Abstract
Endothelial vasomotor function decreases with increasing age. Extracellular superoxide dismutase (ecSOD) protects against vascular dysfunction in several disease states. The purpose of this study was to determine whether endogenous ecSOD protects against endothelial dysfunction in old mice. Vasomotor function of the aorta was studied ex vivo in wild-type (ecSOD+/+) and ecSOD-deficient (ecSOD−/−) mice at 11 (adult) and 29 (old) mo of age. Maximal relaxation to acetylcholine (10−4 M) was impaired in vessels from adult ecSOD−/− mice [75 ± 3% (mean ± SE)] compared with wild-type mice (89 ± 2%, P < 0.05). Maximal relaxation to acetylcholine (10−4 M) was profoundly impaired in aorta from old ecSOD−/− mice (45 ± 5%) compared with wild-type mice (75 ± 4%, P < 0.05). There was a significant correlation between expression of ecSOD and maximal relaxation to acetylcholine in adult and old mice. Tempol (1 mM), a scavenger of superoxide, improved relaxation in response to acetylcholine (63 ± 8%) in old ecSOD−/− mice (P < 0.05), but not wild-type mice (75 ± 4%). Maximal relaxation to sodium nitroprusside was similar in aorta from adult and old wild-type and ecSOD−/− mice. Quantitative RT-PCR showed a decrease in mRNA levels of ecSOD and catalase in aorta of old mice and an increase in levels of TNFα and Nox-4 in aorta of old mice compared with adult mice. The findings support the hypothesis that impaired antioxidant mechanisms may contribute to cumulative increases in oxidative stress and impaired endothelial function in old mice. In conclusion, endogenous ecSOD plays an important role in protection against endothelial dysfunction during aging.
Keywords: oxidative stress, antioxidants, vasomotor function, nitric oxide
extracellular superoxide dismutase (ecSOD) protects against inactivation of nitric oxide (NO) by superoxide and, thereby, protects against endothelial dysfunction. Experimental alteration of ecSOD levels, by adenoviral overexpression or in knockout mice, has little effect on vascular function in normal animals (4, 15), in which levels of superoxide in vessels are low. The protective effect of ecSOD is much greater in the presence of diseases and stimuli that increase oxidative stress (2, 21, 27).
Aging is associated with increased oxidative stress and endothelial dysfunction (1, 3–5, 9, 10). Plasma levels of ecSOD decline with aging (11), and gene transfer of ecSOD improves endothelial function in old rats (4). It is not known, however, whether endogenous ecSOD is sufficient to play an important role in protection against endothelial dysfunction during aging.
An important role for ecSOD in protection of blood vessels during aging is supported by a large study of humans with a gene variant of ecSOD, ecSODR213G, which is associated with increased risk of ischemic heart disease (16). However, cardiovascular risk was not unmasked until the subjects were 70 yr old. The finding implies that ecSOD may have greater protective effects during aging than in youth or, perhaps, that loss of ecSOD results in cumulative increases in oxidative stress that are manifested as a disease state late in life.
The goal of this study was to test the hypothesis, using adult and old wild-type (ecSOD+/+) and ecSOD-deficient (ecSOD−/−) mice, that endogenous ecSOD protects against endothelial dysfunction during aging. In addition, quantitative RT-PCR was used to examine mRNA levels of some genes that are potentially important in relation to endothelial function.
METHODS
Animals.
As described previously (18), we obtained breeding pairs of wild-type and ecSOD−/− mice from Dr. Ralf Brandes. Female wild-type and ecSOD−/− littermates derived from breeding pairs of heterozygous ecSOD+/− mice were used for this study. Mice were euthanized by sevoflurane inhalation followed by exsanguination.
Adult wild-type (n = 8) and adult ecSOD−/− (n = 8) mice were similar in age (11.1 ± 0.3 and 11.3 ± 0.2 mo, respectively). Old wild-type (n = 12) and ecSOD−/− (n = 7) mice were also similar in age (29.3 ± 0.3 and 28.7 ± 0.4 mo, respectively). Adult wild-type and adult ecSOD−/− mice were similar in body weight (30.2 ± 2.3 and 28.3 ± 2.2 g, respectively). Old wild-type and old ecSOD−/− mice were also similar in body weight (21.5 ± 0.8 and 22.6 ± 0.8 g, respectively).
