Abstract
Congenital generalized lipodystrophy (CGL) is a rare auto-somal recessive disorder characterized by extreme paucity of adipose tissue from birth, and early onset of metabolic complications related to insulin resistance. Mutations in three genes, 1-acylglycerol 3-phosphate-O-acyltransferase 2 (AGPAT2), Berardinelli Seip Congenital Lipodystrophy 2 (BSCL2), and Caveolin-1 (CAV1) are associated with the three subtypes of this disorder, CGL1, CGL2 and CGL3, respectively. We report two siblings of Hispanic origin who displayed characteristic features of CGL such as generalized loss of subcutaneous fat from birth, acanthosis nigricans, acromegaloid habitus, umbilical prominence, hepatosplenomegaly, hypoleptinemia, dyslipidemia, and insulin resistance. However, no disease causing variants were detected in the DNA sequence of AGPAT2, BSCL2 or CAV1 genes. Further, whole body magnetic resonance imaging (MRI) in the two siblings revealed marked loss of subcutaneous, intraabdominal and intrathoracic fat like in other patients with CGL, but preservation of bone marrow fat which is invariably lost in all patients with CGL1 and CGL2, but not in the patient reported with CGL3. They also had generalized muscle weakness during infancy and early childhood associated with a nearly fivefold increase in serum creatine kinase (CK) levels, but with normal muscle biopsy and electrophysiologic studies. Both patients were also found to have atlantoaxial dislocation requiring surgical intervention. Thus, this pedigree represents a novel subtype of CGL characterized by generalized loss of body fat but with preservation of bone marrow fat, congenital muscular weakness and cervical spine instability. The genetic basis of this novel subtype remains to be determined.
Keywords: congenital generalized lipodystrophy, adipose tissue, insulin resistance, congenital myopathy, cervical spine instability
INTRODUCTION
Congenital generalized lipodystrophy (CGL) is a rare autosomal recessive disorder (Online Mendelian Inheritance in Man, OMIM no. 269700) characterized by near total absence of adipose tissue from birth. Affected subjects typically have a muscular appearance with acromegaloid features, acanthosis nigricans, umbilical prominence and hepatosplenomegaly [Seip and Trygstad, 1996; Garg, 2004]. Metabolic complications of this syndrome include marked insulin resistance leading to early onset diabetes mellitus, severe hypertriglyceridemia and hepatic steatosis. CGL may also be associated with focal lytic lesions in the long bones [Brunzell et al., 1968; Guell-Gonzalez et al., 1971; Fleckenstein et al., 1992], mild mental retardation [Seip and Trygstad, 1996; Van Maldergem et al., 2002] and cardiomyopathy [Rheuban et al., 1986; Bjornstad et al., 1996; Bhayana et al., 2002] in some patients. Despite similar presentation, considerable phenotypic and genotypic heterogeneity has been noted among CGL patients. Mutations in two genes, the 1-acylglycerol 3-phosphate-O-acyltransferase 2 (AGPAT2) gene on chromosome 9q34 [Agarwal et al., 2002], and the Berardinelli Seip Congenital Lipodystrophy 2 (BSCL2) gene located on chromosome 11q13 [Magre et al., 2001] are associated with the two well characterized subtypes of this disorder, CGL1 and CGL2, respectively. Additional loci for CGL likely exist as some patients do not harbor mutations in either of these two genes [Van Maldergem et al., 2002; Agarwal et al., 2003]. Recently, a third locus for CGL, Caveolin-1 (CAV1) was identified in an 18-year-old female patient belonging to a consanguineous Brazilian pedigree [Kim et al., 2008].We report on two siblings with CGL with a novel pattern of fat loss and congenital muscular weakness, who had no disease causing variants in AGPAT2, BSCL2, or CAV1 genes. While these two siblings show most of the characteristic CGL traits, they also have many unique clinical features distinct from those seen in CGL patients with known mutations, thus suggesting a novel subtype of CGL.
MATERIALS AND METHODS
Clinical Reports
Patient 1
Patient CG 7100.3, a 12-year-old Hispanic girl was born to healthy non-consanguineous parents of Mexican origin, hailing from the same province (Fig. 1). Mother had pregnancy-induced hypertension, but gestation was otherwise normal, and the patient was born at term by spontaneous vaginal delivery with a birth weight of 3.1 kg (25th centile). Feeding difficulties were noted during the neonatal period necessitating prolonged nursery stay for 2 weeks. She had near complete loss of subcutaneous fat from birth, and over the years, she has been noted to have other typical features of CGL including an acromegaloid appearance, acanthosis nigricans, umbilical prominence, hepatosplenomegaly and mild hirsutism (Fig. 2). She had normal growth with both her height and weight generally ranging between the 75th and 90th centile. However, she had developmental delay, not walking until after 2 years of age or talking before 3 years. She had undergone a decompression laminectomy of the posterior fossa for a type 1 Arnold Chiari malformation at the age of 2 years after which she started walking. She continued to have muscular weakness and pain with minimal exertion, and needed physical therapy till the age of 5 years. She preferred to walk on her toes as it was reportedly less painful. Serum creatine kinase (CK) levels were elevated about 3–5 times above normal, and she was suspected to have a congenital myopathy. She was noted to have increased muscle bulk, but decreased power (assessed as 4/5) in both the upper and lower extremities. Biopsy of the left quadriceps muscle at age 4 years was reported to show increased number of pleomorphic mitochondria, suggesting a possible mild mitochondrial myopathy. However, repeat quadriceps biopsy from the right thigh at age 7 years showed no significant histological or ultrastructural abnormalities (Fig. 3). The fiber diameter was in the normal range with no significant variation, size disproportion or other anomalies. Ragged red fibers, ghost fibers or inflammatory or necrotic changes were not noted. Special stains such as Trichrome, Oil Red-O, Cytochrome-c-Oxidase, Periodic Acid Schiff, and for succinate dehydrogenase activity also showed no abnormalities. Immunocytochemistry for lamin and emerin was also normal. Nerve conduction studies and electromyography performed at that time were also normal.
