Abstract
The purpose of this double-blind, randomized controlled pilot study was to compare the effectiveness of four physical therapy interventions in the treatment of primary shoulder impingement syndrome: 1) supervised exercise only, 2) supervised exercise with glenohumeral mobilizations, 3) supervised exercise with a mobilization-with-movement (MWM) technique, or 4) a control group receiving only physician advice. Thirty-three subjects diagnosed with primary shoulder impingement were randomly assigned to one of these four groups. Main outcome measures included 24-hour pain (VAS), pain with the Neer and Hawkins-Kennedy tests, shoulder active range of motion (AROM), and shoulder function (SPADI). Repeated-measures analyses indicated significant decreases in pain, improved function, and increases in AROM. Univariate analyses on the percentage of change from pre- to post-treatment for each dependent variable found no statistically significant differences (P<0.05) between the four groups. Although not significant, the MWM and mobilization groups had a higher percentage of change from pre- to post-treatment on all three pain measures (VAS, Neer, Hawkins-Kennedy). The three intervention groups had a higher percentage of change on the SPADI. The MWM group had the highest percentage of change in AROM, and the mobilization group had the lowest. This pilot study suggests that performing glenohumeral mobilizations and MWM in combination with a supervised exercise program may result in a greater decrease in pain and improved function although studies with larger samples and discriminant sampling methods are needed.
KEYWORDS: Exercise, Glenohumeral Mobilization, Mobilization with Movement, Shoulder Impingement Syndrome
Shoulder impingement syndrome, the most common diagnosis of shoulder dysfunction1, is often described as shoulder pain exacerbated by overhead activities2,3. Primary shoulder impingement occurs when the rotator cuff tendons, long head of the biceps tendon, glenohumeral joint capsule, and/or subacromial bursa become impinged between the humeral head and anterior acromion4. Primary impingement may be due to intrinsic factors: rotator cuff weakness5,6, chronic inflammation of the rotator cuff tendons and/or subacromial bursa7–9, rotator cuff degenerative tendinopathy7,10, and posterior capsular tightness leading to abnormal anterior/superior translation of the humeral head11,12. It may also be due to extrinsic factors: possession of a curved or hooked acromion13–15, acromial spurs16, or postural dysfunction17,18. Secondary shoulder impingement is defined as a relative decrease in the subacromial space due to glenohumeral joint instability or abnormal scapulothoracic kinematics6,19–22. Commonly seen in athletes engaging in overhead throwing activities23,24, secondary impingement occurs when the rotator cuff becomes impinged on the posterior-superior edge of the glenoid rim when the arm is placed in end-range abduction and external rotation7. This positioning becomes pathologic during excessive external rotation, anterior capsular instability, scapular muscle imbalances21,25, and/or upon repetitive overload of the rotator cuff musculature23,26.
Physical therapy has been found to be effective in reducing pain and disability in patients with shoulder impingement. Effective interventions include therapeutic exercises focusing on strengthening the rotator cuff and scapular stabilizing musculature27–34, stretching to decrease capsular tightness35, scapular taping techniques36, and patient education of proper posture37. Studies suggest the incorporation of joint mobilizations to treat shoulder impingement results in superior outcomes compared with therapeutic exercise alone28,29,38. Some researchers propose that a mobilization force can be selectively directed to a specific area of the capsule to restore capsular extensibility29,39. Studies have found that individuals with shoulder impingement often have a tight posterior capsule resulting in altered glenohumeral arthrokinematics11,40 and a decrease in glenohumeral internal rotation range of motion (ROM)6,12,40. Thus, performing grade III or IV mobilizations aimed at restoring posterior capsule mobility in subjects with shoulder impingement may result in increased active ROM and decreased impingement symptoms, whereas all grades of mobilizations (I-IV) may result in pain reduction32.
A manual therapy approach to treating shoulder dysfunction is the Mulligan concept of mobilization with movement (MWM)41,42. The goal of performing MWM is immediate and sustained improvement in joint pain and mobility. Mulligan's techniques entail having the physical therapist apply an accessory mobilization to a peripheral joint while the patient simultaneously generates active movement. During the technique, the therapist must continually monitor the patient to ensure that no pain is recreated. If pain commences, the therapist must investigate different treatment planes and/or grades of accessory motion to ensure pain-free movement. Mulligan believes that failure to improve pain-free ROM indicates that the therapist has not found the correct treatment plane or grade of mobilization, or simply that the technique is not indicated. Mulligan's theory is that joint injury or dysfunction results in a positional fault or chronic state of mal-alignment within the joint, and the techniques may assist in properly aligning the joint or restoring the joint's tracking mechanism41,42. Only two studies have been published supporting the benefits of performing a shoulder MWM technique in treating shoulder dysfunction43,44. One case study using MWM to treat a patient with shoulder impingement reported a decrease in pain, improvement in function, and improvement in shoulder abduction AROM43.
Although therapeutic exercise has been shown to be effective in treating shoulder impingement symptoms27–34, very few studies have evaluated the effectiveness of incorporating glenohumeral joint mobilizations28,29,39,45, and no randomized controlled trials have used a MWM technique to treat shoulder impingement. The purpose of this double-blind, randomized controlled pilot study was to compare the effectiveness of four physical therapy interventions in the treatment of primary shoulder impingement syndrome: supervised exercise only, supervised exercise with glenohumeral mobilizations, supervised exercise with a MWM technique, or a control group receiving only physician advice. A secondary purpose was to examine the appropriateness of the sampling and data collection procedures for a future study with more power.
Methods
Subjects
Thirty-three subjects, 17 men and 16 women, aged 18–74 (mean 46.4 years) and diagnosed with primary shoulder impingement by the referring physician participated in this study. Although 36 subjects enrolled in the study, 3 subjects were later excluded. With two of the subjects, the assessor was unable to obtain baseline measures of ROM due to acute pain and unwillingness to move the extremity; the third subject was mentally ill and displayed inappropriate behaviors rendering participation impossible. Inclusion criteria included superiolateral shoulder pain and two out of four specified objective signs and symptoms: a positive (painful) Neer impingement test, a positive (painful) Hawkins-Kennedy impingement test, painful limitation of active shoulder elevation (flexion, abduction, scaption), and pain or limitation with the functional movement patterns of hand-behind-back or hand-behind-head. Exclusion criteria included a physician diagnosis of adhesive capsulitis, grade III rotator cuff tear, calcific tendonitis confirmed by radiology, systemic or neurological disorder, cervical radiculopathy, a history of shoulder surgery, corticosteroid injection within the past month, and subjects who had received physical therapy treatment for their shoulder within the past three months. A recent systematic review reveals these inclusion and exclusion criteria have been used in many clinical trials46. All subjects signed consent forms approved by the University Institutional Review Board, Committee for the Protection of Human Subjects, at California State University, Northridge.
