Abstract
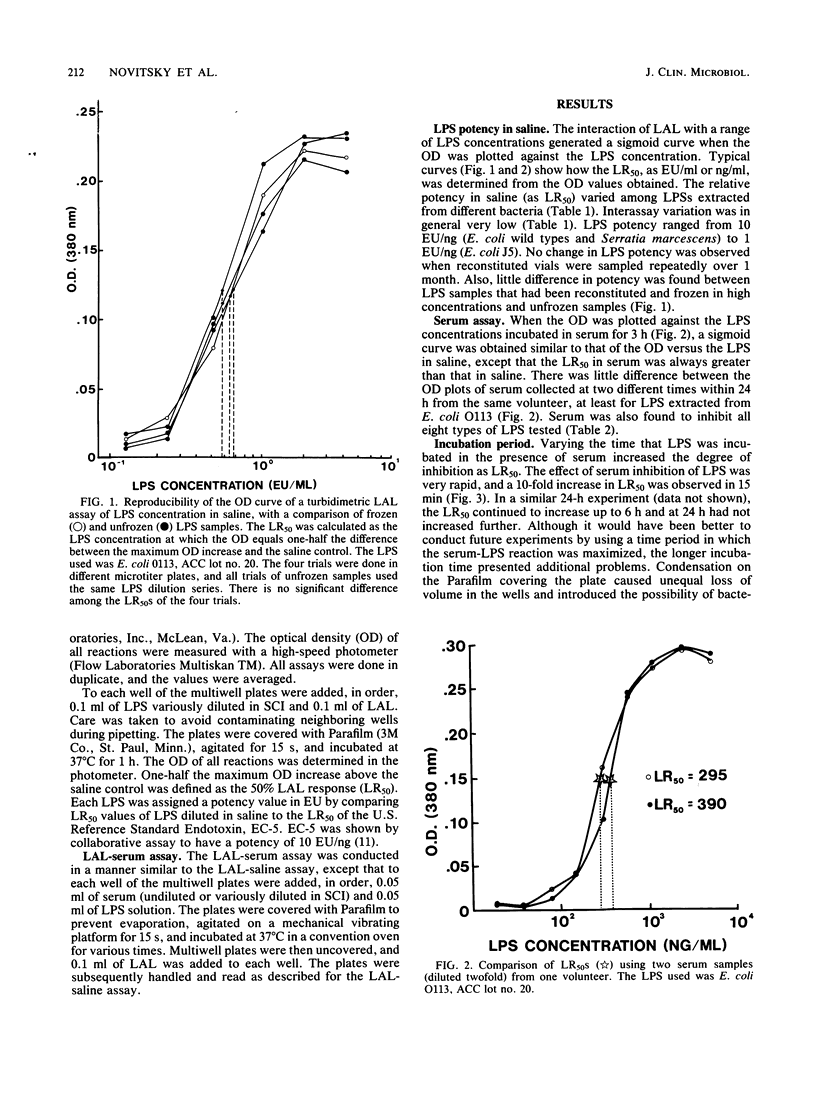
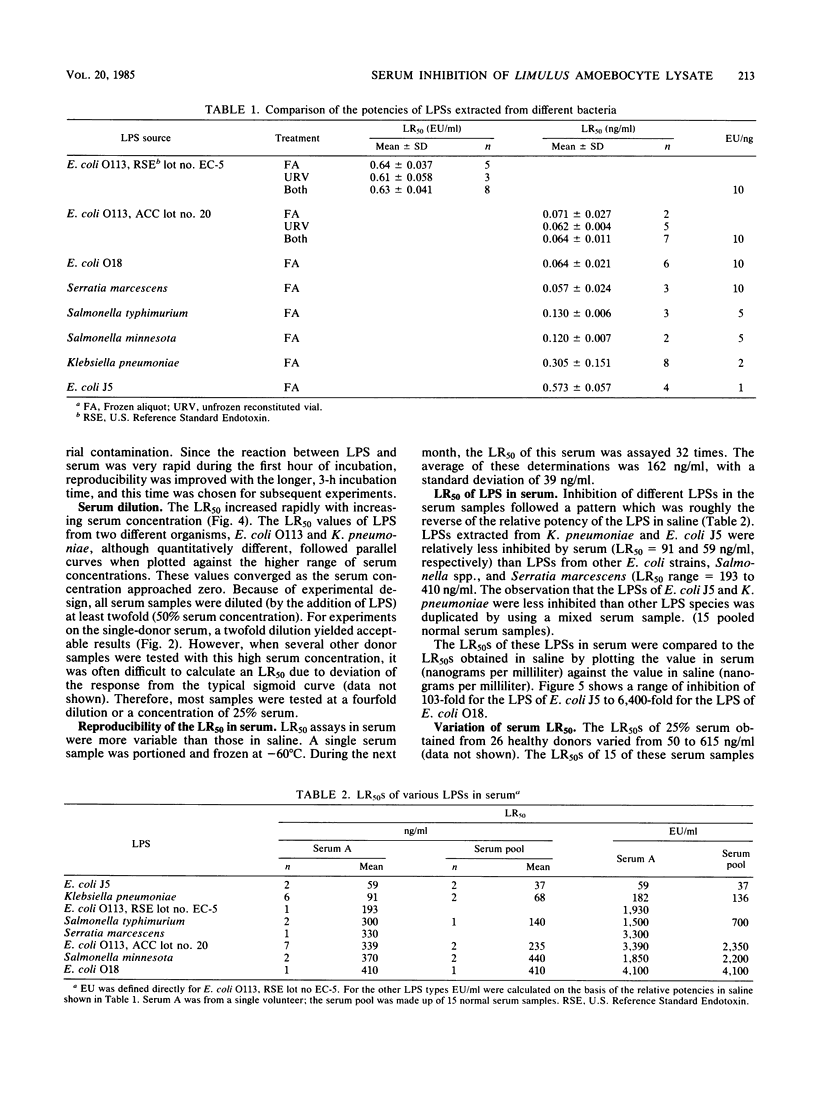
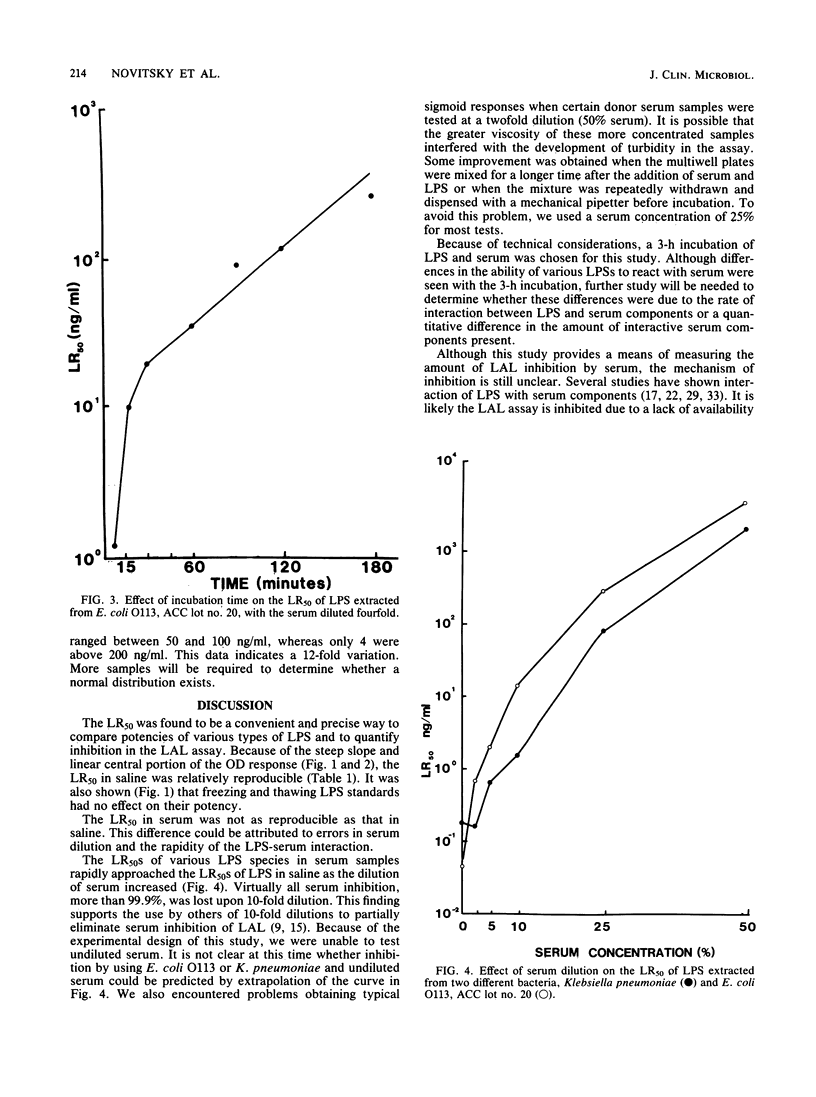
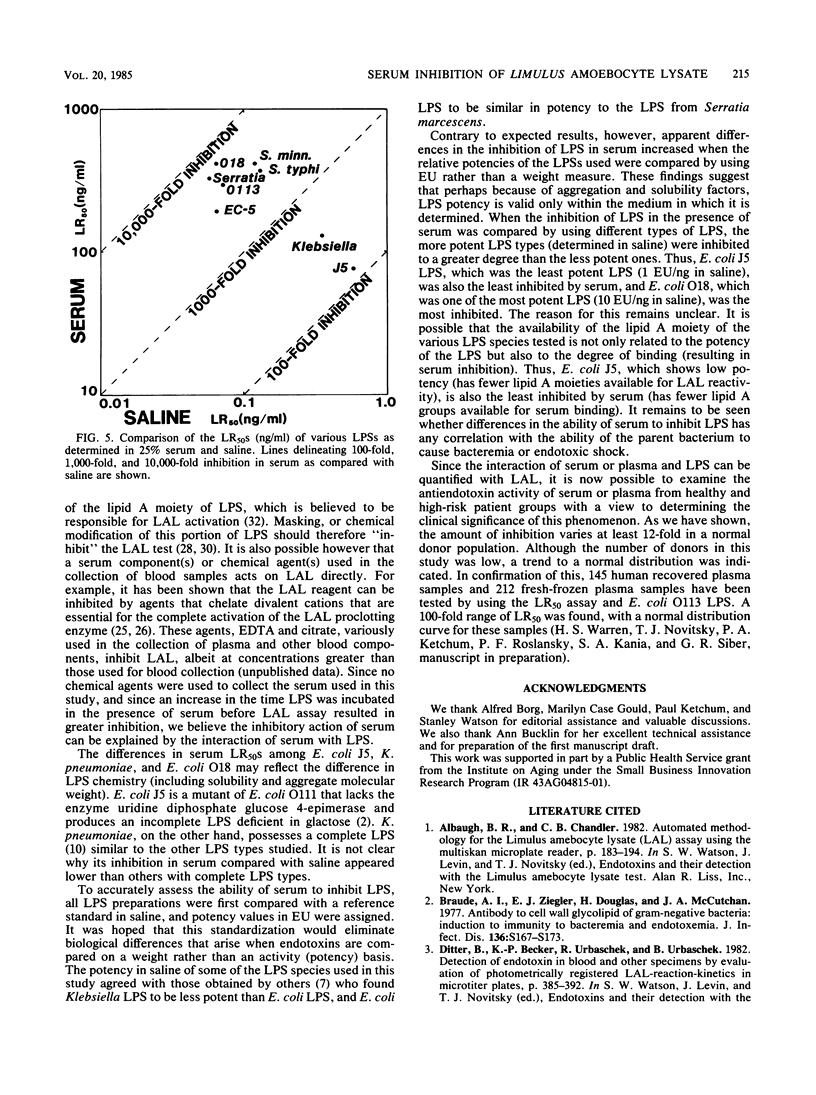
This study describes a method to quantify the inhibition of lipopolysaccharide (LPS) activity by serum with a turbidimetric Limulus amoebocyte lysate assay. Assays were performed in multiwell microplates, and turbidity was measured as the optical density at 380 nm with a microplate spectrophotometer. LPS potency was measured as the 50% maximal Limulus amoebocyte response (LR50) of LPS diluted with saline. By comparing LR50s in saline, LPSs from various species of bacteria were standardized against the U.S. Reference Standard Endotoxin, lot EC-5. The potency of Escherichia coli O113 and O18 and Serratia marcescens LPSs was found to be equal to that of the reference standard EC-5, whereas LPSs from two salmonella species were half as potent. The least potent LPSs tested, obtained from Klebsiella pneumoniae and E. coli rough mutant J5, were 5- and 10-fold less potent, respectively, than EC-5. As a measure of inhibition, the LR50 of LPS in serum was compared to the LR50 of LPS in saline. Serum inhibited the potency of LPS 103- to 6,400-fold compared with saline. A positive correlation was found between standardized potency in saline and serum inhibition of the various LPSs tested. Thus, LPSs from E. coli O113, O18, and EC-5 and S. marcescens, which exhibited the highest potency in saline, were inhibited the most by serum. Likewise, E. coli J5 and K. pneumoniae LPSs, which were the least potent tested, were