Abstract
This anatomical study describes the morphology of the human lumbar multifidus muscle through gross and chemical dissection of fresh cadavers. Previous morphological descriptions were analyzed with regard to fascicular divisions and cleavage planes. Gross dissection was performed on the lumbar multifidus of four fresh adult human cadavers and four preserved cadavers. Gradual chemical dissection using nitric or formic acid was used for connective tissue digestion to enhance the documentation of muscle fiber direction. Results revealed four distinct layers of the lumbar multifidus separated by cleavage planes. The superficial layer was more extensive than previously described with bony attachments at both the origin and insertion at several vertebral levels. The attachments of the second through fourth layers differed in that distinct cleavage planes between the various fascicles were not found with chemical dissection. The lumbar multifidus has a multipennate fiber arrangement, and the fascicles between the various layers inter-attach. Inter-fascicle attachment differs with the description by Macintosh et al of distinct cleavage planes between and within the fascicles of each layer. Accurate anatomical knowledge of the fascicles of the lumbar multifidus is integral for defining the actions of this complex lumbar muscle. This study supports the clinical belief that the multifidus has a significant role in control and stabilization of the lumbar spine in multiple planes of action. The multipennate arrangement of this muscle with fascicular inter-attachment supports the clinical premise that the multifidus is activated in a variety of positions and can potentially produce and mediate intersegmental mobility and provide proprioception.
KEYWORDS: Chemical Dissection, Lumbar Region, Multifidus, Muscles
The morphology of the lumbar multifidus muscle has been described in anatomical texts and in the literature and is of particular importance because of its mechanical functions in the lumbar spine with regard to movement and stabilization. The multifidus is the most medial paraspinal muscle lying over the zygaphophyseal joints and lateral to the spinous processes (SP) of the lumbar spine (Figure 1). It is the largest paraspinal muscle to cross directly over the lumbosacral junction. Attempts to understand the basic function of the lumbar spine and appropriately treat lumbar spine dysfunction have stimulated studies of the multifidus in the areas of electromyography, histology, biomechanics, morphology, and imaging.
FIGURE 1.
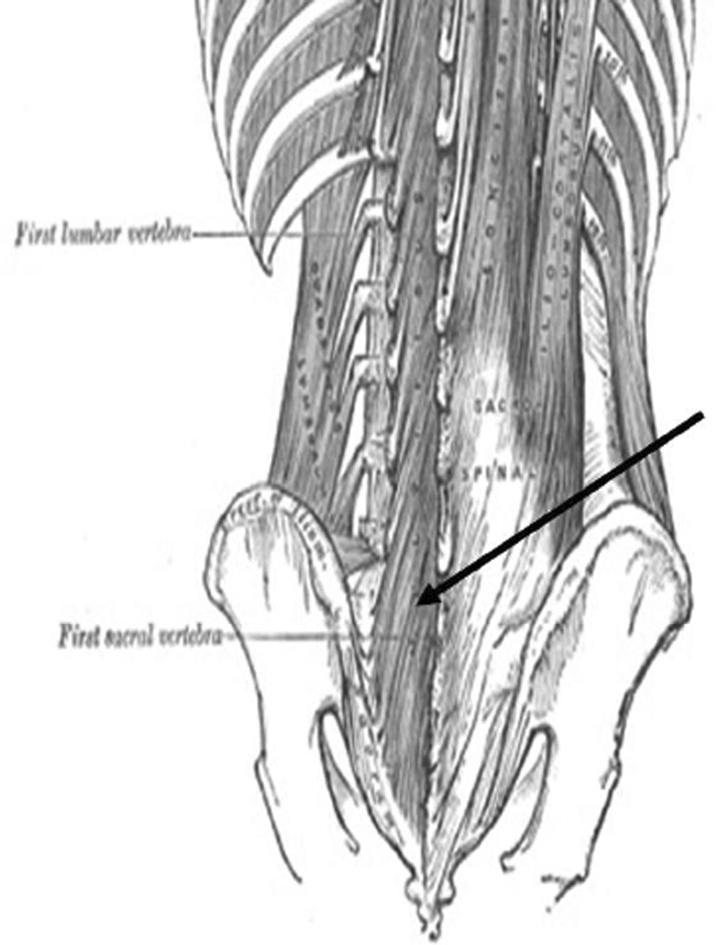
Multifidus as depicted in Gray's Anatomy (1918).
An accurate anatomical description of the multifidus is necessary for biomechanical analysis, rehabilitation, and electromyographic (EMG) evaluation of the lumbar spine. An accurate description of the attachment sites and orientation of its fibers is needed to determine the biomechanical function of the muscle. Understanding the action of the multifidus is inherent to the successful rehabilitation of those with lumbar spine disorders. Associated multifidus dysfunction has been found in patients with low back pain or disc disease1,2. A valid depiction of this complex muscle is also needed for needle placement during EMG evaluation and the interpretation of this EMG data.
