Abstract
Given the importance of stem cells to adult tissues, it has long been postulated that stem cells divide infrequently to preserve their long-term proliferation potential and to prevent the acquisition of errors during DNA replication. Yet, some stem cells must be able to continually churn out progeny in tissues that rapidly turn over or are subject to sudden injuries or growth spurts. This Review explores the challenges that mammalian stem cells face in balancing the competing demands of proliferation and differentiation in tissues.
Introduction
Adult stem cells have the capacity to self-renew and to regenerate tissue(s) long-term, in both homeostasis and wound repair. These remarkable fixtures of longevity place stem cells in an elite class of essential cells of living organisms. Given the importance of stem cells to body tissues, it has long been postulated that stem cells should be used sparingly and tucked safely away into resident niches, guarding them from harm’s way.
Some tissues of the body, such as those in the brain and skeletal muscle, have very little turnover and are well protected, whereas others turnover constantly. Even though the intrinsic properties of stem cells are likely to be similar across tissues, each tissue has its own requisites for homeostasis and regeneration. We lose over 20 billion cells a day, requiring constant replenishment to stay alive. More than a billion of these lost cells come from our blood, necessitating a reservoir of constantly renewing hematopoietic stem cells (Orkin and Zon, 2008). The intestinal epithelium also undergoes constant turnover, taking only 3–5 days for undifferentiated cells at the bottom of the invaginating crypt to proliferate and differentiate into the enterocytes, goblet cells, or enteroendocrine cells of the adsorptive villus (Barker et al., 2008). Analogously, every 4 weeks, we have a brand new epidermis as cells in the basal layer terminally differentiate and are shed from the skin surface (Watt, 2002).
Some stem cells face even greater challenges. During pregnancy, the mammary epithelium undergoes a dramatic change as elaborate glands branch, differentiate, and produce milk. Hair follicles undergo cyclic bouts that entail not only periods of massive destruction and dormancy but also periods of active follicle regeneration and hair growth. Confounding the problem, the hair growth phase, which requires stem cells, is relatively uniform in length, but the resting phase increases with age, leading to extended periods where nothing appears to be happening (Blanpain and Fuchs, 2009). Finally, all of our tissues occasionally face traumatic injuries. Although this is commonplace for some tissues such as the skin epithelium, other tissues, such as the central nervous system, are not so well adjusted.
These sudden demands place a heavy burden on the nearby stem cell niches. All of these considerations mean that stem cells must be able to adjust swiftly in order to maintain a proper balance. When to cycle and how fast to cycle are features that vary considerably among stem cell populations. Moreover within a given tissue, more frequently cycling stem cells seem to function primarily in homeostasis while a reserve of more dormant master stem cells may be set aside for times of injury or unforeseen need. So when is “slow” slow and “fast” fast and what does this mean for maintaining stemness?
Below, I concentrate on three representative populations of adult mammalian stem cells—hematopoietic stem cells, hair follicle stem cells, and intestinal stem cells—and discuss the common themes that have emerged from studying their slow-cycling properties in normal homeostasis and in response to injury. The factors that enter into stem cell longevity are varied and complex and include not only the cellular interactions and stimuli that constitute the environment or “niche” in which stem cells reside but also intrinsic mechanisms governing such diverse processes as telomere length, cell survival, and asymmetric cell division. This Review highlights how the cycling kinetics of stem cells may enter into this medley.
Heterogeneity within the Hematopoietic Stem Cell Niche
The existence of stem cells within the bone marrow was demonstrated nearly 50 years ago by reconstitution of the hematopoietic system following irradiation (Till and McCulloch, 1961). These early serial transplantation studies revealed that less than 1% of bone marrow cells possess the capacity for long-term reconstitution. Detailed cell-cycle analyses have further revealed that most hematopoietic stem cells are quiescent and in the G0 phase of the cell cycle (Cheshier et al., 1999; Kiel et al., 2007; Passegue et al., 2005; Potten et al., 1978; Punzel and Ho, 2001; Spangrude and Johnson, 1990). Over the years, molecular markers have been identified to isolate and purify long-term hematopoietic stem cells (LT-HSCs) that exhibit special longevity (Christensen and Weissman, 2001; Muller-Sieburg et al., 1986; Spangrude et al., 1988). This offers an ideal system for study, as evidenced by the fact that between 20% and 50% of purified (Lin−Sca1+c-kit+CD150+48−) cells possess repopulation activity when serially transplanted in vivo (Challen et al., 2009; Christensen and Weissman, 2001; Foudi et al., 2009; Kiel et al., 2007; Spangrude et al., 1988; Wilson et al., 2008).
The steady-state pool of HSCs has been estimated at ~20,000–100,000. A subset of these are responsible for regenerating the shorter-lived and often rapidly dividing progeny, known as multipotent progenitors (MPPs), which produce nearly a billion circulating blood cells per day (Passegue et al., 2005; Wagers et al., 2002; and references therein). Serial transplantations of these HSCs in mice have been extended up to 5–7 rounds (Harrison and Astle, 1982; Harrison et al., 1978). It is not yet clear whether the inability to carry out serial transfer endlessly is a limitation of the assay or rather reflects a limited self-renewal capacity of these stem cells. However, such in vivo tests for longevity of HSCs are presently superior to those for other adult stem cells.
