Abstract
The availability of antiretroviral medications has transformed living with HIV infection into a manageable chronic illness, and high levels of adherence are necessary. Stigma has been identified as one reason for missing medication doses. The objective of this study was to explore the relationship between perceived HIV stigma and self-reported missed doses of antiretroviral medications in a 12-month, repeated measures cohort study conducted in Lesotho, Malawi, South Africa, Swaziland, and Tanzania. Data were collected from 1457 HIV-positive individuals at three times between January 2006 and March 2007. Participants completed a series of questionnaires. Of the 1457 participants, 698 were taking ARVs during the study and are included in this analysis. There was a significant relationship between perceived HIV stigma and self-report of missed medications over time (t = 6.04, p ≤ 0.001). Individuals who reported missing more ARV medications also reported higher levels of perceived HIV stigma. Individuals reporting fewer medication worries reported decreased stigma over the one year period (t = −4.79, p ≤ 0.001). While those who reported increased symptom intensity also reported increased stigma initially (t = 8.67, p ≤ 0.001) that remained high over time. This study provides evidence of a significant and stable correlation that documents the relationship between perceived HIV stigma and self-reported reasons for missed medications over time. These findings suggest that part of the reason for poor adherence to ARV medications is linked to the stigma experienced by people living with HIV.
Introduction
With the advent of antiretroviral (ARV) medications in the late 1990s, HIV-positive people began living longer and healthier lives. Although ARVs have been available in high-resource countries since approximately 1996, they are only recently reaching lower resource countries, particularly those in Africa. The World Health Organization's HIV treatment initiative “3 × 5” planned to have 3 million individuals on antiretroviral therapy (ART) by 2005, but has not yet been able to achieve that goal. Avert1 has reported that fewer than 20% of the millions of people in need of medications in Africa currently have access to them.
For those people who do have access to ARV medications, adherence is essential. In contrast to other chronic illnesses, HIV requires very high medication adherence rates in order to decrease viral load and increase CD4 counts.2–4 Strict adherence is also required in order to avoid the development of medication resistance, which would render the drugs ineffective at preventing viral replication. The various medication regimens now being used vary in terms of their resiliency to missed doses, with regimens based on non-nucleoside reverse transcriptase inhibitors (NNRTI) requiring higher rates of adherence than protease inhibitor-based regimens.5 In 2006, a 52-study meta-analysis on ARV medication adherence in North America and Africa conducted by Mills and colleagues6 showed an overall proportion of ARV adherence in Africa of 77%. The fact that the primary regimens prescribed in these countries are fixed-dose NNRTI-based regimens makes it apparent that patients with lower levels of adherence to NNRTI therapy are at risk for resistance.
Researchers have investigated stigma as one of a number of factors related to varying ARV medication adherence. Using a three-item assessment to quantify stigma, Rintamaki and colleagues7 found that people in the United States with high HIV stigma concerns were 3.3 times more likely to be nonadherent to their medication regimen than those with low concerns. In their meta-analysis on medication adherence, Mills and colleagues6 identified other factors that influence adherence, such as cost, not having disclosed HIV-status, and alcohol abuse in both Africa and North America. Rao and colleagues8 further suggest that developmental level can be a barrier to adherence. For example, young people were found to be poorer adherers than adults.
HIV stigma in Africa has been documented to be an impediment to disclosure of HIV status. Medley et al.9 summarized 17 studies from peer-reviewed journal articles on barriers to disclosure of HIV status among women in developing countries. These articles suggested that between 3.5% and 14.6% of women reported experiencing a violent reaction from a partner after HIV disclosure. Barriers to disclosure included fear of accusations of infidelity, abandonment, discrimination, and violence. This review builds a strong case for the impact of perceived stigma on fear of disclosure on HIV status. Varga et al.10 reported on HIV disclosure among 31 women from Johannesburg, South Africa. They reported from their in-depth interviews that while many women disclosed to secure adequate infant care, often disclosure resulted in rejection, stigmatization, and the withholding of financial support. Daftary et al.11 interviewed 21 hospitalized patients with tuberculosis (TB)/HIV in Durban, South Africa, exploring the perceived relationship between HIV testing and disclosure. They wrote, “HIV sero status disclosure was impeded by the felt stigma of a ‘discreditable’ infection, manifested by social rejection and discrimination” (p. 572). Wood et al.12 conducted interviews with 56 orphans from Zimbabwe about their experiences with their parents' death from AIDS and their process of bereavement. The stigma experienced by these young people permeates their stories of parental loss.
The traditional definition of stigma is that of Goffman,13 who described it as a trait that is “significantly discrediting” (p. 3). Based on Goffman's work, Alonzo and Reynolds14 expanded the definition of stigma to be “a … powerful discrediting and tainting social label that radically changes the way individuals view themselves and are viewed as persons” (p. 304). Holzemer and colleagues15 applied this prior work on stigma and developed a conceptual model of stigma that is specific to HIV/AIDS in Africa. It describes HIV/AIDS-related stigma as a cyclical process that begins with a trigger, for example a positive HIV test or getting an ARV pharmacy refill, and progresses to stigmatizing behavior such as avoiding or accusing someone. The stigma behaviors ultimately lead to a variety of outcomes such as violence, decreased adherence to medication, or increased morbidity (e.g., wasting), which can in themselves become new stigma triggers. According to this theoretical model, anything that identifies the person as being infected with HIV can be a trigger for stigma. This model hypothesizes that taking ARV pills may result in triggers initiating stigma behaviors that result in negative outcomes, such as hiding medications, failure to disclose, or poor ARV adherence.
Some people consider themselves to be adherent, yet still miss medication doses periodically. Ware et al.16 reported that in 52 HIV-positive, active drug users, taking medications often interfered with their social relationships. These participants in Uganda and Nigeria were more concerned about accidentally disclosing their HIV status by taking ARV medications, than about the impact of being nonadherent. Rao and colleagues8 found that HIV stigma affected adherence for urban youth, who missed medication doses for fear of people finding out their HIV status.
