Abstract
We have recently shown that the inability of repetitive ischemia (RI) to activate p38 MAPK (p38) and Akt in metabolic syndrome [JCR:LA-cp (JCR)] rats was associated with impaired coronary collateral growth (CCG). Furthermore, Akt and p38 activation correlated with optimal O2−· levels and were altered in JCR rats, and redox-sensitive p38 activation was required for CCG. Here, we determined whether the activation of Src, a possible upstream regulator, was altered in JCR rats and whether redox-dependent Src and Akt activation were required for CCG. CCG was assessed by myocardial blood flow (microspheres) and kinase activation was assessed by Western blot analysis in the normal zone and collateral-dependent zone (CZ). RI induced Src activation (∼3-fold) in healthy [Wistar-Kyoto (WKY)] animals but not in JCR animals. Akt inhibition decreased (∼50%), and Src inhibition blocked RI-induced CCG in WKY rats. Src inhibition decreased p38 and Akt activation. Myocardial oxidative stress (O2−· and oxidized/reduced thiols) was measured quantitatively (X-band electron paramagnetic resonance). An antioxidant, apocynin, reduced RI-induced oxidative stress in JCR rats to levels induced by RI in WKY rats versus the reduction in WKY rats to very low levels. This resulted in a significant restoration of p38 (∼80%), Akt (∼65%), and Src (∼90%) activation in JCR rats but decreased the activation in WKY rats (p38: ∼45%, Akt: ∼65%, and Src: ∼100%), correlating with reduced CZ flow in WKY rats (∼70%), but significantly restored CZ flow in JCR rats (∼75%). We conclude that 1) Akt and Src are required for CCG, 2) Src is a redox-sensitive upstream regulator of RI-induced p38 and Akt activation, and 3) optimal oxidative stress levels are required for RI-induced p38, Akt, and Src activation and CCG.
Keywords: signal transduction, oxidative stress, coronary artery disease, syndrome X
cardiac ischemia-reperfusion injury is a biphasic process where the prolonged reduction in blood flow (ischemia) initiates myocardial cell death followed by further injury upon reperfusion, leading to stunning or necrosis. The massive amounts of ROS released during reperfusion have been implicated as the major cause of myocardial tissue death (25). In contrast, transient repetitive ischemia (RI) renders the myocardium tolerant to ischemia-reperfusion (16, 20). The definitive driving force for coronary collateral growth (CCG) is still debated. Ischemia is a well-accepted stimulus for angiogenesis, but elevated fluid shear stress, resulting from occlusion-induced pressure gradients across the coronary circulation, has been proposed to drive collateral remodeling (13, 27, 34, 35). None of these studies have definitively excluded ischemia as the contributing factor to collateral growth. The suggestion that ischemia is not a driving force for CCG rests on a single study (24) in a model of canine coronary occlusion, which observed only epicardial vascular growth while the subendocardium became ischemic.
Several studies now support the view that RI is an important driving force for CCG (7, 10, 14, 17, 23, 42, 46). RI induced CCG, as evident by increased collateral-dependent blood flow and cardiac function recovery, in a model of coronary occlusion in dogs (14), pigs (17), and rats (42). Another study (46) convincingly demonstrated that the exact number and temporal spacing of occlusions, and, thus, ischemic events, was critically important for collateral growth in a canine model of coronary occlusion. In that study (46), three different occlusion protocols produced exactly the same pressure gradient across preexisting collaterals but strikingly different increases in blood flow and cardiac function recovery; thus, this study strongly suggests that pressure gradients by themselves cannot sufficiently dilate preexisting collaterals and demonstrates a critical importance for ischemia in CCG. Furthermore, ischemic periods of 2 min but not 15 s stimulated CCG in dogs (23), providing further evidence for ischemia as a critical factor for CCG.
The specific role of ROS and ROS-sensitive signaling in RI-induced CCG has not been established. N-acetylcysteine (NAC) abrogated RI-induced CCG in a canine model, indicating that ROS generated by RI mediate CCG (11). We (29, 31) have recently shown that complete blockade of myocardial O2−· production abrogated CCG in a healthy rat [Wistar-Kyoto (WKY)] model of RI (29, 31), suggestive of a requirement for some amount of ROS in CCG.
In contrast, a large increase in myocardial O2−· production in healthy animals abrogated CCG (29, 31), suggestive of a detrimental effect of too high levels of oxidative stress on CCG. Elevated oxidative stress is a hallmark of conditions, including metabolic syndrome, that are strong independent risk factors for coronary artery disease. We have recently demonstrated that RI-induced CCG was severely impaired in a rat model of metabolic syndrome [the JCR:LA-cp (JCR) rat] (29) as well as in a rat model of insulin resistance and obesity [the Zucker obese fatty (ZOF) rat]; however, concomitant administration of extracellular SOD (ecSOD) partially restored it (12). The administration of VEGF alone failed to improve CCG in the ZOF rat (12). However, angiotensin II type 1 receptor (AT1R) blockade significantly restored CCG in JCR animals, which was associated with a significant reduction in myocardial O2−· levels (29). Decreasing ROS improved mesenteric collateral development in a rat model of spontaneous hypertension [the sponteneously hypertensive rat (SHR)] (22).
Taken together, these observations are suggestive of a requirement for optimal levels of oxidative stress for CCG. However, the effect of AT1R blockade on CCG may be, at least in part, independent of its effect on O2−· production, and, thus, the direct contribution of ROS to CCG regulation in metabolic syndrome has not been established. It is possible, if not likely, that multiple AT1R-dependent but ROS-insensitive pathways are also involved in the regulation of RI-induced CCG. Likewise, ROS-sensitive but AT1R-independent signaling pathways might be involved in the regulation of CCG. Thus, it is important to alter ROS levels directly to be able to determine the relative contribution of ROS on CCG and the modulation of signaling pathways that regulate it in an animal model of metabolic syndrome. Furthermore, quantitative measurements of oxidative stress have not been conducted in any of the very few studies examining the effects of oxidative stress manipulation in vivo, possibly accounting for the discrepant results between several of these studies.