The roles of the multifidus have been described with much variation. The textbooks of anatomy by Gray and by Cunningham describe it as a contralateral rotator of the spine3,4. Gray's anatomy also describes it as a lateral flexor of the spine. Studies by Morris have described it as a contralateral rotator5,6, while Donisch and Basmajian have described the multifidus as an ipsilateral rotator of the lumbar spine7. Macintosh et al8 disputed the action of the multifidus as a primary ipsilateral rotator of the spine because of the oblique orientation of the muscle fibers and the mechanical limitations of this movement due to impaction of the ipsilateral zygapophyseal joints. They suggested that the primary action of the multifidus is to produce posterior sagittal rotation and that axial rotation is a minor secondary action of this muscle8. EMG studies have supported the action of the multifidus as a rotator but have not specifically examined its possible role as a posterior sagittal rotator5,7,9. EMG studies have attributed a stabilizing role to the multifidus during rotation10. Some authors supported the differentiation of the deep versus superficial fibers of the multifidus with various activities11. These varied actions purported for the multifidus support the need for further study of the morphology of this muscle.
Descriptions of the morphology of the multifidus vary in terms of the number and attachments of the fascicles as well as in the nature of the fascicular differentiation (Table 1). The purpose of this study was to review the previous descriptions of the multifidus morphology based on gross and gradual chemical dissection in fresh cadavers and relate the findings to the function of this important muscle.
TABLE 1.
Descriptions of the morphology of the multifidus.
| AUTHOR | DESCRIPTION (Based on Blunt Dissection) |
|---|---|
| Lewin et al19 | Origin: Mamillary process and superior articular process (SAP). Some fibers merge with the capsule of the facet joint they pass medially and superiorly covering the lateral part of the joint from which they originate. Insertion: Spinous process (SP) of the next higher vertebral level. |
| Gray3 | Fasciculi of varying length; three layers superficial, middle, and deep that extend from four (superficial) to one vertebral level (deep). |
| Paris20 | Bipennate origin and insertion. |
| Origin: Tendinous slip from the mamillary process lateral and inferior to the facet joint, facet joint. | |
| Passes upward and gains muscle attachment. | |
| Insertion: Tendinous slip at the posterior inferior aspect of the SP. | |
| Valencia & Munro21 | Tree layers, regular pattern of attachment. |
| Origin: Posterior-inferior corner of the SP vertebrae as a round tendon at a prominent tubercle and adjacent areas on the SP. | |
| Insertion: Mamillary process of the vertebrae below its origin and the sacrum and ilium at the posterior superior iliac spine (PSIS). | |
| Macintosh et al12 | Five bands divided by distinct cleavage planes. One muscle band at each of the five lumbar vertebral levels. |
| Each band is divided into several fascicles distinguished based on their caudal attachments. | |
| Origin: The deepest fascicles are the shortest and originate on the lamina. All the other fascicles arise from the SP and are continuous with each other from a common tendon that attaches to the caudodorsal corner of the SP. | |
| Insertion: Mamillary processes two to five levels caudal of origin as well as PSIS, posterior sacraliliac joint (SIJ) ligament and sacrum of origin as well as PSIS, posterior SIJ ligament, and sacrum. | |
| De Foa et al22 | Assessed fiber angle for surface electromyographic placement and found fibers run parallel to a line between the PSIS and the L1-L2 interspinous space. |
| Porterfeld & DeRosa23 | Large thick mass arising from the dorsal surface of the sacrum, the aponeruosis of the erector spinae muscle, and the medial surface of the PSIS and the posterior sacroiliac ligaments as well as from the mamillary processes. |
| Biedermann et al24 | Assessed fiber angle including six female cadavers. Found muscle fibers run parallel to the lines described by De Foa et al22; however, a slight increase in obliquity was noted in the females. |
| Shindo25 | Shindo studied 10 adult cadavers and 10 fetuses from 5 to 10 months old. Tree separate layers running from the SP to the facet, mamillary process, and accessory process. No Specifics were mentioned regarding division of layers and Specific attachment sites, but the author did note slight variation in the innervation of the multifidus. Variation was noted in only five fascicles. In these fascicles, the medial branch of the dorsal ramus of a lumbar nerve entered the multifidus muscle that originated from the SP of the lumbar vertebra one level below. |
| Hollinshead & Rosse26 | No clear divisions; single muscle consisting of muscle bundles passing two to four levels in length. |
| Jemmett et al13 | Preliminary study of a single embalmed adult male cadaver using blunt dissection. The authors described five fascicles with each arising from a single SP of the lumbar spine by a common tendon. The first two of the fascicles arose in a bipennate arrangement from the common tendon. The origin at the tip of a SP and insertion of the first fascicle at the mamillary process of L4, the lamina, and the zygoapophyseal joint capsule. The second fascicle originated from the same deep and superficial attachments at the L2 and L3 SPs and inserted into the PSIS and adjacent area near the SIJ. Fascicles 3–5 originated from the base to the tip of their respective SPs and inserted on the posterior surface of the sacrum. A deeper laminar layer was also described with attachments originating from the mamillary process of the superior vertebrae and inserting on to the joint capsule of the inferior vertebrae. |
Review
Previous descriptions of multifidus morphology are presented in Table 1. The most extensive and recent research has been presented by Macintosh et al12 and Jemmett et al13. Macintosh et al12 described the fibers of the multifidus as being arranged in a spino-transverse pattern rather than the more traditional description of transverse-spinal. Using gross dissection methods on preserved cadavers, they found that the multifidus is divided into five bands by distinct cleavage planes12. There is one muscle band at each of the five lumbar vertebral levels. Each band is divided into several fascicles that can be distinguished based on their caudal attachments. The deepest fascicles are the shortest and originate on the lamina. All the other fascicles arise from the SP and originate from a common tendon that attaches to the caudal and most dorsal aspect of the SP.
