aging process and/or aging velocity. The aging rate of the cardiac conduction system may vary among different strains. For some strains, these changes might happen after 18 mo of age, which is out of the time window of our study. Our study showed that the QRS duration increased relatively late in A/J mice, whereas it increased relatively early (at an age equal to 61.7% of their life span) in 129S1 mice (unpublished observations from our aging study).
Genetic contribution to the variation of HR and ECG time intervals.
Heritability is defined as the proportion of the total phenotypic variance that results from genetic factors (39). Broad-sense heritability is the proportion of total phenotypic variation due to all genetic effects. In our study, we based the estimate on r1 and g2, a method developed by Festing (13).
We obtained estimates of ECG heritability in mouse strains that had no pathological differences in their cardiac conduction system (14) and that were housed in an environment that did not affect cardiac conduction. Because our study was a cross-sectional study, we did not record ECGs of the same mice at different ages, and we could not estimate the intrastrain ECG variances of different age groups. Therefore, we could not estimate the quantitative genetic contribution to age-related changes of ECG measurements. The P-R interval seemed to be most influenced by genetic factors: 0.52 (95% CI: 0.48–0.60) for r1 and 0.37 (95% CI: 0.29–0.45) for g2; the QRS complex duration seemed to be the least influenced: 0.31 (95%CI: 0.23–0.39) for r1 and 0.20 (95%CI: 0.13–0.27) for g2 (Table 2). To our knowledge, these are the first evaluations of the heritability of ECG time intervals in mice. Heritability studies in humans are problematic because of the diversity of human populations and the difficulty of controlling their environments (38). Three ECG studies using twins have yielded conflicting results. Mathers et al. (27) measured ECG time intervals in 36 monozygotic and 19 dizygotic twin pairs and concluded that, whereas the QRS duration and QT interval are greatly influenced by genetic factors, the P-R interval and HR are not. Havlik et al. (21) measured ECG time intervals in 355 pairs of middle-aged male twins and found that, whereas the P-R interval and HR are significant affected by genetic factors (heritability estimates: 0.54 and 0.34, respectively), the QRS duration and QT interval are not. Russell et al. (34) measured ECG time intervals in 251 pairs of adult male twins and found that the R-R interval (HR) is significantly influenced by genetic factors (accounting for 77% and 36% of the variability, respectively), whereas the QRS complex duration is not. These inconsistent results may be due to differences in the twin samples used and population-specific differences in gene pools. Most studies with adult twins were limited by small numbers of subjects or relatively high intra-ECG reader variability and low interreader correlation. The small sample size may obscure small but significant heritable components of ECG parameters. Hanson et al. (19) reanalyzed heritability in ECG data pooled from 73 pairs of twins and 2 other studies and reported estimates similar to ours in mice (ranging from 30% to 60%).
Among the ECG time intervals we recorded, the P-R interval was the most significantly heritable component. The genetic influence on P-R interval was much more evident at 6 mo than at 18 mo: the heritability estimates decreased from 0.52 at 6 mo to 0.22 at 18 mo. This change resulted from both the increase of intrastrain variance and the decrease of interstrain variance (Supplemental Tables 3 and 4). Our results indicate that aging increased the environmental variance and also changed the expression of genes related to the P-R interval.
According to Visscher et al. (39), a large heritability implies a strong correlation between phenotype and genotype, so that loci affecting a trait can be more easily detected. We demonstrated that genetic factors contribute significantly to the HR, QRS complex duration, and P-R interval in mice. Aging-related changes in these indexes differed among strains. Our study provides the information necessary for investigators to choose the appropriate mouse strains to cross to identify the genes that regulate HR, ECG time intervals, and aging-related cardiac conduction changes.
GRANTS
This work was funded by National Institute of Aging Nathan Shock Center Grant AG-25707 and by grants from the Ellison Medical Foundation (to B. J. Paigen).
Acknowledgments
The authors are grateful to Maarten van den Berg for critical review of the manuscript and Jesse Hammer and Thomas G. Hampton for assistance with the graphics.
Footnotes
Supplemental material for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Allessie MA, Konings K, Kirchhof CJ, Wijffels M. Electrophysiologic mechanisms of perpetuation of atrial fibrillation. Am J Cardiol 77: 10A–23A, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Arking DE, Chugh SS, Chakravarti A, Spooner PM. Genomics in sudden cardiac death. Circ Res 94: 712–723, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Benchimol A, Li YB, Dimond EG, Voth RB, Roland AS. Effect of heart rate, exercise, and nitroglycerin on the cardiac dynamics in complete heart block. Circulation 28: 510–519, 1963. [DOI] [PubMed] [Google Scholar]
- 4.Berul CI, Bevilacqua LM. Molecular biological and genetic approaches to the evaluation of inherited electrophysiologic disorders. Drugs Today (Barc) 38: 351–364, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Chandler MP, DiCarlo SE. Acute exercise and gender alter cardiac autonomic tonus differently in hypertensive and normotensive rats. Am J Physiol Regul Integr Comp Physiol 274: R510–R516, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Chu V, Otero JM, Lopez O, Morgan JP, Amende I, Hampton TG. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol 1: 6, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. JAMA 288: 3008–3013, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Cole WC, Picone JB, Sperelakis N. Gap junction uncoupling and discontinuous propagation in the heart. A comparison of experimental data with computer simulations. Biophys J 53: 809–818, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craft N, Schwartz JB. Effects of age on intrinsic heart rate, heart rate variability, and AV conduction in healthy humans. Am J Physiol Heart Circ Physiol 268: H1441–H1452, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Cranefield PF, Wit AL, Hoffman BF. Conduction of the cardiac impulse. 3. Characteristics of very slow conduction. J Gen Physiol 59: 227–246, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res 95: 1035–1041, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol Heart Circ Physiol 272: H1053–H1061, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Festing MF Notes on genetic analysis. In: Inbred Strains in Biomedical Research, edited by Festing MF. New York: Oxford Univ. Press, 1979, p. 80–98.