Aortas were quickly removed and placed in cold (4°C) oxygenated Krebs solution (in mM: 133 NaCl, 4.7 KCl, 1.35 NaH2PO2, 16.3 NaHCO3, 0.61 MgSO4, 7.8 glucose, and 2.52 CaCl2). Aortas were trimmed, cleaned, and cut into 5- to 7-mm-long rings. All procedures and handling of animals were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Tissue RNA.
Total RNA was isolated from the aorta using TRIzol (Invitrogen) followed by RNeasy Mini (Qiagen). Reverse transcription was performed as described previously (6). Real-time PCR was performed using the TaqMan method, with primer/probe sets from Applied Biosystems. Relative quantification was performed against β-actin using the cycle threshold (ΔΔCt) method. We measured the three isoforms of SOD, endothelial NO synthase (eNOS), Nox catalytic subunits of NAD(P)H oxidase (Nox-2 and Nox-4), catalase, and Klotho (an aging suppressor gene).
Detection of superoxide.
Superoxide levels were measured by lucigenin-enhanced chemiluminescence as described previously (21). Vessel segments were placed in 0.5 ml of PBS containing 5 μM lucigenin, and relative light units were measured for 5 min. Background counts were determined and subtracted from values for tissue samples, and values were normalized to surface areas. Effects of tiron (a scavenger of superoxide, 10 mM) were determined.
Measurement of superoxide levels with lucigenin requires the entire aorta from a mouse. Thus we used the entire aorta from mice that were different from those used for examination of vasomotor responses in adult wild-type and ecSOD−/− mice. Old mice were not available for measurement of superoxide with lucigenin.
Vasomotor responses.
Aortic rings were mounted on stainless steel hooks at optimal resting tension (0.5 g) in individual organ baths in Krebs bicarbonate solution at 37°C and aerated with 95% O2-5% CO2. Tension was adjusted periodically to the desired level during a 45-min equilibration period. Vascular rings were precontracted with 10−5 mol/l PGF2α and initially tested with 3 × 10−5 acetylcholine and washed. Vascular rings were then contracted to 50% maximal contraction with 10−5 mol/l PGF2α. Responses to acetylcholine (10−9–10−5 mol/l) with and without tempol, an SOD mimetic (1 mM), were measured. Responses to the endothelium-independent vasodilator sodium nitroprusside (10−9–10−5 mol/l) were then examined. Responses to PGF2α (10−9–10−5 mol/l) and serotonin (10−9–10−5 mol/l) were also examined. Contractile responses are expressed as grams of tension, and relaxation is expressed as percentage of contraction produced by PGF2α.
Drugs.
PGF2α, acetylcholine chloride, sodium nitroprusside, serotonin, tempol, and tiron (Sigma) were dissolved in normal saline.
Statistical analysis.
Values are means ± SE. Intergroup comparisons were performed using one-way ANOVA with Bonferroni's multiple comparisons test. Differences were considered to be significant when P < 0.05.
RESULTS
Gene expression.
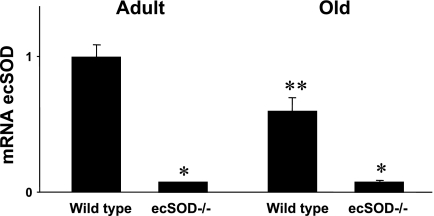
There was minimal expression of ecSOD mRNA in the ecSOD−/− mice compared with wild-type mice (Fig. 1). mRNA for ecSOD is detectable in ecSOD−/− mice, because some mRNA is still produced. As long as primers/probes that target the undisrupted positions are used, as in our experiments, residual expression after disruption of intact ecSOD is detected.
Fig. 1.
Levels of mRNA in aorta of adult and old wild-type mice and extracellular SOD-deficient (ecSOD−/−) mice. Values are means ± SE (n = 7–8). *P < 0.05 vs. wild-type. **P < 0.05 vs. adult wild-type.
Expression of ecSOD was reduced by 40 ± 8% in old wild-type mice compared with adult wild-type mice (Fig. 1). No compensatory increase in expression of other SOD isoforms (Cu,Zn-SOD or Mn-SOD) was observed in ecSOD−/− mice (Table 1). Expression of catalase was similar in wild-type and ecSOD−/− mice but was greatly reduced in old mice. There was also a tendency for eNOS to be reduced in older animals, but differences failed to reach statistical significance.