It has been estimated that two-thirds of the LT-HSCs are in G0 at any one time, a feature that correlates with their ability to function in hematopoietic reconstitution (Passegue et al., 2005). Proliferation kinetics suggest that cell divisions are less frequent in LT-HSCs than in short-tem HSCs and in more committed MPP cells of hematopoietic lineages. In BrdU pulse-chase experiments, ~5%–6% of HSCs still retain label even after 2 months (Cheshier et al., 1999; Foudi et al., 2009; Kiel et al., 2007; Morrison et al., 1997; Wilson et al., 2008). Triple label studies with nucleotide tracers demonstrate that these label-retaining cells (LRCs) do not arise from asymmetric segregation of a master template DNA strand while passing the newly synthesized strand to the committed daughter (Kiel et al., 2007). Such a feature would otherwise skew the use of nucleotide label as a means of monitoring slow-cycling properties of stem cells.
Although the kinetics of BrdU incorporation and retention have long argued for the existence of HSCs that are also LRCs (Cheshier et al., 1999; Kiel et al., 2007), researchers have wondered whether nucleotide label administered to adult mice might fail to mark highly quiescent stem cells if they divide only very rarely. Of further concern is whether BrdU as a mutagen might elicit some cell damage in LRCs. If so, this might prompt snoozing HSCs to awaken, proliferate, and enter a damage-repair mode, diluting nucleotide label in the process. BrdU toxicity also clouds the reliability of testing the stem cell functionality of LRCs.
Some of the possible caveats arising from long-term studies with BrdU have been circumvented by generating mice that harbor an histone 2B-green fluorescent protein (H2B-GFP) transgene driven by a tetracycline-regulatable enhancer element and its requisite transcription factor driven by a tissue-specific promoter (Figure 1) (Tumbar et al., 2004). This method was first devised and tested for the isolation and purification of LRCs of the hair follicle (Tumbar et al., 2004). By constitutively expressing H2B-GFP during embryogenesis, and then switching off expression postnatally in response to tetracycline, cells that retain H2B-GFP label over time can readily be identified and their presumptive progeny traced through 7–10 divisions. This approach is more sensitive than BrdU label retention, ensures initial uniform labeling of cells within the tissue, and affords enhanced sensitivity in monitoring the infrequent division behavior of stem cells (Blanpain et al., 2004; Foudi et al., 2009; Waghmare et al., 2008).
Figure 1. The Histone H2B-GFP Pulse-Chase System.
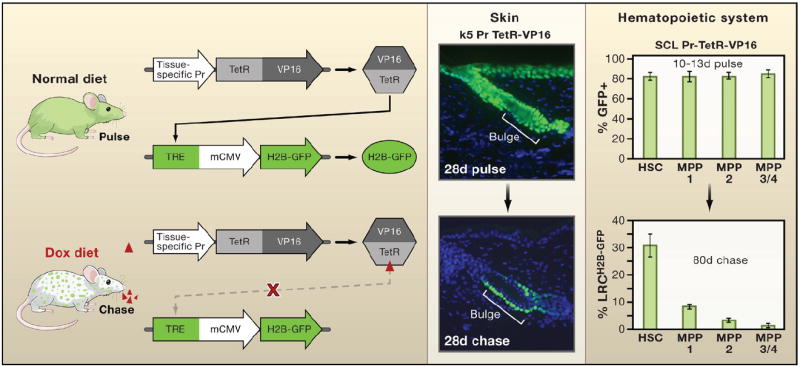
The mating of two parent strains of transgenic mice is needed to make progeny in which expression of a transgene encoding histone H2B-green fluorescent protein (H2B-GFP) can be turned off when tetracycline is added to the animal’s diet. The first parent strain harbors the H2B-GFP transgene under the control of a tetracycline (doxycycline; dox) regulatory element (TRE). The second expresses a transcription factor regulated by tetracycline (TetRVP16) that is under the control of a cell-type-specific promoter. In the first example shown, the keratin 5 (k5) promoter drives expression of TetRVP16 leading to expression of H2B-GFP in the skin epithelium until 4 weeks of age, at which time tetracycline is administered for 4 weeks so that dividing cells dilute out the label and differentiating cells are sloughed from the skin (Tumbar et al., 2004). In the second example, TetRVP16 driven by the promoter of the stem cell leukemia (SCL) gene is used to express H2B-GFP in hematopoietic stem cells (HSCs) and different multipotent progenitors (MPPs) of the hematopoietic system for 10–13 days, after which tetracycline is administered for 80 days to identify the label-retaining cells (LRCs) (Wilson et al., 2008). Schematic adapted from Nowak (2009).
Recently researchers coupled H2B-GFP pulse-chase lineage tracing with 5–6 different molecular markers (Lin−Sca1+c-kit+CD150+48 ± CD34) to unveil a dormant population of G0 LT-HSCs (Figure 1) (Foudi et al., 2009; Wilson et al., 2008). This pool of cells is strongly label retaining and divides on average only once every 4–5 months, or ~5 times per cell within a lifetime. Moreover, ~30% of this HSC population retains H2B-GFP label after several months, as opposed to prior methods involving BrdU where only a few percent exhibit labeling.