the least inhibited. The degree of inhibition of all types of LPS tested increased with increasing serum concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albaugh B. R., Chandler C. B. Automated methodology for the Limulus amebocyte lysate (LAL) assay using the multiskan microplate reader. Prog Clin Biol Res. 1982;93:183–194. [PubMed] [Google Scholar]
- Braude A. I., Ziegler E. J., Douglas H., McCutchan J. A. Antibody to cell wall glycolipid of Gram-negative bacteria: induction of immunity to bacteremia and endotoxemia. J Infect Dis. 1977 Aug;136 (Suppl):S167–S173. doi: 10.1093/infdis/136.supplement.s167. [DOI] [PubMed] [Google Scholar]
- Ditter B., Becker K. P., Urbaschek R., Urbaschek B. Detection of endotoxin in blood and other specimens by evaluation of photometrically registered LAL-reaction-kinetics in microtiter plates. Prog Clin Biol Res. 1982;93:385–392. [PubMed] [Google Scholar]
- DuBose D., Lemaire M., Brown J., Wolfe D., Hamlet M. Survey for positive Limulus amoebocyte lysate test in plasma from humans and common research animals. J Clin Microbiol. 1978 Feb;7(2):139–141. doi: 10.1128/jcm.7.2.139-141.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elin R. J., Robinson R. A., Levine A. S., Wolff S. M. Lack of clinical usefulness of the limulus test in the diagnosis of endotoxemia. N Engl J Med. 1975 Sep 11;293(11):521–524. doi: 10.1056/NEJM197509112931102. [DOI] [PubMed] [Google Scholar]
- Feldman S., Pearson T. A. The Limulus test and gram-negative bacillary sepsis. Am J Dis Child. 1974 Aug;128(2):172–174. doi: 10.1001/archpedi.1974.02110270046009. [DOI] [PubMed] [Google Scholar]