Subjects were asked to decline any other form of treatment for their shoulder during the course of the study including additional physical therapy, chiropractic, acupuncture, or massage therapy to the shoulder, neck, or upper back. Subjects were also instructed to remain on current levels of medication and not to begin any new medications during the course of the study. All participants underwent a follow-up visit with the referring physician after the post-treatment measurements at the study's completion.
Procedure
Participants were randomly assigned to one of four intervention groups according to the block randomization method: Group 1, exercise only; Group 2, exercise and mobilization; Group 3, exercise and MWM; and Group 4, control. Block randomization was used to ensure that an equal number of patients were assigned to each treatment group. As an example, subject #1 had an equal chance of drawing an envelope assigning him/her to group A, B, C, or D. If he/she drew “A,” the card was removed. Subject #2 then had an equal chance of drawing an envelope with group B, C, or D, subject #3 with the remaining two groups, and subject #4 received the final group assignment. Each subject was informed of his/her treatment protocol but remained blinded to other group assignments to avoid subject bias.
One physical therapist with 12 years of clinical experience performed the pre- and post-treatment assessment measurements. This assessor was blinded to group assignment and all intervention protocols. The initial assessment session occurred within 3–4 days of the physician examination. Before commencing the pre-intervention testing session, each subject filled out a demographic survey for statistical reporting of gender, age, hand dominance, symptom duration, medications, and the current as well as past history of shoulder dysfunction (Table 1).
TABLE 1.
Baseline demographics and pre-treatment means (sd) for the dependent variable for each group.
| Control (n=7) | Exercise (n=8) | MOB (n=9) | MWM (n=9) | Sig. | |
|---|---|---|---|---|---|
| Age in Years | 45.6 (13.0) | 47.3 (20.1) | 43.4 (14.7) | 48.9 (13.7) | .90a |
| Gender | 4M, 3F | 4M, 4F | 4M, 5F | 5M, 4F | .95b |
| Involved Shoulder | 6R, 1L | 4R, 4L | 6R, 3L | 4R, 5L | .40b |
| Hand Dominance | 7R | 7R, 1L | 8R, 1L | 9R | .57b |
| Pain Chronicity (months) | 70.0 (92.4) | 32.5 (60.2) | 19.2 (24.6) | 22.6 (17.4) | .26a |
| VAS pre-test | 4.4 (1.2) | 5.7 (3.0) | 6.3 (1.6) | 5.2 (2.5) | .38a |
| NEER pre-test | 3.7 (2.7) | 5.1 (2.1) | 4.8 (2.6) | 2.9 (1.8) | .19a |
| HK pre-test | 2.5 (1.5) | 4.4 (2.4) | 5.0 (2.5) | 3.8 (1.6) | .12a |
| Flexion pre-test | 139.0 (16.2) | 136.3 (15.9) | 152.1 (9.7) | 146.6 (16.0) | .13a |
| Scaption pre-test | 139.6 (28.2) | 134.1 (29.5) | 141.8 (22.1) | 141.8 (20.8) | .91a |
| SPADI pre-test | 49.4 (29.3) | 62.4 (33.0) | 53.1 (23.2) | 48.6 (21.9) | .72a |
Abbreviations: MOB = mobilization group; MWM = mobilization-with-movement group; M = males; F = females; R = right; L = left; VAS = visual analog scale; NEER = Neer impingement test; HK = Hawkins-Kennedy impingement test; SPADI = Shoulder Pain and Disability Index;
One-way ANOVA;
Chi square tests.
The effect of treatment was assessed based on the following dependent variables: maximum pain over the preceding 24-hour period graded by a 10-point visual analog scale (VAS) scale; pain intensity with the Neer test assessed by the same 10-point VAS scale; pain intensity with the Hawkins-Kennedy test via the 10-point VAS; pain-free active ROM measured with a standard goniometer for flexion and scaption; and a measurement of shoulder function assessed with the Shoulder Pain and Disability Index (SPADI). The numerically-scaled SPADI47, a 13-item self-administered instrument measuring shoulder functional status, has been shown to have good test-retest reliability, responsiveness, and/or validity48–50. The SPADI used in this study was modified to facilitate subject understanding by including equal-distanced hashed lines marked 0–10, with zero labeled no pain/no functional limitations and 10 labeled worst pain/unable to perform. If a subject chose to mark between the hashed lines, the question was scored to the nearest 0.25 (for example, 4.25). The instrument was scored 0–130 with 130 representing the worst deficit in function.
Three measures of pain intensity were used in the study: a 24-hour VAS score and pain with the Neer and the Hawkins-Kennedy tests. This focus on pain was deliberate since pain is the primary clinical manifestation of shoulder impingement4,9,51. The Neer impingement test3, conducted by passive forward elevation and internal rotation of the humerus with the scapula stabilized, is deemed positive if the patient reports pain, usually above 120° of shoulder elevation when the critical zone of the rotator cuff tendon is compressed against the subacromial arch8,52. The Neer test has been found to have fair to good sensitivity for determining the presence of shoulder impingement compared with a subacromial injection test or arthroscopy53–55. The Hawkins-Kennedy test2,8 is performed by positioning the arm passively at 90° of shoulder flexion followed by the examiner forcibly internally rotating the arm—a maneuver that also directs the critical zone against the coracoacromial ligament52. The sensitivity of this maneuver has also been found to be good53–55.
Pain-free shoulder flexion and scaption active ROM were measured with a universal goniometer according to a standard procedure56. Scaption was measured in standing by aligning the goniometer axis over the coracoid process, the stationary arm parallel to the thorax and the moving arm midline of the humerus with the medial epicondyle as a guide. Standardized goniometric measurements of glenohumeral motion have been shown to have good intrarater reliability57–60 and validity60.
The principle investigator, who has over 14 years of clinical experience in the orthopaedic setting and who is a Board Certified Orthopaedic Clinical Specialist and Fellow of the American Academy of Orthopaedic Manual Physical Therapy Association, performed all treatment interventions. All subjects in the treatment groups (Groups 1–3) received physical therapy one time a week for 6 weeks according to the following protocols, and each session ended with subjects receiving a cold pack for 10–15 minutes to decrease potential inflammation and delayed muscle soreness. Participants were instructed to perform a home exercise program once a day mimicking the exercises performed in the clinic, and they were required to present a written log of these exercises to the primary investigator at each weekly session. Participants were also educated in the etiology of shoulder impingement syndrome and the importance of proper posture, and they were instructed to modify overhead activities.
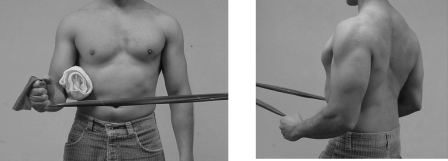
Participants in Group 1, the exercise-only group, performed exercises under the direct one-on-one supervision of the primary investigator. These exercises included posterior capsule stretching, postural correction exercises, and an exercise program focusing on rotator cuff strengthening and scapular stabilization (Figure 1).