Are these dormant cells true molecular Sleeping Beauties or are they merely masquerading in stem cell’s clothing? Transplantation assays with cells from purified populations confirm that dormant hematopoietic LRCs possess long-term repopulation potential under circumstances where more frequently dividing HSCs display only shorter-term repopulation and MMPs achieve little or no repopulation (Figure 2) (Foudi et al., 2009; Wilson et al., 2008). Moreover, these dormant LRCs can be awakened to self-renew with either the jolt of an injury or the stimulus of G-CSF. Although not quite Prince Charming, this enticement to awaken from dormancy and self-renew is reversible, and following HSC replenishment, these reserve LRCs go back into a slumber (Foudi et al., 2009).
Figure 2. Slow-Cycling HSCs Have the Highest Long-Term Stem Cell Potential.
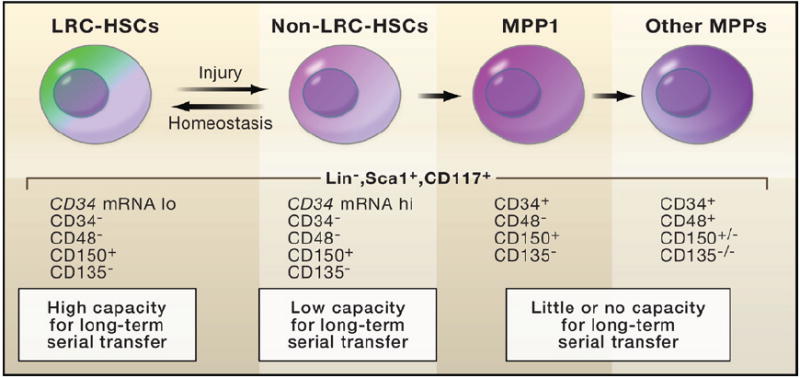
Wilson et al. (2008) coupled the SCL-TetRVP16 pulse-chase experiment (outlined in Figure 1) to fluorescence-activated cell sorting (FACS) in order to purify hematopoietic cell populations. Serial transplant experiments were then used to test the potential of individual hematopoietic cells to reconstitute the bone marrow of X-irradiated mice. The results suggest that cells with the greatest long-term capacity are those that cycle the least frequently. These cells have traditionally been referred to as long-term hematopoietic stem cells (LT-HSCs). They cycle as few as five times per lifetime of the mouse and remain dormant unless challenged by injury or stimuli that induce growth. From these assays, the HSCs that divide slowly but more frequently appear to be the ones that function in normal homeostasis. LRC, label-retaining cells.
These studies suggest a model in which the smaller pool of dormant LT-HSCs functions not in normal homeostasis but rather as a stem cell reserve for times of crisis. This scenario leaves the more plentiful HSCs, which are the LRCs that divide more frequently (at least once a month), as the stem cells that function in normal homeostasis to fuel the production of rapidly but transiently amplifying progenitors. Although it will take time for the field to reach a consensus regarding the precise characteristics of the long-term and shorter-term hematopoietic stem cells, most researchers in the hematopoietic field are currently in agreement that cycling frequency correlates inversely with long-term stem cell activity (Orford and Scadden, 2008).
Heterogeneity within the Hair Follicle Stem Cell Niche
The hair follicle offers an interesting and valuable model for studying stem cells, as it undergoes cyclical bouts of regeneration (anagen), degeneration (catagen), and rest (telogen) and its stem cell lineage is temporally and spatially chronicled (Figure 3). During the initial development of the hair follicle and with each subsequent regenerative phase, the cycling portion (~two-thirds) of the follicle is fueled by rapidly proliferating but transiently amplifying matrix cells that incorporate nucleotide label readily but do not retain it (Cotsarelis et al., 1990). Matrix cells surround and are stimulated by specialized mesenchymal cells, called the dermal papilla (DP), to form the hair bulb at the very bottom of the hair follicle. The matrix supplies cells for the seven concentric upward programs of terminal differentiation that produce the hair and its surrounding channel. By mechanisms poorly understood, the process grinds to a halt during catagen, and as the degenerating follicle retracts, it brings the DP upward until it comes to rest just below the noncycling portion of the follicle. Residing just below the sebaceous gland, this region is frequently referred to as the bulge.
Figure 3. Cycles of Hair Follicle Growth, Destruction, and Rest.