There have been several studies on ARV medication adherence in southern Africa that have demonstrated the challenge of maintaining high medication adherence as well as stigma as one of the significant barriers to medication adherence. Nachega et al.17 documented a strong relationship between failure to refill ARV medication prescriptions and survival in a large sample of patients in South Africa. It is possible that refilling a prescription is a trigger that results in increased stigma. Wolfe et al.18 interviewed 112 patients in Botswana and explored the relationship between HIV stigma and adherence to ARV medications. Ninety-four percent of the patients reported keeping their HIV status secret from their community and 69% from family members. Forty percent reported they delayed getting tested for HIV. Much of the failure to disclose was described to be related to perceived HIV-related stigma. Nachega et al.19 examined adherence in a sample of 66 patients with HIV in Soweto, South Africa. They reported that adherence decreased considerably with fear of being stigmatized by a sexual partner. Weiser et al.20 examined barriers to medication adherence in a sample of 109 patients using both qualitative and quantitative research methods. Principal barriers to medication adherence were financial constraints (44%), stigma (15%), travel/migration (10%), and side effects (9%).
The aim of the study reported here was to explore the potential relationship between perceived HIV stigma and ARV medication adherence in Lesotho, Malawi, South Africa, Swaziland, and Tanzania. This was part of a larger study on HIV/AIDS and stigma.15,21–29
Methods
Research design
A repeated measures cohort study followed people living with HIV infection for one year. Data were collected at baseline, 6 months, and 12 months from participants in Lesotho, Malawi, South Africa, Swaziland, and Tanzania. The analysis presented here included only people who were taking antiretroviral medications.
Protection of human subjects
Prior to the study, the research protocol was reviewed and approved by the appropriate bodies at each of the universities involved, as well as at the local and central governmental levels (when necessary). Each potential participant was oriented to the purpose of the study, the requirements for participation (amount of time), and the fact that the study was completely voluntary and that they could withdraw at any time. They were also assured that all data collected would remain confidential. If the person agreed to participate, s/he signed a consent form. Participants were consented and the surveys were conducted either in English or in the local language (Sesotho in Lesotho, Chichewa in Malawi, Tswana in South Africa, Swazi in Swaziland, and Kiswahili in Tanzania).
Settings and sample
The research team collected data from January 2006 to March 2007. Each country principal investigator sought to enroll 300 HIV-positive people into the study at baseline, and then to follow-up with them at 6 months and 12 months. Researchers recruited participants from HIV support groups, clinics, as well as through flyers in the community and word of mouth. Once enrolled, participants completed a set of questionnaires either by themselves, or with assistance from the researchers in their language of choice. They received lunch, and their transportation expenses were reimbursed. The total sample consisted of 1457 HIV-positive people in the overall study. This analysis reports on the 698 participants who reported taking ARV medications at the time of the data collection.
Instruments
Each participant completed a survey booklet that included the following five instruments:
Demographic questionnaire.
This questionnaire elicited data on demographics and illness. It included questions related to country, gender, age, education, marital status, work for pay, setting, years known HIV-positive, go to bed hungry at night, attended a support group in the past 3 months, and had clinic visit clinic in the past 3 months.
HIV/AIDS Stigma Instrument–PLWA (HASI-P).24
The HASI-P contains 33 items that measure 6 aspects of HIV/AIDS-related stigma (verbal abuse, negative self-perception, health care neglect, social isolation, fear of contagion, workplace stigma). For the scale scores, the Cronbach α reliability coefficients range from 0.76 to 0.90, and is 0.94 for the total scale. The instrument was developed based upon data from focus group discussion with HIV-positive people and nurses in each of the five participating countries. The stem for the instrument poses the following question: “Please tell me how often it happened to you because of your HIV status.”
The rating scale is: 0 = never, 1 = once or twice, 2 = several times, 3 = most of the time. The instrument is scored by taking the mean of the total item score.
HIV/AIDS Targeted Quality of Life Instrument (HAT-QoL).30,31
The HAT-QoL consists of 34 items. It is a disease-specific measure of quality of life with 9 dimensions: overall function, life satisfaction, health worries, financial worries, medication worries, HIV mastery, disclosure worries, provider trust, and sexual function. The dimensions are scored to produce a final dimension score of 0 to 100 (0 is worst, 100 is best). Holmes and Shea30,31 have reported on the development and validation of the scale. As this study reports on ARV adherence, only the medication worries subscale of the HAT is reported. The Cronbach α reliability coefficient of this 5–item subscale was 0.86 at baseline, and 0.96 and 0.95 for the two subsequent assessment periods indicating excellent internal consistency reliability of the items.
The Revised Sign and Symptom Checklist for Persons with HIV Disease (SSC-HIVrev).32
The SCC-HIVrev measures the intensity and frequency of 72 common signs and symptoms of HIV disease. The instrument includes 45 HIV-related physical and psychological symptoms (eleven factors and a total score; reliability estimates from 0.76–0.91), 19 HIV-related symptoms that do not cluster but may be clinically useful, and eight gynecological symptoms for women. Only the 64 symptoms that are relevant to both males and females were used in this analysis. The HIV Sign & Symptom check-List (rev) has been used extensively in Southern Africa.33
Reasons for missed medications.
The AIDS Clinical Trials Group's instrument (ACTG-Rev)34 initially consisted of 14 self-reported reasons for missing medications, such as wanted to avoid side effects, felt depressed, forgot. In 2006, Holzemer and colleagues35 reported on additional factor analyses conducted to reduce the ACTG-Rev to a 9-item instrument with a one-factor solution and Cronbach reliability estimate of 0.96. Two scores are calculated from the revised 9-item scale. Respondents rate how often in the past month they have missed their ARV medications for a particular reason, on a scale of 1 to 4 (never to often). The resulting score ranges from 9 to 36, where higher scores mean the person missed more doses. For this analysis, participants' scores were dichotomized into having missed at least one ARV dose in the past month (1 = yes) and missing no ARV medication doses (0 = no).