FIGURE 1.
A. FIGURE 1B. Performing supervised exercises of shoulder external rotation with band resistance and scapular retraction.
Participants in Group 2, the exercise + mobilization group, received the standard exercise protocol as per Group 1 with the addition of glenohumeral joint mobilization techniques. Glenohumeral joint anterior, posterior, and inferior glides, and long-axis distraction passive accessory motions (PAM) were evaluated and graded using a 0–6 accessory motion scale61,62. The intrarater reliability using this scale to access spinal passive intervertebral motion (PIVM) was found to be good in one published study63 although interrater reliability and accuracy has been found to be poor63–65. The amount of pain or joint reactivity during passive accessory motion testing was graded on a 0–3 point scale with 0 = no reactivity, 1 = minimal, 2 = moderate, and 3 = severe reactivity, respectively. Studies have found good to fair intrarater reliability when assessing the onset of pain during PIVM testing in the spine65,66, and one study reported excellent validity when using PIVM testing compared to a fluoroscopy-guided nerve block to detect cervical segmental involvement based on reproduction of pain during passive motion67.
Given the results of these reliability and validity studies, the grade of joint mobility and reactivity were not used as a dependent variable, but were used to determine the direction and intensity of the mobilization. Anterior, posterior, inferior glides, or long-axis distraction grade I-IV joint mobilizations were applied accordingly (Figure 2). For situations where there was reactivity within the capsular ROM, grade I-II mobilizations were applied. For situations where there was no reactivity but capsular hypomobility, grade III-IV accessory motions were applied. Each mobilization was applied for 30 seconds at a rate of approximately one mobilization every 1 to 2 seconds, followed by a 30-second rest. The 30-second mobilization and resting sessions were repeated 2 additional times for a total of 3 sets of 30-second mobilizations.
FIGURE 2.
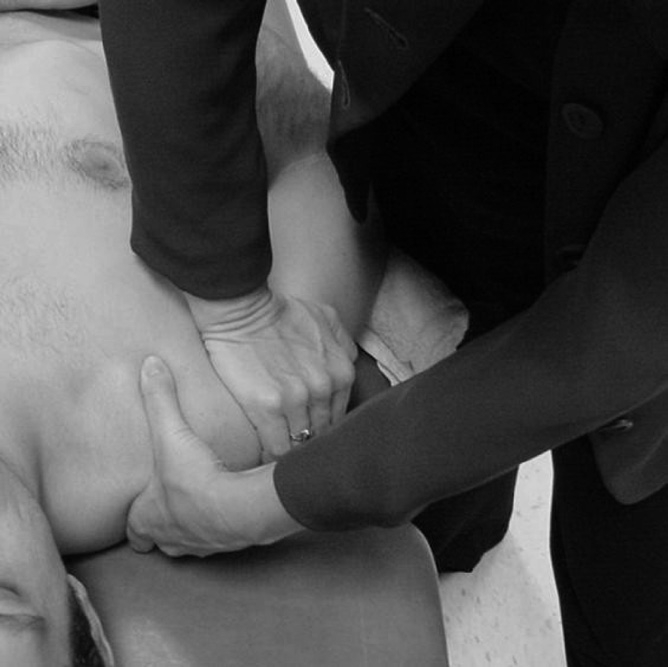
Glenohumeral posterior glide mobilization.
Participants in Group 3, the exercise plus MWM group, received the standard exercise protocol as per Groups 1 and 2, plus a glenohumeral joint MWM technique as described by Mulligan41. This technique involved the therapist applying a sustained posterior accessory glide to the glenohumeral joint while the subject simultaneously actively flexed the shoulder to the pain-free endpoint and applied a gentle overpressure force using the contralateral arm (Figure 3). Total abolition of pain during the technique was mandatory; if the patient started to experience pain during active motion, the therapist would investigate different force planes and/or grades of force until pain-free motion was restored. This procedure was repeated for a total of 3 sets of 10 repetitions as long as pain-free motion was sustained; if pain commenced during any repetition of any set, the technique was terminated.
FIGURE 3.
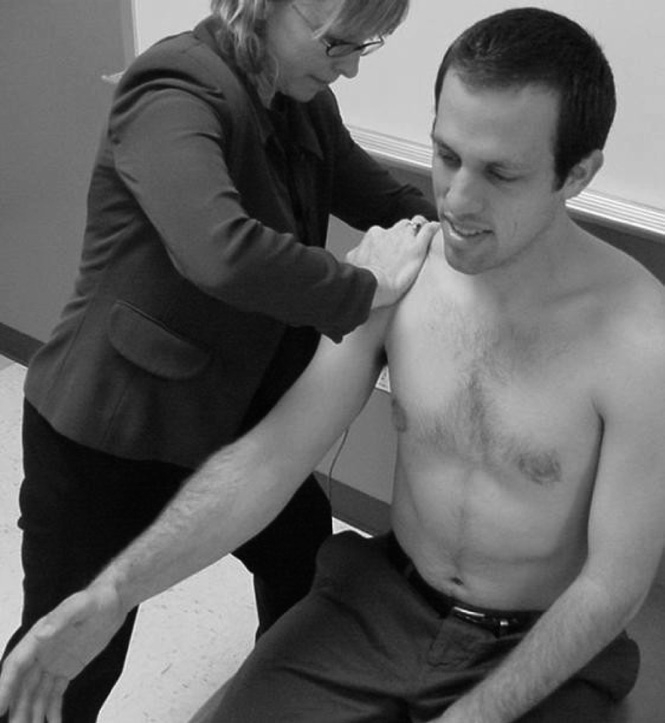
Shoulder mobilization-with-movement technique sustaining posterior glide with active shoulder flexion.
Participants in Group 4 served as the control group. Subjects in this group received patient education on postural awareness and limitation of overhead activities by the referring physician during his/her initial examination session. The physician also provided the subject with a standard shoulder impingement home exercise program without any input from the physical therapist. Thus, subjects in this group did not receive physical therapy intervention, nor were they instructed in a home exercise program by a physical therapist during the course of the study. After the final testing session and completion of the study, subjects in the control group were allowed to discuss treatment options and a home exercise program with the investigating physical therapist.
Statistical Analyses
Subject baseline characteristics are presented in Table 1. Chi square analyses and univariate analyses (ANOVA) were conducted to determine whether the four groups differed on the demographic characteristics and pre-treatment measures. Next, repeated-measures ANOVAs with Tukey's post hoc test were conducted for each of the dependent variables with the pre-treatment and post-treatment scores as the within-subjects variable and the four groups as the between-subjects variable.