Table 1.
Gene expression (mRNA) in adult and old wild-type and ecSOD-deficient mice
| n | Cu,Zn-SOD | Mn-SOD | CAT | eNOS | Nox-2 | Nox-4 | TNFα | Klotho | |
|---|---|---|---|---|---|---|---|---|---|
| Adult | |||||||||
| ecSOD+/+ | 7 | 1.00±0.04 | 1.00±0.17 | 1.00±0.13 | 1.00±0.12 | 1.00±0.12 | 1.00±0.11 | 1.00±0.18 | 1.00±0.31 |
| ecSOD−/− | 8 | 1.19±0.10 | 1.34±0.64 | 1.03±0.24 | 1.19±0.26 | 0.90±0.08 | 1.19±0.23 | 0.69±0.21 | 0.86±0.19 |
| Old | |||||||||
| ecSOD+/+ | 11 | 0.86±0.11 | 0.76±0.08 | 0.36±0.06* | 0.75±0.10 | 1.28±0.09 | 1.79±0.24* | 1.75±0.35 | 0.40±0.10* |
| ecSOD−/− | 7 | 0.83±0.17 | 0.95±0.20 | 0.22±0.04† | 0.64±0.08 | 0.82±0.11 | 1.56±0.40 | 1.33±0.14 | 0.23±0.06† |
Values are means ± SE. ecSOD, extracellular SOD; CAT, catalase; eNOS, endothelial nitric oxide synthase.
P < 0.05 vs. adult ecSOD+/+.
P < 0.05 vs. adult ecSOD−/−.
Expression of Nox-2 was unchanged across all groups (Table 1). Levels of Nox-4 and TNFα tended to be increased in aortas of old mice compared with adult mice (Table 1). Expression of Klotho, an age-related gene, was similar in wild-type and ecSOD−/− mice but was profoundly reduced in old mice.
Superoxide.
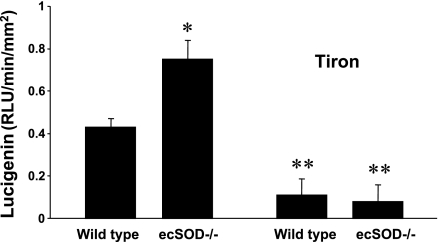
Superoxide was measured with lucigenin in separate groups of adult wild-type (n = 7) and adult ecSOD−/− (n = 7) mice. Superoxide was significantly greater in aortas from adult ecSOD−/− mice than wild-type mice (Fig. 2), and the lucigenin signal was abolished by tiron in both groups, which indicates specificity for superoxide. Tiron eliminated the difference between aortas from ecSOD−/− and wild-type mice (Fig. 2).
Fig. 2.
Superoxide levels (lucigenin) in aorta of adult wild-type and ecSOD−/− mice, with and without tiron. Values are means ± SE (n = 7–8). RLU, relative light units. *P < 0.05 vs. wild-type. **P < 0.05 vs. without tiron.
Vasomotor responses.
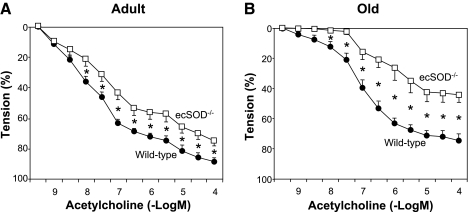
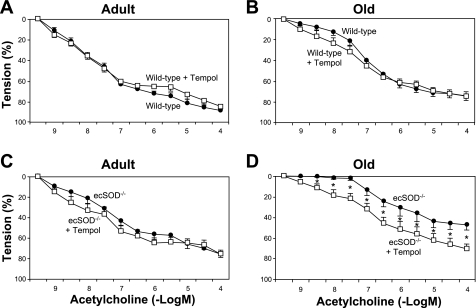
In adult mice, maximal relaxation to acetylcholine (10−4 M) was significantly less in aortas from ecSOD−/− mice than wild-type mice (75 ± 4 vs. 89 ± 2%; Fig. 3A). In old mice, maximal relaxation to acetylcholine (10−4 M) was much less in ecSOD−/− mice than wild-type mice (45 ± 5 vs. 75 ± 4%; Fig. 3B).
Fig. 3.