- Firca J. R., Rudbach J. A. Reference endotoxin: a practical rationale. Prog Clin Biol Res. 1982;93:121–300. [PubMed] [Google Scholar]
- Fossard D. P., Kakkar V. V., Elsey P. A. Assessment of limulus test for detecting endotoxaemia. Br Med J. 1974 Jun 1;2(5917):465–468. doi: 10.1136/bmj.2.5917.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. A., Reller L. B., Wang W. L. Limulus amebocyte lysate test in neonates. Am J Clin Pathol. 1976 Dec;66(6):1012–1015. doi: 10.1093/ajcp/66.6.1012. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Ohta M., Mori M., Nakashima I., Kato N. The Klebsiella O3 lipopolysaccharide isolated from culture fluid: structure of the polysaccharide moiety. Microbiol Immunol. 1983;27(8):683–694. doi: 10.1111/j.1348-0421.1983.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Hochstein H. D., Mills D. F., Outschoorn A. S., Rastogi S. C. The processing and collaborative assay of a reference endotoxin. J Biol Stand. 1983 Oct;11(4):251–260. doi: 10.1016/s0092-1157(83)80013-4. [DOI] [PubMed] [Google Scholar]
- Homma R., Kuratsuka K., Akama K. Application of the LAL test and the chromogenic substrate test to the detection of endotoxin in human blood products. Prog Clin Biol Res. 1982;93:301–317. [PubMed] [Google Scholar]
- Levin J., Poore T. E., Young N. S., Margolis S., Zauber N. P., Townes A. S., Bell W. R. Gram-negative sepsis: detection of endotoxemia with the limulus test. With studies of associated changes in blood coagulation, serum lipids, and complement. Ann Intern Med. 1972 Jan;76(1):1–7. doi: 