Data from the questionnaires were entered into Statistical Package for the Social Sciences (SPSS) for Windows Version 15.0 software (SPSS, Inc., Chicago, IL). The data were then reviewed, cleaned, and scored.
Data analysis
Multilevel growth modeling is a statistical model development methodology that is appropriate to analyze variable change over time. The method does not require equal waves of data as in repeated measures analysis of variance (ANOVA).36 An important feature of the five-country cohort design of this study was that the reported stigma scores may vary by country.
The distribution of the dependent variable of stigma was normalized using the natural log (ln) transformation. In deriving the final model (Model D), the variables identified in Tables 1, 2, and 3 were entered in the model for their impact in the initial baseline status and the variable's interaction with time was modeled. Variables that were non-significant at the 0.95 level were dropped from the model to maintain a parsimonious model. All of the demographic variables were entered into the model, but most were dropped due to their nonsignificant relationship with the dependent variable. Time-varying (level one) covariates were modeled as randomly varying and the variables that did not significantly improve the model fit assessed by the chi-squared difference between the log likelihood deviance statistic were removed from the analysis.
Table 1.
Sample Sizesa and Totals by Country for Individuals Taking Antiretroviral Medications
| Time 1 n (%) | Time 2 n (%) | Time 3 n (%) | |
|---|---|---|---|
| Country | |||
| Lesotho | 155 (22.2%) | 139 (19.4%) | 101 (21.6%) |
| Malawi | 186 (26.6%) | 208 (29.1%) | 148 (31.7%) |
| South Africa | 080 (11.4%) | 107 (14.9%) | 065 (13.9%) |
| Swaziland | 153 (22.0%) | 115 (16.1%) | 073 (15.6%) |
| Tanzania | 124 (17.8%) | 145 (20.3%) | 080 (17.1%) |
| Total | 698 (100%) | 714 (100%) | 467 (100%) |
The increasing and decreasing sample sizes over the three time periods is due to a combination of the antiretroviral (ARV) roll out and participant attrition over time.
Table 2.
Sample Characteristics and Attrition Analysis Comparing Participants who Left the Study with Those who Stayed
| Variable | Baseline total | End of study total | Left study total | Test value | df | p |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 70% (n = 484) | 70% (n = 324) | 70% (n = 160) | 0.01 | 1 | 0.99 |
| Male | 30% (n = 212) | 30% (n = 142) | 30% (n = 70) | |||
| Missing | (n = 2) | (n = 1) | ||||
| Age | ||||||
| n = 672 | n = 451 | n = 220 | ||||
| Mean = 36.9 yrs | Mean = 37.0 yrs | Mean = 36.5 yrs | 0.67 | 699 | 0.50 | |
| SD = 8.8 | SD = 8.6 | SD = 9.2 | ||||
| Range = 17–71 | ||||||
| Postschool education | ||||||
| No postschool | 72% (n = 506) | 73% (n = 341) | 71% (n = 164) | 0.68 | 2 | 0.71 |
| Certificate | 23% (n = 159) | 23% (n = 106) | 23% (n = 53) | |||
| Diploma/advanced | 5% (n = 33) | 4% (n = 20) | 6% (n = 13) | |||
| Marital Status | ||||||
| Never married | 26% (n = 175) | 25% (n = 113) | 29% (n = 62) | |||
| Married | 34% (n = 231) | 33% (n = 151) | 36% (n = 79) | 3.60 | 4 | 0.47 |
| Widowed | 26% (n = 174) | 28% (n = 127) | 22% (n = 47) | |||
| Divorced | 10% (n = 66) | 10% (n = 45) | 10% (n = 21) | |||
| Cohabiting | 4% (n = 27) | 4% (n = 19) | 4% (n = 8) | |||
| Work for pay | ||||||
| Yes | 29% (n = 199) | 28% (n = 129) | 31% (n = 70) | 0.65 | 1 | 0.42 |
| No | 71% (n = 495) | 72% (n = 336) | 69% (n = 158) | |||
| Setting | ||||||
| Urban | 37% (n = 250) | 36% (n = 169) | 36% (n = 80) | |||
| Peri-urban | 30% (n = 203) | 32% (n = 145) | 26% (n = 58) | 4.21 | 2 | 0.12 |
| Rural | 33% (n = 225) | 32% (n = 146) | 38% (n = 85) | |||
| Years HIV positive | ||||||
| n = 678 | n = 456 | n = 223 | ||||
| Mean = 3.2 yrs | Mean = 3.3 yrs | Mean = 2.9 yrs | 1.75 | 677 | 0.08 | |
| SD = 2.7 | SD = 2.7 | SD = 2.7 | ||||
| Range = 0–18 | ||||||
| Go to bed hungry at night | ||||||
| Yes | 29% (n = 197) | 30% (n = 137) | 27% (n = 60) | 0.69 | 1 | 0.41 |
| No | 71% (n = 487) | 70% (n = 322) | 73% (n = 164) | |||
| Stigma total score | ||||||
| n = 695 | n = 466 | n = 229 | ||||