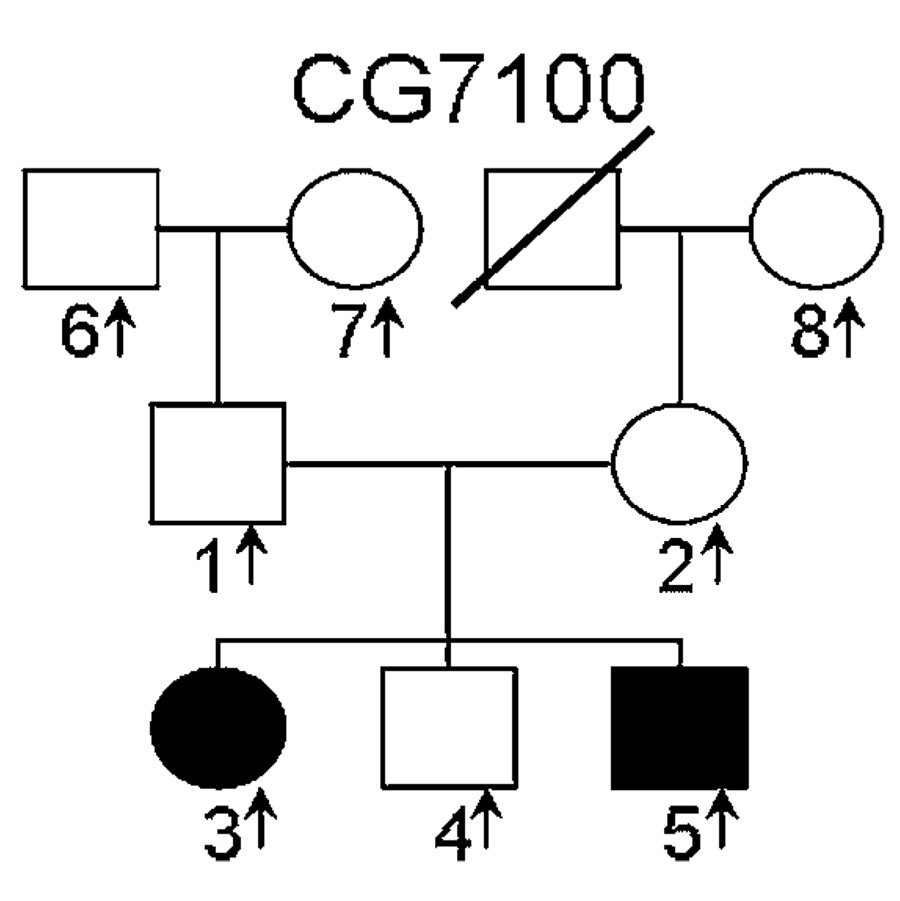
FIG. 1.
CG 7100 Pedigree. Affected individuals are shown as filled black symbols, unaffected subjects as unfilled symbols, and deceased individuals are indicated by a diagonal line. Arrows indicate subjects for whom DNA is available. Squares represent males and circles represent females.
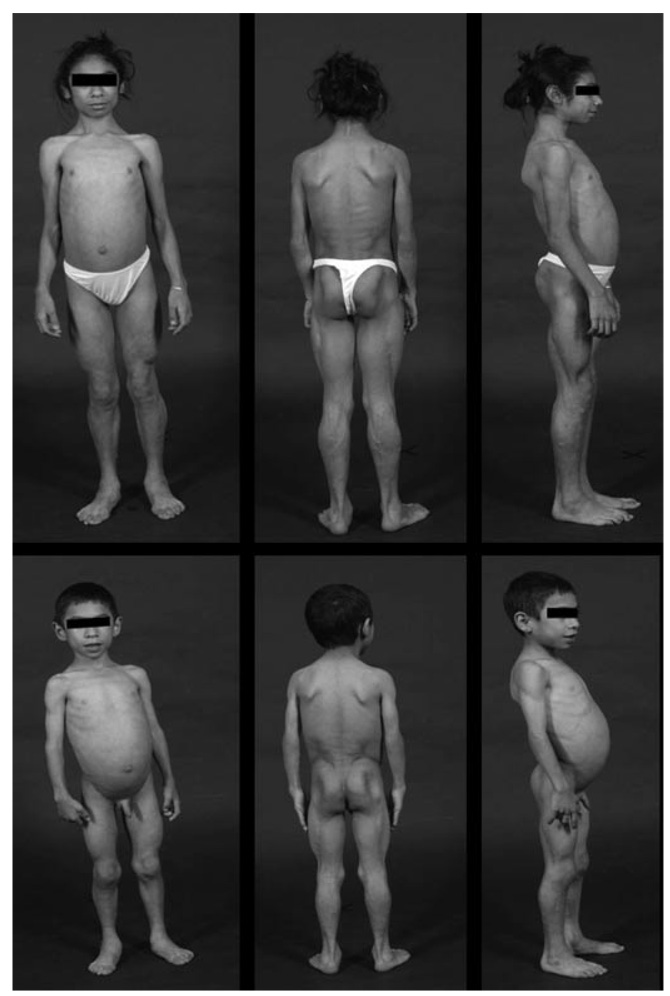
FIG. 2.
