Abstract
We hypothesized that the interaction between tumor necrosis factor-α (TNF-α)/nuclear factor-κB (NF-κB) via the activation of IKK-β may amplify one another, resulting in the evolution of vascular disease and insulin resistance associated with diabetes. To test this hypothesis, endothelium-dependent (ACh) and -independent (sodium nitroprusside) vasodilation of isolated, pressurized coronary arterioles from mLeprdb (heterozygote, normal), Leprdb (homozygote, diabetic), and Leprdb mice null for TNF-α (dbTNF−/dbTNF−) were examined. Although the dilation of vessels to sodium nitroprusside was not different between Leprdb and mLeprdb mice, the dilation to ACh was reduced in Leprdb mice. The NF-κB antagonist MG-132 or the IKK-β inhibitor sodium salicylate (NaSal) partially restored nitric oxide-mediated endothelium-dependent coronary arteriolar dilation in Leprdb mice, but the responses in mLeprdb mice were unaffected. The protein expression of IKK-α and IKK-β were higher in Leprdb than in mLeprdb mice; the expression of IKK-β, but not the expression of IKK-α, was attenuated by MG-132, the antioxidant apocynin, or the genetic deletion of TNF-α in diabetic mice. Leprdb mice showed an increased insulin resistance, but NaSal improved insulin sensitivity. The protein expression of TNF-α and NF-κB and the protein modification of phosphorylated (p)-IKK-β and p-JNK were greater in Leprdb mice, but NaSal attenuated TNF-α, NF-κB, p-IKK-β, and p-JNK in Leprdb mice. The ratio of p-insulin receptor substrate (IRS)-1 at Ser307 to IRS-1 was elevated in Leprdb compared with mLeprdb mice; both NaSal and the JNK inhibitor SP-600125 reduced the p-IRS-1-to-IRS-1 ratio in Leprdb mice. MG-132 or the neutralization of TNF-α reduced superoxide production in Leprdb mice. In conclusion, our results indicate that the interaction between NF-κB and TNF-α signaling induces the activation of IKK-β and amplifies oxidative stress, leading to endothelial dysfunction in type 2 diabetes.
Keywords: coronary microcirculation, cytokines, inflammation, nitric oxide, vasodilation
the prevalence of obesity and diabetes has led to a significant increase in complications associated with cardiovascular disease. Many of these complications can be linked to a dysregulation of the body's immune system (16, 33). Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine implicated in cardiovascular diseases (14, 34). We previously found that an increased TNF-α expression induces the production of reactive oxygen species (ROS), leading to endothelial dysfunction in type 2 diabetes (15). Importantly, TNF-α activates the transcription nuclear factor-κB (NF-κB), which regulates the expression of genes involved in inflammation, oxidative stress, and endothelial dysfunction (15). TNF-α also initiates signaling cascades via the inhibition of the NF-κB (IκB) kinase (IKK) complex, which contains IKK-α and IKK-β. The inhibitory protein IκB-α is phosphorylated, ubiquitinated, and degraded by proteasomes, releasing NF-κB to translocate into the nucleus. Under normal physiological conditions, the inflammatory response is terminated by binding NF-κB with the inhibitory protein IκB (24, 36). An investigation of the TNF-α-mediated NF-κB pathway is needed to identify specific proinflammatory agents in type 2 diabetes.
Salicylates, including aspirin and sodium salicylate (NaSal), are common nonsteroidal anti-inflammatory drugs, which have antiplatelet and anti-inflammatory effects. Aspirin inhibits prostaglandin production, but NaSal has a prostaglandin-independent effect. NaSal inhibits the activity of IKK-β, which is required for the activation of NF-κB. IKK-β, the molecular target of NaSal, is associated with the rise of insulin resistance (7, 43). Recent work suggests that a specific antagonism of the NF-κB inflammatory pathway through the inhibition of IKK-β reduces acute myocardial damage following ischemia-reperfusion injury (26) and that insulin action plays a critical role in reducing myocardial infarct size by increasing cardiac myocyte metabolism (5, 8, 29). We previously found that TNF-α-activated Jun NH2-terminal kinase (JNK), which mediates O2− production and impairs endothelium-dependent, nitric oxide (NO)-mediated vasodilation in coronary arterioles (45). NaSal prevents TNF-α-induced JNK activation from blocking insulin signaling via a serine phosphorylation (18). Therefore, we evaluated 1) whether reciprocal interactions between TNF-α and NF-κB via the activation of IKK-β accentuate the evolution of vascular disease in type 2 diabetes and 2) whether NaSal restores endothelial dysfunction in coronary arterioles and insulin resistance by inhibiting IKK-β/NF-κB activity, thereby preventing JNK activation in type 2 diabetic mice.
MATERIALS AND METHODS
Animals.
The procedures followed were approved by and in accordance with the guidelines of the Laboratory Animal Care and Use Committee at Texas A&M University. Heterozygote controls (mLeprdb), homozygote type 2 diabetic (Leprdb), and Leprdb mice null for TNF (dbTNF−/dbTNF−) were purchased from Jackson Laboratory and maintained on a normal rodent chow diet. mLeprdb mice are normal in body weight, blood glucose, and plasma insulin. Our studies used 12–16-wk-old, 15–25-g mLeprdb, 25–50-g Leprdb, and dbTNF−/dbTNF− mice of either sex. We used the same strain (C57BL/6J) of mLeprdb and dbTNF−/dbTNF− mice to match the backgrounds of Leprdb mice, type 2 diabetic models that are characterized by leptin resistance and obesity. The cross (dbTNF−/dbTNF−) of Leprdb with TNF knockout is heterozygous for Leprdb and homozygous for TNF knockout (TNF−/−). The dbTNF−/dbTNF− mice show the phenotypes of hyperglycemia and obesity that are consistent with diabetes and the penetrance of the leptin receptor mutation. The obese mice from the second round of breeding of the Leprdb and TNF−/− mice were used in experimentation.