The mechanisms by which these ROS generated in response to transient ischemia regulate CCG are unclear. Redox-dependent regulation of signal transduction in cardiac and vascular cells and in vitro angiogenesis assays has been well documented (9, 18, 38). However, neither signal transduction nor its redox dependency have been extensively studied in response to RI or in collateral growth in vivo. Our previous studies have demonstrated that inhibition of SOD and of flavin-containing oxidases blocked p38 activation in healthy WKY animals (31) and that AT1R inhibition-mediated reduction or an angiotensin II-mediated increase in myocardial O2−· resulted in altered p38 and Akt activation (29). However, no study has examined the effect of direct reduction in oxidative stress on Akt or Src activation in CCG, collateral growth, or angiogenesis. A single study (19) has reported that the genetic knockout of the immediate upstream regulator of Akt, phosphatidylinositol 3-kinase, exhibited reduced collateral growth induced by hindlimb ischemia. We (31) have previously demonstrated that p38 is required for RI-induced CCG (31). We (29) have also shown that RI activated p38 and Akt in WKY rats but failed to activate these kinases in JCR rats, and treatments that restored CCG and lowered oxidative stress in JCR rats were associated with the restoration of kinase activation. These results, however, do not conclusively demonstrate a requirement for Akt activation in CCG. Neither RI-induced activation of Src nor its possible involvement in the regulation of collateral growth has been studied to date. Furthermore, no study except our previous study (29) has examined alterations in RI-mediated signal transduction in an animal model of metabolic syndrome, diabetes, or hypertension; thus, the signaling profile for Src in JCR animals is unknown.
Therefore, in the present study, we determined 1) the effect of lowering of oxidative stress on RI-induced CCG in healthy (WKY) versus metabolic syndrome (JCR) animals, 2) O2−· concentrations and the myocardial redox state in WKY versus JCR animals quantitatively by X-band electron paramagnetic resonance (EPR), 3) whether Akt and Src were required for coronary collateral development, and 4) the effect of lowering of oxidative stress on RI-induced p38, Akt, and Src activation in WKY versus JCR animals.
MATERIALS AND METHODS
Materials.
Apocynin and the Src inhibitor (PP2) were from Invitrogen, and the Akt inhibitor [1L6-hydroxymethyl-chiro-inositol-2-(R)-2-O-methyl-3-O-octadecyl-sn-glycerocarbonate] was from Calbiochem. Dominant negative (DN; Thr308Ala and Ser473Ala) Akt adenovirus (DN-Akt-Adv) was a generous gift from Dr. Hanjoong Joo (30).
Rat model of CCG and RI.
Male WKY or JCR rats (3–4 mo old) were used for 10-day implantation of a pneumatic occluder over the left anterior descending coronary artery (LAD) as previously described (42). The RI protocol consisted of eight 40-s occlusions once every 20 min (2 h, 20 min total) followed by a rest period of 5 h, 40 min. This 8-h cycle was repeated 3 times/day for 10 days. JCR (Russell) rat (Charles River Laboratories) is a cross between the lean LA/N and spontaneously hypertensive obese rat developed in the laboratory of Dr. Carl Hansen at the National Institutes of Health (NIH), sent to Dr. Jim Russell at the University of Alberta in Edmonton, ON, Canada, in 1978, and to Charles River Laboratories in 2003 (http://www.criver.com/en-US/ProdServ/ByType/ResModOver/ResMod/Pages/JCRRat.aspx). At 3–4 mo, the JCR rat is hypertensive [mean arterial blood pressure: 156 ± 11 mmHg vs. the WKY rat (105 ± 12 mmHg)], obese [body weight: 650 ± 23 g vs. the WKY rat (320 ± 12 g)], hyperlipidemic [triglycerides: 2.85 ± 0.05 mmol/l vs. the WKY rat (0.76 ± 0.06mmol/l)] (33), hyperglycemic [glucose: 567 ± 44 mg/dl vs. the WKY rat (83 ± 6 mg/dl)], and insulin resistant [insulin: 1,100 ± 36 nmol/l vs. the WKY rat (250 ± 20 nmol/l)] (33), thus mimicking human metabolic syndrome. All surgical procedures were performed in accordance with the Animal Welfare Act and were approved by the Institutional Animal Care and Use Committees of the University of South Alabama and Louisiana State University Health Sciences Center.
Myocardial and collateral-dependent blood flow measurements.
Microspheres (5 × 105) labeled with 57Co [at day 0 of RI (initial surgery)] or 103Ru (at day 10 of RI) were injected into the left ventricle (LV) during LAD occlusion according to Buckberg's rules (3), so that >400 microspheres were present in each tissue sample (∼1,500 for JCR rats and ∼2,000 for WKY rats). To ensure that microspheres were well mixed at the site of injection, an arterial reference blood sample (carotid) was obtained for every microsphere measurement (days 0 and 10 of RI). In some animals, kidneys were also collected, and radioactivity was measured to ensure equal distribution between the two kidneys. Blood pressure and heart rate were monitored during and for 10 min postmicrosphere injection to ensure that no hemodynamic changes occurred due to microsphere injection. Blood flows to the normal zone (NZ; in ml·min−1·g−1) and collateral-dependent zones (CZ; in ml·min−1·g−1) were calculated from the following formula: blood flow = [(radioactive counts in myocardial tissue) × (blood reference withdrawal rate)/(radioactive count in blood reference)]/(weight of myocardial tissue). All experiments were n = 8 except for DN-Akt-Adv, where n = 3. Data were analyzed by two-way ANOVA followed by a t-test. P < 0.05 determined statistical significance. CZ and NZ flows were measured in WKY and JCR rats of the following groups: sham (animal was instrumented but did not undergo RI) (29), RI (29), RI + apocynin (0.25 mg/ml in drinking water for 10 days), RI + SOD (100 U/g by intravenous injection, Sigma); and in WKY animals only of the following groups: RI + Akt inhibitor (5.8 mg·kg−1·day−1 by intraperitoneal injection every day of the RI protocol), RI + DN-Akt-Adv (2 × 1012 plaque-forming units by direct injection into the LV cavity at the initial surgery during a 20-s aortic occlusion followed by a 40-s LAD occlusion), and RI + Src inhibitor [3 mg·kg−1·day−1 by intraperitoneal injection every day of the RI protocol (10 days)].
In additional groups of WKY rats (RI and RI + apocynin) and JCR rats (RI and RI + apocynin) (n = 3 animals/group), blood flow was measured before and after the intravenous administration of adenosine (5 × 105 M) to exclude possible confounding effects of vascular reactivity on the assessment of CCG.
Myocardial oxidative stress measurements [O2−· and myocardial redox state (oxidized-to-reduced thiol ratio)].
O2−· production was evaluated using dihydroethidium (DHE) and X-band EPR. DHE was injected into the LV (60 μg/kg) as previously described (29, 31). DHE fluorescence was visualized on 5-μm cryosections [n = 5 hearts/group, 5 consecutive sections/heart (excitation/emission: 518/605 nm)]. A Bruker EMX spectrometer was used for X-band EPR measurements of O2−· using 1-hydroxy-3-carboxy-pyrrolidine (CP-H, Alexis) as a spin trap. Animals underwent two consecutive periods of ischemia-reperfusion and were then killed, the heart was removed, and the CZ separated from the NZ. CP-H (238 μg/100 mg tissue) was added to the tissue samples immediately. The tissue was then homogenized by sonication on ice and frozen in liquid nitrogen until EPR measurements. The O2−· concentration was calculated from arbitrary units (AU; 3.4 × 106 AU/nM). P < 0.05 determined statistical significance. Measurements of oxidized-to-reduced thiol ratios were obtained in an identical manner except that bis-(2,2,5,5-tetramethyl-3-imidazoline-1-oxyl-4-yl)disulfide (RSSR; 10 μg/100 mg tissue, Alexis) was used as a spin trap, and the oxidized-to-reduced thiol ratio was calculated from a standard curve of known amounts of oxidized/reduced glutathione (GSH/GSSG). EPR measurements were from n = 5 animals/group. Data were analyzed by two-way ANOVA followed by a t-test. P < 0.05 determined statistical significance.