Anterior, posterior and side views of the two patients CG7100.3 (top) and CG7100.5 (bottom). Note the generalized loss of subcutaneous fat leading to prominence of musculature and subcutaneous veins. Large hands (acromegaloid appearance) and prominent umbilicus are visible.
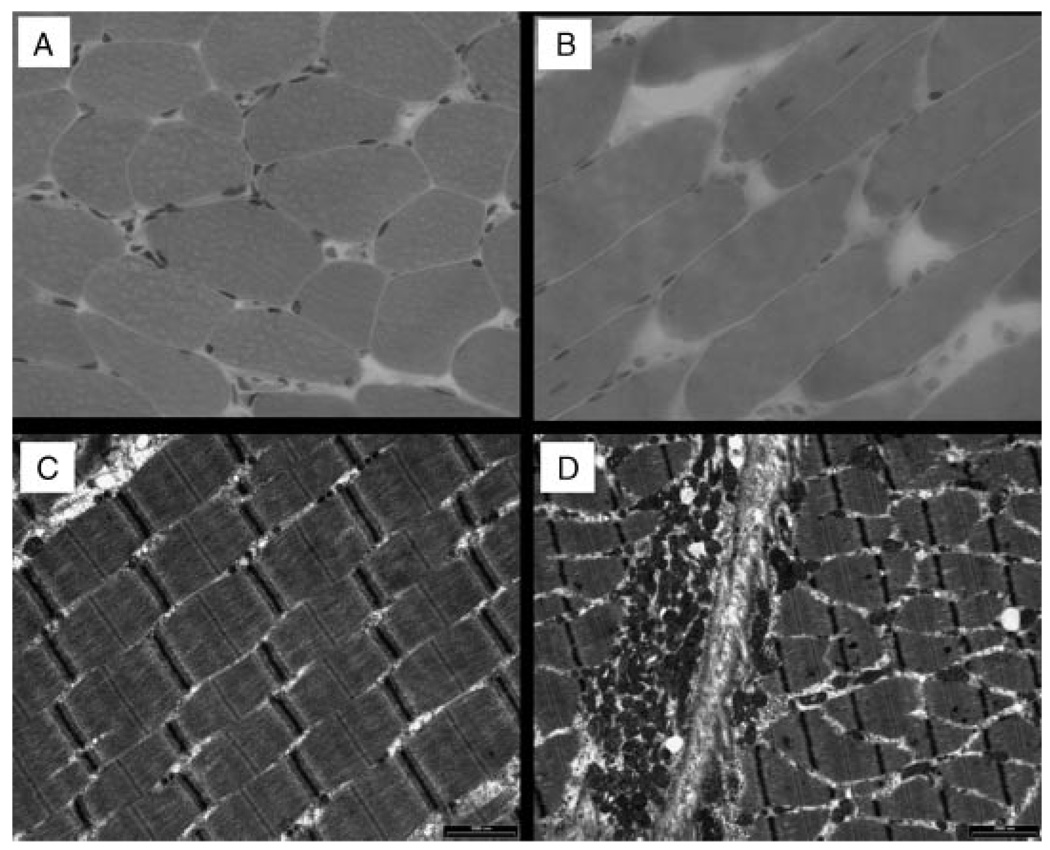
FIG. 3.
Hematoxylin and eosin (H&E) stained light microscopic sections (A, B) and electron micrographs (C, D) of the right quadriceps femoris muscle from patient CG7100.3 at the age of 7 years. Normal fiber diameter and nuclear morphology is evident on the H&E stains, while electron microscopy revealed normal sarcomeric pattern (C) and only mild unremarkable pleomorphic changes in the mitochondria (D).
At age 9 years, she was evaluated for neck pain following a motor vehicle accident, and was found to have atlanto-axial subluxation (Fig. 4), for which she underwent posterior spinal fusion. She was diagnosed with hypertension at age 10 years and started on Lisinopril 2.5 mg twice a day. Renal sonogram revealed duplication of the left collecting system, and increased size of the kidneys, but no hydronephrosis or other anomalies. Renal function tests, serum renin and aldosterone levels were normal. After 1–1.5 years of therapy, she stopped Lisinopril, and her blood pressure has remained below 125/80 mm Hg. Electrocardiogram initially showed features of left ventricular hypertrophy, but subsequent echocardiography revealed normal size and function of both the ventricles. She had learning difficulties at school, and had to repeat a year in kindergarten. However, her subsequent academic performance improved, and she is currently doing well at school obtaining A–B grades. At the time of current evaluation, she also had no muscular weakness or neurological deficits but complained of poor exercise tolerance. Her height was 151 cm (50th centile), weight was 40.7 kg (50th centile), and blood pressure was 125/62mmHg. She had generalized loss of subcutaneous fat except over the palms and soles besides other features of CGL such as acanthosis nigricans, hepatosplenomegaly and umbilical prominence. Clinodactyly of the middle toes was noted. She had prominent musculature and an acromegaloid appearance. No focal deficits were apparent on detailed neurological examination. Muscle strength and coordination was normal in all groups. Deep tendon reflexes were symmetrical and normal, and a normal flexor plantar reflex was seen bilaterally. She had tight heel cords possibly due to shortening of the Achilles tendon, but had no fixed contractures. Gait was normal, and she could walk on her toes but not on her heels. No dysmetria, ataxia or other cerebellar signs were noted. She was pre-pubertal with Tanner stage 2 breast and pubic hair development, but no axillary hair. Clitoromegaly was noted on genital examination.