Measurement of blood parameters: blood glucose, cholesterol level, insulin level, and homeostasis model assessment-insulin resistance.
Blood was obtained from the vena cava after anesthesia with pentobarbital sodium (50 mg/kg ip) and the exposure of the vein. Whole blood samples were spun at 3,000 rpm for 10 min, and serum was stored at −80°C until analysis. We used a OneTouch Ultramini glucometer (LifeScan) for measuring blood glucose in mLeprdb, Leprdb, and Leprdb mice treated with NaSal at the same time (8:00 am–10:00 am) throughout the protocol. The serum cholesterol level was measured with the Cholesterol/Cholesteryl Ester Quantitation Kit (Biovision), and insulin was measured with the use of a commercial kit, insulin (Mouse) Ultrasensitive EIA (ALPCO Diagnostics) using spectrophotometry (Multiskan MCC, Fisher Scientific). Insulin resistance (IR) was determined by utilizing the homeostasis model assessment (HOMA) by using the following formula: HOMA-IR = {[nonfasting glucose (in mmol/l)] × [nonfasting insulin (in mU/l)]}/22.5.
Measurement of superoxide by electron paramagnetic resonance spectroscopy.
Superoxide (O2−) quantitation from the electron paramagnetic resonance (EPR) spectra was determined in the homogenates of 4–6 isolated coronary arterioles by a double integration of the peaks, with reference to a standard curve from horseradish peroxidase generation of the anion from a standard solutions of H2O2, using p-acetamidophenol as the cosubstrate (32, 45), and then normalized to the mean value of mLeprdb mice.
Treatment with TNF-α neutralization.
The neutralizing antibody to TNF (21) is 2E2 monoclonal antibody (2E2 MAb, 94021402, NCI Biological Resources Branch). At 12–16 wk of age, all mice received anti-TNF (2E2 MA, 0.625 mg·ml−1·kg−1·day−1 ip for 3 days); the dosage was based on our estimates of TNF-α expression, which was estimated to be in the low nanogram or picogram range. This amount was able to neutralize 10–100 fold more than the estimated levels of TNF-α.
Treatment with MG-132, apocynin, NaSal, or SP-600125.
MG-132 tripeptide [Z-Leu-Leu-Leu-aldehyde (Sigma) dissolved in dimethyl sulfoxide (DMSO)] is a peptide aldehyde proteasome inhibitor. Ultimately preventing IκB-α degradation by the proteasome restricts NF-κB activity in vitro (13, 23, 24). Leprdb mice were injected (10 mg·kg−1·day−1 ip for 3 days) (25). The oxidative stress was assessed by treating mice with the NAD(P)H oxidase inhibitor apocynin (100 mg·kg−1·day−1 ip for 3 days) (28). We administered NaSal (6 mg/ml in drinking water for 7–10 days, pH 7; Calbiochem) to Leprdb and mLeprdb mice to determine whether the blocking of IKK-β affects their blood glucose level. To determine whether the blockage of JNK affects insulin receptor substrate-1 (IRS-1) activity, we also administered the JNK inhibitor SP-600125 (10 mg·kg−1·day−1 ip in 0.05 ml DMSO for 7 days; CalBiochem) to Leprdb mice (3).
Functional assessment of isolated coronary arterioles.
The techniques for the identification and the isolation of coronary microvessels were previously described in detail (20, 45). Briefly, coronary arterioles (40–100 μm in diameter) from murine hearts were carefully dissected for in vitro study. Acetylcholine (ACh) is an endothelium-dependent vasodilator, and sodium nitroprusside (SNP) is endothelium-independent vasodilator. Flow-induced vasodilation is NO mediated, endothelial dependent, but agonist independent. To determine whether NF-κB or IKK-β played a role in endothelial injury in type 2 diabetes, ACh (0.1 nmol/l to 10 μmol/l)-, SNP (0.1 nmol/l to 10 μmol/l)-, and flow (4–60 cmH2O)-induced vasodilation were assessed in the coronary arterioles in mLeprdb, Leprdb, and Leprdb mice treated with MG-132 or NaSal. Flow was established by the production of a pressure drop across the vessel and linearly related to the pressure drop (ΔP).
Insulin tolerance test to nonfasting mice.
The tail was nicked with a fresh razor blade by a horizontal cut of the tip, and the OneTouch Ultramini glucometer was used to measure baseline blood glucose. A total of 0.75 U/kg body wt of diluted insulin from porcine pancreas (Sigma) was injected into the intraperitoneal cavity. Blood samples were taken for glucose determinations at 0, 30, 60, and 120 min later. The blood was sampled from the tail of each mouse by gently massaging a small drop of blood onto the glucometer strip.
Protein expression by Western blot analyses.
Protein concentrations were assessed using a BCA Protein Assay Kit (Pierce), and equal amounts of protein (15 μg) were separated by SDS-PAGE and transferred to nitrocellulose (Hybond, Amersham) or polyvinylidene difluoride membranes (Pierce Biotechnology). IKK-α (Santa Cruz), IKK-β (Abcam), phosphorylated (p)-IKK-β (Cell Signal), IκB-α (Santa Cruz), p-IκB-α (Santa Cruz), NF-κB (Santa Cruz), TNF-α (Santa Cruz), N-Tyr (Abcam, an indicator for peroxynitrite-mediated tissue injury), JNK, p-JNK (Abcam), IRS-1, and p-IRS-1 (Millipore) antibodies were used to assess the protein expression and protein modification in mLeprdb, Leprdb, and Leprdb mice treated with anti-TNF (0.625 mg·ml−1·kg−1·day−1 ip for 3 days), apocynin (100 mg·kg−1·day−1 ip for 3 days), NF-κB antagonist MG-132 (10 mg·kg−1·day−1 ip for 3 days), NaSal (6 mg/ml in drinking water), or SP-600125 (10 mg·kg−1·day−1 ip for 7 days). Our results were presented as fold changes normalized to the internal control (β-actin), compared with normal control mice (defined as 100 for control). Signals were visualized by enhanced chemiluminescence (Santa Cruz), scanned with a Fuji LAS3000 densitometer, and quantified by Multigauge software (Fuji film). The relative amounts of protein expression were quantified and normalized to those of the corresponding internal reference and β-actin and then normalized to corresponding mLeprdb control, which were set to a value of 1.0.