Western blot analysis.
Hearts were excised, the LV was dissected, and the CZ was separated from the NZ and snap frozen in liquid nitrogen before homogenization in lysis buffer containing 0.1% SDS and 1% Triton X-100 as previously described (29). Equal amounts of protein (30 μg) were separated by SDS-PAGE and transferred to Hybond-ECL nitrocellulose membranes. Phospho-specific anti-p38 (Thr180/Tyr182), Akt (Ser473), and Src (Tyr416); anti-total p38, total Akt, and total Src (Cell Signaling); and anti-PCNA (Cell Signaling) and anti-smooth muscle (SM)-specific α-actin (Sigma Aldrich) were used for Western blot analysis. Bands were visualized by ECL (Amersham) and quantified using NIH Image software. For phospho-Akt, phospho-p38, and phospho-Src, data were normalized to protein expression, which did not change in any treatment group. For PCNA and SM α-actin, data were normalized to GAPDH expression, which did not change in any group. Experiments were n = 4 animals/group (n = 3 animals/group for PCNA and SM α-actin) and analyzed by two-way ANOVA followed by a t-test. P < 0.05 determined statistical significance.
Immunohistochemistry.
Paraffin-embedded cardiac tissue was cut into 5-μm sections and deparaffinized. Anti-SM α-actin antibody or anti-PCNA antibody (the same antibodies as used for Western blot analysis) followed by Alexa 568-labeled (Invitrogen) or horseradish peroxidase-labeled (Cell Signaling) secondary antibodies were used for immunohistochemistry. Representative images were obtained on either Nikon fluorescent (×10) or light (×40) microscopes.
RESULTS
Decreased oxidative stress compromises CCG in healthy (WKY) rats but improves CCG in metabolic syndrome (JCR) rats.
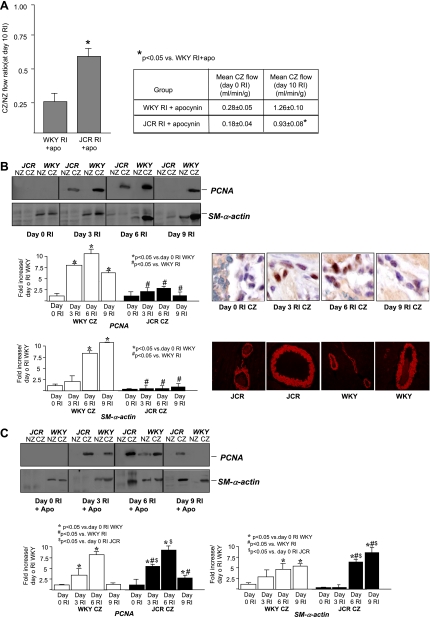
Our recent study (29) has demonstrated that RI induced CCG in healthy WKY animals [at day 10 of RI, mean collateral-dependent (CZ) flow was 1.84 ± 0.07 vs. 0.28 ± 0.09 ml·min−1·g−1 in WKY sham rats] but completely failed to induce CCG in JCR animals (at day 10 of RI, CZ flow was 0.20 ± 0.09 ml·min−1·g−1). In the present study, an antioxidant, apocynin [0.25 mg/ml in drinking water for 14 days (2 days before occluder implantation, 2 days of recovery, and 10 days of RI)], greatly decreased collateral-dependent blood flow in WKY rats (Fig. 1A). In contrast, in JCR animals, which completely fail to grow collaterals in response to RI (29), apocynin significantly increased collateral-dependent blood flow (Fig. 1A). Thus, apocynin partially but significantly restored RI-induced CCG in the JCR rat but abrogated CCG in the WKY rat. Since a characteristic of the JCR phenotype is elevated blood pressure, and a recent study (45) has suggested that apocynin lowers blood pressure, we measured systemic blood pressure in WKY and JCR animals for the duration of treatment, and observed no effect of apocynin (data not shown).
Fig. 1.
A: rats underwent the 10-day repetitive ischemia (RI) protocol and were treated with apocynin (Apo; 0.25 mg/ml in drinking water) as shown. Left: coronary flow was measured in the collateral-dependent zone (CZ) and normal zone (NZ) using radioactive microspheres during left anterior descending coronary artery (LAD) occlusion and expressed as the ratio of CZ to NZ flows at day 10 of RI. Right: coronary flow was measured on days 0 and 10 of the RI protocol, and flow in the LAD-dependent zone (CZ) during LAD occlusion was expressed in milliliters per gram per minute. WKY, Wistar-Kyoto (healthy) rats; JCR, JCR:LA-cp (metabolic syndrome) rats. *P < 0.05 vs. WKY rats in the RI + Apo group. B, top left: Western blots with anti-PCNA and anti-smooth muscle (SM) α-actin antibodies in the NZ and CZ of WKY and JCR rats on days 0, 3, 6, and 9 of RI. Bottom left, cumulative data as area × density [in arbitrary units (AU)] in the CZ. *P < 0.05 vs. WKY rats in the sham group; #P < 0.05 vs. WKY rats in the RI group. Top right, immunohistochemistry in sections of the arterial wall using anti-PCNA antibody in the CZ of WKY rats at days 0, 3, 6, and 9 of RI. Bottom right, immunohistochemistry with anti-SM α-actin antibody in WKY and JCR myocardium on day 0 on RI. C, top: Western blots with anti-PCNA and anti SM-α-actin antibodies in the NZ and CZ of WKY and JCR rats on days 0, 3, 6, and 9 of RI + Apo. Bottom, cumulative data as area × density (in AU) in the CZ. *P < 0.05 vs. WKY rats in the sham + Apo group; #P < 0.05 vs. WKY rats in the RI + Apo group; $P < 0.05 vs. JCR rats in the RI group on day 0.
To exclude possible confounding effects of vascular reactivity on the assessment of CCG, separate groups of animals were treated with adenosine (5 × 105 M). Although systemic blood pressure decreased by an average of 26 ± 4 mmHg, there were no significant differences in blood flow in WKY rats (RI and RI + apocynin groups) or in JCR rats (RI and RI + apocynin groups) (n = 3 animals/group) before versus after the administration of adenosine (after adenosine, the CZ-to-NZ ratio was 0.82 ± 0.08 for WKY rats in the RI group, 0.28 ± 0.06 for WKY rats in the RI + apocynin group, 0.1 ± 0.06 for JCR rats in the RI group, and 0.64 ± 0.05 for JCR rats in the RI + apocynin group). Flows (in ml·min−1·g−1) in the NZ and CZ were, likewise, nearly identical before and after the addition of adenosine.