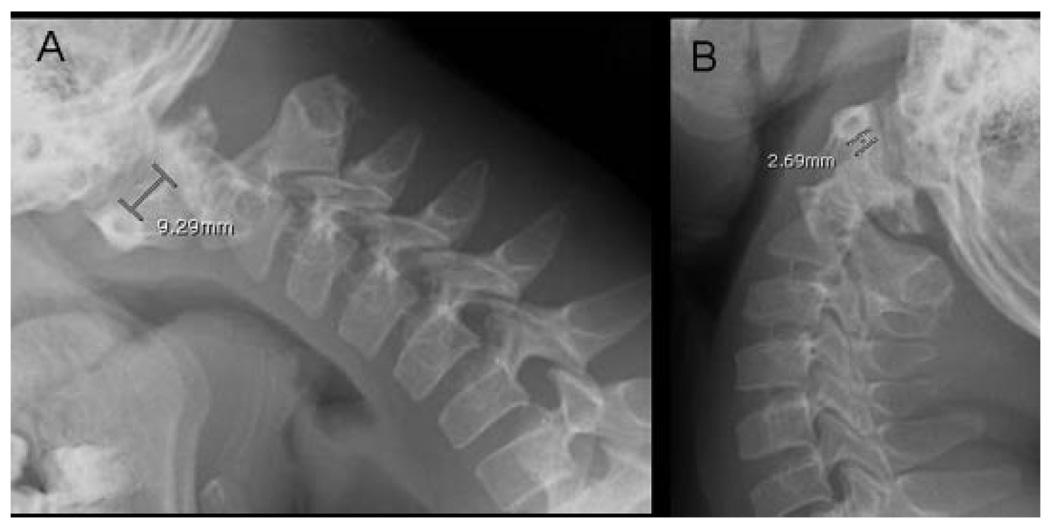
FIG. 4.
Lateral cervical spine roentgenograms of patient CG7100.3 in flexion (A) and extension (B) position of the neck showing more than 5mmseparation between the first and second cervical vertebrae, suggestive of atlantoaxial instability.
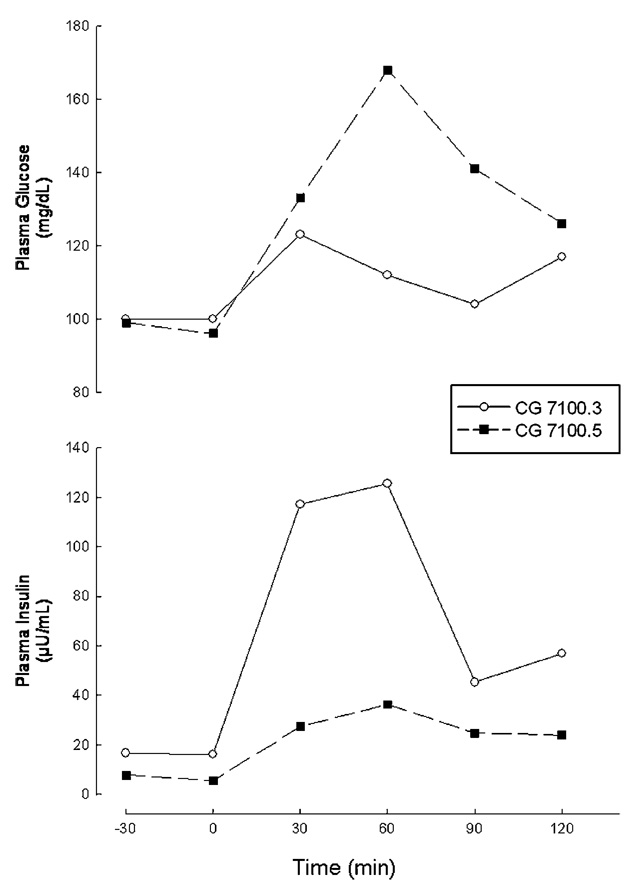
The physical and biochemical characteristics are summarized in Table I. She had decreased body fat as measured by dual energy X-ray absorptiometry (DEXA) and low serum leptin levels, consistent with generalized lipodystrophy. Fasting plasma glucose and glucose tolerance (Fig. 5) were normal, but insulin resistance was apparent from the marked fasting hyperinsulinemia, high homeostasis assessment model for insulin resistance (HOMA-IR) and low composite insulin sensitivity index (ISI) which were calculated as described before [Matthews et al., 1985; Matsuda and DeFronzo, 1999]. Further, serum triglycerides were above the 95th percentile of age and sex matched controls and high density lipoprotein (HDL) cholesterol levels were below the 5th percentile [Tamir et al., 1981]. A four to fivefold elevation in serum CK levels was also noted (normal range, 38–174 IU/L). Slight elevations in serum alanine aminotransferase (46 IU/L, normal range, 0–40 IU/L) and serum aspartate aminotransferase (49 IU/L, normal range, 10–42 IU/L) levels were noted. Serum calcium level was normal, while phosphorus and alkaline phosphatase levels were slightly increased as expected in growing children.