Macintosh et al12 reported variations in the pattern at each level, but the archetypical pattern of fascicles is present at the L1 vertebral level. Their description consists of a series of repeating fascicles that originate from the SP and laminae and insert caudally in consistent patterns. The laminar layer is made up of the deepest fascicles, which insert on the mamillary process of the vertebrae two levels caudally. The fascicles from the lateral surface of the SP insert onto the mamillary process of the vertebrae three levels caudal. The fascicles from the tubercle of the SP insert onto the mamillary processes of the vertebrae four and five levels caudal. Another long slender fascicle arises from the common tendon and inserts into the caudomedial aspect of the posterior superior iliac spine (PSIS). Some of the more superficial fibers arising from the common tendon attach to the deep surface of the erector spinae aponeurosis over the sacrum. The fibers from the band at the L2 vertebral level demonstrate a pattern similar to that of the L1 vertebrae with the exception that the three groups of fibers that extend down to the mamillary process will attach to the mamillary processes of L5 and S1 and then to the posterior sacroiliac ligament and medial to the PSIS12.
Macintosh et al12 also described fascicles from the L3 vertebral level again including the laminar fascicles inserting two levels caudal on the mamillary process of L5. The fascicle from the lateral surface of the SP inserts onto the S1 mamillary process while the fascicles from the common tendon attach to the posterior sacroiliac ligament and across the lateral third of the upper three to four sacral segments and PSIS. The most superficial fibers from the SP also insert into the deep surface of the erector spinae aponeurosis12.
The fascicles from the L4 vertebral level include the laminar fascicles that insert on the mamillary process of S1. The fascicles from the common tendon of the SP insert on the posterior sacroiliac ligament and laterally on the upper four levels of the sacrum. The fascicles from the laminae and lateral SP of the L5 level insert on the upper medial corner of the dorsal surface of the sacrum. The fibers from the tip of the L5 SP insert on the medial third of the upper three sacral levels.
The homogeneous appearance of the multifidus is created by the overlapping of the fibers in a systematic pattern from lateral to medial and from deep to superficial. The description of the multifidus as a spino-transverse muscle is justified by these authors according to the structural pattern and innervation of the fascicles.
Jemmett et al13 performed blunt dissection of a single embalmed adult male cadaver to analyze the group architecture of the lumbar musculature as it applies to segmental motion. They described five fascicles, with each arising from a single SP of the lumbar spine by a common tendon. The first two fascicles arose in a bipennate arrangement from the common tendon. These fascicles were made of a superficial and deep portion that attached, respectively, to the caudolateral tip of a SP and the caudolateral base of the next lower SP. The insertion of the first fascicle was described as the mamillary process of L4, the lamina, and the zygapophyseal joint capsule. The second fascicle originated from the same deep and superficial attachments at the L2 and L3 SPs. The second fascicle inserted into the PSIS and adjacent area near the sacroiliac joint (SIJ). Fascicles 3–5 originated from the base to the tip of their respective SPs and inserted on the posterior surface of the sacrum with fascicle 3 occupying the largest and most lateral area of insertion and fascicle 5 the smallest and most medial. A deeper laminar layer was also described with attachments originating from the mamillary process of the superior vertebrae and inserting onto the joint capsule of the vertebrae.
The innervation of the multifidus is important to consider in this review because it has established the categorization scheme for the multifidus as either spino-transverse or transverse-spinal. Bogduk et al14 have described the innervation of this muscle as unisegmental. Muscle fibers that attach to the vertebral body of L1 will be innervated by the L1 segment. In that study, the authors reported that the medial branch of the dorsal ramus supplies the fascicles that arise from the SP and lamina of the vertebrae with the same segmental number as the nerve. While some authors have supported this description15, some discrepancies have been noted in the recent literature that question the concept of unisegmental innervation16,17.