In addition, since a salient feature of true collateral vessels (vs. endothelial tubes) is the existence of a medial vascular smooth muscle layer, and thus CCG, like angiogenesis, is characterized by endothelial cell proliferation but, in contrast to angiogenesis, is also characterized by vascular smooth muscle cell proliferation and a 10-fold increase in SM-specific α-actin content in rodents (4, 34), we assessed cell proliferation as well as SM α-actin content quantitatively on successive days (days 0, 3, 6, and 9) of the RI protocol in these groups. Proliferation, assessed by anti-PCNA Western blot analysis and immunohistochemistry, was restricted specifically to the CZ in all groups, maximal at day 6 of RI, and much greater in WKY versus JCR animals at all time points (Fig. 1B). Apocynin significantly decreased RI-induced cell proliferation in WKY animals but significantly increased it in JCR animals (Fig. 1C). SM α-actin expression was significantly lower in JCR versus WKY animals at baseline (day 0 of RI; Fig. 1B). RI greatly increased SM α-actin expression in WKY animals with a maximal increase at day 9 of RI (Fig. 1B). In contrast, no increase was observed in JCR animals at any day of the RI protocol (Fig. 1B). Apocynin significantly decreased the RI-induced increase in SM α-actin expression in WKY animals but increased it in JCR animals (Fig. 1C). To verify the selectivity of the SM α-actin antibody for vascular smooth muscle cells, we performed immunohistochemistry on cardiac sections and found specific staining of the coronary vascular smooth muscle (Fig. 1B).
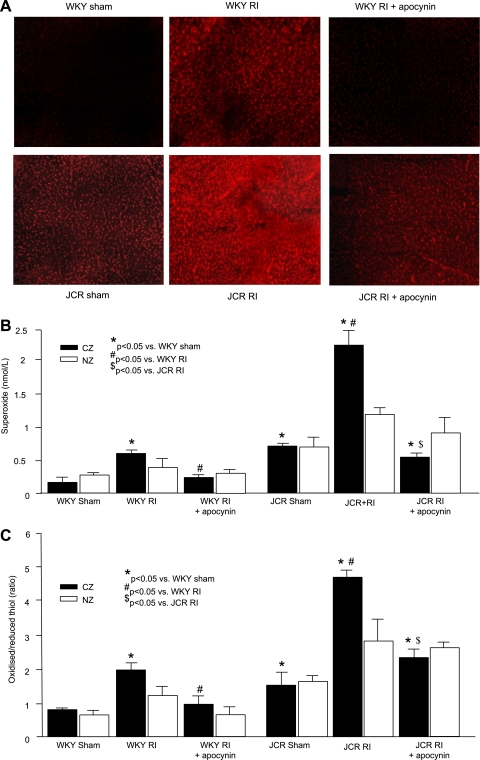
We initially verified that in vivo apocynin treatment was indeed effective in reducing myocardial O2−· in the CZ of WKY and JCR animals by DHE (Fig. 2A). Next, we used X-band EPR to obtain quantitative measurements of two separate indexes of myocardial oxidative stress: 1) O2−· levels and 2) oxidized-to-reduced thiol ratios as indicative of the myocardial redox state in response to apocynin compared with baseline (sham) and those generated by RI. In accordance with our recently published results (29) on a small sample size (n = 3) (29), basal O2−· levels in the CZ were significantly higher in JCR rats compared with WKY rats (0.75 ± 0.08 vs. 0.12 ± 0.04 nmol/l), and RI induced an increase that was much greater in JCR versus WKY animals (2.32 ± 0.25 vs. 0.61 ± 0.08 nmol/l; Fig. 2B). Our newly acquired results showed that the oxidized-to-reduced thiol ratio followed a similar trend (JCR rat in the sham group: 1.5 ± 0.04 vs. WKY rats in the sham group: 0.8 ± 0.1 and JCR rats in the RI group: 4.8 ± 0.2 vs. WKY rats in the RI group: 2.0 ± 0.2; Fig. 2C). Apocynin reduced myocardial O2−· levels and the oxidized-to-reduced thiol ratio in both WKY and JCR animals (WKY rats in the RI + apocynin group: 0.25 ± 0.04 nmol/l O2−· and 1.0 ± 0.3 oxidized-to-reduced thiol ratio vs. JCR rats in the RI + apocynin group: 0.58 ± 0.07 nmol/l O2−· and 2.3 ± 0.3 oxidized-to-reduced thiol ratio; Fig. 2, B and C). Strikingly, apocynin treatment in JCR animals resulted in O2−· levels and oxidized-to-reduced thiol ratios that were similar (not statistically different) to those found in WKY animals in response to RI, which allowed for maximal CCG. O2−· levels and oxidized-to-reduced thiol ratios in the NZ were also measured, and although values for both parameters followed the trend observed in the CZ, changes in the NZ were much smaller in magnitude and, in the case of oxidized-to-reduced thiol ratios, failed to achieve significance at P < 0.05.
Fig. 2.
A: dihydroethidium fluorescence in the LAD-dependent zone on day 3 of RI after two periods of ischemia (40 s)-reperfusion (20 min) in WKY/JCR rats in the sham group, WKY/JCR rats in the RI group, and WKY/JCR rats in the RI + Apo group. B: O2−· levels measured by X-band electron paramagnetic resonance (EPR) in the CZ and NZ. C: oxidized-to-reduced thiol ratios measured by X-band EPR in the CZ and NZ. *P < 0.05 vs. WKY rats in the sham group; #P < 0.05 vs. WKY rats in the RI group; $P < 0.05 vs. JCR rats in the RI group.
Finally, to distinguish whether extracellular or intracellular alterations in oxidative stress were the primary determinants of CCG, instead of apocynin, n = 3 WKY animals in the RI group and JCR animals in the RI group received cell-impermeable SOD for 10 days. In contrast to apocynin, SOD administration had no effect on CCG in either group (CZ/NZ: 0.83 ± 0.06 in WKY rats in the RI + SOD group vs. 0.84 ± 0.02 in WKY rats in the RI group and 0.11 ± 0.04 in JCR rats in the RI + SOD group vs. 0.12 ± 0.02 in JCR rats in the RI group). The efficacy of SOD delivery was assessed by X-band EPR measurements of O2−· levels in blood and myocardial tissue. Intravenous SOD administration significantly reduced O2−· levels in blood (WKY rats in the RI + SOD group: 0.19 ± 0.04 nmol/l vs. WKY rats in the RI group: 0.64 ± 0.16 nmol/l and JCR rats in the RI + SOD group: 0.47 ± 0.12 nmol/l vs. JCR rats in the RI group: 2.39 ± 0.05 nmol/l) but not in myocardial tissue (WKY rats in the RI + SOD group: 0.59 ± 0.18 vs. WKY rats in the RI group: 0.61 ± 0.08 nmol/l and JCR rats in the RI + SOD group: 2.34 ± 0.16 vs. JCR rats in the RI group: 2.32 ± 0.25 nmol/l).