Due to the wide variability between subjects on pre-treatment scores, some subjects had greater room for improvement and some had relatively little room for improvement. Hence, a percentage of change score from pre- to post-treatment was calculated for each dependent variable. For the VAS, Neer, Hawkins-Kennedy, and SPADI measures, the following formula was used: Percentage of change = [(pre-treatment score—post-treatment score) / pre-treatment score] ∗ 100. For flexion and scaption active ROM, the pre- and post-treatment scores were subtracted from full ROM of 180° using the following formula: Percentage of change = [((180—pre-treatment score)—(180—post-treatment score)) / (180—pre-treatment score)] ∗ 100. The difference scores and percentage of change scores were analyzed using univariate analyses with Tukey's post hoc test.
All statistical analyses were performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL). Differences were considered statistically significant when the P < 0.05. For the univariate analyses, post hoc power estimates (i.e., observed power) were determined using the SPSS power option.
Results
Chi square analyses indicated no statistically significant differences between the four groups on gender, involved shoulder, or hand dominance. One-way ANOVA analysis indicated no statistically significant differences between the groups on age, pain chronicity, or baseline pretreatment scores.
Repeated-measures univariate analyses indicated that all groups had statistically significant decreases in pain intensity from pre- to post-treatment on the (a) VAS test [F(1,29)=28.5, P<.001, ηp2=.50, observed power=.99]; (b) NEER test [F(1,29)=35.2, P<.001, ηp2=.55, observed power=1.0]; and (c) Hawkins-Kennedy test [F(1,29)=31.1, P<.001, ηp2=.52, observed power=1.0]. The analyses also indicated that there were statistically significant increases in (a) pain-free active ROM from pre- to post-treatment on both flexion [F(1,29)=19.7, P<.001, ηp2=.40, observed power=.99]; and scaption [F(1,29)=18.8, P<.001, ηp2=.39, observed power=.99] as well as (b) shoulder function measured with the SPADI [F(1,29)=47.7, P<.001, ηp2=.62, observed power=1.0]. However, no statistically significant differences were found on the interaction between the four groups and mean change from pre- to post-treatment: (a) VAS test [F(3,29)=.26, P=.85, ηp2=.03, observed power=.09]; (b) NEER test [F(3,29)=.19, P=.90, ηp2=.02, observed power=.08]; (c) Hawkins-Kennedy test [F(3,29)=.79, P=.51, ηp2=.08, observed power=.20]; (d) flexion [F(3,29)=1.20, P<=.33, ηp2=.411, observed power=.29]; (e) scaption [F(3,29)=.98, P=.41, ηp2=.09, observed power=.24]; and (f) SPADI [F(3,29)=.35, P=.79, ηp2=.04, observed power=.11].
Next, univariate analyses were conducted on the percentage of change from pre- to post-treatment. Again, no statistically significant differences (P<.05) were found between the four groups. Table 2 shows the percentage of change, F value, effect size (i.e., omega squared), and observed power for each analysis. Examination of the percentages of change in Table 2 revealed a pattern. Specifically, the mobilization and the MWM groups both had a higher percentage of change on all three pain intensity measures than the control group and the exercise-only group. Also, all three of the intervention groups had a higher percentage of change than the control group on the SPADI. In regards to flexion and scaption AROM, the MWM group had a higher percentage of change than the other three groups and the effect size (i.e., omega squared) was .05; the mobilization group had the lowest percentage of change.
TABLE 2.
Percentage of change from pre- to post-treatment (sd) for the dependent variables for each group.
| DVs | Control (n=7) | Exercise (n=8) | MOB (n=9) | MWM (n=9) | Fa | ω2 | Power |
|---|---|---|---|---|---|---|---|
| VAS | 14.4 (119.8) | 20.8 (112.3) | 44.2 (38.6) | 55.2 (31.9) | .45 | .00 | .13 |
| Neer | 46.4 (49.5) | 44.0 (57.2) | 57.6 (38.7) | 66.5 (36.6) | .44 | .00 | .13 |
| HK | 11.2 (130.7) | 39.5 (54.9) | 52.1 (62.9) | 60.2 (43.3) | .60 | .00 | .16 |
| Flexion | 42.6 (15.8) | 27.6 (41.7) | −15.9 (116.6) | 46.7 (31.9) | 1.54 | .05 | .36 |
| Scaption | 29.8 (49.0) | 19.8 (70.3) | 2.5 (88.8) | 66.5 (28.1) | 1.60 | .05 | .37 |
| SPADI | 34.2 (58.9) | 61.6 (35.9) | 56.7 (29.8) | 55.5 (20.1) | .78 | .00 | .20 |
Abbreviations: MOB = mobilization group; MWM = mobilization-with-movement group; ω2 = Omega squared; VAS = visual analog scale; NEER = Neer impingement test; HK = Hawkins-Kennedy impingement test; SPADI = Shoulder Pain and Disability Index.
No statistically significant differences were found between groups.
Discussion
Repeated-measures analyses indicated that subjects in all four groups had significant decreases in pain, significant improvement in function, and significant increases in active ROM. Hence, time, exercise, and joint mobilization appeared to have an effect on recovery from shoulder impingement. No significant differences were found between the four groups on the dependent variables. However, results suggest that the two groups receiving manual therapy in combination with supervised exercise had a higher percentage of change on all three of the pain intensity measures (VAS, Neer, and Hawkins-Kennedy) compared to the supervised exercise group and the control group. Conroy and Hayes29 and Bang and Deyle28 found statistically significant reductions in pain measures with subjects who received joint mobilizations in combination with supervised exercise compared to those receiving exercise alone; however, no control group was used in either study. It is likely that the passive movement produced by both manual techniques resulted in pain reduction through activation of mechanoreceptors inhibiting nociceptive stimuli through the gate-control mechanism68,69 or through facilitation of synovial fluid nutrition70. In addition to these hypoalgesic effects, it can further be speculated that the mobilization and MWM techniques used in this study resulted in capsular stretching and/or restoration of normal glenohumeral arthrokinematics.
Results also revealed that all three intervention groups had a higher percentage of change than the control group on the SPADI function test, but these changes were not statistically significant. The only other study with which this finding can be roughly compared is the study by Bang and Deyle28 reporting that the group receiving mobilization techniques aimed at the shoulder, shoulder girdle, cervical spine, and/or upper thoracic spine had a statistically significant increase in function as assessed with a questionnaire modified from the Oswestry. Since the SPADI function test is based on shoulder pain with functional activities, it makes sense that interventions resulting in pain reduction would also result in an improved SPADI score.
A noteworthy finding in the present study is that the MWM group showed the highest percentage of change in decreasing pain and improving function from pre- to post-treatment. This may be attributed to the fact that the MWM technique is designed specifically for decreasing shoulder pain during active shoulder motion, and the amount of manual force applied is dependent on the ability of the technique to decrease pain with active movement. Studies using the MWM technique have reported improved pain-free motion, function, and/or pressure thresholds in patients with elbow lateral epicondylalgia71–73, de Quervain's74, and ankle sprains75,76. Paungmali et al73 found that performing MWM for chronic lateral epicondylalgia produced hypoalgesic effects and concurrent sympathetic nervous system effects including increased heart rate, blood pressure, and cutaneous activity. An additional explanation as to why the MWM technique was better than glenohumeral mobilizations in decreasing pain and improving function is that MWM has the additional benefit of being performed throughout AROM, which may engage additional proprioceptive tissues, such as the Golgi tendon organs activated by tendon stretch.