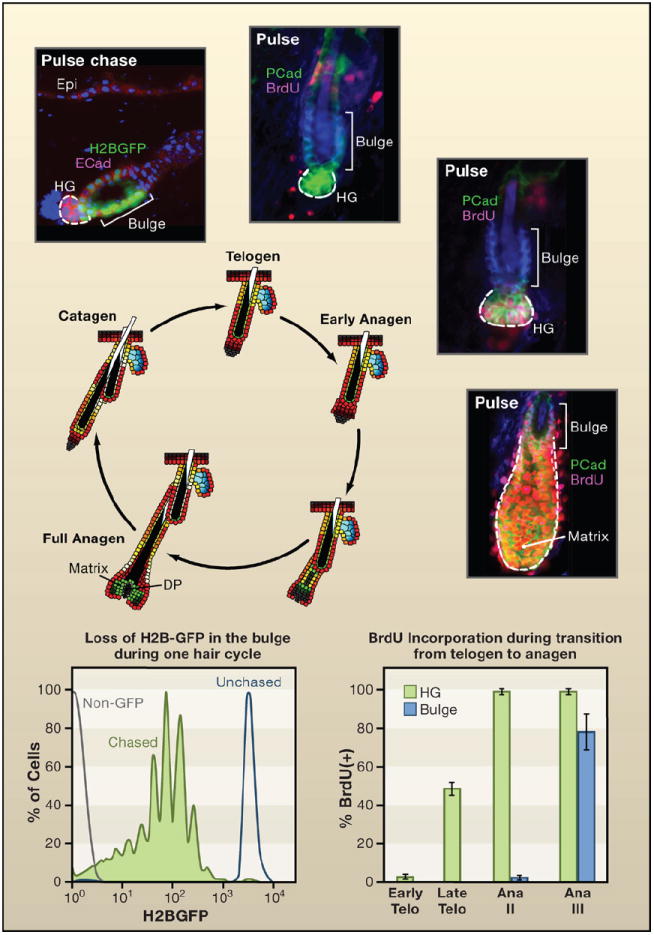
Shown is a schematic of the hair cycle, accompanied by the proliferation state of the stem cell niche. The bulge is a niche for cells that divide relatively infrequently. It is found at the base of the permanent segment of the hair follicle. After the destructive phase (catagen), the hair germ (HG) emerges as a small cluster of cells. The HG is thought to arise from bulge cells that migrate out to “meet” the dermal papilla (DP) that contracts upward (Ito et al., 2004). Histone H2B-GFP pulse-chase experiments show that the upper portion of the HG is label retaining but typically less so than many other bulge cells. HG cells express P-cadherin more strongly than bulge cells (shown). During the resting phase (telogen), both HG and bulge are quiescent. Short BrdU pulses reveal little activity throughout this phase, which can last for many weeks (Greco et al., 2009). Late in telogen and transitioning to early anagen, the HG becomes mobilized prior to the bulge. A few days later (Ana III), the HG has rapidly expanded to form the early matrix of transiently amplifying cells that form the hair shaft and its channel (Greco et al., 2009). At this time, the bulge begins to proliferate. By monitoring reductions in H2B-GFP, the bulge cells divide on average three times per hair cycle, approximately once per week (Waghmare et al., 2008). Thus in contrast to some stem cell niches, such as the intestinal niche, the cycling rates of stem cells are highly sensitive to the relative stage of the hair cycle (Blanpain et al., 2004; Greco et al., 2009; Jaks et al., 2008; Nowak et al., 2008; Waghmare et al., 2008). Schematic and data adapted from Nowak (2009).
Nucleotide tracing experiments in the 1990s and H2B-GFP pulse-chase studies in this decade show that slow-cycling follicle LRCs concentrate in the bulge (Cotsarelis et al., 1990; Morris and Potten, 1999; Tumbar et al., 2004). The extended dormant phase of the hair cycle led to the initial rationalization of this infrequently dividing population of cells. However, even during embryonic development, long before the entry into dormancy, cells with slower cycling kinetics begin to form in the upper portion of the hair follicle, just below where the sebaceous gland will emerge (Nowak et al., 2008).
Nucleotide label in some of these slow-cycling cells can be chased for 3 weeks to the adult bulge, confirming their identity as early bulge LRCs. Moreover, early bulge cells are also marked by expression of four key transcription factors: Sox9, Tcf3, Lhx2, and NFATc1 (Nowak et al., 2008). Within several weeks of postnatal life, bulge cells acquire expression of two markers widely used in studying adult bulge cells: CD34 and a lacZ transgene under the control of the Keratin 15 promoter (K15-lacZ) (Cotsarelis et al., 1990; Morris et al., 2004). Thus early in development, a population of infrequently cycling cells with many molecular characteristics of stem cells appears to be set aside to provide the engine that later drives the hair cycle. A fascinating question for the future is how environmental and intrinsic cues converge to achieve this fertile stem cell ground in the noncycling portion of the follicle.
An additional distinction in the intricate hierarchy of hair follicle cells with proliferative capacity is the hair germ (HG), a small cluster of cells that extend down from the bulge and abut the DP (Figure 3). First emerging toward the end of catagen, HG cells have been proposed to arise from the bulge, and indeed some of its residents are LRCs (Ito et al., 2004). Although distinctive, the pattern of gene expression in HG cells is more similar to the bulge than to transiently amplifying matrix (Greco et al., 2009). Notable genes whose expression spans across bulge and HG include K15, Sox9, and Lgr5, which encodes an orphan G protein-coupled receptor that is commonly used as a marker of intestinal stem cells. Genetic lineage tracing with any of these promoters shows that the bulge region (encompassing bulge and HG) harbors stem cells that give rise to all cells of the follicle and sebaceous gland (Jaks et al., 2008; Morris et al., 2004; Nowak et al., 2008). Functional analyses with Sox9 further underscore the physiological relevance of these stem cells. Without Sox9, follicle LRCs are not maintained, and there is an arrest in hair follicle and sebaceous gland morphogenesis and homeostasis (Nowak et al., 2008; Vidal et al., 2005).