| Mean = 0.43 | Mean = 0.41 | Mean = 0.47 | 1.47 | 693 | 0.14 | |
| SD = 0.47 | SD = 0.44 | SD = 0.51 | ||||
| Range = 0–3 | Range = 0–3 | Range = 0–3 | ||||
| Symptom intensity | ||||||
| n = 696 | n = 466 | n = 230 | ||||
| Mean = 31.42 | Mean = 29.7 | Mean = 35.0 | 2.27 | 693 | 0.02 | |
| SD = 29.51 | SD = 28.8 | SD = 30.7 | ||||
| Range = 0–192 | Range = 0–192 | Range = 0–192 | ||||
| Symptom frequency | ||||||
| n = 696 | n = 466 | n = 230 | ||||
| Mean = 15.66 | Mean = 17.38 | Mean = 17.38 | 2.38 | 693 | 0.02 | |
| SD = 13.15 | SD = 14.37 | SD = 14.37 | ||||
| Range = 0–64 | Range = 0–64 | Range = 0–64 | ||||
| Missing medications (lower is better) | ||||||
| n = 591 | n = 400 | n = 191 | ||||
| Mean = 4.51 | Mean = 3.58 | Mean = 6.471 | 4.61 | 300 | ≤ 0.001 | |
| SD = 6.63 | SD = 5.86 | SD = 7.67 | ||||
| Range = 0–30 | Range = 0–30 | Range = 0–30 | ||||
| HAT-QOL medication worries (higher is better) | ||||||
| n = 656 | n = 441 | n = 214 | ||||
| Mean = 64.22 | Mean = 62.3 | Mean = 68.4 | 2.05 | 456 | 0.04 | |
| SD = 37.0 | SD = 37.9 | SD = 34.7 | ||||
| Range = 0–100 | Range = 0–100 | Range = 0–100 | ||||
| Support group use in past 3 months | ||||||
| Never = 34.4% | Never = 25.8% | Never = 37.8% | ||||
| (n = 240) | (n = 180) | (n = 86) | ||||
| Once = 8.5% | Once = 6.0% | Once = 11.0% | ||||
| (n = 59) | (n = 42) | (n = 25) | ||||
| Twice = 11.2% | Twice = 4.4% | Twice = 14.0% | 14.4 | 4 | 0.006 | |
| (n = 78) | (n = 31) | (n = 32) | ||||
| Three = 12.8 | Three = 6.0% | Three = 13.6% | ||||
| (n = 89) | (n = 42) | (n = 31) | ||||
| >Three = 32.4% | >Three = 23.9% | >Three = 23.7% | ||||
| (n = 226) | (n = 167) | (n = 54) | ||||
SD, standard deviation.
Table 3.
Study Variables Measured at Three Points in Time
| Variable | Time 1 | Time 2 | Time 3 |
|---|---|---|---|
| Stigma total score | n = 696 | n = 581 | n = 466 |
| Mean = 0.43 | Mean = 0.44 | Mean = 0.31 | |
| SD = 0.47 | SD = 0.47 | SD = 0.41 | |
| Range = 0–3 | Range = 0–3 | Range = 0–3 | |
| α 0.93 | α = 0.94 | α = 0.96 | |
| Missing medications (lower is better) | |||
| n = 592 | n = 494 | n = 410 | |
| Mean = 4.51 | Mean = 2.55 | Mean = 2.31 | |
| SD = 6.63 | SD = 5.29 | SD = 4.62 | |
| Range = 0–27 | Range = 0–31 | Range = 0–27 | |
| α = 0.97 | α = 0.92 | α = 0.86 | |
| HAT-QOL medication worries (Higher is better) | |||
| n = 656 | n = 496 | n = 410 | |
| Mean = 64.22 | Mean = 61.68 | Mean = 82.01 | |
| SD = 37.0 | SD = 36.87 | SD = 26.26 | |
| Range = 0–100 | Range = 0–100 | Range = 0–100 | |
| α = 0.86 | α = 0.96 | α = 0.95 | |
| Symptom intensity | |||
| n = 696 | n = 507 | n = 466 | |
| Mean = 31.42 | Mean = 28.27 | Mean = 27.42 | |
| SD = 29.51 | SD = 31.51 | SD = 25.41 | |
| Range = 0–192 | Range = 0–192 | Range = 0–192 | |
| Symptom frequency | |||
| n = 696 | n = 507 | n = 466 | |
| Mean = 15.66 | Mean = 18.72 | Mean = 17.38 | |
| SD = 13.15 | SD = 20.56 | SD = 14.37 | |
| Range = 0–64 | Range = 0–64 | Range = 0–64 | |
| Time taking ARVs | |||
| < 3 months 3.6% | |||
| n = 23 | |||
| — | — | 3–6 months 5.2% | |
| n = 33 | |||
| 6 mos–1 yr 18.9% | |||
| n = 121 | |||
| 1–2 yrs 42.7% | |||
| n = 273 | |||
| > 2 yrs 29.6% | |||
| n = 189 | |||
| Support group use in past 3 months | Never = 34.4% | Never = 34.7% | Never = 25.8% |
| (n = 240) | (n = 242) | (n = 180) | |
| Once = 8.5% | Once = 7.3% | Once = 6.0% | |
| (n = 59) | (n = 51) | (n = 42) | |
| Twice = 11.2% | Twice = 8.3% | Twice = 4.4% | |
| (n = 78) | (n = 58) | (n = 31) | |
| Three = 12.8% | Three = 7.7% | Three = 6.0% | |
| (n = 89) | (n = 54) | (n = 42) | |
| >Three = 32.4% | >Three = 24.2% | >Three = 23.9% | |
| (n = 226) | (n = 169) | (n = 167) | |
| Clinic use in past 3 months | |||
| Never = 16.1% | Never = 25.4% | Never = 21.9% | |
| (n = 111) | (n = 146) | (n = 102) | |
| Once = 22.2% | Once = 24.2% | Once = 28.8% | |
| (n = 153) | (n = 139) | (n = 134) | |
| Twice = 17.1% | Twice = 16.0% | Twice = 15.1% | |
| (n = 118) | (n = 92) | (n = 70) | |
| Three = 23.7% | Three = 20.3% | Three = 21.1% | |
| (n = 163) | (n = 117) | (n = 98) | |
| >Three = 21.0% | >Three = 14.1% | >Three = 13.1% | |
| (n = 144) | (n = 81) | (n = 61) | |
SD, standard deviation; ARVs, antiretrovirals.