It is interesting to note that there was no statistically significant difference in pain measures and function between the two manual therapy groups. MWM was applied with a force not specified nor measured but with a sufficient posterior-inferior force needed for pain reduction throughout glenohumeral active ROM. In contrast, specific grades of force and direction of joint mobilizations were applied according to pre-testing assessment of capsular mobility and pain. Since theoretically any grade mobilization has analgesic effects, this may explain why both manual therapy groups improved in pain intensity and function, but there was no significant difference between these two groups.
Another possible explanation as to why there was little outcome difference between the two manual therapy groups is that all the joint mobilizations in the mobilization group were performed with the glenohumeral joint in the loose-packed position. Performing joint mobilizations at mid-range may not provide sufficient capsular stretch in subjects with capsular hypomobility to result in capsular elongation and associated mechanoreceptor activation. Studies have suggested that if there is restricted ROM, it is more effective to perform glenohumeral mobilization techniques at end-range, resulting in a more optimal stretch of the ROM limiting tissues (capsule and corresponding glenohumeral ligaments)77–80. Cadaver studies also suggest that therapists may need to use larger mobilization forces for a greater duration in order to achieve elastic region capsular stretch81.
Results also revealed no appreciable percent change difference between groups in scaption and flexion AROM measurements. This finding is consistent with Conroy and Hayes29, who reported no significant difference in AROM between subjects receiving glenohumeral joint mobilizations compared to a group receiving standardized therapy interventions. It is interesting to note that although the MWM group had a higher percentage of change in AROM than the other three groups, the mobilization group had the lowest change. If pain is the primary factor limiting glenohumeral AROM in individuals with shoulder impingement, the MWM technique may be more effective at decreasing pain, resulting in better ROM outcomes. Additionally, the MWM technique may result in improved ROM as it is performed during active shoulder motion. This finding might suggest that capsular hypomobility, which would be addressed with grade III-IV mobilizations, was not the primary factor limiting glenohumeral AROM.
Although the percentage of change from pre- to post-treatment revealed statistically significant patterns of improvement for all four groups, there were no significant differences between groups in any outcome measure. This is possibly attributed to the low power of the study due to small sample size, which can thwart the ability to detect differences and/or magnify the negative influence of a few subjects who demonstrate a poor response to treatment. Another consideration is the amount of activity performed immediately prior to the post-treatment measurements. As an example, two of the subjects who reported more pain at the end of the study admitted that in actuality, one to two days before their post-treatment session, their shoulders had felt “so good” that they had performed activities they had been unable to do before being involved in the study (cleaning out a garage and cleaning out closets). Thus, although their participation in the study resulted in a substantial reduction in their shoulder pain, this diminution in pain permitted them to perform high-function activities that they had previously been unable to perform and led to an increase in their shoulder pain immediately prior to the post-treatment assessment session. Again, given the small number of subjects in this study, these individuals had a negative influence on the results. This may be one reason why improvement as assessed with the SPADI may be more indicative of overall functional improvement as it is evaluated by the individual as a general or average over the previous few days.
While this pilot study provides preliminary data regarding the incorporation of glenohumeral joint mobilizations and a MWM technique to treat individuals with primary shoulder impingement, several limitations and considerations warrant further discussion:
The small participant sample size resulted in low statistical power. Decreased power magnifies the influence of a few subjects who demonstrate unusually poor or good response to treatment. For example, in the mobilization group, 2 subjects out of 9 had a decrease in AROM after intervention. With a small sample size, it is difficult to tell if these two are outliers or not.
Three subjects were removed from the study due to inability to obtain baseline measures and inappropriate behavior. This may potentially introduce an element of bias into the study design.
Another limitation in this study is that the four groups were unbalanced at pre-treatment. Specifically, the control group had more room for growth on almost all the variables compared to the other groups. A future recommendation is to conduct a stratified random assignment into groups after pre-treatment data are collected wherein subjects are assigned based on low, medium and high pain. Hence, each group would get equal numbers of subjects with low, medium, and high pain at pre-treatment.
The subject inclusion criteria may not have been specific enough. One consideration is that the investigators should have controlled for acuity or chronicity of symptoms. Also, all inclusion criteria dealt with pain, potentially leading to inaccurate physician diagnosis. As an example, patients with a pain-causing pathology in the rotator cuff that is etiologically unrelated to impingement may be misdiagnosed during a standardized provocation maneuver. Thus, improved sub-grouping of patients with impingement syndrome based on more restrictive objective findings is warranted, and more specific inclusion criteria for participation based on these measures would further strengthen the study. As examples, subjects should be selected who meet specific qualifications in regards to chronicity of symptoms and severity of symptoms as well as more specific objective findings leading to the shoulder impingement, including scapular dyskinesia or posterior capsule tightness. Since a method for measuring posterior capsule tightness has been found to be reliable and valid in a pilot study82, inclusion criteria could specify that subjects exhibit this objective finding, aiding in continuity of patient inclusion. One must consider, however, that admitting subjects for participation based on posterior capsule accessory hypomobility is difficult as little evidence exists that supports the reliability of grading capsular accessory motion63, and thus joint hypomobility cannot be conclusively determined.
Since this study lacks a long-term follow-up, it is suggested that future studies seek to provide this information.
Even with these limitations, the present study is unique in that this is the only controlled, double-blind clinical trial investigating the outcomes of using a MWM technique on the shoulder to treat shoulder impingement syndrome with a true control group that did not receive any physical therapy intervention. Also, the pilot study demonstrates the importance of using larger sample sizes in each group, establishing appropriate inclusionary and exclusionary criteria, and randomly assigning subjects to the groups after the pre-treatment data are collected.
Conclusion
In summary, the physical therapy interventions of glenohumeral mobilizations and MWM in combination with a supervised exercise program resulted in a higher percentage of change (but not statistically significant) from pre- to post-treatment in decreasing pain and improving function compared to the supervised exercise only and control groups. This study, albeit a pilot, provides preliminary evidence that these manual therapy techniques can be an important adjunct to supervised exercise in the treatment of individuals with shoulder impingement syndrome. However, other studies with larger sample sizes are needed to ascertain whether these trends in improvement are consistent and statistically significant.
Acknowledgements
The authors would like to acknowledge the following physical therapist students for their contributions to this study with planning and participating in data collection: Marc Phillips, Edward Zambrano, Allison Smith, Sarah Menning, Melissa Redmond, and Laura Morton.
Footnotes
Supported by the California State University, Northridge Research, Scholarship and Creative Activity Award. No authors had any potential for material gain in this study.