Wu et al17 examined the multifidus of four complete Frankel A paraplegic subjects (T10 or T11) through EMG testing. The EMG demonstrated voluntary motor unit action potentials in the multifidi three to seven segments below the level of spinal cord injury. The authors tested this information through a direct approach by generating lesions in specific lumbar nerve roots and then following up with EMG of the multifidus18. In another study performed by Wu et al18, the authors performed bilateral percutaneous radiofrequency neurotomy of the medial branches of the L3 dorsal rami in a 49-year-old male. EMG examination was performed in the L2–5 multifidi both prior to and 3 weeks after the procedure. Positive sharp waves and fibrillations appeared in the L3-L5 multifidi after the neurotomy. The authors concluded that this study provides electrophysiological evidence in the human lumbar spine that the medial branch of the lumbar root innervates the multifidus muscle at multiple levels; i.e., the lumbar multifidus muscle is polysegmentally innervated18.
Indahl et al16 studied the porcine spine through stimulation of the annulus fibrosus. Stimulation of the disc produced muscular contraction bilaterally and at multiple levels of the most medial fibers of the multifidus, producing motor unit action potentials at the levels from L2–4. This finding supports the possibility that the multifidus receives neural reference from multiple spinal levels.
As a result of the varied descriptions of the morphology of the lumbar multifidus and its function in the lumbar spine, the present study was performed to qualify previous descriptions of the muscular morphology of the lumbar multifidus and further study the cleavage planes of this important muscle by chemical dissection. The description of the anatomy is intended to provide information of value for future biomechanical and EMG analyses.
Methods
The muscular morphology of the lumbar multifidus in eight human cadavers was studied by both gross and chemical dissection. Four were fresh cadavers dissected using gross and chemical dissection technique. Two male cadavers, ages 75 and 84 years, and two female cadavers, ages 73 and 84 years were dissected within 12 to 96 hours after death. Four preserved specimens were dissected by gross dissection technique; the two male cadavers were aged 78 and 80 years, and the two female cadavers were aged 85 and 90 years. All cadavers were Caucasian. The cause of death was available for only two of the cadavers, but there were no signs of trauma or surgery in any of the specimens.
Gross dissection began with resection of the thoracolumbar fascia and lumbar erector spinae. Care was taken to observe the attachments of the multifidus to the erector spinae aponeurosis and to resect them in a manner that would preserve this layer. After inspection of this superficial layer of the multifidus was completed, it was resected at its cephalic attachment on the SP and its caudal attachment was noted for each vertebral level. This process was repeated for the two intermediate layers and the deep layer of the multifidus.
Gross dissection alone was performed on cadaver #1 in order to assess the various levels of the multifidus and compare the attachments and fascicles to previously described literature. Cadavers #2 and #3 were chemically dissected with formic acid and cadaver #4 with nitric acid prior to the gross dissection27–29. Formic acid was less caustic to the muscle tissue and more selective for dissection of the connective tissue cleavage planes; thus, it did not macerate the muscular fibers, leaving the muscular planes more apparent. The time requirements for dissection were longer with the formic acid. Blunt dissection only was performed on cadavers #5–8 with observations of fiber direction and proximal and distal attachments noted.
The procedure for chemical dissection consisted of an en bloc dissection wherein the spine was severed at the T10 level such that the specimen retained its normal position in regard to the lumbar spine, sacrum, and ilium. All lateral and anterior muscle tissue that was not part of the multifidus was removed prior to chemical soaking. The procedure for chemical dissection as described by Loeb and Gans recommends the use of formic acid for chemical dissection of fresh material27. The specimen was placed en bloc in a 25% formic acid solution for 2 hours, then rinsed and evaluated for amount of connective tissue dissection. It was then placed in the same percentage solution for another 2 hours to allow for more connective tissue digestion. At this point the specimen was stored in a 7% formic acid solution, refrigerated for 12 hours, and then stored in glycerol to reduce any further chemical dissection. Chemical dissection with 20% nitric acid solution28,29 was performed initially for 2 hours, then 8, 12, 24, and 36 hours with gross dissection performed as connective tissue dissection occurred. Each specimen was photographed at each stage of dissection to assess cleavage planes, architecture of the muscle fibers, and attachments of the fascicles. The anatomy was recorded by photographs throughout the various stages of dissection.
The overall appearance of the multifidus in a fresh specimen does create the illusion of a homologous muscle group (Figure 2). However, four layers of the multifidus were evident with blunt dissection alone of the fresh cadaver. These layers were arranged from superficial to deep. The superficial and second layers were separated by a cleavage plane. The second layer, which was similarly described by Macintosh et al18, originates by a common tendon from a tubercle situated at the most caudal and lateral aspect of the SP. The third layer originates underneath this tubercle on the lateral aspect of the SP by a muscular attachment to the bone. The fourth layer originates on the lamina of the SP and lies beneath the fibers of the third layer.
FIGURE 2.