Data analysis.
At the end of each experiment, the vessel was relaxed with 100 μmol/l SNP to obtain its maximal diameter at 60 cmH2O intraluminal pressure (45). All diameter changes to pharmacological agonists were normalized to the control diameter and expressed as a percentage of control diameters. All data are presented as means ± SE, except the protein expression that is presented as means ± SD. Statistical comparisons of vasomotor responses under various treatments were performed with two-way ANOVA, and intergroup differences were tested with Bonferonni inequality. Significance was accepted at P < 0.05.
RESULTS
Body weight, abdominal girth, serum concentration of glucose, cholesterol, insulin, and insulin resistance.
Body weight and serum parameters were measured at 12–16 wk for the different strains of mice (Table 1). Leprdb mice were treated with NaSal for 7–10 days, and serum was collected from nonfasting mice in the morning. Nonfasting measurements were required since the Leprdb murine model was intolerant to a fasting protocol.
Table 1.
Baseline serum parameters
| mLeprdb | Leprdb | mLeprdb + NaSal | Leprdb + NaSal | |
|---|---|---|---|---|
| Body weight, g | 25.82±1.5 | 50.53±3.17* | 24.1±0.6 | 47.81±4.7* |
| Abdominal girth, cm | 8.05±0.5 | 11.9±0.4* | 8.3±0.4 | 11.75±0.6* |
| Glucose (nonfasting), mg/dl | 139±17.26 | 442±52.38* | 122.25±16 | 234.5±57.08† |
| Cholesterol level, μg/μl | 1.12±0.23 | 1.8±0.4* | 1.24±0.32 | 1.8±0.25* |
| Insulin (nonfasting), ng/ml | 2.234±0.73 | 2.58±0.52 | 2.8±0.45 | 2.8±0.24 |
| HOMA-IR | 18.3±6.1 | 67.5±12.3* | 20.3±4.4 | 38.9±9.2† |
Values are means ± SD; n = 8 mice. Cholesterol level was higher in Leprdb (homozygote, diabetic) and Leprdb mice treated with sodium salicylate (NaSal) than in mLeprdb (heterozygote, normal) mice. Glucose concentration was higher in Leprdb, but the treatment of NaSal attenuated blood glucose in Leprdb mice vs. mLeprdb mice. Abdominal girth and body weight were higher in Leprdb and Leprdb mice treated with NaSal vs. mLeprdb on the day of surgery, but weight loss in diabetic mice was 2.3 ± 2.5 mg in Leprdb mice treated with NaSal in drinking water (6 mg/ml for 7–10 days). Nonfasting insulin level was identical in Leprdb and mLeprdb mice before and after treatment with NaSal; however, homeostasis model assessment-insulin resistance (HOMA-IR) was higher in diabetic Leprdb mice vs. control, and NaSal significantly attenuated HOMA-IR in Leprdb mice.
P < 0.05 vs. mLeprdb control mice;
P < 0.05 vs. Leprdb.
Roles of IKK-α and IKK-β in type 2 diabetes.
To evaluate the roles of IKK-α and IKK-β in diabetes, Leprdb mice were treated with anti-TNF, apocynin, and MG-132, which plays a role in the selective proteolysis of IκB-α (13, 23, 24). The protein expression of IKK-α and IKK-β was increased in Leprdb mice (Fig. 1). IKK-β expression was significantly attenuated in Leprdb mice treated with MG-132 or apocynin, or in dbTNF−/dbTNF− mice versus Leprdb mice. However, the alteration of IKK-α expression was not observed in Leprdb mice treated with a neutralizing antibody to TNF-α or in dbTNF−/dbTNF− mice. These findings suggest a link among IKK-β, NF-κB, oxidative stress, and TNF-α in type 2 diabetic mice.
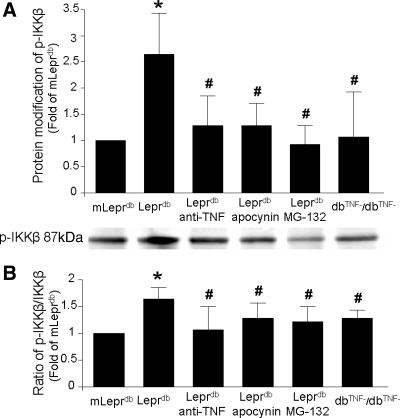
Fig. 1.
Western blot analysis of protein expression of IKK subunit from heart extract. A: protein expression of IKK-α was slightly higher in Leprdb (homozygote, diabetic) than in mLeprdb (heterozygote, normal) mice. Leprdb mice treated with anti-TNF, apocynin, MG-132, and the genetic deletion of TNF-α in diabetic mice (dbTNF−/dbTNF−) did not show significantly changes in IKK-α expression. B: protein expression of IKK-β was higher in Leprdb than in mLeprdb mice. dbTNF−/dbTNF− mice and Leprdb treatment with MG-132 or antioxidant apocynin significantly decreased IKK-β expression in Leprdb mice. Data represent means ± SD; n = 4 separate experiments. *P < 0.05 vs. mLeprdb; #P < 0.05 vs. Leprdb.
Activation of IKK-β, NF-κB, and degradation of IκB-α induced by TNF-α.