Decreased oxidative stress abrogates RI-induced p38, Akt, and Src activation in WKY rats but induces their activation in JCR rats.
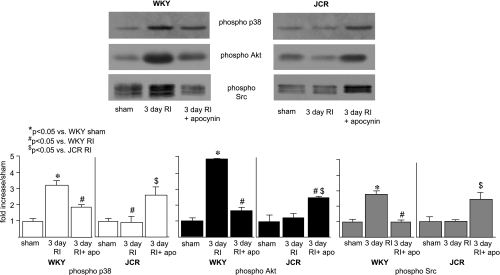
Next, we sought to elucidate some of the signaling mechanisms by which oxidative stress may modulate RI-induced CCG. We (29) have recently shown that 3 days of RI induced maximal p38 and Akt activation in WKY animals but completely failed to induce the activation of either kinase in JCR animals, which correlated with CCG versus no CCG, respectively. We (31) have also previously shown that RI-induced p38 and Akt activation was redox sensitive in WKY animals. In the present study, we first determined whether p38 and Akt activation, as well as that of Src, their known upstream redox-sensitive regulator in many cell types (24) including cardiac myocytes (40) and vascular smooth muscle cells (9), were redox sensitive. Apocynin significantly decreased p38 [1.9 ± 0.1- vs. 3.4 ± 0.25-fold (RI)] and to an even greater extent Akt [1.7 ± 0.2- vs. 4.85 ± 0.02-fold (RI)] activation and completely blocked Src [1.0 ± 0.2- vs. 2.8 ± 0.2-fold (RI)] activation in the CZ of WKY animals at the time point of their maximal activation by RI (day 3; Fig. 3). In contrast, apocynin partially but significantly restored p38 [2.8 ± 0.3- vs. 0.95 ± 0.3-fold (RI)], Akt [2.7 ± 0.2- vs. 1.2 ± 0.2-fold (RI)], and Src [2.48 ± 0.3- vs. 1.0 ± 0.1-fold (RI)] activation in the CZ of JCR animals at day 3 of RI (Fig. 3). These results demonstrate that RI-induced p38, Akt, and Src activation is ROS dependent and, moreover, requires some optimal amount of ROS (WKY rats in the RI group and JCR rats in the RI + apocynin group) that is neither too high (JCR rats in the RI group) nor too low (WKY rats in the RI + apocynin group). p38, Akt, and Src were not activated in the NZ, and their expression was not altered in either the CZ or NZ of any group (data not shown).
Fig. 3.
Top left: Western blots with phospho-p38, phospho-Akt, and phospho-Src antibodies in WKY rats in the sham group, WKY rats in the RI group on day 3, WKY rats in the RI + Apo group on day 3. Top right: same as top left but in JCR rats in the sham group, JCR rats in the RI group on day 3, and JCR rats in the RI + Apo group on day 3. Bottom: cumulative data as area × density (in AU) for phospho-p38 (left), phosopho-Akt (middle), and phospho-Src (right). *P < 0.05 vs. WKY rats in the sham group; #P < 0.05 vs. WKY rats in the RI group; $P < 0.05 vs. JCR rats in the RI group.
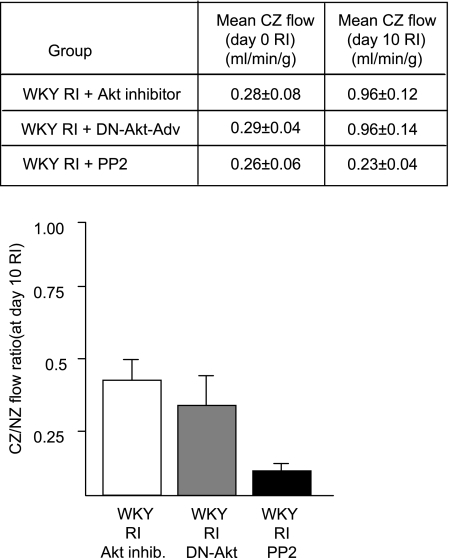
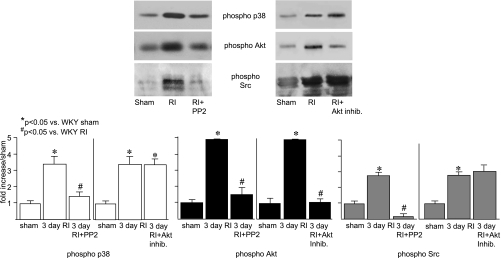
To determine the specific relevance of these findings, it was important to determine whether the activation of these kinases was in fact essential for CCG. We (31) have previously shown that p38 activation is required for RI-induced CCG since treatment with SB-203580 significantly attenuated RI-induced CCG (at day 10 of RI CZ flow was 0.90 ± 0.11 vs. 1.84 ± 0.07 ml·min−1·g−1 in WKY rats in the RI group). Here, we used specific inhibitors of Akt (Akt inhibitor) and Src (PP2) to determine their requirement for CCG. Akt inhibition resulted in a 50% decrease in CCG, and Src inhibition completely blocked CCG in WKY animals (Fig. 4). Neither treatment altered myocardial oxidative stress (as assessed by X-band EPR) or affected Src, Akt, or p38 expression (data not shown). To confirm the efficacy and specificity of PP2 and the Akt inhibitor, we analyzed Src, Akt, and p38 activation on day 3 of RI by Western blot analysis. The Akt inhibitor blocked ∼80% of Akt activation and had no effect on either p38 or Src activation. PP2 completely inhibited Src and partially blocked Akt (∼70%) and p38 (∼60%) activation (Fig. 5), implicating Src as an important upstream regulator of p38 and Akt during CCG. Finally, we used DN-Akt-Adv to confirm the effect of pharmacological Akt inhibition in a smaller sample of n = 3 WKY rats. Our administration protocol resulted in >80% myocardial transduction, as assessed by enhanced green fluorescent protein (EGFP) fluorescence, and an ∼11-fold increase in myocardial Akt expression, assessed by Western blot analysis, which persisted throughout the duration of the RI protocol (data not shown). DN-Akt-Adv decreased CCG to an extent nearly identical (not statistically different) to the Akt inhibitor [the CZ-to-NZ flow ratio on day 10 of RI was 0.38 ± 0.1 vs. 0.42 ± 0.05 in WKY rats in the RI + Akt inhibitor group vs. 0.84 ± 0.02 in WKY rats in the RI group; Fig. 4]. DN-Akt-Adv completely blocked the phosphorylation of Akt on Ser473 and Ser308 as well as the activation of p70S6 kinase, a direct downstream target of Akt, indicative of its function as a DN construct, and had no effect on p38, ERK1/2, or Src expression, activation, or myocardial oxidative stress (data not shown). Administration of EGFP-Adv in an identical manner had no effect on CCG, Akt expression, Akt, p38, or Src activation, or oxidative stress (data not shown).