Results
Sample characteristics
At baseline, the average age for the sample participants (n = 698) was 36.9 years (standard deviation [SD] = 8.8, range, 17–71); 70.0% (n = 484) were female. The participants were from Lesotho (22.2%, n = 155), Malawi (26.6%, n = 186), South Africa (11.4%, n = 80), Swaziland (22.0%, n = 153), and Tanzania (17.8%, n = 124; Table 1). Thirty-seven percent (n = 250) of these participants resided in an urban setting, 30.0% (n = 203) in a per-urban setting, and 33.0% (n = 225) lived in a rural setting. Seventy-two percent (n = 506) had no post school (beyond grade 12) education, 23% (n = 159) had a certificate level of education, and 5% (n = 33) had a diploma or advanced education. Twenty-nine percent (n = 199) of participants identified themselves as employed for pay. Thirty-four percent of participants (n = 231) were married, 26.0% (n = 175) were never married, an additional 26.0% (n = 174) were widowed, 10.0% (n = 66) divorced, and 4% (n = 27) reported cohabiting. The HIV illness characteristics show the mean years living with HIV to be 3.2 years (SD = 2.7, range 0–18 years). Almost a third of participants (29.0%, n = 197) reported having gone to bed hungry in the past week (Table 2).
Participants varied in terms of how long they had been taking ARV medications: less than 3 months (3.6%, n = 23), 3 to 6 months (5.2%, n = 33), 6 to 12 months (18.9%, n = 121), 1 to 2 years (42.7%, n = 273), and more than 2 years (29.6%, n = 189; Table 3).
Attrition analysis
The overall participant loss during the study was 33% (n = 231) over the 1-year period. Reasons for participant attrition included death, loss to follow-up, and refusal to participate in the study survey. The attrition percentages varied by country with Lesotho losing 35% (n = 54) of their participants, Tanzania losing 35% (n = 44), Swaziland losing 52% (n = 80), Malawi losing 20% (n = 38) and South Africa losing 19% (n = 15) of their participants over the course of the year. Participant loss generally occurred between the second and third assessment periods (Table 2).
Mixed model growth analysis
This section reports on four models describing the iterative process in model assessment using mixed models growth analysis. The modeled trajectories of stigma change over time and the independent variables contributing to change over time are presented. The first model (Table 4, Model A) tested each individual's initial total stigma score status at baseline and its associated variation from the mean total stigma score. This model is designed to show the variation in the initial stigma scores only and to quantify the total variation in the stigma scores. The model estimated the natural log (ln) of the mean total stigma score to be 0.30, upon conversion of the log value, the mean value estimate is 0.35. This model revealed significant variation in an individual's initial status of stigma scores about the mean value (t = 34.4, p ≤ 0.001). The estimate of the proportion of total stigma variation between individuals (the intra-class correlation coefficient) for the within-person and between-person variation was calculated to be 0.33 (0.028/0.028 + 0.056) indicating that one third of the total variation in the total stigma score is attributable to differences among the participants.36
Table 4.
Mixed Model Growth Analysis—Dependent Variable, Natural Logarithm Stigma
| |
Unconditional means model |
Unconditional growth model |
Growth model missing medications |
Growth model with missing medications, health worries, symptoms & social support with country |
|
|---|---|---|---|---|---|
| Fixed Effectsa | Model A | Model B | Model C | Model D | Guide to coefficients in Model D |
| Initial status—Intercept (standard error [SE]) | 0.300 (0.009) | 0.330 (0.010) | 0.290 (0.015) | 0.206 (0.024) | 1. Significant variation in individual initial stigma scores about the mean score of 0.23 (ln 0.206). |
| Initial status—Missed medications yes/no | 0.041 (0.020) | 0.046 (0.018) | 2. Significantly higher initial stigma scores in individual who reported missing at least one ARV dose in the past month. | ||
| Initial status—Medication worries | ns | 3. No significant difference in report of worries about taking medications support on initial stigma scores. | |||
| Initial status—Uses support group | 0.053 (0.014) | 4. Individuals who participated in support group more frequently reported significantly higher initial stigma scores. | |||
| Initial status—Symptom intensity | 0.039 (0.005) | 5. For every unit increase in the report of symptom intensity, there is a significant increase in the baseline report of stigma. | |||
| Initial status—Country—Lesotho | 0.141 (0.028) | 6. Lesotho participants reported higher than average initial stigma scores. | |||
| Initial status—Malawi | −0.120 (0.012) | 7. Malawi participants reported lower than average initial stigma scores. | |||
| Initial status—South Africa | ns | 8. South African participants reported initial stigma scores that were near the group average. | |||
| Initial status—Swaziland | −0.029 (0.006) | 9. Swaziland participants reported lower than average initial stigma scores. | |||
| Initial status—Tanzania | ns | 10. Tanzanian participants reported initial stigma scores that were near the group average. | |||
| Rate of change—Intercept | −0.037 (0.007) | −0.057 (0.011) | ns | 11. There is no significant change in the average stigma group scores over the 1-year period. | |
| Rate of change—Missed medications yes/nob time | 0.057 (.016) | 0.032 (0.014) | 12. Individuals who reported missing at least one ARV dose in the past month report significantly higher stigma scores over time. | ||
| Rate of change—Medication worriesb time | −0.009 (0.003) | 13. Individuals who reported fewer worries about their medication taking had significantly decreased mean stigma scores over time. | |||
| Rate of change—Support groupb time | ns | 14. There was no significant interaction of support group use on reported stigma over time. | |||
| Rate of change—Symptom intensityb time | ns | 15. There was no significant interaction of symptom intensity on the report of stigma over time. | |||
| Rate of change—Lesotho | −0.107 (0.018) | 16. Lesotho participants reported significantly decreasing reports of stigma over time. | |||