10.7326/0003-4819-76-1-1. [DOI] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- Levin J., Tomasulo P. A., Oser R. S. Detection of endotoxin in human blood and demonstration of an inhibitor. J Lab Clin Med. 1970 Jun;75(6):903–911. [PubMed] [Google Scholar]
- Martinez L. A., Quintiliani R., Tilton R. C. Clinical experience on the detection of endotoxemia with the limulus test. J Infect Dis. 1973 Jan;127(1):102–105. doi: 10.1093/infdis/127.1.102. [DOI] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Dietschy J. M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect Immun. 1981 Dec;34(3):835–843. doi: 10.1128/iai.34.3.835-843.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold R. B., Fine J. A technique for quantitative measurement of endotoxin in human plasma. Proc Soc Exp Biol Med. 1971 May;137(1):334–340. doi: 10.3181/00379727-137-35572. [DOI] [PubMed] [Google Scholar]
- Rudbach J. A., Akiya F. I., Elin R. J., Hochstein H. D., Luoma M. K., Milner E. C., Milner K. C., Thomas K. R. Preparation and properties of a national reference endotoxin. J Clin Microbiol. 1976 Jan;3(1):21–25. doi: 10.1128/jcm.3.1.21-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKARNES R. C., ROSEN F. S., SHEAR M. J., LANDY M. Inactivation of endotoxin by a humoral component. II. Interaction of endotoxin with serum and plasma. J Exp Med. 1958 Nov 1;108(5):685–699. doi: 10.1084/jem.108.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifele D. W., Melton P., Whitchelo V. Evaluation of the Limulus test for endotoxemia in neonates with suspected sepsis. J Pediatr. 1981 Jun;98(6):899–903. doi: 10.1016/s0022-3476(81)80582-3. [DOI] [PubMed] [Google Scholar]