The phosphorylation of IKK-β activates IκB-α to decrease the NF-κB inhibitory action of IκB-α. In Leprdb mice, the protein modification of p-IKK-β was higher, but the protein modification of p-IKK-β was attenuated by anti-TNF, MG-132, apocynin, or the genetic deletion of TNF-α in diabetic mice (Fig. 2). In Leprdb mice, the protein expression of IκB-α was lower versus mLeprdb mice (Fig. 3A). However, the protein modification of p-IκB-α was higher in Leprdb versus mLeprdb mice. The protein modification of p-IκB-α was attenuated in dbTNF−/dbTNF− and Leprdb mice treated with anti-TNF, apocynin, or MG-132 versus mLeprdb mice (Fig. 3B). In Leprdb mice, the protein expression of NF-κB was higher, but apocynin, MG-132, or the genetic deletion of TNF-α presented a reduced protein expression of NF-κB (Fig. 3C).
Fig. 2.
Protein modification of phosphorylated (p)-IKK-β (A) and ratio of p-IKK-β to IKK-β (B) was higher in Leprdb vs. mLeprdb mice heart extract. Anti-TNF, apocynin, NF-κB inhibitor MG-132, and genetic deletion of TNF-α attenuated phosphorylation of IKK-β in Leprdb mice. Data represent means ± SD; n = 4 separate experiments. *P < 0.05 vs. mLeprdb; #P < 0.05 vs. Leprdb.
Fig. 3.
Protein expression of IκB-α (A) was higher in mLepr than in Lepr mice heart extract. In contrast, p-IκB-α (B) was higher in Leprdb vs. mLeprdb mice. Anti-TNF, antioxidant apocynin, NF-κB inhibitor MG-132, and genetic deletion of TNF-α in Leprdb mice attenuated the phosphorylation of IκB-α, thereby reducing the protein expression of NF-κB (C) as well. Data represent means ± SD; n = 4 separate experiments. *P < 0.05 vs. mLeprdb; #P < 0.05 vs. Leprdb.
Role of oxidative stress and NF-κB activation in type 2 diabetes-induced vascular dysfunction.
The vasodilation to the endothelium-dependent vasodilator ACh was significantly impaired in Leprdb versus mLeprdb mice (Fig. 4A), but NF-κB antagonist MG-132 partially restored NO-mediated coronary arteriolar dilation in Leprdb mice. The EPR results showed that O2− production was elevated and that MG-132 decreased O2− production in Leprdb versus mLeprdb mice (Fig. 4B).
Fig. 4.
A: Leprdb mice impaired but MG-132 treatment significantly restored ACh-induced endothelium-dependent vasodilation in Leprdb mice. Data represent means ± SE; n = 4 mice. B: O2− production from isolated coronary arterioles was higher in Leprdb mice vs. mLeprdb mice, and MG-132 attenuated O2− production in Leprdb mice. Values were normalized to the mean value of mLeprdb. Data represent means ± SD; n = 5 separate experiments. *P < 0.05 vs. mLeprdb; #P < 0.05 vs. Leprdb.
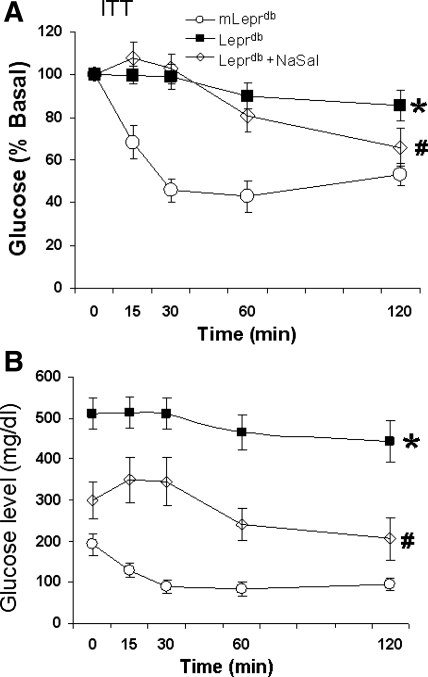
Effect of NaSal on improvement of insulin sensitivity in the heart.
Blood glucose, body weight, abdominal girth, lipid levels, insulin levels, and HOMA-IR were higher in Leprdb versus mLeprdb mice. There were no significant differences in body weight, abdominal girth, lipid level, and insulin level before and after treatment with NaSal (See Table 1). Lower values of glucose concentration and HOMA-IR were found in Leprdb mice treated with NaSal. The average body weight loss of Leprdb mice was 2.3 ± 2.5 mg after 7 days administration with 6 mg/ml NaSal in drinking water. Insulin tolerance testing (Fig. 5) showed a significant difference in the glucose clearance rate at 120 min after insulin administration in Leprdb mice treated with NaSal compared with Leprdb mice.
Fig. 5.
Insulin was injected (0.75 U/kg ip) and blood samples were taken for glucose determinations 0, 30, 60, and 120 min later. A: insulin tolerance test (ITT) showed that the glucose clearance rate at 120 min after insulin administration in Leprdb mice treated with sodium salicylate (NaSal) was significantly higher compared with Leprdb mice. The value was normalized to the basal glucose level. B: actual glucose level. Each value represents the mean ± SE from 6–8 mice. *P < 0.05 vs. mLeprdb; #P < 0.05 vs. Leprdb.
Role of NaSal in vascular dysfunction in type 2 diabetes.
To establish the role of IKK-β in the signaling pathway, we studied whether the blockade of IKK-β improves the endothelium-dependent vasodilation in Leprdb mice. Functional results (Fig. 6) showed that NaSal partially restored the ACh- and flow-induced NO-mediated vasodilation in Leprdb mice. SNP-induced vasodilation was equivalent in mLeprdb and Leprdb mice, indicating that the function of smooth muscle was preserved.
Fig. 6.