| Rate of change—Malawi | 0.043 (0.008) | 17. Malawi participants reported significantly increasing reports of stigma over time. | |||
| Rate of change—South Africa | −0.019 (0.007) | 18. South African participants reported significantly decreasing stigma over time. | |||
| Rate of change—Swaziland | ns | 19. Swaziland participants reported stigma scores were consistently lower and nonvarying over time. | |||
| Rate of change—Tanzania | ns | 20. Tanzania participants reported stigma scores were near the group mean and nonvarying over time. | |||
| Variance components Within-person variation | 0.056 (0.002) | 0.049 (0.003) | 0.044 (0.003) | 0.042 (0.003) | 21. There is significant remaining unexplained within-person variation in stigma scores. |
| Initial status of stigma | 0.028 (0.003) | 0.041 (0.005) | 0.040 (0.006) | 0.017 (0.004) | 22. There is significant remaining unexplained variation in the initial total stigma scores. |
| Level 2 in rate of change of stigma | 0.006 (0.002) | 0.005 (0.002) | 0.0004 ns | 23. There is no significant remaining unexplained variation in the rates of change in stigma scores over time. | |
| Within-person variation of stigma | 10% | 4.5% | 24. There is a total of 14.5% explained variance in the within person variation from model B by adding the level 2 predictors to the model. | ||
| Initial status variation of stigma | 2% | 57% | 25. There is a total of 59% additional explained variance in initial stigma scores by adding the level 2 predictors to the model. | ||
| Rate of change variation of stigma | 17% | 92% | 26. 92% of the variation in change over time in reported stigma is explained by adding the level 2 predictors to the model. | ||
| Goodness of Fit (lower is better) | |||||
| Deviance statistic | 475 | 439b | 257b | 46b | 27. The addition of level 2 predictor variables to the unconditional growth model (Model B) significantly improved the model fit. |
| Akaike Information Criterion (AIC) | 481 | 451 | 273 | 14 | |
| Bayesian Information Criterion (BIC) | 498 | 484 | 316 | 70 | |
DV, dependent variable; ln, natural logarithm; ns, not significant; ARV, antiretroviral.
All parameters are significant at the p < 0.01 level except where noted.
χ2 significant improvement in model fit.
The second model (Table 4, Model B) estimated the change over time in stigma for the group by assessment of the individual growth trajectories in stigma and the between-person differences in the growth trajectories. In this model, the natural log of the mean total stigma score at baseline was 0.33 and over time on average, the entire sample, showed a steady decrease in stigma scores to 0.29 at the 1-month follow-up assessment and 0.26 at the 2-month follow-up assessment. The variance parameters associated with the stigma intercept trajectories over the 1-year period showed significant variation, indicating stigma change often differs for each person. This is evidenced by the between-person variability in initial status (0.041) and rate of change (0.006). The estimates of variation from these two unconstrained models were used for subsequent model comparisons to assess any change in variance and improved model fit by adding level two variables that further describe the sample and explain variation.
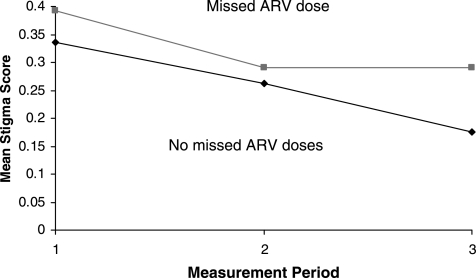
The third model (Table 4, Model C) shows results of testing for differences in the initial report of stigma and change over time in participants. There was significant variation in individual initial stigma scores about the mean score of 0.33 (ln 0.29). There is a significant difference in the change trajectories of participants who reported missing at least one ARV dose in the last month compared to those who reported having missed no doses of medications. The mean initial stigma score was higher in participants who reported having missed at least one ARV dose in the last month (0.39 in the missed dose group versus 0.33 in the not missed group) and while both groups' stigma scores decreased over time, those who reported not missing taking their ARV doses had a significantly steeper decrease in stigma scores over time compared to those who did miss medication doses (Fig. 1). The addition of the missing medications variable to the model revealed improved reductions in variance in the between individual trajectories by 10%, the average initial variation in stigma scores by 2%, and a 17% explanation of variance in the average change over time. The goodness of fit indices compared to the previous model showed improved model fitness.
FIG. 1.
Effect of missing medications on stigma. Individuals reporting missing at least one antiretroviral (ARV) dose in the past month had significantly higher reports of stigma initially and over time.
The final model (Table 4, Model D) shows the effects of adding additional level 2 dependent variables to further explain the variation in initial stigma score status and rate of change over time. As in prior models, there is significant variation in the initial average stigma scores about the mean score of 0.23 (ln 0.206) and again this model reveals higher mean initial stigma scores for those missing medication doses 0.29 (ln 0.252) compared to those who did not miss medication doses in the last month 0.23 (ln 0.206). There is with no significant difference in the report of the medication worries on initial stigma scores. However, over time those individuals who reported fewer worries about their medication taking had lower mean stigma scores. The relationship between symptom intensity and initial stigma scores showed that those who had increased symptom intensities reported increased stigmatization and this increased effect of symptom intensity stayed high over time and did not change significantly. The improved goodness of fit statistics in the consecutive models indicate that the addition of level 2 predictor variables significantly improved the model fit.
Use of support groups
Individuals who participated in support groups more frequently reported significantly higher initial mean stigma scores of 0.33 indicating that, for every unit increase in support group visits in the last month, there was a 0.05 (ln 0.053) unit increase in stigma holding all other variables constant. The stigma scores remained constant throughout the study period and did not vary significantly.