- Skarnes R. C. Host defense against bacterial endotoxemia: mechanism in normal animals. J Exp Med. 1970 Aug 1;132(2):300–316. doi: 10.1084/jem.132.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumacher R. J., Kovnat M. J., McCabe W. R. Limitations of the usefulness of the Limulus assay for endotoxin. N Engl J Med. 1973 Jun 14;288(24):1261–1264. doi: 10.1056/NEJM197306142882402. [DOI] [PubMed] [Google Scholar]
- Sullivan J. D., Jr, Watson S. W. Inhibitory effect of heparin on the Limulus test for endotoxin. J Clin Microbiol. 1976 Aug;2(2):151–151. [PMC free article] [PubMed] [Google Scholar]
- Thomas L. L., Sturk A., Kahlé L. H., ten Cate J. W. Quantitative endotoxin determination in blood with a chromogenic substrate. Clin Chim Acta. 1981 Oct 8;116(1):63–68. doi: 10.1016/0009-8981(81)90169-8. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Harrison S. J. Limulus amebocyte lysate - a means to monitor inactivation of lipopolysaccharide. Prog Clin Biol Res. 1979;29:367–378. [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster C. J. Principles of a quantitative assay for bacterial endotoxins in blood that uses Limulus lysate and a chromogenic substrate. J Clin Microbiol. 1980 Nov;12(5):644–650. doi: 10.1128/jcm.12.5.644-650.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]
- Young L. S. Opsonizing antibodies, host factors, and the limulus assay for endotoxin. Infect Immun. 1975 Jul;12(1):88–92. doi: 10.1128/iai.12.1.88-92.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