A: sodium nitroprusside-induced dilation in coronary arterioles was identical between mLeprdb and Leprdb mice. NaSal did not affect sodium nitroprusside-induced vasodilation in Leprdb mice. B: ACh-induced vasodilation in isolated mice coronary arterioles was blunted in Leprdb vs. mLeprdb mice, and NaSal partially restored this dilation in Leprdb mice. C: NaSal partially restored flow-induced vasodilation in Leprdb mice. Data represent means ± SE; n = 5 mice. *P < 0.05 vs. mLeprdb; #P < 0.05 vs. Leprdb. ΔP, change of pressure.
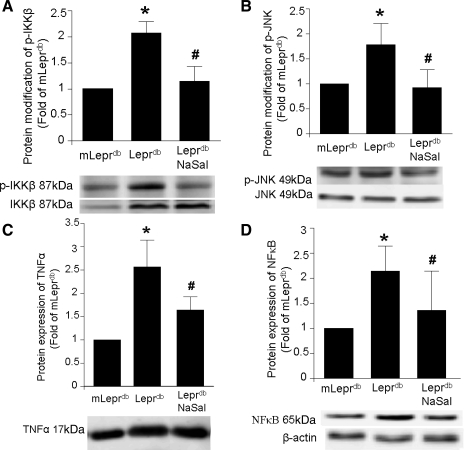
Insulin resistance enhanced activity of JNK and IKK-β in type 2 diabetes.
Western blot analysis (Fig. 7) showed that the protein expression of TNF-α and NF-κB and the protein modification of p-IKK-β and p-JNK were greater in Leprdb mice, but NaSal attenuated the protein expression of TNF-α and NF-κB and the protein modification of the phosphorylation of IKK-β and the phosphorylation of JNK in Leprdb mice.
Fig. 7.
Protein modification of p-IKK-β (A) and p-JNK (B) was greater in Leprdb mice vs. mLeprdb mice heart extract, but NaSal decreased p-IKK-β and p-JNK without affecting the expression of IKK-β and JNK. Protein expression of TNF-α (C) and NF-κB (D) was greater in Leprdb mice vs. mLeprdb mice heart extract, but NaSal decreased TNF-α and NF-κB. Data represent means ± SD; n = 5 separate experiments. *P < 0.05 vs. mLeprdb; #P < 0.05 vs. Leprdb.
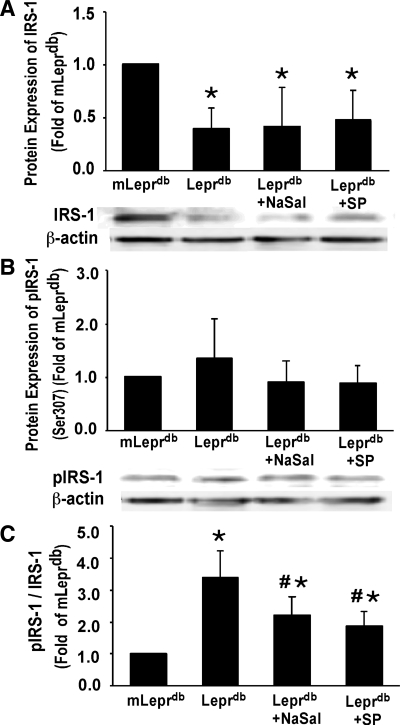
The phosphorylation of IRS-1 on the serine site blocks insulin signal transduction in diabetes. The protein expression of IRS-1 (Fig. 8A) was significantly reduced in Leprdb, Leprdb treated with NaSal, and Leprdb treated with JNK inhibitor SP-600125 versus mLeprdb mice. The protein modification of p-IRS-1 (Ser307) was unaffected (Fig. 8B) in Leprdb, Leprdb treated with NaSal, and Leprdb mice treated with JNK inhibitor SP-600125 versus mLeprdb mice. However, the ratio of p-IRS-1 to IRS-1 was significantly higher in Leprdb. Leprdb mice treated with NaSal and Leprdb mice treated with JNK inhibitor SP-600125 showed decreased p-IRS-1/IRS-1.
Fig. 8.
A: total insulin receptor substrate-1 (IRS-1) protein expression was decreased in Leprdb vs. mLeprdb mice heart extract; NaSal and SP-600125 (SP) treatment did not increase IRS-1 expression. B: p-IRS-1 (Ser307) protein modification was elevated in Leprdb compared with mLeprdb control mice heart extract, and both NaSal and SP-600125 treatment appeared to reduce p-IRS-1 protein modification although there was no significant difference between groups. C: ratio of p-IRS-1 to total IRS-1 (p-IRS-1/IRS-1) was significantly higher in Leprdb vs. mLeprdb heart extract. NaSal and SP-600125 treatment reduced p-IRS-1/IRS-1. Data represent means ± SD; n = 8 mice. *P < 0.05 vs. mLeprdb; #P < 0.05 vs. Leprdb.
DISCUSSION
Our results suggest that the interaction of NF-κB and TNF-α signaling induces the activation of IKK-β in insulin-resistant Leprdb mice heart, which amplifies oxidative stress, leading to endothelial dysfunction in the coronary arterioles of Leprdb mice. Our findings support the concept that a feed-forward signaling of TNF-α and NF-κB via the IKK-β pathway induces insulin resistance and coronary arteriolar dysfunction in type 2 diabetes based on the following observations: NF-κB antagonist MG-132, or IKK-β inhibitor NaSal, restored NO-mediated endothelium-dependent coronary arteriolar dilation in Leprdb mice, but the responses in mLeprdb mice were unaffected. The protein expression of IKK-α and IKK-β were higher in Leprdb than in mLeprdb mice; the expression of IKK-β, but not the expression of IKK-α, was attenuated by MG-132, antioxidant apocynin, or the genetic deletion of TNF-α in diabetic mice. Insulin resistance was increased in Leprdb mice, and NaSal improved insulin sensitivity. The protein expression of TNF-α, NF-κB, and protein modification of p-IKK-β and p-JNK were greater in Leprdb mice, but NaSal attenuated TNF-α, NF-κB, p-IKK-β, and p-JNK in Leprdb mice. The p-IRS-1-to-IRS-1 ratio was elevated in Leprdb compared with mLeprdb mice. Both NaSal and the JNK inhibitor SP-600125 reduced the p-IRS-1-to-IRS-1 ratio in Leprdb mice. MG-132 (Fig. 4B) or anti-TNF-α reduced O2− production in Leprdb mice. NF-κB induces TNF-α signaling to accentuate oxidative stress and endothelial dysfunction via an IKK-β-dependent mechanism, which may be associated with inflammatory and insulin signaling pathways in type 2 diabetes. Our findings are consistent with NF-κB involvement, by interacting with TNF-α, thereby inducing IKK-β and oxidative stress, in endothelial dysfunction in type 2 diabetes. The present molecular results further support our previous physiological observations that the increases in TNF-α expression induce the production of ROS, leading to endothelial dysfunction in type 2 diabetes (15).