During the hair cycle, the levels of label retention, cycling activity, and the pattern of gene expression vary markedly within the bulge (Figure 3) (Blanpain et al., 2004; Jaks et al., 2008; Nowak et al., 2008). Throughout most of telogen, which can last for weeks, both HG and bulge appear largely quiescent: they do not take up BrdU during short nucleotide pulses nor do they express proliferative markers (Greco et al., 2009). Toward the end of this phase, the HG cells at the bulge base are the first to proliferate (Greco et al., 2009; Panteleyev et al., 2003). At this time, a few cells begin to divide (Greco et al., 2009; Taylor et al., 2000; Tumbar et al., 2004). As they do, they exhibit signs of nuclear β-catenin (the downstream effector of Wnt signaling) and downregulation of the bone morphogenetic protein (BMP) pathway (Greco et al., 2009; reviewed by Blanpain and Fuchs, 2009). Several days later, ~10% of bulge cells show active incorporation of BrdU within a 24 hr pulse, accompanied by changes in gene expression expected for a Wnt-activated state (Blanpain et al., 2004; Lowry et al., 2005). As this transition to anagen progresses, more than 50% of Lgr5+ cells within the emerging hair follicle enter S phase (Jaks et al., 2008). Thus in contrast to the tortoise, a snapshot of the hair gives a very different picture of its behavior when it is resting versus racing to the finish line.
Most bulge cells remain slow cycling but active throughout the growing phase, dividing ~3 times during the 3 week growth phase (Figure 3). Double and triple labeling with nucleotides and histones document the slow-cycling activity of bulge cells during the growth phase and refute the immortal strand hypothesis as a means of explaining the presence of LRCs in the hair follicle (Waghmare et al., 2008; Sotiropoulou et al., 2008). However, in vivo, the overall size of the bulge remains constant (Blanpain et al., 2004), suggesting that its progeny participate in the growth of new hair.
To understand how, Barrandon and colleagues conducted a landmark study by surgically preparing chimeric rat whisker follicles containing genetically marked bulge cells and monitoring their fate following engraftment (Oshima et al., 2001). Bulge progeny were progressively detected along the outer layer of the follicle, then to the matrix, and finally to the upward terminally differentiating cells of the hair shaft. Similar patterns have been observed for murine hair coat follicles, initially by short pulse-chase experiments (Alonso et al., 2005) and then by developmental genetic tracing with Sox9-Cre LacZ reporter mice (Nowak et al., 2008). Through these interactions between different classes of lineage progenitors, the system is able to channel a steady flow of differentiated cells into the growing hair (Legue and Nicolas, 2005).
The cycling kinetics of the genetically marked bulge cells used by Oshima and Barrandon were not evaluated. That said, accompanying culture studies estimate that each whisker bulge contains ~1000 clonogenic stem cells (Oshima et al., 2001), and Morris and Potten (1999) described similar behavior for cultured LRCs. Moreover, when engrafted to immunocom-promised mice, clonogenic cultures derived from individual bulge cells of either rat whiskers or mouse backskins can regenerate epidermis, sebaceous glands, and cycling hair follicles (Blanpain et al., 2004; Claudinot et al., 2005), which are features expected of stem cells.
For the rat whisker bulge, multiple serial engraftments have been achieved after culture, suggestive of long-term stem cell potential (Claudinot et al., 2005). Recently, long-term genetic lineage tracing has also documented the maintenance of hair follicle cells marked by Lgr5 expression (Jaks et al., 2008). Like follicle stem cell transcription factors Sox9, Tcf3, and Lhx2, Lgr5 is not uniquely expressed by bulge cells but rather extends to the HG and lower outer root sheath of cycling follicles. In this regard, it is noteworthy that during the cycling phase of the follicle, cells with clonogenic potential can reside outside the bulge niche (Oshima et al., 2001). As additional follicle stem cell genes are functionally defined and as the point of no return within the stem cell lineage of the hair follicle is increasingly delineated, future prospects for pinpointing and characterizing bona fide follicle stem cells and evaluating their relation to LRCs are promising (Blanpain and Fuchs, 2009).
How do the slow-cycling cells of the hair follicle and hematopoietic system compare? The resting phase of the hair cycle gets increasingly longer with age, and given that little activity exists during this time, it can take months for some LRCs to fall below the threshold limits for detection. Overall, however, the cycling kinetics of follicle LRCs during anagen most closely resemble those of the ~20% of HSCs that retain label for up to several months and participate in blood homeostasis (Cheshier et al., 1999; Foudi et al., 2009; Wilson et al., 2008).
Interestingly, a small subset (1–4 per hair follicle) of bulge LRCs are dormant LRCs (>100 d/cycle), and as such, these are not likely to function in normal hair follicle homeostasis (Morris and Potten, 1999). It is not clear whether these dormant LRCs can be mobilized in wound repair and whether they might represent long-term follicle stem cells analogous to the dormant stem cells recently reported for the bone marrow. On the one hand, follicle LRCs can mobilize, divide, and move upward following injury or stimulation with TPA (tumor-promoting antigen), as shown by double-label pulse-chase experiments (Taylor et al., 2000) and by monitoring the fate of H2B-GFP labeled bulge cells (Tumbar et al., 2004; Waghmare et al., 2008). On the other hand, genetic lineage tracing in adult mice suggests that bulge/HG stem cells only repair epidermal wounds transiently until epidermal stem cells can move into the wound site (Ito et al., 2007; Levy et al., 2007). Without the ability to conduct single-cell serial transplantations and without further resolution of the molecular heterogeneity within the bulge/hair germ, it may be some time before this issue is resolved for the hair follicle.