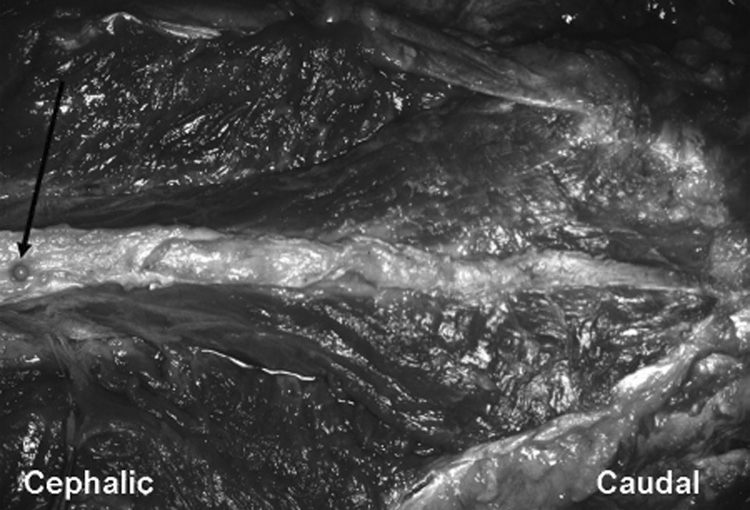
Dorsal view of multifidus in fresh cadaver-blunt dissection only.
Arrow pointing to pin on L1 spinous process.
The architecture of the multifidus was similar bilaterally and between genders, but the size of the fascicles did vary from cadaver to cadaver. The fascicle size of the male cadavers was approximately 25% larger than in the female. This change in size may have been due to a functional adaptation.
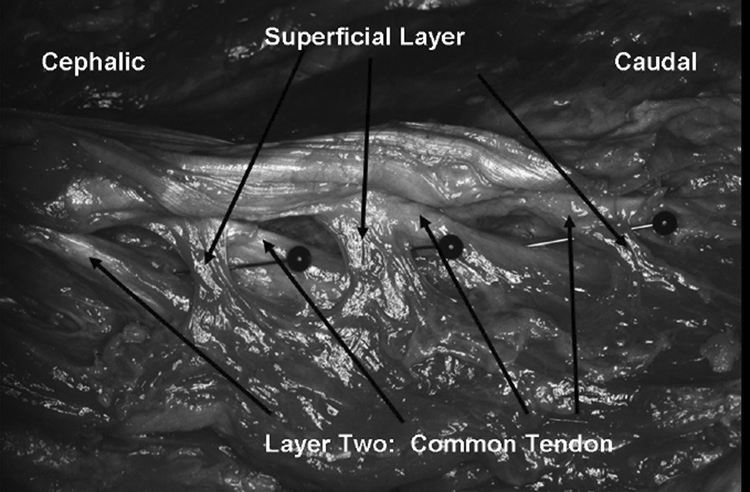
The superficial layer is best described as arising from the most lateral and superficial aspects of each mamillary process (shown in Figure 3) and the superior articular process (SAP). It passes dorsally upwards and medially to insert by muscular fibers to the two SPs and supraspinous ligaments above it (see Figures 4, 4A, 4B, and 5). This layer also attaches by tendinous slips and muscle tissue that extends dorsally to the overlying erector spinae.
FIGURE 3.
Lateral views of multifidus-blunt dissection.
FIGURE 4.
Superficial layer: SP=Spinous Process, SAP=Superior Articular Process.
FIGURE 4A.
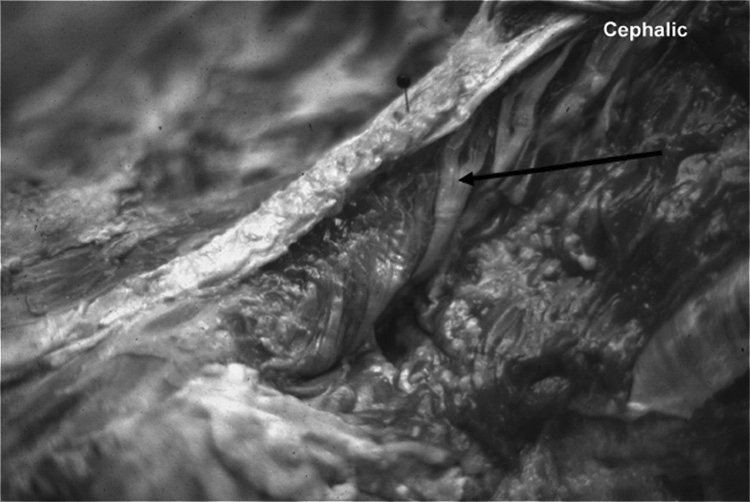
Superficial layer; origin: mamillary process; insertion: spinous processes.
FIGURE 4B.

Resection of superficial layer; fresh cadaver.
FIGURE 5.
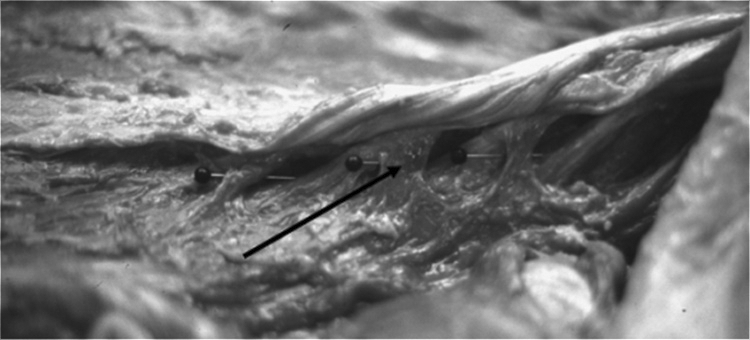
Superficial layer; pins depicting the natural separation of layers one and two.