Roles of interaction of TNF-α and NF-κB signaling in type 2 diabetes.
The inducible transcription factor NF-κB regulates the expression of genes encoding oxidants, cytokines, chemokines, and adhesion molecules, which are associated with inflammation and activated by the gene products of NF-κB, e.g., a feed-forward interaction (12, 15, 19, 30). These proinflammatory agents play a detrimental role in the vascular pathology, and TNF-α initiates signaling cascades that converge on the IKK complex via the NF-κB signaling pathway. TNF-α is an inducer of IKK-β and IκB-α in the classical NF-κB pathway. Thus TNF-α initiates signaling cascades, predominantly acting through IKK-β (6, 22). TNF-α induces oxidative stress by activating NAD(P)H oxidase, which is the primary source of O2− in vascular tissue. O2− is dismutased into oxygen and H2O2 by O2− dismutase (SOD) in mitochondria. Mitochondria-derived H2O2 diffuses through the cellular membrane and then is involved in the regulation of endothelial NF-κB by activating the IKK complex (10, 11, 37). In this study, we examined whether a feed-forward interaction between TNF-α and NF-κB, via the IKK-β pathway, contributes to the evolution of vascular disease and insulin resistance in type 2 diabetes. Our Western blot analysis results showed that MG-132, apocynin, or the genetic deletion of TNF-α attenuated the expression of IKK-β but not the expression of IKK-α. This points to a linkage among IKK-β, NF-κB, oxidative stress, and TNF-α in contributing to type 2 diabetes.
Our results showed that the protein expression of IκB-α was lower. However, the protein expression of p-IκB-α was higher in Leprdb mice compared with mLeprdb mice. In dbTNF−/dbTNF− and Leprdb mice treated with anti-TNF, apocynin, or MG-132, the protein expression of p-IκB-α was attenuated. In Leprdb mice, the protein expression of NF-κB was higher, but apocynin, MG-132, or dbTNF−/dbTNF− mice had a reduced protein expression of NF-κB, indicating that TNF-α, oxidative stress, and IκB-α degradation may increase the activity of NF-κB. NF-κB contributes to the endothelial inflammation in ROS-dependent activation of the IKK complex (11), and this supports the concept that TNF-α induces oxidative stress by activating NAD(P)H oxidase, which then activates NF-κB.
Our functional results showed that ACh-induced vasodilation was impaired in Leprdb mice versus mLeprdb mice, but NF-κB antagonist MG-132 partially restored ACh-induced, NO-mediated coronary arteriolar dilation in Leprdb mice. EPR results showed that O2− production was elevated and that MG-132 decreased O2− production in Leprdb mice, which indicates that the blocking of NF-κB transcriptional action markedly attenuated O2− production in isolated coronary arterioles in Leprdb mice. This supports the concept that IKK-β is a putative activator of NF-κB, which is thought to alter subsequent gene expression in this pathway (12, 19, 30).
Role of IKK-β in vascular dysfunction in type 2 diabetes.
NaSal is an IKK-β inhibitor that interrupts the cascade of molecular events leading to inflammation. NaSal inhibited the expression of inducible NO synthase, which causes endothelial dysfunction and inflammation in cardiac fibroblasts (40) and TNF-α production, which is dependent on NF-κB activity, in lipopolysaccharide-stimulated macrophage (39). NaSal may interfere with NF-κB activity through a mitogen-activated protein kinase-dependent process (39).
Yuan et al. (43) showed that reduced signaling through the IKK-β pathway inhibition by NaSal in obese mice is accompanied by improved insulin sensitivity. Recent studies noted that NF-κB blockade by inhibiting IKK-β activity decreased myocardial injury and preserved cardiac function following ischemia-reperfusion (26), which supports the concept that the inflammatory response participates in the development of heart failure (42). Our results show that p-IKK-β increases in diabetes, but the blockade of NF-κB and oxidative stress and the genetic deletion of TNF-α attenuated p-IKK-β, suggesting that the phosphorylation of IKK-β increases in diabetic mice by the activation of oxidative stress and TNF-α.
The pathogenesis of endothelial dysfunction in diabetes is initially related to a decrease in NO synthesis or the inactivation of NO due to an increased endothelial production of ROS (31). An underlying mechanism proposed for TNF-α-induced endothelial dysfunction is that TNF-α signaling leads to oxidative stress via NAD(P)H activation, which in turn may lead to reduced NO bioactivity (15, 28). We identified a link between vascular dysfunction and insulin resistance: the treatment with NaSal in coronary microcirculation resulted in an improvement of endothelial dysfunction in diabetic Leprdb mice. The NO donor SNP induced an identical vasodilation in Leprdb and mLeprdb mice, but ACh-induced vasodilation was impaired in isolated coronary arterioles in diabetic Leprdb mice, which indicates that endothelial-dependent and NO-mediated vasodilation is impaired in diabetes. Most importantly, NF-κB antagonist MG-132 or IKK-β inhibitor NaSal partially restored NO-mediated coronary arteriolar dilation in type 2 diabetes. These results further support our hypothesis that TNF-α and NF-κB signaling via an IKK-β-dependent mechanism plays a key role in endothelial dysfunction in diabetes.