Heterogeneity within the Intestinal Stem Cell Niche
The small intestine offers an interesting example of a rapidly and continuously regenerating epithelium. It can be subdivided into units, composed of a relatively undifferentiated crypt at the base and contiguous progeny organized into villi composed of differentiated epithelial cells (adsorptive enterocytes, secretory goblet cells, and hormone-producing enteroendocrine cells) (Figure 4). Within the crypt, the only terminally differentiated cells are Paneth cells, which secrete antibacterial peptides. Based upon ultrastructure the least differentiated columnar cells within the small intestine are those that are interspersed among Paneth cells.
Figure 4. The Intestinal Stem Cell Zone Replenishes the Adsorptive Villus.
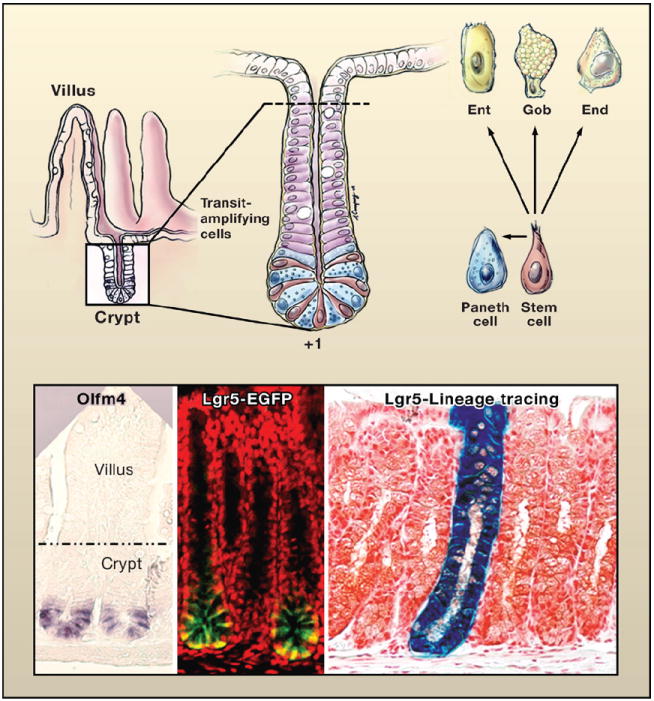
(Top left) Schematic of the crypt-villus architecture of the small intestine. (Top right) Magnified view of the crypt. At the base of the crypt is the putative stem cell zone (Bjerknes and Cheng, 1981; Cheng and Leblond, 1974). This zone contains undifferentiated cells sharing a similar ultrastructure that are interspersed with highly differentiated antibacterial Paneth cells. The undifferentiated columnar cells, purported to be stem cells, extend from a position +1 at the very base of the crypt to +4, marking the boundary between the Paneth cells and the transiently amplifying, rapidly dividing cells that give rise to the terminally differentiating enterocytes (Ent), goblet cells (Gob), and enteroendocrine cells (End) of the villus. Drawing by A. Canapary. (Lower panels) In situ hybridization with a probe for Olfm4, enriched in the stem cell zone (van der Flier et al., 2009). The expression of an Lgr5-EGFP-CreER transgene marks the stem cell zone (Barker et al., 2007). To the right is a lineage tracing of a bitransgenic mouse expressing Lgr5-EGFP-CreER and Rosa26-fl-stop-fl-LacZ. Cells in which the Lgr5 promoter is active are marked by EGFP expression and express tamoxifen-regulatable Cre recombinase. Following activation of Cre, floxed sequences recombine, excising the stop codon and activating LacZ. With time, the entire crypt-villus structure displays β-galactosidase expressing progeny, revealing that individual Lgr5-positive stem cells can regenerate the entire niche (Barker et al., 2007). Images reprinted by permission from Macmillan Publishers Ltd: Nature (Barker et al., 2007), copyright 2007.
Cheng and Leblond (1974) conducted a series of lineage-tracing experiments, remarkable for its time, that coupled tritiated thymidine pulse-chase with the ability of these undifferentiated basal crypt cells to phagocytose dead Paneth cells and hence become marked with Paneth cell remnants identifiable by their ultrastructure. The conclusion from these early lineage-tracing studies was that the crypt base cells are multipotent stem cells that are able to give rise to all four differentiated lineages of the intestine (Cheng and Leblond, 1974). Moreover, their results led to the conclusion that the stem cells are mitotically active and give rise to committed cells of each lineage. These cells transiently divide as they either move further up the crypt and into the villus to generate enterocytes, enteroendocrine cells, and goblet cells or move downward toward the base of the crypt to form the fully differentiated Paneth cells (Cheng and Leblond, 1974).
Counting cells from the base of the crypt (position 1), the stem cells were initially purported to exist from positions 1–4 to create a “stem cell zone” (Bjerknes and Cheng, 1981), now more typically referred to as a niche. The flux of migration in the intestinal epithelium is fast, with a transit time of ~3 days from the time a stem cell exits at the base of the crypt until its most distal progeny reach the top (Cheng and Leblond, 1974). Although Cheng and Leblond recognized that the stem cells take longer to exit their niche than their transit-amplifying progeny, Potten and colleagues showed that position +4 of the niche is particularly enriched for nucleotide LRCs (Marshman et al., 2002). At the border of the putative niche, these cells also have a distinctive program of gene expression and notably are marked by enriched expression of Bmi1. Bmi1 is a component of the polycomb complex of chromatin-mediated repression that is involved in the self-renewal of hematopoietic and neuronal stem cells (Lessard and Sauvageau, 2003; Molofsky et al., 2003).