Fig. 4.
Rats underwent the 10-day RI protocol and were treated with the Akt inhibitor (5.8 mg·kg−1·day−1 ip), dominant negative Akt adenovirus (DN-Akt-Adv; 2 × 1012 plaque-forming units), or PP2 (3 mg·kg−1·day−1 ip) as shown. Bottom: coronary flow was measured in the CZ and NZ using radioactive microspheres during LAD occlusion and expressed as the ratio between CZ and NZ flows at day 10 of RI. Top: coronary flow was measured on days 0 and 10 of the RI protocol, and flow in the LAD-dependent zone (CZ) during LAD occlusion was expressed in milliliters per gram per minute.
Fig. 5.
Top: Western blots with phospho-p38, phospho-Akt, and phospho-Src antibodies in WKY rats in the sham group, WKY rats in the RI group on day 3, WKY rats in the RI + PP2 group on day 3, and WKY rats in the RI + Akt inhibitor group on day 3. Bottom: cumulative data as area × density (in AU) for phospho-p38 (left), phosopho-Akt (middle), and phospho-Src (right). *P < 0.05 vs. WKY rats in the sham group; #P < 0.05 vs. WKY rats in the RI group.
DISCUSSION
The present study is the first to 1) examine the effect of directly lowering oxidative stress on CCG in healthy (WKY) versus metabolic syndrome (JCR) animals; 2) quantitatively determine two separate indexes of myocardial oxidative stress (myocardial O2−· concentrations and the oxidized-to-reduced thiol ratio) in WKY versus JCR animals; 3) determine the effect of oxidative stress manipulation on RI-induced p38, Akt, and Src activation during CCG in WKY versus JCR animals; and 4) demonstrate the requirement for Akt and Src activation for CCG.
Our results show that in healthy animals (WKY rats), lowering of oxidative stress to baseline levels (without RI) critically impairs RI-induced CCG, whereas in an animal model of metabolic syndrome (JCR rats), which completely fails to grow coronary collaterals in response to RI alone (29), lowering of oxidative stress to levels approximately equal to those found in healthy WKY animals in response to RI greatly enhances CCG (Figs. 1 and 2). Furthermore, the magnitude of these effects in both phenotypes suggests that alterations in myocardial oxidative stress are, although not the only, a highly significant factor in the regulation of CCG.
In agreement with our results demonstrating a beneficial effect of reduction in oxidative stress in JCR animals, Miller et al. (22) demonstrated that mesenteric collateral growth, impaired in SHRs, was significantly improved by apocynin. Since a common characteristic of the JCR (29) and SHR (22) phenotype is elevated oxidative stress, these results indicate that lowering oxidative stress in phenotypes where it is elevated is critical for the restoration of collateral growth. This is also in agreement with our previous study (12) in which the administration of ecSOD to ZOF rats significantly improved RI-induced CCG when VEGF alone failed to do so. Interestingly, a study (2) in portal hypertensive rats has indicated that NAD(P)H oxidase inhibition significantly decreased portosystemic collateral development. Although seemingly contradictory, these findings may in fact emphasize the importance of differences between vascular beds and animal disease models. In addition, perhaps significantly, oxidative stress was not evaluated in the study (2).
On the other hand, our results demonstrating a detrimental effect of oxidative stress reduction on CCG in healthy WKY animals (Fig. 1) indicate that some amount of ROS is required for this process. Several studies support this idea. NAC abrogated CCG in a canine model of repetitive ischemia (11). Neovascularization in the ischemic hindlimb was impaired in mice lacking a critical component of the oxidase (gp91phox−/−) as well as in wild-type mice treated with the antioxidant ebselen (41). Apocynin inhibited angiogenesis in a chicken embryo chorioallantoic membrane assay (28). Angiopoetin-1-induced vessel sprouting from aortic rings was decreased in mice deficient in another critical component of the oxidase (p47phox−/−) (5, 6). In contrast, in a mouse model of hindlimb ischemia, blood flow recovery, collateral vessel formation, and capillary density were inhibited in ecSOD−/− mice compared with wild-type mice (15). There are several potential explanations for this discrepancy. First, nitic oxide radical (NO•−) levels were significantly lower in ecSOD−/− mice (15). A previous study (21) has indicated a critical importance of NO•− in collateral growth in the hindlimb ischemia model; thus, NO•− may be the critical regulator of collateral growth in this model, and it is possible that any decrease in NO•− is sufficient to negatively affect this process. Second, although relative measurements have indicated lower O2−· levels in ecSOD−/− mice, there are no quantitative measurements of oxidative stress in this study (15); thus, it is difficult to compare these levels with those induced by RI in our model. In addition, ecSOD deletion, in theory, blocks H2O2 production, which may be critical for the regulation of signal transduction involved in collateral growth.
Our recent studies (29, 31) have suggested the critical importance of an optimal amount of ROS, neither too low nor too high, in the regulation of CCG. Here, we addressed this idea by determining the effect of an antioxidant, apocynin, on two separate indexes of myocardial oxidative stress: myocardial O2−· concentrations and the oxidized-to-reduced thiol ratio, as assessed quantitatively by X-band EPR. We show that, in JCR animals, apocynin lowered both parameters to levels not statistically different from those generated by RI in WKY animals, which were compatible with CCG (Fig. 2). In combination with our recent study examining the effects of AT1R blockade on myocardial oxidative stress and CCG, these results, while still not specifically defining the limits of the redox window permissive for CCG, support that concept.
Whether intracellular or extracellular ROS and/or ROS scavengers contribute to various vascular remodeling processes, including CCG, is an open question. Our results demonstrating that lowering intracellular but not extracellular oxidative stress affected CCG in both JCR (improved) and WKY (abrogated) animals suggest that intracellular oxidative stress, at least in myocytes (since our measurements most likely reflect myocyte and not vascular cell oxidative stress), plays a far more important role in the regulation of RI-induced CCG. This is in agreement with a previous study (39) that identified intracellular sources of ROS to be critical for the regulation of signaling pathways leading to a variety of effects, including the synthesis and secretion of growth factors and extracellular matrix-degrading enzymes.