Second Layer
The second layer arises from the posteroinferior lateral aspect of the SPs by a tendon. The tendon was round in two of the cadavers, and in the other two, it arose as a flat sheath; no predilection for tendon type was noted in the male or female cadavers. From the SP, the tendon passes downwards and angles laterally (oblique) with the degree of angulation decreasing at lower lumbar levels. The tendon extends to the level of the lamina below, where it continues as a fascicle (2F) of muscle (Figure 6). This fascicle is independent of surrounding muscle tissue until it reaches approximately two vertebral levels inferior to its origin. At this inferior level, the fibers of this fascicle (2F) fan outwardly in a pinnate pattern. These radiating fibers attach to a similar fascicle (2FA) originating from the tendon that is one vertebral level above and another similar fascicle (2FB) from one vertebral level below (Figures 6 and 7). Each of these fascicles (2F) also attach inferiorally into the third layer of muscle tissue (Figure 6).
FIGURE 6.
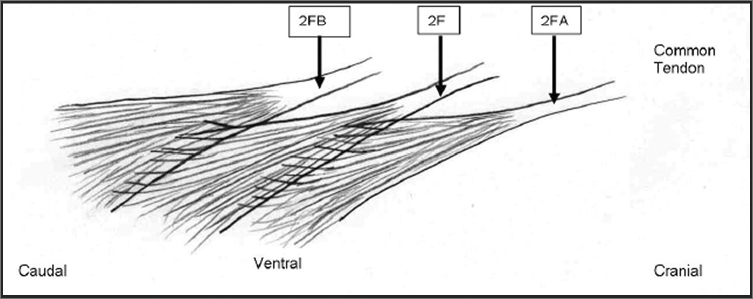
Fascicles of the second layer of the multifidus.
2F=Tendon and fascicle of second layer originating from L2 spinous process.
2FA=Tendon and fascicle of second layer originating from L1 spinous process. Fascicles from 2FA interdigitate with fascicle 2F.
2FB=Tendon and fascicle of second layer originating from L3 spinous process receiving fascicles from 2F.
Not pictured: Interdigitation of muscle fibers to the layer ventral to the second layer or 3F fascicles.
FIGURE 7.
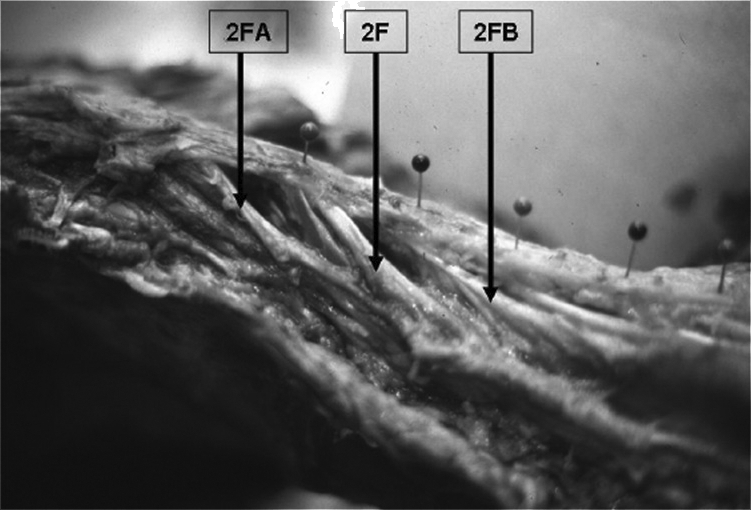
Layer 2 post-chemical dissection fresh cadaver.
2F=Tendon and fascicle of second layer originating from L1 spinous process.
2FA=Tendon and fascicle of second layer origination from T12 spinous process. Fascicles from 2FA interdigitate with fascicle 2F.
2FB=Tendon and fascicle of second layer originating from L2 spinous process receiving fascicles from 2F.
The fascicles interdigitate from the vertebral levels above and below as well as to the ventral layer or 3F fascicle.
With gross and chemical dissections, no distinct fascial planes were found separating the distal portions of the three interdigitating fascicles. A distinct distal insertion site of the fascicles of the second layer could not be established as one might expect in a strap muscle or a distinct band of muscle. However, a pattern of insertion did exist for the pinnate muscle fibers from the second and third layers when considered as a group (Figure 8). The distal aspect of muscle fibers that originated from the fascicle from L1 insert into the facet capsule and mamillary process of L4. The distal aspect of muscle fibers that originated from the fascicle from L2 insert into the facet capsule and mamillary process of L5 while those from L3 and more caudal levels insert into the iliac crest, sacroiliac joint, and the sacrum itself.
FIGURE 8.