Table I.
Physical and Biochemical* Characteristics of Patients With Novel Subtype of Congenital Generalized Lipodystrophy
| Characteristic | CG7100.3 | CG7100.5 |
|---|---|---|
| Age (year)/sex | 12/female | 7/male |
| BMI (kg/m2) | 17.9 | 15.1 |
| Body fat (% of body mass)a | 4.9 | 8.9 |
| Serum leptin (ng/ml) | 0.07 | 0.27 |
| Serum glucose (mmol/L) | 5.5 | 5.1 |
| Serum insulin (µU/ml) | 36.5 | 6 |
| HOMA-IR | 8.9 | 1.36 |
| ISI(composite) | 2.76 | 7.04 |
| Serum cholesterol (mg/dl) | 125 | 154 |
| Serum HDL cholesterol (mg/dl) | 27 | 26 |
| Serum triglycerides (mg/dl) | 325 | 198 |
| Creatine kinase (IU/L) | 944 | 714 |
BMI, body mass index.
All biochemical analyses were performed on a fasting blood sample.
Body fat determined by dual energy X-ray absorbtiometry; HOMA-IR, homeostatic model assessment of insulin resistance; ISI, insulin sensitivity index; HDL, high density lipoprotein.
FIG. 5.
Plasma glucose and insulin responses in patients CG7100.3 and CG7100.5 during a standard (1.75 g/kg body weight glucose administration) oral glucose tolerance test preformed at ages 10.5 and 5 years, respectively.
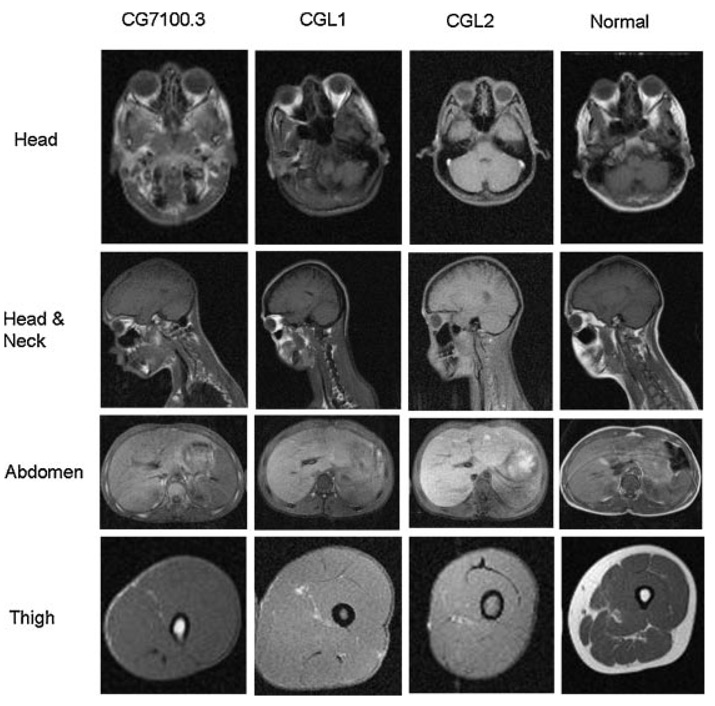
Skinfold thickness, measured by a Lange skinfold caliper at six sites on the extremities and five on the trunk, were below the 10th centile. T1 weighted whole body magnetic resonance imaging (MRI), performed as per previously described specifications [Simha and Garg, 2002], showed generalized loss of subcutaneous, intra-abdominal and intra-thoracic fat as seen in all CGL patients compared to normal subjects (Fig. 6). Similar to CGL1 patients, mechanical fat was preserved in the retro-orbital area, peri-articular regions, and in the palms and soles. However, unlike both CGL1 and CGL2 patients, bone marrow fat was preserved.
FIG. 6.
T1-weighted MR images of Patient CG7100.3 in comparison with a representative CGL1 (16 years, female) and CGL2 (15 years, female) patient and a normal subject (24 years, female) at different levels as indicated. When compared to the normal subject, all the three CGL patients have marked loss of subcutaneous fat. Retro-orbital fat is preserved in patient with CGL1 and to some extent in CG7100.3. Note the preservation of bone marrow fat in CG7100.3 (femur at the level of the thigh) which is very distinct from patients with CGL1 and CGL2. Minimal amounts of subcutaneous fat can also be seen in the dorsocervical region in this patient unlike the CGL1 and CGL2 patients.
Patient 2
Patient CG 7100.5 was the 7-year-old younger brother of Patient 1 who presented with similar features of CGL and developmental delay. He was also born at term gestation after an uncomplicated pregnancy with a birth weight of 3 kg (25th centile), but was admitted to the neonatal intensive care unit for 3 days because of transient tachypnea and respiratory distress. He had feeding difficulties during infancy and early childhood due to difficulty with chewing and swallowing, requiring tube feeding for some time. During this period he had frequent ear and respiratory tract infections, and gastro esophageal reflux with aspiration. He had muscle weakness similar to his sister, and serum CK levels were elevated three to fivefold. He had normal growth with weights between 25th and 50th centile and heights between 50th and 75th centile, but developmental milestones were delayed. He could roll over at 8 months, walk at 30 months, and speak at 34 months. With physical therapy and speech therapy, he improved remarkably, and at the time of current evaluation, had no motor or functional deficits except for some learning difficulties at school. He had attention problems, did not know his alphabet, and could count only up to 20. Interestingly, like his sister, he was also noted to have atlanto-axial dislocation, for which he has had a posterior cervical spine fusion at the age of 4 years. However, he did not have an Arnold Chiari malformation. He was noted to have elevated blood pressure on some occasions, but has not been treated with any other medications. Renal sonogram revealed slightly enlarged kidneys for his age, but no hydronephrosis, duplication of the collecting system or other anomalies. At 7 years his height was 125 cm (75th centile), weight was 23.6 kg (50th centile) and blood pressure was 112/63 mm Hg. He had similar features of CGL as his sister (Fig. 2) including generalized paucity of subcutaneous fat except at the palms and soles, prominent musculature, mild hepatomegaly and umbilical prominence, besides clinodactyly of the middle toes. No focal neurological deficits were apparent, with normal muscle bulk and strength, and deep tendon reflexes. He was pre-pubertal with no axillary or pubic hair.