Relaxation of aorta to acetylcholine in adult wild-type and ecSOD−/− (n = 8 each) mice (A) and old wild-type (n = 11) and ecSOD−/− (n = 7) mice (B). Values are means ± SE. *P < 0.05 vs. wild-type.
The response to acetylcholine was less in old wild-type and ecSOD−/− mice (Fig. 3B) than adult mice (Fig. 3A). In wild-type mice, there was mild impairment in maximum relaxation to acetylcholine during aging (89 ± 2 and 75 ± 4% in adult and wild-type mice, respectively, P < 0.05; Fig. 3). Impairment of the response to acetylcholine was exaggerated in old ecSOD−/− mice compared with adult mice (45 ± 5 and 75 ± 4%, respectively; Fig. 3).
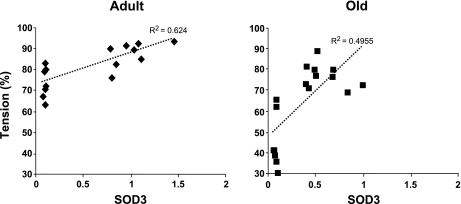
The response to acetylcholine was compared with gene expression of ecSOD in adult and old wild-type and ecSOD−/− mice (Fig. 4). There was a positive correlation between relaxation to acetylcholine and expression of ecSOD in adult and old mice (P < 0.05). The regression line was significantly steeper in old mice than in adult mice.
Fig. 4.
Relationship of expression of ecSOD (mRNA) to maximal relaxation of aorta to acetylcholine in adult and old wild-type and ecSOD−/− mice. In adult mice, there was a significant correlation between expression of ecSOD (SOD3) and relaxation to acetylcholine (P < 0.05, slope = 15.2). In old mice, there also was a significant correlation (P < 0.05, slope = 43.9), which was significantly different from the slope in adult mice (P < 0.05).
In old ecSOD−/− mice, tempol improved responses to acetylcholine (63 ± 8%; Fig. 5D). Tempol did not improve responses to acetylcholine in old wild-type mice or adult wild-type or ecSOD−/− mice.
Fig. 5.
Effects of tempol on relaxation of aorta to acetylcholine in adult wild-type (n = 8; A), old wild-type (n = 11; B), adult ecSOD−/− (n = 8; C), and old ecSOD−/− (n = 7; D) mice. Values are means ± SE. *P < 0.05, tempol vs. control.
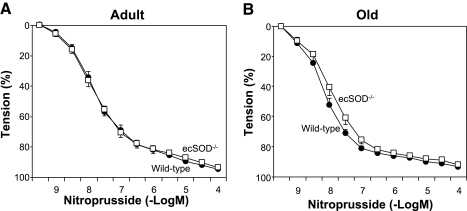
Responses to nitroprusside were not altered in adult or old ecSOD−/− mice (Fig. 6).
Fig. 6.
Relaxation of aorta to sodium nitroprusside in adult wild-type and ecSOD−/− (n = 8 each) mice (A) and old wild-type (n = 11) and ecSOD−/− (n = 7) mice (B). Values are means ± SE.
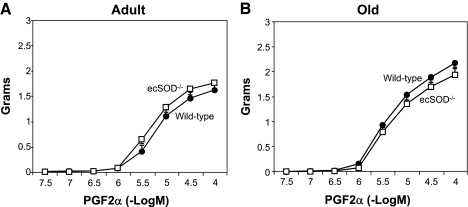
Contraction to PGF2α was similar in adult and old ecSOD−/− and wild-type mice (Fig. 7). Contractile responses to serotonin also were similar in ecSOD−/− and wild-type adult and old mice (data not shown).
Fig. 7.
Contraction of aorta to PGF2α in adult wild-type and ecSOD−/− (n = 8 each) mice (A) and old wild-type (n = 11) and ecSOD−/− (n = 7) mice (B). Values are means ± SE.
DISCUSSION
The major new finding of this study is that although endogenous ecSOD in the aorta declines substantially during aging, relaxation to acetylcholine is impaired much more in old ecSOD−/− mice than adult ecSOD−/− mice. Endothelial dysfunction in old ecSOD−/− mice was reversed in part with tempol. These findings suggest that residual levels of endogenous ecSOD during aging play a critical role in protection against endothelial dysfunction.
Vasorelaxation.