Blood glucose, body weight, abdominal girth, lipid level, insulin level, and HOMA-IR were higher in Leprdb versus mLeprdb mice. However, the lower values of glucose concentration and HOMA-insulin resistance found in Leprdb mice treated with NaSal indicate that NaSal improved insulin sensitivity in Leprdb mice (Table 1). MG-132 or anti-TNF antibody did not change blood glucose or body weight. Insulin tolerance testing (Fig. 5) shows that there was a significant difference in the glucose clearance rate at 120 min after insulin administration in Leprdb mice treated with NaSal compared with Leprdb mice. Our results indicate that hyperglycemia and insulin resistance in Leprdb mice were amended by a short-term treatment with NaSal via a downregulation of IKK-β and NF-κB signaling. An increased phosphorylation of IKK-β may also contribute to the development or progression of vascular disease and hyperglycemia in type 2 diabetes.
Link between inflammation-induced insulin resistance and vascular dysfunction.
The heart is an insulin-responsive organ, and disorders of insulin action, such as diabetes and obesity, can have profound effects on cardiac performance (1). Coronary artery disease causes acute myocardial infarction, which is associated with significant morbidity and mortality in patients with diabetes (9). The inflammation-induced insulin resistance, but genetic disruption of NF-κB and JNK signaling pathways, has been shown to improve insulin resistance (33). Human type 2 diabetes is currently characterized by defects in both insulin action and insulin secretion, which lack the focus needed to identify a single molecular abnormality underlying these features, if indeed one exists. IRS proteins may be involved in type 2 diabetes (41) and tyrosine phosphorylation activates insulin IRS-1, which further leads to the translocation of glucose transporters (GLUT4) to the cell surface (38). IKK-β may affect the glucose metabolism by altering NF-κB transcriptional action to express GLUT4 (4). JNK is a main regulatory molecule that contributes to coronary arteriolar dysfunction and insulin resistance (44, 35). Whereas IRS-1 tyrosine phosphorylation by Akt induces insulin signal transduction (18), serine phosphorylation by JNK can downregulate or inactivate IRS-1. The impaired activation of Akt and the enhanced activation of JNK by TNF-α are correlated with insulin resistance (2, 17, 35). Although NaSal had no obvious effect on the activation of IKK-β or JNK, the reduced signaling by inhibiting IKK-β in obese mice is accompanied by an improved insulin sensitivity (43). Consistent with the findings described above, the inhibition of IKK-β or JNK activity by NaSal significantly reduced the blood glucose level and augmented insulin signaling in cardiac tissue. Our results showed that the protein expression of TNF-α and NF-κB were higher in diabetic mice but that NaSal attenuated the protein expression of TNF-α and NF-κB. Specifically, NaSal attenuated the phosphorylation of IKK-β and JNK without altering the protein expression of IKK-β and JNK. This suggests that NaSal interrupts the phosphorylation of IKK-β and the phosphorylation of JNK. TNF-α activates the IKK-β (Fig. 1B) and JNK signaling pathways (44), and, conversely, the expression of the TNF-α gene is regulated by NF-κB through the activation of IKK-β (Fig. 7D). NaSal attenuates TNF-α; however, the complete role of NaSal in this process remains to be elucidated.
To further elucidate the signaling pathway of defective insulin action in the cardiac tissue of Leprdb mice, we tested whether IRS-1 serine phosphorylation is attenuated by SP-600125 and NaSal. p-IRS-1 protein modification was elevated in Leprdb compared with mLeprdb mice; both NaSal and JNK inhibitor SP-600125 reduced the p-IRS-1 protein modification in Leprdb mice (Fig. 8). Cardiac myocyte metabolism occurs through paracrine mechanisms by activating insulin receptors in the heart (5), thereby decreasing myocardial infarct size and increasing cardiac contractility (8, 29). Because insulin signaling influences numerous functions within the heart, an altered insulin action can have many significant indirect effects on the heart that may lead to vascular dysfunction. Our results indicate that TNF-α, the phosphorylation of JNK and IKK-β, and NF-κB contribute to insulin resistance and vascular dysfunction putatively via the activation of IKK-β in type 2 diabetes. NaSal and JNK inhibitor SP-600125 sensitize insulin signaling by preventing serine phosphorylation on the IRS-1 of IKK-β and JNK. This indicates that NaSal inhibits the phosphorylation of IKK-β and JNK in insulin-resistant diabetic murine hearts.
In summary, our results demonstrate that TNF-α is correlated with IKK-β to induce NF-κB activation. IKK-β inhibitor NaSal or NF-κB antagonist MG-132 restored ACh-induced endothelium-dependent vasodilation in coronary arterioles in diabetes. Diabetic mice treated with NaSal show a decrease in glucose level and a reversal of insulin resistance. Both IKK-β and JNK activation are increased in Leprdb mice, and NaSal attenuates their activation. Overall, our molecular and functional results show that IKK-β is a crucial component of the biochemical pathway responsible for vascular dysfunction, inflammation, and insulin resistance in type 2 diabetic murine hearts. The blockade of IKK-β activity not only preserves coronary arteriolar vasodilation but also prevents insulin resistance in type 2 diabetic mice. Although the current study confirms that NaSal amends whole body insulin sensitivity and demonstrates that IKK-β and NF-κB signaling is associated with insulin resistance and inflammation in diabetic murine hearts, further validation is needed to show how a direct insulin signaling in coronary arterioles affects vessel function.
GRANTS
This study was supported by an American Heart Association Scientist Development Grant 110350047A; a Pfizer Atorvastatin Research Award 2004-37; and National Heart, Lung, and Blood Institute Grants RO1-HL-077566 and RO1-HL-085119 (to C. Zhang).