Country level differences in the report of stigma
A large amount of model variation in initial stigma ratings and in change over time is attributed to country level differences. Lesotho participants reported initial stigma scores that were on average 13% higher than the group's initial stigma score average, and this difference was significant. Furthermore, Lesotho participants' reports of stigma had decreased 10% by the conclusion of the study, and this too was significant.
The initial mean stigma scores for Malawi and Swaziland participants were significantly lower than average. Neither South Africa nor Tanzania's initial mean stigma scores differed significantly from the group average. However, Malawi participants reported significantly increasing reports of stigma over time whereas South Africa participants reported a significant decrease in stigma over time. The stigma scores of Swaziland and Tanzania participants were consistently lower and non-varying over time compared to the group mean stigma score trajectories.
Discussion
The attrition rate over the three measurement periods shows that those who left the study had a higher level of missed medicines and knew about their HIV-positive status for a shorter time than those who remained in the study. This could be due to the level of understanding about the complexity of the ARV regimen and adherence thereof, as well as stigma related to accessing services, as highlighted by Rintamaki et al.7
There is a demonstrated overall decrease in stigma scores over time, with those who reported having messed medication doses also reporting higher stigma. The work of Edwards37 and others support this result, showing that HIV stigma is one factor that interferes with medication adherence.
These results demonstrate that participants who reported more symptoms, more medication worries, and more missed medication doses had significantly higher reports of stigma. This may demonstrate the relationship between being noticeably sick, reflected by the increased symptom intensity, and the experience of increased stigmatization. Rintamaki et al.7 state that social stigma influences medication practices and consequently treatment efficacy and health outcomes. These results support those findings. The findings of this study are further enhanced by the work of Mills et al.6 and Edwards,37 which state that medication worries could be patient related, reflecting a patient's fear of potential side effects and a lack of understanding regarding the treatment regimen. Fear may also be reflective of a concern that accessibility to the medications can change. Other authors have reported medication worries related to stigma as some patients fear to disclose having HIV/AIDS and hence would miss medications since they cannot take it in front of relatives or friends.
Individuals who were participating in regular support groups reported experiencing greater stigma. This finding is somewhat counterintuitive and suggests that support groups might consider including stigma in their work and exploring strategies on how to manage stigma, since stigma may be one reason they are participating in the support group in the first place. This is supported in the work of Rintamaki et al.7 who suggests that social, psychological and educational factors about ARV treatment foster acceptance of illness and thus do not pose a threat to treatment through social stigma. Perceived HIV stigma has been demonstrated to be a significant correlate of missed medication doses—those who missed medications more frequently had higher stigma scores over time. These findings support the concept that health care providers should discuss HIV-related stigma issues with their patients before patients are placed on an antiretroviral regimen.
This study had several limitations. Because there was no sampling frame at the country level and subjects selected were a convenience sample, it is not possible to generalize the findings too broadly. Because of the limitation in sampling, it is somewhat difficult to interpret the observed country-level differences because for Lesotho and Swaziland, the samples were much more representative than for Malawi, Tanzania, or South Africa simply due to sample size selected.
Acknowledgments
This work was supported by National Institutes of Health Research Grant #R01 TW06395 funded by the Fogarty International Center, the National Institute of Mental Health, and the Health Resources and Services Administration, U.S. Government, and T32NRD007969 funded by the National Institute of Nursing Research.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Avert. HIV & AIDS in Africa. www.avert.org/aafrica.htm. [Jun 12;2008 ]. www.avert.org/aafrica.htm
- 2.Bangsberg DR. Hecht FM. Clague H, et al. Provider assessment of adherence to HIV antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;26:435–442. doi: 10.1097/00126334-200104150-00005. [DOI] [PubMed] [Google Scholar]
- 3.Cohn SE. Kammann E. Williams P. Currier JS. Chesney MA. Association of adherence to Mycobacterium avium complex prophylaxis and antiretroviral therapy with clinical outcomes in Acquired Immunodeficiency Syndrome. Clin Infect Dis. 2002;34:1129–1136. doi: 10.1086/339542. [DOI] [PubMed] [Google Scholar]
- 4.Paterson DL. Swindells S. Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 6.Mills EJ. Nachega JB. Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: A meta-analysis. JAMA. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 7.Rintamaki LS. Davis TC. Skripkauskas S. Bennett CL. Wolf MS. Social stigma concerns and HIV medication adherence. AIDS Patient Care STDs. 2006;20:359–368. doi: 10.1089/apc.2006.20.359. [DOI] [PubMed] [Google Scholar]
- 8.Rao D. Kekwaletswe TC. Hosek S. Martinez J. Rodriguez F. Stigma and social barriers to medication adherence with urban youth living with HIV. AIDS Care. 2007;19:28–33. doi: 10.1080/09540120600652303. [DOI] [PubMed] [Google Scholar]