Genetic lineage tracing with a Rosa26-lacZ reporter mouse expressing Bmi1-CreER transgene marked the +4 cells at early times after tamoxifen induction and entire crypts and villi after 12 months (Sangiorgi and Capecchi, 2008). After 5 days some crypts had only 1–2 labeled cells, an expected feature of slow-cycling cells within an epithelium that otherwise turns over rapidly. Moreover, when β-catenin, the downstream effector of Wnt signaling, is conditionally ablated using Bmi1-CreER crypt maintenance is abrogated. These studies make a compelling argument in support of the LRCs as a population of stem cells within the small intestine.
Equally compelling are similar lineage-tracing studies of Clevers and colleagues (Barker et al., 2007), who find that Lgr5 (Lgr5-EGFP-CreER) is expressed within the stem cell zone that spans +1 to +4, with the strongest labeling at the base where Wnt signaling is highest (Figure 4). Within 2 months of Lgr5-CreER induction, some individual villus-crypt units scored positive for bacterial β-galactosidase activity, indicating that the Lgr5-expressing cells can regenerate all intestinal lineages. Most recently, Clevers and coworkers have elegantly shown that single Lgr5+ intestinal stem cells can even regenerate self-renewing, functional crypt-like structures in vitro when exposed to the appropriate milieu of key extracellular matrix and signaling factors (Sato et al., 2009). Similar lineage-tracing studies have also been conducted with Prominin 1, and both Prominin 1 and Lgr5 appear to mark stem cells that upon mutagenesis are able to initiate intestinal tumors (Barker et al., 2009; Zhu et al., 2009). When taken together, these studies suggest that the entire stem cell zone described by early morphologists may be a residence of multipotent intestinal stem cells. However, recent advances in purifying intestinal stem cells reveal that like the hair follicle stem cell niche, there seems to be some heterogeneity, not only in cycling kinetics but also in molecular features. Cells residing deeper within the stem cell zone exhibit somewhat higher levels of nuclear β-catenin, Lgr5, Sox9, CD133, and Olfm4 (Barker et al., 2007; van der Flier et al., 2009). By contrast, stem cells residing near or at the +4 position are flanked on one side by differentiating Paneth cells and on the other by the transit-amplifying cells of the villus lineages; these +4 cells exhibit higher levels of Bmi1 and several other molecular markers (such as phosphorylation of Dcamkl1, Mapk14, and AKT) and show reduced cycling activity relative to the stem cells at the crypt base (Giannakis et al., 2006; He et al., 2004; Sangiorgi and Capecchi, 2008). Now that the roadblock to isolating and transcriptionally profiling intestinal stem cells has finally been overcome, as it has for HSCs and hair follicle SCs, the pace of advances in the study of intestinal stem cells should continue to accelerate.
The Relation of Label Retention to Stemness
As new and improved tools become available and new adult stem cell populations emerge, it is becoming increasingly apparent that LRCs are a frequent, albeit not necessarily essential, feature of stem cell niches. The limbus of the eye is a niche that harbors LRCs and is enriched for stem cells with a high capacity for regeneration, a useful feature in therapy for corneal blindness (Cotsarelis et al., 1989; Pellegrini et al., 1999). That said, some evidence suggests that at least in some animals, the cornea is also a source of self-renewing clonogenic corneal stem cells with long-term regenerative potential (Majo et al., 2008 and references therein). In contrast to the limbus, the corneal epithelium does not show signs of label retention over prolonged periods of time.
In comparing the niches of epithelial LRCs of the intestinal crypt, the bulge, and the limbus, all are complex junctures where different cell types converge. Moreover, adjacent to these LRC niches are cells that appear to be molecularly quite similar to their LRC counterparts but are considerably more proliferative. Although more research is needed to fully address the issue, the evidence to date favors the notion that for each of these tissues, label retention is a feature of some but not all of its stem cells.
Are stem cells that divide infrequently equivalent to those that divide more rapidly? The answer to this question is more difficult to address, and even for the well-studied hematopoietic stem cells, controversy still surrounds the question. That said, if, as recently suggested, the slowest cycling HSCs are ones that function primarily in repair of injury or tissue damage, this might also explain why the limbus is the major source of stem cells that repair corneal wounds (Majo et al., 2008; Wilson et al., 2008), and why anticancer treatments lead to temporary but not permanent hair loss.
It makes sense that master stem cells should undergo as few rounds of DNA replication as possible to prevent the genome from experiencing unnecessary risk. A study of injury repair, however, reveals a few interesting departures from this paradigm. For example, when slow-cycling follicle bulge cells are depleted by repeated hair plucking, the more active hair germ cells appear to repopulate the bulge niche (Ito et al., 2004). Additionally, when skin is catastrophically wounded, actively cycling epidermal cells contribute to follicle regeneration and can recreate the bulge niche (Ito et al., 2007). The ability to regenerate a niche of slow-cycling bulge stem cells from engraftments of proliferating cultured bulge stem cells has also been documented (Blanpain et al., 2004). Whether these extreme adaptive behaviors reflect the special need for skin stem cells to continually adjust to natural bouts of catastrophic degeneration and regeneration awaits future investigation.