REFERENCES
- 1.Abel ED Insulin signaling in heart muscle: lessons from genetically engineered mouse models. Curr Hypertens Rep 6: 416–423, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277: 1531–1537, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Assi K, Pillai R, Gómez-Muñoz A, Owen D, Salh B. The GTPase Rac regulates the proliferation and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Mol Med 13: 297–304, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin RL, Rune A, Bouzakri K, Zierath JR, Krook A. siRNA-mediated reduction of inhibitor of nuclear factor-κB kinase prevents tumor necrosis factor-α-induced insulin resistance in human skeletal muscle. Diabetes 57: 2066–2073, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest 109: 629–639, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, Jegga AG, Aronow BJ, Ghosh G, Rickert RC, Karin M. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:p52 dimers. EMBO J 23: 4202–4210, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 11: 183–190, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai W, Wu Y, Li G, Cao W, Yang Z, Liu Z. Activation of p38 mitogen-activated protein kinase abolishes insulin-mediated myocardial protection against ischemia-reperfusion injury. Am J Physiol Endocrinol Metab 294: E183–E189, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Chatham JC, Gao ZP, Bonen A, Forder JR. Preferential inhibition of lactate oxidation relative to glucose oxidation in the rat heart following diabetes. Cardiovasc Res 43: 96–106, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783–797, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging : role of NF-κB. J Appl Physiol 105: 1333–1341, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.dela Paz NG, Simeonidis S, Leo C, Rose DW, Collins T. Regulation of NF-κB-dependent gene expression by the POU domain transcription factor Oct-1. J Biol Chem 282: 8424–8434, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Elliott PJ, Zollner TM, Boehncke WH. Proteasome inhibition: a new anti-inflammatory strategy. J Mol Med 81: 235–245, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 83: 847–850, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-α induces endothelial dysfunction in Leprdb mice. Circulation 115: 245–254, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton SJ, Chew GT, Watts GF. Therapeutic regulation of endothelial dysfunction in type 2 diabetes mellitus. Diab Vasc Dis Res 4: 89–102, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Jiang G, Dallas-Yang Q, Liu F, Moller DE, Zhang BB. Salicylic acid reverses phorbol 12-myristate-13-acetate (PMA)- and tumor necrosis factor α (TNFα)-induced insulin receptor substrate 1 (IRS1) serine 307 phosphorylation and insulin resistance in human embryonic kidney 293 (HEK293) cells. J Biol Chem 278: 180–186, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-κB: its role in health and disease. J Mol Med 82: 434–448, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol Heart Circ Physiol 255: H1558–H1562, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Lattime EC, Stutman O. Thymic lymphomas mediate non-MHC-restricted, TNF-dependent lysis of the murine sarcoma WEHI-164. Cell Immunol 136: 69–79, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence T, Bebien M, Liu GY, Nizet V, Karin M. IKKα limits macrophage NF-κB activation and contributes to the resolution of inflammation. Nature 434: 1138–1143, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol 8: 397–403, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289: 2350–2354, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letoha T, Somlai C, Takács T, Szabolcs A, Rakonczay Z Jr, Jármay K, Szalontai T, Varga I, Kaszaki J, Boros I, Duda E, Hackler L, Kurucz I, Penke B. The proteasome inhibitor MG132 protects against acute pancreatitis. Free Radic Biol Med 39: 1142–1151, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Moss NC, Stansfield WE, Willis MS, Tang RH, Selzman CH. IKKβ inhibition attenuates myocardial injury and dysfunction following acute ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 293: H2248–H2253, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Olson AL, Pessin JE. Transcriptional regulation of the human GLUT4 gene promoter in diabetic transgenic mice. J Biol Chem 270: 23491–23495, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-α induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res 99: 69–77, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Ren J, Sowers JR, Walsh MF, Brown RA. Reduced contractile response to insulin and IGF-I in ventricular myocytes from genetically obese Zucker rats. Am J Physiol Heart Circ Physiol 279: H1708–H1714, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Rimbach G, Valacchi G, Canali R, Virgili F. Macrophages stimulated with IFN-γ activate NF-κB and induce MCP-1 gene expression in primary human endothelial cells. Mol Cell Biol Res Commun 3: 238–242, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Russo G, Leopold JA, Loscalzo J. Vasoactive substances: nitric oxide and endothelial dysfunction in atherosclerosis. Vascul Pharmacol 38: 259–269, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Salvemini D, Cuzzocrea S. Superoxide, superoxide dismutase and ischemic injury. Curr Opin Investig Drugs 3: 886–895, 2002. [PubMed] [Google Scholar]
- 33.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 132: 2169–2180, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature 409: 307–312, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Sugita M, Sugita H, Kaneki M. Increased insulin receptor substrate 1 serine phosphorylation and stress-activated protein kinase/c-Jun N-terminal kinase activation associated with vascular insulin resistance in spontaneously hypertensive rats. Hypertension 44: 484–489, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Tran K, Merika M, Thanos D. Distinct functional properties of IκB α and IκB β. Mol Cell Biol 17: 5386–5399, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Virkamäki A, Ueki K, Kahn CR. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest 103: 931–943, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vittimberga FJ, McDade TP, Perugini RA, Callery MP. Sodium salicylate inhibits macrophage TNF-alpha production and alters MAPK activation. J Surg Res 84: 143–149, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Brecher P. Salicylate inhibition of extracellular signal-regulated kinases and inducible nitric oxide synthase. Hypertension 34: 1259–1264, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391: 900–904, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-kappaB in myocardium of patients with congestive heart failure. Circulation 98: 100–103, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted distruption of IKKβ. Science 293: 1673–1677, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Zhang C, Hein TW, Wang W, Ren Y, Shipley RD, Kuo L. Activation of JNK and xanthine oxidase by TNF-α impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol 40: 247–257, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian WM. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 26: 475–480, 2006. [DOI] [PubMed] [Google Scholar]