In blood vessels, NO is released from the endothelium and diffuses to smooth muscle to produce relaxation. In vessels with high levels of superoxide, NO may be degraded and fail to reach smooth muscle cells, thereby producing endothelial dysfunction. ecSOD, which constitutes a large portion of total SOD in the vessel wall, is strategically located to protect NO during diffusion to smooth muscle (25). Thus ecSOD plays a key role in protection against endothelial dysfunction.
The finding that vascular responses are further impaired in old ecSOD−/− mice compared with adult ecSOD−/− mice is novel. Modest impairment of aortic relaxation to acetylcholine in adult ecSOD−/− mice is also consistent with previous reports (14, 15). Vessel size appears to influence susceptibility to alteration of endothelial function in adult mice, since endothelial function of mesenteric arteries (in contrast to aorta) was not impaired in adult ecSOD−/− mice (14). Impaired relaxation of arteries to acetylcholine with increasing age is consistent with previous studies in rats (4) and, presumably, is related to elevated levels of superoxide in arteries with aging (4, 5, 10, 15). The present data, with ecSOD deficiency, also suggest that superoxide may be an important mediator of impaired endothelium-dependent relaxation with aging.
Relaxation to nitroprusside, which acts as a direct NO donor, was not affected by deficiency of ecSOD or by increasing age in these experiments. These findings suggest that impaired responses to acetylcholine are due primarily to a defect in endothelial function and bioavailability of NO, rather than impairment of smooth muscle function in the aorta.
Gene transfer of ecSOD protects against endothelial dysfunction in rats during aging (4). Gene transfer of ecSOD in old rats reduced superoxide levels to levels similar to those in younger animals and restored vascular function. Although high levels of ecSOD, generated by gene transfer, support a role for superoxide in endothelial dysfunction during aging, the role of endogenous ecSOD during aging was not addressed (4). The present study indicates that even though levels of ecSOD are reduced with aging, the residual, endogenous levels of ecSOD play a key role in protection against endothelial dysfunction during aging.
There is a positive correlation between responses to acetylcholine and gene expression of ecSOD in adult and old mice. The finding suggests that ecSOD may be important in reducing oxidative stress and preserving endothelial function in adult and old animals. The regression line was significantly steeper in old than in adult mice, which supports the conclusion that the protective effect of ecSOD against endothelial dysfunction is greater in old than in adult mice. It is important to acknowledge, however, that these correlations indicate an association but do not establish a causal relationship.
Deficiency of Cu,Zn-SOD or Mn-SOD produces vascular dysfunction with increasing age (5, 10). Responses to acetylcholine are normal in young heterozygous Cu,Zn-SOD+/− and Mn-SOD+/− mice, but levels of superoxide are increased and responses to acetylcholine are impaired in old mice (5, 10). Thus SOD in each cellular and extracellular compartment plays an important protective role during aging.
Superoxide levels.
Our data support previous findings (15) that levels of superoxide are elevated in adult ecSOD−/− mice. We did not measure superoxide in old mice, because measurement of superoxide with lucigenin in mice requires the whole aorta, and we did not have enough old mice to measure superoxide. Thus we do not have direct evidence that endogenous ecSOD is a major determinant of vascular superoxide dismutation in old mice, and we recognize that this is an important limitation of the study. We did observe, however, that tempol (an SOD mimetic) improved relaxation to acetylcholine in old ecSOD−/− mice in this study, which suggests that superoxide was increased in the aorta. In addition, we previously observed elevated superoxide levels in aortas of old rats (4) and showed phenotypic “rescue” with adenoviral overexpression of ecSOD and acute incubation with SOD mimetics.
Gene expression.
There are several new findings from real-time RT-PCR quantification of mRNA levels in mouse aorta.
First, eNOS tended to be reduced in old wild-type and ecSOD−/− mice. Reduction of eNOS expression in old animals has been demonstrated in carotid arteries of mice and in aortas and carotid and coronary arteries of rats (9, 26, 29). This finding may explain in part impaired vasomotor function in old mice. The findings of the present study, that deficiency in ecSOD impairs the response to acetylcholine in adult mice (slightly) and old mice (severely) and that impairment could be reversed in part by tempol, suggest that the role of eNOS deficiency in endothelial dysfunction in old animals may be less than the major role of increased levels of extracellular superoxide.
Second, TNFα in the aorta tends to increase in old mice compared with both wild-type and ecSOD−/− adult mice. This finding suggests augmented inflammation in the aorta of old mice, as well as old rats (8), as reported previously (9).