Our results using adenosine to achieve maximal systemic vasodilation demonstrate that vasomotor activity (vasodilation) cannot explain the observed increases in blood flow in the CZ, since there were no differences in coronary blood flow before or after vasodilation. This is in agreement with our previous study (42) that showed that dilation with dipyridamole did not change coronary blood flow, as well as with several other studies. Unthank et al. (44) have previously shown that even though the microvasculature distal to the occlusion is able to increase flow by dilation both initially and 1 wk later in a rat model of hindlimb ischemia, the primary reason for the increases in flow at 1 wk is collateral growth. Scholtz et al. (34) asserts that observed large increases in collateral-dependent perfusion (40–80%) cannot be achieved by either vasodilation or angiogenesis (capillary growth) and are primarily due to collateral growth (arteriogenesis).
Our results supporting the idea that RI is an important driving force for CCG are in agreement with multiple studies (7, 10, 14, 17, 23, 42, 46). Elevated fluid shear stress, resulting from occlusion-induced pressure gradients across the coronary circulation, has also been proposed to drive collateral remodeling (13, 27, 34, 35). However, these conclusions are largely derived from animal models of hindlimb ischemia (4, 13, 27) or mesenteric artery occlusion (43). First, anatomic differences between the hindlimb ischemia and coronary occlusion models may explain this view. In models of hindlimb ischemia, the major site of ischemia (the femoral artery) is distant from the major area of collateral growth (lower limb); the ischemic zone in the myocardium is in direct proximity to the zone of major collateral remodeling. Second, in some studies (4, 35), capillary densities and velocities of flow but not total blood flow or arterial density were measured. Also, some studies were conducted in models of prolonged ischemia followed by reperfusion versus transient RI models (35). The suggestion that ischemia is not a driving force for CCR rests on one study (34) in a model of canine coronary occlusion, which observed only epicardial vascular growth while the subendocardium became ischemic. Importantly, none of these studies have excluded ischemia as the contributing factor to collateral growth. A study (7) using microembolization to cause ischemia in the left circumflex perfusion territory demonstrated that myocardial ischemia in dogs without alterations in pressure gradients between large epicardial coronary arteries was a sufficient stimulus for the initiation but not the propagation of CCG, suggesting that ischemia initiates CCG, but elevated shear stress may contribute to continuation of collateral remodeling.
To elucidate some of the mechanisms by which oxidative stress may modulate RI-induced CCG, we examined how apocynin affected RI-induced Akt, p38, and Src activation. Apocynin significantly decreased p38 and Akt activation and completely blocked Src activation in WKY animals but partially restored p38, Akt, and Src activation in the CZ of JCR animals, in which RI alone failed to activate any of the three kinases (Fig. 3). These results demonstrate, for the first time, that RI-induced activation of p38, Akt, and Src is redox dependent in an in vivo model of CCG and is responsible, at least in part, for the compromised CCG in metabolic syndrome.
Importantly, our results also conclusively demonstrate that Akt and Src are required for CCG. Akt inhibition, like p38 inhibition (31), partially but significantly attenuated and Src inhibition completely blocked RI-induced CCG (Fig. 4). Several studies examining angiogenesis, a process distinct from collateral growth, which may nevertheless share some common mechanisms, support our findings. Reduced endothelial cell tube formation in Matrigel in vitro has been reported in cells derived from Src−/− mice (32), and Src has been shown to play a key role in tumor angiogenesis, since Src inhibitors reduce tumor vascular density (26). Expression of DN Akt inhibited tumor angiogenesis (8). VEGF-mediated endothelial cell tube formation as well as ischemia-induced hindlimb angiogenesis were compromised in Akt1−/− mice (1). Prolonged Akt activation (6 wk) resulted in pathological cardiac hypertrophy characterized by decreased angiogenesis (36); however, 2-wk Akt activation resulted in physiological hypertrophy characterized by increased myocardial angiogenesis in mice (36). While these studies suggest a critical importance for the duration of Akt activation, they support the notion that Akt activation is required for angiogenesis. Paradoxically, increased angiogenesis in Matrigel plug assays was observed in Akt1−/− mice (37). The reasons for these disparate findings are unclear but may indicate significant differences in the signal transduction-mediated regulation of angiogenesis, and, by implication, collateral growth, between different experimental models. Since 80% of cells, by volume, in the whole heart are cardiac myocytes, the observed redox-dependent alterations in myocardial oxidative stress and p38, Akt, and Src activation profiles in our study are likely to be primarily in myocytes and, thus, directly related to their potential roles in secretion of growth factor and matrix-degrading enzymes from myocytes rather than survival, proliferation, and migration of vascular cells, processes that are also critical for CCG. Redox-sensitive activation of p38, Akt, and Src has indeed been associated with matrix metalloprotease and VEGF secretion from solid tumors (26) and cardiac myocytes (40). The investigation of these possibilities and identification of additional signaling pathways that may regulate CCG were beyond the scope of this study.
An important finding in the present study, that lowering of myocardial oxidative stress to a concentration range compatible with CCG in an animal model of metabolic syndrome restores, to a great extent, RI-induced CCG, may have direct clinical relevance for this high-risk patient population. In fact, the extent of restoration is at least equivalent to that achieved by clinically important AT1R blockade (20) (with a nonstatistically significant trend toward a greater effect), implicating a critical role for oxidative stress. Furthermore, we provide an insight into a molecular signaling mechanism by which ROS may mediate these effects. While oxidative stress reduction in metabolic syndrome resulted in signaling profiles approaching those observed in healthy animals, these were not identical (p38, Akt, and Src activation were not completely restored). Since we have identified these kinases to be required for CCG, this may provide an underlying reason as to why oxidative stress reduction alone could not completely restore CCG in metabolic syndrome and indicates the importance of further elucidating the signal transduction pathways involved in the regulation of CCG as well as additional components responsible for their modulation, especially in pathologies where CCG is compromised, such as in metabolic syndrome.
GRANTS
This work was supported by American Heart Association Grant SDG-0630285N and by National Institutes of Health Center of Biomedical Research Excellence Grants RR-018766 and R01-HL-093052.