Body fat distribution, as assessed by skinfold thickness measurements and whole body MRI was very similar to that in Patient 1 with generalized loss of subcutaneous fat and preservation of bone marrow fat. Body fat content and serum leptin levels were likewise decreased. Glucose and insulin levels were however normal, though his serum triglycerides were also above the 95th percentile of age and sex matched controls. Elevations in serum CK and liver enzymes were similar to his sister, and he was also eucalcemic.
METHODS
Both patients, along with their healthy male sibling and parents, were evaluated in the General Clinical Research Center at the University of Texas South-western Medical Center at Dallas. Informed consent was obtained from the subjects and parents, and the study protocol was approved by the Institutional Review Board. Blood was collected after a 12 hr overnight fast for analysis of serum lipoproteins, insulin, leptin, glucose, and a chemistry profile as previously described [Garg, 2000] and for genetic studies.
Genetic analysis
GenomicDNAwas isolated from the blood sample, and exons and splice-site junctions of genes associated with CGL or childhood onset generalized lipodystrophy, AGPAT2, BSCL2, CAV1, and Lamin A/C (LMNA) were amplified. The PCR product was purified and sequenced using ABI PRISM 3100 (Applied Biosystems, Foster City, CA). Sequences were compared using Vector NTi Suite 6 software (InforMax, Inc., Bethesda, MD) and by visual inspection. RNA transcripts of AGPAT2 and BSCL2 were also analyzed for presence of disease causing variants. The details of the procedure have been described before [Agarwal et al., 2003]. We also studied linkage to the AGPAT2 and BSCL2 loci using specific dinucleotide repeat markers or single nucleotide polymorphisms flanking these loci in the 9q34 and 11q13 regions, respectively [Agarwal et al., 2003]. The PCR products were analyzed on ABI 377, and linkage analysis was performed manually.
Genetic Results
Genetic studies did not reveal the etiology of this syndrome. Sequencing of the exons and splice sites of the three CGL loci AGPAT2, BSCL2 and CAV1 showed no disease causing variants. Similarly, there were no mutations noted in the LMNA gene which is associated with lipodystrophy and muscular dystrophy. Linkage analysis showed linkage to the 9q34 region, but not to the 11q13 region. RNA transcript analysis of both AGPAT2 and BSCL2 was normal.
DISCUSSION
Phenotypic and genotypic heterogeneity in CGL has been well recognized [Rajab et al., 2002; Van Maldergem et al., 2002; Agarwal et al., 2003]. All patients present with marked loss of subcutaneous adipose tissue from birth and manifest features of extreme insulin resistance from early childhood. However, subtle differences in body fat distribution [Simha and Garg, 2003], and in the prevalence of associated comorbidities such as cardiomyopathy, mental retardation and skeletal defects have been described among patients with the two well known subtypes of CGL, CGL1 and CGL2. Recently, another novel subtype of CGL, CGL3, associated with hypocalcemia, and due to homozygous null mutation in CAV1 has been described [Kim et al., 2008] further illustrating the genetic and phenotypic heterogeneity of this syndrome, and the important role of caveolae in lipid storage and synthesis [Garg and Agarwal, 2008]. Since some CGL patients do not show mutations in the known loci, it is likely that additional subtypes of CGL exist, and more CGL loci remain to be identified. The two siblings reported in this communication represent one such novel subtype of CGL.
Both patients exhibited characteristic traits of CGL. They had extreme lack of subcutaneous fat from birth, acromegaloid appearance with prominent musculature, acanthosis nigricans, hepatomegaly and umbilical prominence. Patient 1 also demonstrated severe insulin resistance based on fasting hyper-insulinemia, high HOMA-IR and low ISI(composite) derived from OGTT. Her HOMA-IR value was nearly threefold higher than that reported for age matched Mexican American children in the National Health and Nutrition Examination Survey 1999–2002 [Lee et al., 2006], and at least 50% higher than that observed in Mexican–American children with acanthosis nigricans and other risk factors for type 2 diabetes mellitus [Urrutia-Rojas et al., 2004]. Patient 2 did not yet show similar impairment in insulin sensitivity, but it is likely that he may do so with increasing age. Both patients already had significant dyslipidemia and hypoleptinemia. Slight elevation of blood pressure was also noted along with renal enlargement and duplication of the collecting system in one of the patients. While hypertension is associated with certain partial lipodystrophies, it is uncommon in children with CGL. Mild visceromegaly is a well known feature of CGL, but whether this novel subtype of CGL is associated with hypertension and subtle renal abnormalities is not clear.