Third, Klotho, an aging suppressor gene (19), was reduced in the aorta of old mice compared with adult mice, independent of ecSOD genotype. Reduction of mRNA expression of Klotho has been demonstrated in the liver of old rats (28). In Klotho-knockout mice, eNOS expression in vessels was undetectable (19, 24). The mechanism for the relationship between Klotho and eNOS is not known.
We found that mRNA levels of ecSOD, but not Cu,Zn-SOD or Mn-SOD, are significantly reduced in old wild-type mice compared with adult wild-type mice. This finding is consistent with a previous report of no compensatory upregulation of protein expression of Cu,Zn-SOD or Mn-SOD in adult ecSOD−/− mice (14). A recent study reported that when ecSOD−/− mice were challenged with pressure overload, cardiac Cu,Zn-SOD activity was reduced without a change in Cu,Zn-SOD protein levels (21). This finding suggests that extracellular oxidative stress may affect intracellular status. Reduced levels of ecSOD have been reported in plasma of old patients (11). Mechanisms for reduction of ecSOD with aging are not clear, but inflammation has been reported to decrease ecSOD in lungs (17). Because eNOS is a positive regulator for ecSOD expression (12) and eNOS tended to be reduced in old mice in the present study, reduction of eNOS may also contribute to decreased expression of ecSOD.
Expression of catalase was significantly less in old mice than adult mice, independent of ecSOD genotype. Reduction of expression of catalase has been reported in old rats in aortas and other tissues, including heart, kidney, and liver (28, 30). Reduction of catalase in the aorta of old mice may increase levels of hydrogen peroxide in blood vessels, which can contribute to decreased NO availability by inhibiting SOD activity (13) and by activating NAD(P)H oxidase (20). However, since tempol, an SOD mimetic, improved vascular function, we suggest that superoxide is more important than hydrogen peroxide as a determinant of vascular dysfunction during aging.
An important limitation of these findings is that although mRNA levels were quantified, protein or enzymatic activity was not measured because of constraints imposed by scarcity of tissues. Nevertheless, the findings, with accurate quantification that is not attainable by other measurements, lead us to speculate about mechanisms that contribute to impaired endothelial function during aging.
Implications.
ecSOD may play an important role in vasomotor function with aging. Because of its location in the vessel wall, ecSOD may be critical in protecting NO from degradation after release from the endothelium, especially with advanced age. These findings may have clinical implications in relation to a gene polymorphism (R213G) found in humans in the heparin-binding site of ecSOD (16, 23, 31). This gene variant impairs binding of ecSOD to the vessel wall and, thus, impairs protection of vessels in the presence of increased levels of superoxide. We speculate that the present findings, which suggest that endogenous ecSOD protects against endothelial dysfunction during aging, may explain in part why the risk of ischemic heart disease in subjects with ecSODR213G is confined to people over 70 years old (16).
GRANTS
This work was supported by National Institutes of Health Grants HL-62984 and NS-24621, funds from the Department of Veterans Affairs, and funds from the Carver Trust Research Program of Excellence of the University of Iowa.
Acknowledgments
We thank Arlinda LaRose and Cindy Evans for secretarial assistance.