REFERENCES
- 1.Millar AL, Jasheway PA, Eaton W, Christensen F. A retrospective, descriptive study of shoulder outcomes in outpatient physical therapy. J Orthop Sports Phys Ther. 2006;36:403–414. doi: 10.2519/jospt.2006.2101. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8:151–158. doi: 10.1177/036354658000800302. [DOI] [PubMed] [Google Scholar]
- 3.Neer CS. Impingement lesions. Clin Orthop. 1983;173:70–77. [PubMed] [Google Scholar]
- 4.Neer CS. Anterior acromioplasty for the chronic impingement syndrome in the shoulder: A preliminary report. J Bone Joint Surg Am. 1972;54:41–50. [PubMed] [Google Scholar]
- 5.Leroux J-L, Codine P, Thomas E, Pocholle M, Mailhe D, Blotman F. Isokinetic evaluation of rotational strength in normal shoulders and shoulders with impingement syndrome. Clin Orthop. 1994;304:108–115. [PubMed] [Google Scholar]
- 6.McClure PW, Michener LA, Karduna AR. Shoulder function and 3-dimensional scapular kinematics in people with and without shoulder impingement syndrome. Phys Ther. 2006;86:1075–1090. [PubMed] [Google Scholar]
- 7.Bigliani LU, Levine WN. Current concepts review: Subacromial impingement syndrome. J Bone Joint Surg Am. 1997;79:1854–1868. [PubMed] [Google Scholar]
- 8.Hawkins RJ, Abrams JS. Impingement syndrome in the absence of rotator cuff tear (stages 1 and 2) Orthop Clin North Am. 1987;18:373–382. [PubMed] [Google Scholar]
- 9.Rahme H, Solem-Bertoft E, Westerberg C-E, Lundberg E, Sorensen S, Hilding S. The subacromial impingement syndrome. Scand J Rehabil Med. 1998;30:253–262. doi: 10.1080/003655098444002. [DOI] [PubMed] [Google Scholar]
- 10.Ogata S, Uhthoff HK. Acromial enthesopathy and rotator cuff tear: A radiologic and histologic postmortem investigation of the coracoacromial arch. Clin Orthop. 1990;254:39–48. [PubMed] [Google Scholar]
- 11.Harryman DT, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg Am. 1990;72-a:1334–1343. [PubMed] [Google Scholar]
- 12.Tyler TF, Nicholas Sj, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28:668–673. doi: 10.1177/03635465000280050801. [DOI] [PubMed] [Google Scholar]
- 13.Morrison DS, Greenbaum BS, Einhorn A. Shoulder impingement. Orthop Clin North Am. 2000;31:285–293. doi: 10.1016/s0030-5898(05)70148-6. [DOI] [PubMed] [Google Scholar]
- 14.Toivonen DA, Tuite MJ, Orwin JF. Acromial structure and tears of the rotator cuff. J Shoulder Elbow Surg. 1995;4:376–383. doi: 10.1016/s1058-2746(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 15.Tuite MJ, Toivonen DA, Orwin JF, Wright DH. Acromial angle on radiographs of the shoulder: Correlation with the impingement syndrome and rotator cuff tears. Am J Roentgenol. 1995;165:609–613. doi: 10.2214/ajr.165.3.7645479. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson GP, Goodman DA, Flatow EL, Bigliani LU. The acromion: Morphologic condition and age-related changes. A study of 420 scapulas. J Shoulder Elbow Surg. 1996;5:1–11. doi: 10.1016/s1058-2746(96)80024-3. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JS, Wright C, Green A. Subacromial impingement syndrome: The effect of changing posture on shoulder range of movement. J Orthop Sports Phys Ther. 2005;35:72–87. doi: 10.2519/jospt.2005.35.2.72. [DOI] [PubMed] [Google Scholar]
- 18.Borstad JD, Ludewig PM. The effect of long versus short pectoralis minor resting length on scapular kinematics in healthy individuals. J Orthop Sports Phys Ther. 2005;35:227–238. doi: 10.2519/jospt.2005.35.4.227. [DOI] [PubMed] [Google Scholar]
- 19.Endo K, Ikata T, Takeda Y. Radiographic assessment of scapular rotational tilt in chronic shoulder impingement syndrome. J Orthop Sci. 2001;6:3–10. doi: 10.1007/s007760170017. [DOI] [PubMed] [Google Scholar]
- 20.Ludewig PM, Cook TM. Translations of the humeral in persons with shoulder impingement symptoms. J Orthop Sports Phys Ther. 2002;32:248–259. doi: 10.2519/jospt.2002.32.6.248. [DOI] [PubMed] [Google Scholar]
- 21.Lukasiewicz AC, McClure P, Michener L, Pratt N, Sennett B. Comparison of 3-dimensional scapular position and orientation between subjects with and without shoulder impingement. J Orthop Sports Phys Ther. 1999;29:574–583. doi: 10.2519/jospt.1999.29.10.574. [DOI] [PubMed] [Google Scholar]
- 22.Lin L-J, Hanten WP, Olson SL, et al. Shoulder dysfunction assessment: Self-report and impaired scapular movements. Phys Ther. 2006;86:1065–1074. [PubMed] [Google Scholar]
- 23.Belling Sorensen AK, Jorgensen U. Secondary impingement in the shoulder. Scand J Med Sci Sports. 2000;10:266–278. doi: 10.1034/j.1600-0838.2000.010005266.x. [DOI] [PubMed] [Google Scholar]
- 24.Glousman RE. Instability versus impingement syndrome in the throwing athlete. Orthop Clin North Am. 1993;24:89–99. [PubMed] [Google Scholar]
- 25.Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80:276–291. [PubMed] [Google Scholar]
- 26.Paulson MM, Watnik NF, Dines DM. Coracoid impingement syndrome, rotator interval reconstruction, and biceps tenodesis in the overhead athlete. Orthop Clin North Am. 2001;32:485–493. doi: 10.1016/s0030-5898(05)70217-0. [DOI] [PubMed] [Google Scholar]
- 27.Ludewig PM, Borstad JD. Effects of a home exercise program on shoulder pain and functional status in construction workers. Occup Environ Med. 2003;60:841–849. doi: 10.1136/oem.60.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bang MD, Deyle GD. Comparison of supervised exercise with and without manual physical therapy for patients with shoulder impingement syndrome. J Orthop Sports Phys Ther. 2000;30:126–137. doi: 10.2519/jospt.2000.30.3.126. [DOI] [PubMed] [Google Scholar]
- 29.Conroy DE, Hayes KW. The effect of joint mobilization as a component of comprehensive treatment for primary shoulder impingement syndrome. J Orthop Sports Phys Ther. 1998;28:3–14. doi: 10.2519/jospt.1998.28.1.3. [DOI] [PubMed] [Google Scholar]