In closing, we are left with the view that whether in homeostasis or in wound repair, adult stem cells can adjust their cell-cycle properties to the circumstances that surround them. In this regard, their rate and timing of proliferation may not directly be linked to their stemness, but rather to their microenvironment (Weigelt and Bissell, 2008). A graphic illustration of this is the ability of rat bulge cells to be stimulated to proliferate for >140 divisions in vitro without losing their multipotent long-term potential when subsequently engrafted in vivo (Claudinot et al., 2005). The abilities of most hair follicle LRCs (Cotsarelis et al., 1990) or hematopoietic LRCs (Foudi et al., 2009; Wilson et al., 2008) to undergo rapid proliferation in response to injury and then return to their state of quiescence offer additional examples.
As the molecular characteristics intrinsic to tissue-specific stem cells are increasingly well-defined, the mechanisms controlling extrinsic features, such as variation in proliferative rates, should continue to unfold. With this knowledge comes the promise of new and improved methods for clinical treatments. Two cases in point are cord blood, which offers a possible source of HSCs for clinics, and limbal epithelial stem cells, which offer hope for treating corneal degenerative disorders that lead to blindness. A prerequisite to overcoming the present barriers in advancing these therapies is to devise new and improved methods of expanding the long-term self-renewing potential of these stem cells outside of their native niches.
Acknowledgments
I thank T. Tumbar, C. Blanpain, V. Horsley, M. Schober, and M. Baron for their critical reading of the manuscript and H. Clevers, N. Barker, A. Canapary, J. Nowak, V. Greco, and T. Tumbar for their contributions to the figures. E.F. is an HHMI Investigator and the recipient of grants from the NIH and the Starr Foundation.
References
- Alonso L, Okada H, Pasolli HA, Wakeham A, You-Ten AI, Mak TW, Fuchs E. Sgk3 links growth factor signaling to maintenance of progenitor cells in the hair follicle. J Cell Biol. 2005;170:559–570. doi: 10.1083/jcb.200504131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. IV. Effects of resecting 30% of the small intestine. Am J Anat. 1981;160:93–103. doi: 10.1002/aja.1001600108. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Challen GA, Boles N, Lin KK, Goodell MA. Mouse hematopoietic stem cell identification and analysis. Cytometry A. 2009;75:14–24. doi: 10.1002/cyto.a.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci USA. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Foudi A, Hochedlinger K, Van Buren D, Schindler JW, Jaenisch R, Carey V, Hock H. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol. 2009;27:84–90. doi: 10.1038/nbt.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakis M, Stappenbeck TS, Mills JC, Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam M, Brent MR, Gordon JI. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281:11292–11300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, dela Cruz-Racelis J, Fuchs E. Dissecting the hair cycle activation step in the stem cell niche. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Astle CM. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J Exp Med. 1982;156:1767–1779. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Astle CM, Delaittre JA. Loss of proliferative capacity in immunohemopoietic stem cells caused by serial transplantation rather than aging. J Exp Med. 1978;147:1526–1531. doi: 10.1084/jem.147.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legue E, Nicolas JF. Hair follicle renewal: organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development. 2005;132:4143–4154. doi: 10.1242/dev.01975. [DOI] [PubMed] [Google Scholar]
- Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21:1–9. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456:250–254. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112:470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci USA. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Sieburg CE, Whitlock CA, Weissman IL. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986;44:653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JA. PhD. thesis. Rockefeller University; New York, NY: 2009. Specification and function of early hair follicle stem cells. [Google Scholar]
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Panteleyev AA, Mitchell PJ, Paus R, Christiano AM. Expression patterns of the transcription factor AP-2alpha during hair follicle morphogenesis and cycling. J Invest Dermatol. 2003;121:13–19. doi: 10.1046/j.1523-1747.2003.12319.x. [DOI] [PubMed] [Google Scholar]
- Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Ranno R, Stracuzzi G, Bondanza S, Guerra L, Zambruno G, Micali G, De Luca M. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68:868–879. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell. 1978;15:899–906. doi: 10.1016/0092-8674(78)90274-x. [DOI] [PubMed] [Google Scholar]
- Punzel M, Ho AD. Divisional history and pluripotency of human hematopoietic stem cells. Ann N Y Acad Sci. 2001;938:72–81. doi: 10.1111/j.1749-6632.2001.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009 doi: 10.1038/nature07935. Published online March 19, 2009. 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Candi A, Blanpain C. The majority of multipotent epidermal stem cells do not protect their genome by asymmetrical chromosome segregation. Stem Cells. 2008;26:2964–2973. doi: 10.1634/stemcells.2008-0634. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Johnson GR. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci USA. 1990;87:7433–7437. doi: 10.1073/pnas.87.19.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Till JE, McCulloch E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- Waghmare SK, Bansal R, Lee J, Zhang YV, McDermitt DJ, Tumbar T. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 2008;27:1309–1320. doi: 10.1038/emboj.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18:311–321. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]