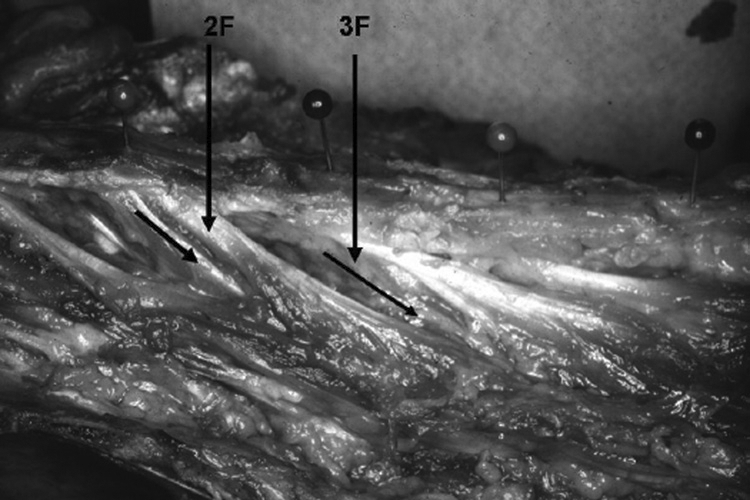
Second and third layers post-chemical dissection fresh cadaver.
2F=Second-layer tendon and fascicle.
3F=Third-layer fascicle, muscular in origin from spinous process.
In three of the four cadavers, a small fascicle arose at multiple but varying lumbar levels. These small fasciculi began at the junction of the tendon and the second layer fasciculi at the same vertebral level (Figure 7).
Third Layer
The third layer lies deep to the second layer. Its fasciculi (3F) originate on the inferior and lateral aspect of the SP directly beneath the round or flat tendinous attachment of the second layer (Figures 9, 10, and 11). This third-layer fascicle (3F) joins the second-layer muscle fascicle (2F) dorsally, superiorally, and inferiorally as well as the tendon of the second-layer fascicle from the same vertebral level. The third-layer fascicle (3F) radiates outward in a pinnate pattern to attach to the third-layer fascicle (3F) from the vertebrae one level above and the third-layer fascicle from the vertebra below. This interdigitating pattern of the third-layer fascicles is similar to that found for the second-layer fascicles. No distinct fascial planes were found between the distal portion of the second- (2F) and third- (3F) layer fascicles. A single site of distal insertion of 3F was not distinct, but the 2F and 3F fascicles attached as a single group of fibers to the mamillary process two levels inferior to the SP from which it originated.
FIGURE 9.
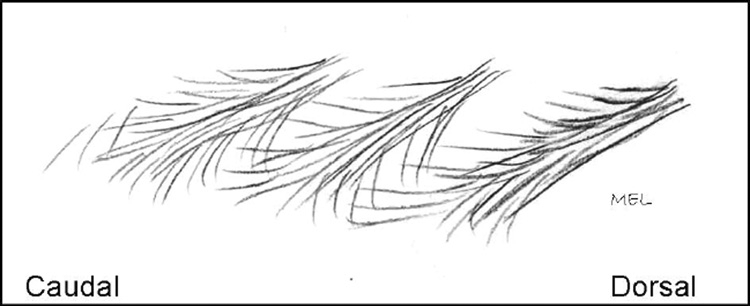
Layer 3 fascicles interdigitating with fascicles above and below.
FIGURE 10.
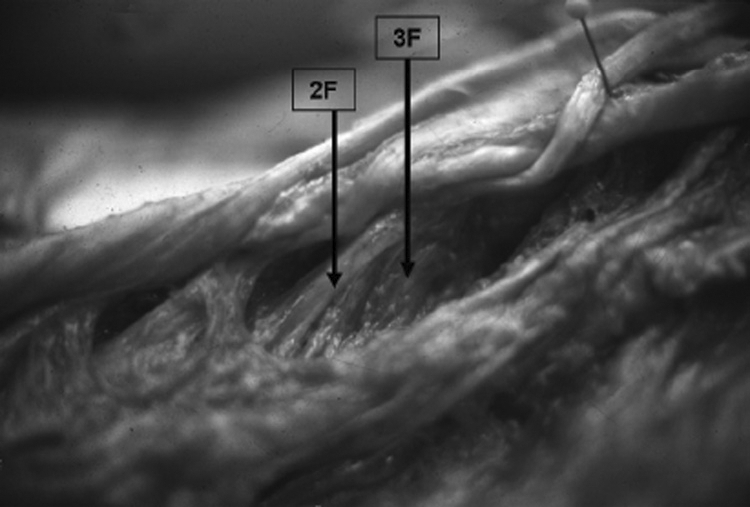
Second- and third-layer fresh cadaver.
2F=Second-layer tendon and fascicle.
3F=Third-layer fascicle, muscular in origin from spinous process.
FIGURE 11.