- 14.Festing MFW Origins and characteristics of inbred strains of mice. In: Genetic Variants and Strains of the Laboratory Mouse (3rd ed.), edited by Lyon MF, Rastan S, Brown SDM, and the International Committee on Standardized Genetic Nomenclature for Mice. New York: Oxford Univ. Press, 1996, p. 1537–1576.
- 15.Fleg JL, Das DN, Wright J, Lakatta EG. Age-associated changes in the components of atrioventricular conduction in apparently healthy volunteers. J Gerontol 45: M95–M100, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Gardner PI, Ursell PC, Fenoglio JJ Jr, Wit AL. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation 72: 596–611, 1985. [DOI] [PubMed] [Google Scholar]
- 17.Go AS The epidemiology of atrial fibrillation in elderly persons: the tip of the iceberg. Am J Geriatr Cardiol 14: 56–61, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Goldbarg AN, Hellerstein HK, Bruell JH, Daroczy AF. Electrocardiogram of the normal mouse, Mus musculus: general considerations and genetic aspects. Cardiovasc Res 2: 93–99, 1968. [DOI] [PubMed] [Google Scholar]
- 19.Hanson B, Tuna N, Bouchard T, Heston L, Eckert E, Lykken D, Segal N, Rich S. Genetic factors in the electrocardiogram and heart rate of twins reared apart and together. Am J Cardiol 63: 606–609, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Hartzler GO, Maloney JD, Curtis JJ, Barnhorst DA. Hemodynamic benefits of atrioventricular sequential pacing after cardiac surgery. Am J Cardiol 40: 232–236, 1977. [DOI] [PubMed] [Google Scholar]
- 21.Havlik RJ, Garrison RJ, Fabsitz R, Feinleib M. Variability of heart rate, P-R, QRS and Q-T durations in twins. J Electrocardiol 13: 45–48, 1980. [DOI] [PubMed] [Google Scholar]
- 22.Howden R, Liu E, Miller-Degraff L, Keener HL, Walker C, Clark JA, Myers PH, Rouse DC, Wiltshire T, Kleeberger SR. The genetic contribution to heart rate and heart rate variability in quiescent mice. Am J Physiol Heart Circ Physiol 295: H59–H68, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jay PY, Harris BS, Buerger A, Rozhitskaya O, Maguire CT, Barbosky LA, McCusty E, Berul CI, O'Brien TX, Gourdie RG, Izumo S. Function follows form: cardiac conduction system defects in Nkx2-5 mutation. Anat Rec A Discov Mol Cell Evol Biol 280: 966–972, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104: 569–580, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Lakatta EG Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 73: 413–467, 1993. [DOI] [PubMed] [Google Scholar]
- 26.London B, Jeron A, Zhou J, Buckett P, Han X, Mitchell GF, Koren G. Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel. Proc Natl Acad Sci USA 95: 2926–2931, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathers JA, Osborne RH, Degeorge FV. Studies of blood pressure, heart rate, and the electrocardiogram in adult twins. Am Heart J 62: 634–642, 1961. [DOI] [PubMed] [Google Scholar]
- 28.Meijler FL, Strackee J, Stokhof AA, Wassenaar C. Scaling of atrioventricular transmission in mammalian species: an evolutionary riddle! J Cardiovasc Electrophysiol 13: 826–830, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol Heart Circ Physiol 274: H747–H751, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Oosthoek PW, Viragh S, Lamers WH, Moorman AF. Immunohistochemical delineation of the conduction system. II: the atrioventricular node and Purkinje fibers. Circ Res 73: 482–491, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Paigen K One hundred years of mouse genetics: an intellectual history. II. The molecular revolution (1981–2002). Genetics 163: 1227–1235, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohr S, Kucera JP, Kleber AG. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res 83: 781–794, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115: e69–e171, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Russell MW, Law I, Sholinsky P, Fabsitz RR. Heritability of ECG measurements in adult male twins. J Electrocardiol Suppl 30: 64–68, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Seed WA, Noble MI, Oldershaw P, Wanless RB, Drake-Holland AJ, Redwood D, Pugh S, Mills C. Relation of human cardiac action potential duration to the interval between beats: implications for the validity of rate corrected QT interval (QTc). Br Heart J 57: 32–37, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turakhia M, Tseng ZH. Sudden cardiac death: epidemiology, mechanisms, and therapy. Curr Probl Cardiol 32: 501–546, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol 31: 593–601, 1998. [DOI] [PubMed] [Google Scholar]
- 38.van Asselt KM, Kok HS, van der Schouw YT, Peeters PH, Pearson PL, Grobbee DE. Role of genetic analyses in cardiology: part II: heritability estimation for gene searching in multifactorial diseases. Circulation 113: 1136–1139, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era–concepts and misconceptions. Nat Rev Genet 9: 255–266, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Walker KE, Lakatta EG, Houser SR. Age associated changes in membrane currents in rat ventricular myocytes. Cardiovasc Res 27: 1968–1977, 1993. [DOI] [PubMed] [Google Scholar]