- 9.Medley A. Garcia-Moreno C. McGill S. Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: Implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82:299–307. [PMC free article] [PubMed] [Google Scholar]
- 10.Varga CA. Sherman GG. Jones SA. HIV-disclosure in the context of vertical transmission: HIV-positive mothers in Johannesburg, South Africa. AIDS Care. 2006;18:952–960. doi: 10.1080/09540120500356906. [DOI] [PubMed] [Google Scholar]
- 11.Daftary A. Padayatchi N. Padilla M. HIV testing and disclosure: A qualitative analysis of TB patients in South Africa. AIDS Care. 2007;19:572–577. doi: 10.1080/09540120701203931. [DOI] [PubMed] [Google Scholar]
- 12.Wood K. Chase E. Aggleton P. 'Telling the truth is the best thing': Teenage orphans' experiences of parental AIDS-related illness and bereavement in Zimbabwe. Soc Sci Med. 2006;63:1923–1933. doi: 10.1016/j.socscimed.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Goffman E. Stigma: Notes on the Management of Spoiled Identity. Englewood Cliffs, NJ: Prentice Hall; 1963. [Google Scholar]
- 14.Alonzo AA. Reynolds NR. Stigma, HIV and AIDS: An exploration and elaboration of a stigma trajectory. Soc Sci Med. 1995;41:303–315. doi: 10.1016/0277-9536(94)00384-6. [DOI] [PubMed] [Google Scholar]
- 15.Holzemer WL. Uys L. Makoae L, et al. A conceptual model of HIV/AIDS stigma from five African countries. J Adv Nurs. 2007;58:541–551. doi: 10.1111/j.1365-2648.2007.04244.x. [DOI] [PubMed] [Google Scholar]
- 16.Ware NC. Wyatt MA. Bangsberg DR. Examining theoretic models of adherence for validity in resource-limited settings. A heuristic approach. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S18–22. doi: 10.1097/01.qai.0000248343.13062.4b. [DOI] [PubMed] [Google Scholar]
- 17.Nachega JB. Hislop M. Dowdy DW, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43:78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe WR. Weiser SD. Bangsberg DR, et al. Effects of HIV-related stigma among an early sample of patients receiving antiretroviral therapy in Botswana. AIDS Care. 2006;18:931–933. doi: 10.1080/09540120500333558. [DOI] [PubMed] [Google Scholar]
- 19.Nachega JB. Stein DM. Lehman DA, et al. Adherence to antiretroviral therapy in HIV-infected adults in Soweto, South Africa. AIDS Res Hum Retroviruses. Oct. 2004;20:1053–1056. doi: 10.1089/aid.2004.20.1053. [DOI] [PubMed] [Google Scholar]
- 20.Weiser S. Wolfe W. Bangsberg D, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr. 2003;34:281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Uys LR. Chirwa M. Dlamini P, et al. Eating plastic, winning the lotto, joining the WWW: Descriptions of HIV/AIDS in Africa. J Assoc Nurses AIDS Care. 2005;16:11–21. doi: 10.1016/j.jana.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Kohi T. Makoae L. Chirwa M, et al. HIV and AIDS stigma violates human rights in five African countries. Nurs Ethics. 2006;13:405–414. doi: 10.1191/0969733006ne865oa. [DOI] [PubMed] [Google Scholar]
- 23.Naidoo J. Uys L. Greeff M, et al. Urban and rural differences in HIV/AIDS stigma in five African countries. Afr J AIDS Res. 2007;6:17–23. doi: 10.2989/16085900709490395. [DOI] [PubMed] [Google Scholar]
- 24.Holzemer WL. Uys LR. Chirwa ML, et al. Validation of the HIV/AIDS Stigma Instrument–PLWA (HASI-P) AIDS Care. 2007;19:1002–1012. doi: 10.1080/09540120701245999. [DOI] [PubMed] [Google Scholar]
- 25.Dlamini PS. Kohi TW. Uys LR, et al. Verbal and physical abuse and neglect as manifestations of HIV/AIDS stigma in five African countries. Public Health Nurs. 2007;24:389–399. doi: 10.1111/j.1525-1446.2007.00649.x. [DOI] [PubMed] [Google Scholar]
- 26.Greeff M. Phetlhu R. The meaning and effect of HIV/AIDS stigma for people living with AIDS and nurses involved in their care in the North West Province, South Africa. Curationis. 2007;30:12–23. doi: 10.4102/curationis.v30i2.1066. [DOI] [PubMed] [Google Scholar]
- 27.Greeff M. Phetlhu R. Makoae LN, et al. Disclosure of HIV status: experiences and perceptions of persons living with HIV/AIDS and nurses involved in their care in Africa. Qual Health Res. 2008;18:311–324. doi: 10.1177/1049732307311118. [DOI] [PubMed] [Google Scholar]
- 28.Makoae LN. Greeff M. Phetlhu RD, et al. Coping with HIV-related stigma in five African countries. J Assoc Nurses AIDS Care. 2008;19:137–146. doi: 10.1016/j.jana.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uys LR. Holzemer WL. Chirwa ML, et al. The development and validation of the HIV/AIDS Stigma Instrument-Nurse (HASI-N) AIDS Care. 2009;21:150–159. doi: 10.1080/09540120801982889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes WC. Shea JA. A new HIV/AIDS-targeted quality of life (HAT-QoL) instrument: Development, reliability, and validity. Med Care. 1998;36:138–154. doi: 10.1097/00005650-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Holmes WC. Shea JA. Two approaches to measuring quality of life in the HIV/AIDS population: HAT-QoL and MOS-HIV. Qual Life Res. 1999;8:515–527. doi: 10.1023/a:1008931006866. [DOI] [PubMed] [Google Scholar]
- 32.Holzemer WL. Hudson A. Kirksey KM. Hamilton MJ. Bakken S. The Revised Sign and Symptom Check-List for HIV (SSC-HIVrev) J Assoc Nurses AIDS Care. 2001;12:60–70. doi: 10.1016/s1055-3290(06)60263-x. [DOI] [PubMed] [Google Scholar]
- 33.Phaladze NA. Human S. Dlamini SB, et al. Quality of life and the concept of “living well” with HIV/AIDS in sub-Saharan Africa. J Nurs Scholarsh. 2005;37:120–126. doi: 10.1111/j.1547-5069.2005.00023.x. [DOI] [PubMed] [Google Scholar]
- 34.Chesney MA. Ickovics JR. Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 35.Holzemer WL. Bakken S. Portillo CJ, et al. Testing a nurse-tailored HIV medication adherence intervention. Nurs Res. 2006;55:189–197. doi: 10.1097/00006199-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Singer J. Willett J. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 37.Edwards L. Perceived social support and HIV/AIDS medication adherence among African American women. Qual Health Res. 2006;16:679–691. doi: 10.1177/1049732305281597. [DOI] [PubMed] [Google Scholar]