REFERENCES
- 1.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton R, Galasso G, Birnbaum M, Walsh K, Sessa W. Akt1/PKBalpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 115: 2119–2127, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angermayr B, Fernandez M, Mejias M, Garcia-Sancho J, Garcia-Pagan J, Bosch J. NAD(P)H oxidase modulates angiogenesis and the developemnt of portosystemic collaterals and splenic hyperaemia in portal hypertensive rats. Gut 56: 560–564, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckberg G Studies of regional coronary flow using radioactive microspheres. Ann Thorac Surg 20: 46–51, 1975. [DOI] [PubMed] [Google Scholar]
- 4.Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin (Shanghai) 40: 681–692, 2008. [PubMed] [Google Scholar]
- 5.Chen J, Zeng H, Lawrence M, Blackwell T, Meyrick B. Angiopoetin-1-induced angiogenesis is modualted by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol 291: H1563–H1572, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zeng H, Yu H, Meyrick B, Aschner J. NADPH oxidase modulates myocardial Akt, ERK1/2 activation, and angiogenesis after hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol 292: H1664–H1674, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chilian W, Mass H, Williams S, Layne S, Smith E, Scheel K. Microvascualr occlusions promote coronary collateral growth. Am J Physiol Heart Circ Physiol 258: H1103–H1111, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Fang J, Ding M, Yang L, Liu L, Jiang B. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signal 19: 2487–2497, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griendling K, Sorescu D, Lassègue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol 20: 2175–2183, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Gu J, Wang Y, Li J, Wang J, Jin T. Proteomic analysis of left ventricular tissues following intermittent myocardial ischemia during coronary colalteralization in rabbits. Int J Cardiol 131: 326–335, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Gu W, Weihrauch D, Tanaka K, Tessmer J, Pagel P, Kersten J, Chilian W, Warltier D. Reactive oxygen species are critical mediators of coroanry collaterla development in a canine model. Am J Physiol Heart Circ Physiol 285: H1582–H1589, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Hattan N, Chilian W, Park F, Rocic P. Restoration of coroanry collaterla growth in the Zucker obese rat: impact of VEGF and ecSOD. Basic Res Cardiol 102: 217–223, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Heil M, Schaper W. Influence of mechanical, cellualr and molecualr factors on collateral artery growth (arteriogenesis). Circ Res 95: 449–458, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Kersten J, Warltier D. Modulation of coroanry colalteral angiogenesis; a canine model of neovascualrization induced by chronic ischemia. J Card Surg 10: 354–357, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Lin A, Guldberg R, Ushio-Fukai M, Fukai T. Essential role of extracellualr SOD in reperative neovascualrization induced by hindlimb ischemia. Circ Res 101: 409–419, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Koerselman J, van der Graaf Y, de Jaegere P, Grobbee D. Coronary collaterals: an important and underexposed aspect of coronary artery disease. Circulation 107: 2507–2511, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Wu T, Huang P, Lin S, Qiu F, Meng X, Gao J, Li J. Effect and mechanism of intermittent myocardial ischemia induced by exercies on coroanry collateral formation. Am J Phys Med Rehabil 87: 803–814, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Lyle A, Griendling K. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology 21: 269–280, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Madeddu P, Kraenkel N, Barcelos L, Siragusa M, Campagnolo P, Oikawa A, Caporali A, Herman A, Azzolino O, Barberis L, Perino A, Damilano F, Emanueli C, Hirsch E. Phosphoinositide 3-kinase gama gene knockout impairs postischemic neovascualrization and endothelial progenitor cell function. Arterioscler Thromb Vasc Biol 28: 68–76, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maulik N, Das D. Potentiation of angiogenic response by ischemic and hypoxic preconditioning of the heart. Cell Mol Med 6: 13–24, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendoza M, Robles H, Romo E, Rios A, Escalante B. Nitric oxide-dependent neovascularization role in the lower extremity disease. Curr Pharm Des 13: 3591–3596, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Miller S, Norton L, Murphy M, Dalsing M, Unthank J. The role of the renin-angiotensin system and oxidative stress in spontaneously hypertensive rat mesanteric collateral growth impairment. Am J Physiol Heart Circ Physiol 292: H2523–H2531, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Mohri M, Tomoike H, Noma M, Inoue T, Hisano K, Nakamura M. Duration of ischemai is vital for collateral development: repeated brief coronary artery occlusions in conscious dogs. Circ Res 64: 287–296, 1989. [DOI] [PubMed] [Google Scholar]
- 24.Monteiro H, Arai R, Travassos L. Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling. Antioxid Redox Signal 10: 843–890, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Park J, Lucchesi B. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg 68: 1905–1912, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Park S, Shaha A, Zhang J, Gallick G. Regulation of angiogensis and vascualr premiability by Src family kinases: opportunities for therapeutic treatment of solid tumors. Expert Opin Ther Targets 11: 1207–1217, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Pipp F, Boehm S, Cai W, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol 24: 1664–1668, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Polytarchou C, Papadimitriou E. Antioxidents inhibit angiogenesis in vivo through down-regulation of nitric oxide synthase expression and activity. Free Radic Res 38: 501–508, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coroanry colalterla growth. Arterioscler Thromb Vasc Biol 28: 61–67, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Rocic P, Jo H, PAL. A role for PYK2 in ANG II-dependent regulation of the PHAS-1-eIF4E complex by multiple signaling cascades in vascular smooth muscle. Am J Physiol Cell Physiol 285: C1437–C1444, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Rocic P, Kolz C, Reed R, Potter B, Chilian W. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol 292: H2729–H2736, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Schlessinger J New roles for Src kinases in control of cell survival and angiogenesis. Cell 100: 293–296, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Schneider D, Absher P, Neimane D, Russell J, Sobel B. Fibrinolysis and atherogenesis in the JCR:LA-cp rat in relation to insulin and triglyceride concentrations in blood. Diabetologia 41: 141–147, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Scholtz D, Cai W, Schaper W. Arteriogenesis, a new concept of vascualr adaptation in occlusive disease. Angiogenesis 4: 247–257, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Scholtz D, Schaper W. Preconditioning of arteriogenesis. Cardiovasc Res 65: 513–523, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Shiojima I, Sato K, Izumiya Y, Schiokofer S, Ito M, Liao R, Colucci W, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115: 2108–2118, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Somanath P, Razorenova O, Chen J, Byzova T. Akt1 in endothelial cell angiogenesis. Cell Cycle 5: 512–518, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugden P, Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid Redox Signal 8: 2111–2124, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Svineng G, Ravuri C, Rikardsen O, Huseby NE, Winberg J. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect Tissue Res 49: 197–202, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Takano H, Zou Y, Hasegawa H, Akazawa H, Nagai T, Komuro I. Oxidative stress-induced signal transduction pathways in cardiac myocytes: involvement of ROS in heart diseases. Antioxid Redox Signal 5: 789–794, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Tojo T, Ushio-Fukai M, Yamaoka-Toyo M, Ikeda S, Patrushev N, Alexander W. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111: 2347–2355, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Toyota E, Warltier D, Brock T, Ritman E, Kolz C, O'Malley P, Rocic P, Focardi M, Chilian W. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation 112: 2108–2113, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Tuttle J, Hahn T, Sanders B, Witzmann F, Miller S, Dalsing M, Unthank J. Impaired collateral development in mature rats. Am J Physiol Heart Circ Physiol 283: H146–H155, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Unthank J, Nixon J, Lash J. Early adaptations in collateral and microvascular resistance after ligation of the rat femoral artery. J Appl Physiol 79: 73–82, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Williams H, Griendling K. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol 50: 9–16, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Yamanishi A, Fujita M, Ohno A, Sasayama S. Importance of myocardial ischemia for recruitment of coroanry collateral circulation in dogs. Cardiovasc Res 24: 271–277, 1990. [DOI] [PubMed] [Google Scholar]