- 30.Desmeules F, Cote CH, Fremont P. Therapeutic exercise and orthopedic manual therapy for impingement syndrome: A systematic review. Clin J Sport Med. 2003;13:176–182. doi: 10.1097/00042752-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Ginn KA, Herbert RD, Khouw W, Lee R. A randomized, controlled clinical trial of a treatment for shoulder pain. Phys Ther. 1997;77:802–811. doi: 10.1093/ptj/77.8.802. [DOI] [PubMed] [Google Scholar]
- 32.Holmes CF, Fletcher JP, Blaschak MJ, Schenck RC. Management of shoulder dysfunction with alternative model of orthopaedic physical therapy intervention: A case report. J Orthop Sports Phys Ther. 1997;26:347–354. doi: 10.2519/jospt.1997.26.6.347. [DOI] [PubMed] [Google Scholar]
- 33.McClure P, Bialker J, Neff N, Williams G, Karduna A. Shoulder function and 3-dimensional kinematics in people with shoulder impingement syndrome before and after a 6-week exercise program. Phys Ther. 2004;84:832–848. [PubMed] [Google Scholar]
- 34.Brox JI, Gjengedal E, Uppheim G, et al. Arthroscopic surgery versus supervised exercises in patients with rotator cuff disease (stage II impingement syndrome): A prospective, randomized, controlled study in 125 patients with 2.5 year follow-up. J Shoulder Elbow Surg. 1999;8:102–111. doi: 10.1016/s1058-2746(99)90001-0. [DOI] [PubMed] [Google Scholar]
- 35.D'Hespeel CG. Current concepts: Rehabilitation of patients with shoulder impingement and tight posterior capsule. Orthop Pract. 2004;16:9–13. [Google Scholar]
- 36.Host HH. Scapular taping in the treatment of anterior shoulder impingement. Phys Ther. 1995;75:803–812. doi: 10.1093/ptj/75.9.803. [DOI] [PubMed] [Google Scholar]
- 37.Borstad JD. Resting position variables at the shoulder: Evidence to support a posture-impairment association. J Orthop Sports Phys Ther. 2006;86:549–557. [PubMed] [Google Scholar]
- 38.Winters JC, Jorritsma W, Groenier KH, Sobel JS, Meyboom-De Jong B, Arendzen HJ. Treatment of shoulder complaints in general practice: Long-term results of a randomized, single blind study comparing physiotherapy, manipulation, and corticosteroid injection. Br Med J. 1999;318:1395–1396. doi: 10.1136/bmj.318.7195.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sole G. A multi-structural approach to treatment of a patient with sub-acromial impingement: A case report. J Man Manip Ther. 2003;11:49–55. [Google Scholar]
- 40.Grossman MG, Tibone JE, McGarry MH, Schneider DJ, Veneziani S, Lee TQ. A cadaveric model of the throwing shoulder: A possible etiology of superior labrum anteriorto-posterior lesions. J Bone Joint Surg Am. 2005;87–A:824–831. doi: 10.2106/JBJS.D.01972. [DOI] [PubMed] [Google Scholar]
- 41.Mulligan BR. Manual Therapy “Nags”, “Snags”, “Mwm”, etc, 4th ed. Wellington, NZ: Plane View Series Ltd; 1999. [Google Scholar]
- 42.Exelby L. Peripheral mobilizations with movement. Man Ther. 1996;1:118–126. doi: 10.1054/math.1996.0259. [DOI] [PubMed] [Google Scholar]
- 43.DeSantis L, Hasson SM. Use of mobilization with movement in the treatment of a patient with subacromial impingement: A case report. J Man Manip Ther. 2006;14:77–87. [Google Scholar]
- 44.Mulligan BR. The painful dysfunctional shoulder: A new treatment approach using “mobilisation with movement”. New Zealand J Physiother. 2003;31:140–142. [Google Scholar]
- 45.Winters JC, Sobel JS, Groenier KH, Arendzen HJ, Meyboom-De Jong B. Comparison of physiotherapy, manipulation, and corticosteroid injection for treating shoulder complaints in general practice: Randomized, single blind study. Br Med J. 1997;314:1320–1325. doi: 10.1136/bmj.314.7090.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michener LA, Walsworth MK, Burnet EN. Effectiveness of rehabilitation for patients with subacromial impingement syndrome: A systematic review. J Hand Ther. 2004;17:152–164. doi: 10.1197/j.jht.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Williams JW, Holleman DR, Simel DL. Measuring shoulder function with the shoulder pain and disability index. J Rheumatol. 1995;22:727–732. [PubMed] [Google Scholar]
- 48.Cloke DJ, Lynn SE, Watson H, Steen IN, Purdy S, Williams JR. A comparison of functional, patient-based scores in subacromial impingement. J Shoulder Elbow Surg. 2005;14:380–384. doi: 10.1016/j.jse.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Roddey TS, Olson SL, Cook KF, Gartsman GM, Hanten W. Comparison of the University of California-Los Angeles Shoulder Scale and the Simple Shoulder Test with the Shoulder Pain and Disability Index: Singleadministration reliability and validity. Phys Ther. 2000;80:759–768. [PubMed] [Google Scholar]
- 50.Michener LA, Leggin BG. A review of selfreport scales for the assessment of functional limitation and disability of the shoulder. J Hand Ther. 2001;14:68–76. doi: 10.1016/s0894-1130(01)80036-3. [DOI] [PubMed] [Google Scholar]
- 51.Van der Heijden GJMG, van der Windt DAWM, de Winter AF. Physiotherapy for patients with soft tissue disorders: A systematic review of randomized clinical trials. Br Med J. 1997;315:25–30. doi: 10.1136/bmj.315.7099.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valadie AL, Jobe CM, Pink MM, Ekman EF, Jobe FW. Anatomy of provocative tests for impingement syndrome of the shoulder. J Shoulder Elbow Surg. 2000;9:36–46. doi: 10.1016/s1058-2746(00)90008-9. [DOI] [PubMed] [Google Scholar]
- 53.Calis M, Akgun K, Birtane M, Karacan I, Calis H, Tuzun F. Diagnostic values of clinical diagnostic tests in subacromial impingement syndrome. Ann Rheum Dis. 2000;59:44–47. doi: 10.1136/ard.59.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacDonald PB, Clark P, Sutherland K. An analysis of the diagnostic accuracy of the Hawkins and Neer subacromial impingement signs. J Shoulder Elbow Surg. 2000;9:299–301. doi: 10.1067/mse.2000.106918. [DOI] [PubMed] [Google Scholar]