MRI and DEXA scan confirmed fat loss, but also revealed a distinct pattern of fat distribution. Preservation of mechanical fat in the retroorbital and periarticular regions, and over the palms and soles was reminiscent of fat distribution in CGL1 patients. However, these patients also showed preservation of bone marrow fat which is not seen in either CGL1 or CGL2 patients. Bone marrow fat was reportedly preserved in the CGL3 patient too [Kim et al., 2008], but unlike this patient, our patients did not have hypocalcemia or CAV1 mutations, thus suggesting a unique pattern of lipodystrophy. Also noted was a small amount of subcutaneous fat in the dorsocervical region in both the patients which was not seen in other CGL patients. There were subtle but definite indicators of a distinct pattern of fat loss in the two siblings.
Furthermore, both patients had generalized muscle weakness from birth which is also not associated with any of the CGL subtypes. The muscle weakness persisted during infancy and early childhood, and was associated with elevated CK levels. Despite extensive investigations, the cause for the weakness and CK elevations are not clear. Based on the clinical characteristics, congenital myopathy was strongly suspected, but two muscle biopsies failed to show any characteristic features associated with structural congenital myopathy. They also did not have progressive muscle weakness and atrophy, or other histologic features of muscular dystrophies. Given the presence of lipodystrophy, a metabolic disorder, they could have a ‘metabolic myopathy’ due to impaired energy production in the muscle. A similar myopathy has, however, not been noted in other patients with lipodystrophy, and they did not have features of encephalopathy or hepatic dysfunction commonly noted in patients with other metabolic myopathies. Mitochondrial abnormalities noted on one of the biopsies were mild and nonspecific. It remains to be determined if they have a novel type of “non-structural” congenital or metabolic myopathy. Physical therapy has helped to restore muscle strength but asymptomatic CK elevation persists.
Another interesting feature observed in both patients was atlantoaxial dislocation requiring surgical intervention. Cervical spine instability has been associated previously with Down syndrome [Caird et al., 2006], mucopolysaccharidosis [Dickerman et al., 2004], or in association with other skeletal defects such as in the Dyggve-Melchoir-Clausen syndrome [Kandziora et al., 2002]. We are not aware of any previous reports of CGL patients with spinal or other joint instabilities. It is well known that that defective connective tissue development leads to laxity of the ligaments and joint instability in Down syndrome. If a similar defect were to have caused atlantoaxial dislocation in the patients described in this report, it might suggest that in this novel subtype of CGL, development of muscle and connective tissue is also affected besides adipose tissue. Identification of the underlying genetic defect would help in clarifying the pathogenesis of these defects.
In conclusion, this pedigree represents a novel subtype of CGL, characterized by preservation of bone marrow fat, and associated with congenital muscular weakness and cervical spine instability, thus suggesting further genetic heterogeneity in CGL.
ACKNOWLEDGMENTS
We are grateful to Dr. Dennis Burns for reviewing the muscle biopsy slides, to Sarah Gilmore for technical assistance, and to the nursing services of the General Clinical Research Center at the University of Texas Southwestern Medical Center, Dallas for patient care. The study was supported in part by the National Institutes of Health grants R01-DK54387 and MO1-RR00633, and by the Southwestern Medical Foundation.
REFERENCES
- Agarwal AK, Arioglu E, De Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31:21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Simha V, Oral EA, Moran SA, Gorden P, O’Rahilly S, Zaidi Z, Gurakan F, Arslanian SA, Klar A, Ricker A, White NH, Bindl L, Herbst K, Kennel K, Patel SB, Al-Gazali L, Garg A. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88:4840–4847. doi: 10.1210/jc.2003-030855. [DOI] [PubMed] [Google Scholar]
- Bhayana S, Siu VM, Joubert GI, Clarson CL, Cao H, Hegele RA. Cardiomyopathy in congenital complete lipodystrophy. Clin Genet. 2002;61:283–287. doi: 10.1034/j.1399-0004.2002.610407.x. [DOI] [PubMed] [Google Scholar]
- Bjornstad PG, Foerster A, Ihlen H. Cardiac findings in generalized lipodystrophy. Acta Paediatr Suppl. 1996;413:39–43. doi: 10.1111/j.1651-2227.1996.tb14264.x. [DOI] [PubMed] [Google Scholar]
- Brunzell JD, Shankle SW, Bethune JE. Congenital generalized lipodystrophy accompanied by cystic angiomatosis. Ann Intern Med. 1968;69:501–516. doi: 10.7326/0003-4819-69-3-501. [DOI] [PubMed] [Google Scholar]
- Caird MS, Wills BP, Dormans JP. Down syndrome in children: The role of the orthopaedic surgeon. J Am Acad Orthop Surg. 2006;14:610–619. doi: 10.5435/00124635-200610000-00003. [DOI] [PubMed] [Google Scholar]