REFERENCES
- 1.Blackwell KA, Sorenson JP, Richardson DM, Smithe LA, Suda O, Nath K, Katusic ZS. Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol 287: H2448–H2453, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bowler RP, Nicks M, Tran K, Taner G, Chang LY, Young SK, Worthen GS. Extracellular superoxide dismutase attenuates lipopolysaccharide-induced neutrophilic inflammation. Am J Respir Cell Mol Biol 31: 432–439, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res 66: 286–294, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Brown KA, Chu Y, Lund DD, Heistad DD, Faraci FM. Gene transfer of extracellular superoxide dismutase protects against vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 290: H2600–H2605, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Brown KA, Didion SP, Andersen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol 27: 1941–1946, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mRNA for endothelial synthase in mouse blood vessels by real-time polymerase chain reaction. Arterioscler Thromb Vasc Biol 22: 611–616, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Colston JT, de la Rosa SD, Strader JR, Anderson MA, Freeman GL. H2O2 activates Nox 4 through PLA2-dependent arachidonic acid production in adult cardiac fibroblasts. FEBS Lett 579: 2533–2540, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Csiszar A, Labinsky N, Smith K, Rivera A, Orosz A, Ungvari A. Vasculoprotective effects of anti-tumor necrosis factor-α treatment in aging. Am J Pathol 170: 388–398, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM. Heterozygous CuZn superoxide dismutase produces a vascular phenotype with aging. Hypertension 48: 1072–1079, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Di Massimo C, Lo Presti R, Corbacelli C, Pompei A, Scarpelli P, De Amicis D, Caimi G, Tozzi Ciancarelli MG. Impairment of plasma nitric oxide availability in senescent healthy individuals: apparent involvement of extracellular superoxide dismutase activity. Clin Hemorheol Microcirc 35: 231–327, 2006. [PubMed] [Google Scholar]
- 12.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest 105: 1631–1639, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstone AB, Liochev SI, Fridovich I. Inactivation of copper, zinc superoxide dismutase by H2O2: mechanism of protection. Free Radic Biol Med 41: 1860–1863, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension 48: 473–481, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res 93: 622–629, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Juul K, Tybjaerg-Hansen A, Marklund S, Heegaard NH, Steffensen R, Sillesen H, Jensen G, Nordestgaard BG. Genetically reduced antioxidative protection and increased ischemic heart disease risk: The Copenhagen City Heart Study. Circulation 109: 59–65, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Kinnula VL, Hodgson UA, Lakari EK, Tan RJ, Sormunen RT, Soini YM, Kakko SJ, Laitinen TH, Oury TD, Paakko PK. Extracellular superoxide dismutase has a highly specific localization in idiopathic pulmonary fibrosis/usual interstitial pneumonia. Histopathology 49: 66–74, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitayama J, Chu Y, Faraci FM, Heistad DD. Modulation of dilator responses of cerebral arterioles by extracellular superoxide dismutase. Stroke 37: 2802–2806, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Kuro-OM, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the klotho gene leads to a syndrome resembling aging. Nature 390: 45–51, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Li WG, Miller FJ Jr, Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H2O2-induced O2 production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem 276: 29251–29256, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu A, Xu X, Hu X, Shu G, Zhang P, van Deel ED, French JP, Fassett JT, Oury TD, Bache RJ, Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure-induced left ventricular hypertrophy and dysfunction. Hypertension 51: 19–25, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund DD, Chu Y, Brooks RM, Faraci FM, Heistad DD. Effects of a common human gene variant of extracellular superoxide dismutase on endothelial function after endotoxin in mice. J Physiol 584: 583–590, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marklund SL, Nilsson P, Israelsson K, Schampi I, Peltonen M, Asplund K. Two variants of extracellular-superoxide dismutase: relationship to cardiovascular risk factors in an unselected middle-aged population. J Intern Med 242: 5–14, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Saito Y, Ohyama Y, Masuda H, Sumino H, Kuro-OM, Nabeshima Y, Nagai R, Kurabayashi M. Production of nitric oxide, but not prostacyclin, is reduced in klotho mice. Jpn J Pharmacol 89: 149–156, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase: a regulator of nitric oxide bioavailability. Lab Invest 75: 617–636, 1996. [PubMed] [Google Scholar]
- 26.Pimiento JM, Maloney SP, Tang PCY, Muto A, Westvik TS, Fitzgerald TN, Fancher TT, Tellides G, Dardik A. Endothelial nitric oxide synthase stimulates aneurysm growth in aged mice. J Vasc Res 45: 251–258, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Sheng H, Brady TC, Pearlstein RD, Crapo JC, Warner DS. Extracellular superoxide dismutase deficiency worsens outcome from focal ischemia in the mouse. Neurosci Lett 267: 13–16, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Shih PH, Yen GC. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology 8: 71–80, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Tschudi MR, Barton M, Bersinger NA, Moreau P, Cosentino F, Noll G, Malinski T, Luscher TF. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J Clin Invest 98: 899–905, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia E, Rao G, Van Remmen H, Heydari AR, Richardson A. Activities of antioxidant enzymes in various tissues of male Fischer 344 rats are altered by food restriction. J Nutr 125: 195–201, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Yamada H, Yamada Y, Adachi T, Fukatsu A, Sakuma M, Futenma A, Kakumu S. Protective role of extracellular superoxide dismutase in hemodialysis patients. Nephron 84: 218–223, 2000. [DOI] [PubMed] [Google Scholar]