- 55.Park HB, Yokota A, Gill H, El Rassi G, McFarland E. Diagnostic accuracy of clinical tests for the different degrees of subacromial impingement syndrome. J Bone Joint Surg Am. 2005;87:1446–1455. doi: 10.2106/JBJS.D.02335. [DOI] [PubMed] [Google Scholar]
- 56.Norkin CC, White DJ. Measurement of Joint Motion: A Guide to Goniometry, 3rd ed. Philadelphia, PA: F. A. Davis; 2003. [Google Scholar]
- 57.Boone DC, Azen SP, Lin C-M, Spence C, Baron C, Lee L. Reliability of goniometric measurements. Phys Ther. 1978;58:1355–1360. doi: 10.1093/ptj/58.11.1355. [DOI] [PubMed] [Google Scholar]
- 58.Riddle DL, Rothstein JM, Lamb RL. Goniometric reliability in a clinical setting: Shoulder measurements. Phys Ther. 1987;67:668–673. doi: 10.1093/ptj/67.5.668. [DOI] [PubMed] [Google Scholar]
- 59.Mayerson NH, Milano RA. Goniometric measurement reliability in physical medicine. Arch Phys Med Rehabil. 1984;65:92–94. [PubMed] [Google Scholar]
- 60.Marx RG, Bombardier C, Wright JG. What do we know about the reliability and validity of physical examination tests used to examine the upper extremity? J Hand Surg. 1999;24a:185–193. doi: 10.1053/jhsu.1999.jhsu24a0185. [DOI] [PubMed] [Google Scholar]
- 61.Kaltenborn FM. Manual Mobilization of the Joints: The Kaltenborn Method of Joint Examination and Treatment, 5th ed. Vol. 1. Minneapolis, MN: OPTP; 1999. [Google Scholar]
- 62.Paris SV. Mobilization of the spine. Phys Ther. 1979;59:988–995. doi: 10.1093/ptj/59.8.988. [DOI] [PubMed] [Google Scholar]
- 63.Gonnella C, Paris SV, Kutner M. Reliability in evaluating passive intervertebral motion. Phys Ther. 1982;62:436–444. doi: 10.1093/ptj/62.4.436. [DOI] [PubMed] [Google Scholar]
- 64.Binkley J, Stratford PW, Gill C. Interrater reliability of lumbar accessory motion mobility testing. Phys Ther. 1995;75:786–795. doi: 10.1093/ptj/75.9.786. [DOI] [PubMed] [Google Scholar]
- 65.Maher C, Adams R. Reliability of pain and stiffness assessments in clinical manual lumbar spine examination. Phys Ther. 1994;74:801–809. doi: 10.1093/ptj/74.9.801. [DOI] [PubMed] [Google Scholar]
- 66.Matyas T, Bach T. The reliability of selected techniques in clinical arthrometrics. Aust J Physiother. 1985;31:175–199. doi: 10.1016/S0004-9514(14)60633-4. [DOI] [PubMed] [Google Scholar]
- 67.Jull G, Bogduk N, Marsland A. The accuracy of manual diagnosis for cervical zygapophysial joint pain syndromes. Med J Aust. 1988;148:233–236. doi: 10.5694/j.1326-5377.1988.tb99431.x. [DOI] [PubMed] [Google Scholar]
- 68.Melzack R, Wall PD. Pain mechanisms: A new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 69.Wall PD. The gate control theory of pain mechanisms: An examination and a restatement. Brain. 1978;101:1–18. doi: 10.1093/brain/101.1.1. [DOI] [PubMed] [Google Scholar]
- 70.Threlkeld AJ. The effects of manual therapy on connective tissue. Phys Ther. 1992;72:893–902. doi: 10.1093/ptj/72.12.893. [DOI] [PubMed] [Google Scholar]
- 71.Abbott JH, Patla CE, Jensen RH. The initial effects of an elbow mobilization with movement technique on grip strength in subjects with lateral epicondylalgia. Man Ther. 2001;6:163–169. doi: 10.1054/math.2001.0408. [DOI] [PubMed] [Google Scholar]
- 72.McLean S, Naish R, Reed L, Urry S, Vicenzino B. A pilot study of the manual force levels required to produce manipulationinduced hypoalgesia. Clin Biomech. 2002;17:304–308. doi: 10.1016/s0268-0033(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 73.Paungmali A, O'Leary S, Souvlis T, Vicenzino B. Hypoalgesic and sympathoexcitatory effects of mobilization with movement for lateral epicondylalgia. Phys Ther. 2003;83:374–383. [PubMed] [Google Scholar]
- 74.Backstrom KM. Mobilization with movement as an adjunct intervention in a patient with complicated DeQuervain's tenosynovitis: A case report. J Orthop Sports Phys Ther. 2002;32:86–97. doi: 10.2519/jospt.2002.32.3.86. [DOI] [PubMed] [Google Scholar]
- 75.Collins N, Teys P, Vicenzino B. The initial effects of a Mulligan's mobilization with movement technique on dorsiflexion and pain in subacute ankle sprains. Man Ther. 2004;9:77–82. doi: 10.1016/S1356-689X(03)00101-2. [DOI] [PubMed] [Google Scholar]
- 76.Vicenzino B, Branjerdporn M, Teys P, Jordan K. Initial changes in posterior talar glide and dorsiflexion of the ankle after mobilization with movement in individuals with recurrent ankle sprain. J Orthop Sports Phys Ther. 2006;36:464–471. doi: 10.2519/jospt.2006.2265. [DOI] [PubMed] [Google Scholar]
- 77.Hsu A-T, Hedman T, Chang JH, et al. Changes in abduction and rotation range of motion in response to simulated dorsal and ventral translational mobilization of the glenohumeral joint. Phys Ther. 2002;82:544–556. [PubMed] [Google Scholar]
- 78.Hsu A-T, Ho L, Ho S. Joint position during anterior-posterior glide mobilization: Its effect on glenohumeral abduction range of motion. Arch Phys Med Rehabil. 2000;81:210–214. doi: 10.1016/s0003-9993(00)90143-6. [DOI] [PubMed] [Google Scholar]
- 79.Hsu A-T, Ho L, Ho S, Hedman T. Immediate response of glenohumeral abduction range of motion to a caudally directed translational mobilization: A fresh cadaver simulation. Arch Phys Med Rehabil. 2000;81:1511–1516. doi: 10.1053/apmr.2000.9389. [DOI] [PubMed] [Google Scholar]
- 80.O'Brien SJ, Schwartz RS, Warren RF, Torzilli PA. Capsular restraints to anterior-posterior motion of the abducted shoulder: A biomechanical study. J Shoulder Elbow Surg. 1995;4:298–308. doi: 10.1016/s1058-2746(05)80024-2. [DOI] [PubMed] [Google Scholar]
- 81.Hsu A-T, Ho L, Chang JH, Chang G-L, Hedman T. Characterization of tissue resistance during a dorsally directed translational mobilization of the glenohumeral joint. Arch Phys Med Rehabil. 2002;83:360–366. doi: 10.1053/apmr.2002.30621. [DOI] [PubMed] [Google Scholar]
- 82.Tyler TF, Roy T, Nicholas SJ, Gleim GW. Reliability and validity of a new method of measuring posterior shoulder tightness. J Orthop Sports Phys Ther. 1999;29:262–274. doi: 10.2519/jospt.1999.29.5.262. [DOI] [PubMed] [Google Scholar]