- Dickerman RD, Colle KO, Bruno CA, Jr, Schneider SJ. Craniovertebral instability with spinal cord compression in a 17-month-old boy with Sly syndrome (mucopolysaccharidosis type VII): A surgical dilemma. Spine. 2004;29:E92–E94. doi: 10.1097/01.brs.0000112074.48566.fa. [DOI] [PubMed] [Google Scholar]
- Fleckenstein JL, Garg A, Bonte FJ, Vuitch MF, Peshock RM. The skeleton in congenital, generalized lipodystrophy: Evaluation using whole-body radiographic surveys, magnetic resonance imaging and technetium-99 m bone scintigraphy. Skeletal Radiol. 1992;21:381–386. doi: 10.1007/BF00241817. [DOI] [PubMed] [Google Scholar]
- Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety) J Clin Endocrinol Metab. 2000;85:1776–1782. doi: 10.1210/jcem.85.5.6605. [DOI] [PubMed] [Google Scholar]
- Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- Garg A, Agarwal AK. Caveolin-1: A new locus for human lipodystrophy. J Clin Endocrinol Metab. 2008;93:1183–1185. doi: 10.1210/jc.2008-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell-Gonzalez JR, Mateo de Acosta O, Alavez-Martin E, Arce-Hidalgo B, Navarro-Lauten A, Diaz-Diaz O. Bone lesions in congenital generalised lipodystrophy. Lancet. 1971;2:104–105. doi: 10.1016/s0140-6736(71)92085-x. [DOI] [PubMed] [Google Scholar]
- Kandziora F, Neumann L, Schnake KJ, Khodadadyan-Kloster-mann C, Rehart S, Haas NP, Mittlmeier T. Atlantoaxial instability in Dyggve-Melchior-Clausen syndrome. Case report and review of the literature. J Neurosurg. 2002;96:112–117. doi: 10.3171/spi.2002.96.1.0112. [DOI] [PubMed] [Google Scholar]
- Kim CA, Delepine M, Boutet E, El Mourabit H, Le Lay S, Meier M, Nemani M, Bridel E, Leite CC, Bertola DR, Semple RK, O’Rahilly S, Dugail I, Capeau J, Lathrop M, Magre J. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93:1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: A population-based study. Diab Care. 2006;29:2427–2432. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- Magre J, Delepine M, Khallouf E, Gedde-Dahl T, Jr, Van Maldergem L, Sobel E, Papp J, Meier M, Megarbane A, Bachy A, Verloes A, d’Abronzo FH, Seemanova E, Assan R, Baudic N, Bourut C, Czernichow P, Huet F, Grigorescu F, de Kerdanet M, Lacombe D, Labrune P, Lanza M, Loret H, Matsuda F, Navarro J, Nivelon-Chevalier A, Polak M, Robert JJ, Tric P, Tubiana-Rufi N, Vigouroux C, Weissenbach J, Savasta S, Maassen JA, Trygstad O, Bogalho P, Freitas P, Medina JL, Bonnicci F, Joffe BI, Loyson G, Panz VR, Raal FJ, O’Rahilly S, Stephenson T, Kahn CR, Lathrop M, Capeau J BSCL Working Group. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28:365–370. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diab Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Rajab A, Heathcote K, Joshi S, Jeffery S, Patton M. Heterogeneity for congenital generalized lipodystrophy in seventeen patients from Oman. AmJ Med Genet. 2002;110:219–225. doi: 10.1002/ajmg.10437. [DOI] [PubMed] [Google Scholar]
- Rheuban KS, Blizzard RM, Parker MA, Carter T, Wilson T, Gutgesell HP. Hypertrophic cardiomyopathyin total lipodystrophy. J Pediatr. 1986;109:301–302. doi: 10.1016/s0022-3476(86)80389-4. [DOI] [PubMed] [Google Scholar]
- Seip M, Trygstad O. Generalized lipodystrophy, congenital and acquired (lipoatrophy) Acta Paediatr Suppl. 1996;413:2–28. doi: 10.1111/j.1651-2227.1996.tb14262.x. [DOI] [PubMed] [Google Scholar]
- Simha V, Garg A. Body fat distribution and metabolic derangements in patients with familial partial lipodystrophy associated with mandibuloacral dysplasia. J Clin Endocrinol Metab. 2002;87:776–785. doi: 10.1210/jcem.87.2.8258. [DOI] [PubMed] [Google Scholar]
- Simha V, Garg A. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy caused by mutations in the AGPAT2 or seipin genes. J Clin Endocrinol Metab. 2003;88:5433–5437. doi: 10.1210/jc.2003-030835. [DOI] [PubMed] [Google Scholar]
- Tamir I, Heiss G, Glueck CJ, Christensen B, Kwiterovich P, Rifkind BM. Lipid and lipoprotein distributions in white children ages 6–19 yr. The Lipid Research Clinics Program Prevalence Study. J Chronic Dis. 1981;34:27–39. doi: 10.1016/0021-9681(81)90079-5. [DOI] [PubMed] [Google Scholar]
- Urrutia-Rojas X, Menchaca J, Wadley W, Ahmad N, Lacko A, Bae S, Spellman C, Kudchodkar B, Kudolo G, McConathy W. Cardiovascular risk factors in Mexican-American children at risk for type 2 diabetes mellitus (T2DM) J Adolesc Health. 2004;34:290–299. doi: 10.1016/j.jadohealth.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Van Maldergem L, Magre J, Khallouf TE, Gedde-Dahl T, Jr, Delepine M, Trygstad O, Seemanova E, Stephenson T, Albott CS, Bonnici F, Panz VR, Medina JL, Bogalho P, Huet F, Savasta S, Verloes A, Robert JJ, Loret H, De Kerdanet M, Tubiana-Rufi N, Mégarbané A, Maassen J, Polak M, Lacombe D, Kahn CR, Silveira EL, D’Abronzo FH, Grigorescu F, Lathrop M, Capeau J, O’Rahilly S. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet. 2002;39:722–733. doi: 10.1136/jmg.39.10.722. [DOI] [PMC free article] [PubMed] [Google Scholar]