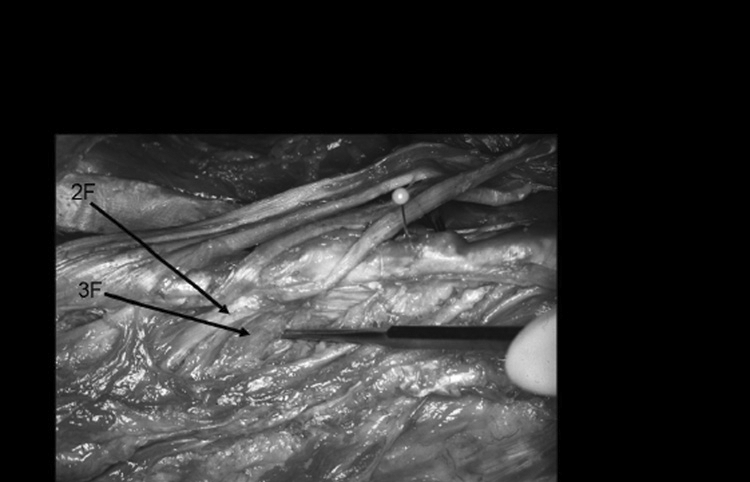
Layer 3 fascicle-muscular attachment.
2F= Second-layer tendon and fascicle.
3F= Third-layer fascicle, muscular in origin from spinous process.
Fourth Layer
The fourth and deepest layer of the multifidus consists of the interlaminar fibers that arise from the SAP and lamina of the vertebra below. These fibers insert into the facet capsule and lamina of the vertebra just above (Figure 11).
Discussion
This morphological description of the multifidus differs from others in three distinct ways. First the attachments differ, second the architecture and pinnate nature differs, and third is the lack of cleavage planes between the various fascicles of the multifidus as described by Macintosh et al12.
In this study, the superficial layer originated from the mamillary process to insert onto the tip of two SPs and supraspinous ligaments at the same vertebral level and one above. Tendinous slips and muscle tissue extended dorsally to the overlying erector spinae aponeurosis. Macintosh et al12 described these superficial fibers as arising from the common tendon to their attachment on the deep surface of the erector spinae aponeurosis over the sacrum12. No other studies noted attachment into the erector spinae aponeurosis. The descriptions differ within this layer through the distinction between the layers as noted by cleavage plane analysis versus the layer as arising from the common tendon.
The second layer, which originated from the posteroinferior lateral aspect of the SP as a common tendon, was similar in description of origin to the reviewed studies. The difference appeared to be in our description of the interdigitation of the fascicles from the vertebral levels above and below. Jemmett et al13 described this layer as bipennate, while Macintosh et al12 described a single fascicle originating from the tubercle that inserts onto the mamillary processes of the vertebrae four and five levels caudal.
Our description would be more consistent with a multipennate arrangement due to the interdigitation from the same layer above and below as well as from the third-layer muscular interdigitation from the ventral aspect. This interdigitation was evident after the chemical dissection. Jemmett et al13 described the SP attachment of this layer as the insertion for the primary fascicle at each level. The distinct distal insertion of the fascicles of the second layer that originated at each SP cannot be established as it could be for a strap muscle or a distinct band of muscle as described by Macintosh et al12.
The third layer, which we have described as the muscular layer, originates from the lateral aspect of the inferior aspect of the SP as a muscular band of origin. Jemmett et al13 and Paris20 have described a bipennate orientation in this layer in relation to the common tendon as well as the distal musculature. With gross dissection, it would be easy to attribute this fascicle as attaching to the common tendon; however, the chemical dissection noted a cleavage plane between the tendon and muscle sheath at the superior aspect of its origin on the SP. The inferior aspect of this fascicle was interdigitated with the same muscular layer above and below.
Other authors have described a fourth layer or interlaminar fibers (Jemmett et al13 and Macintosh et al12). In this study, the interlaminar fibers are described as arising from the SAP and lamina of the vertebra below to insert into the facet capsule and lamina of the vertebra just above. Jemmett et al13 described a similar pattern of attachment, while Macintosh et al12 described the laminar fascicles as inserting two levels caudal on the mamillary process.
The differences in architectural structure have been described as multipennate, bipennate, and multipennate but strap-like in nature due to the singular or strap-like nature of the individual fascicles. This study describes interdigitation of fascicles from four layers of musculature in a multipennate fashion. The chemical dissection using formic acid was faster and appeared to penetrate through the entire muscle better than the nitric acid.
The description of the lumbar multifidus by Macintosh et al12 has been the primary and widely accepted description in the literature and served as the basis for their biomechanical analysis of this muscle. Macintosh et al12 analyzed the multifidus based on individual fascicles and bands, thereby reporting that the principal action of the multifidus is posterior sagittal rotation. They reported that any axial rotation produced by the multifidus is a secondary role.
Our study indicates that the fascicles must be considered together to interpret the actions of the multifidus. The interweaving of the muscle fibers between fascicles of the various layers suggests a complex functional interaction among the fascicles and a complex pattern of muscle action. Based on our findings, the multifidus is a multifunctional muscle that can move the lumbar spine in three planes; it stabilizes the lumbar spine and possibly minimizes compression and shear on the articular facets.
All four layers of the lumbar multifidus may act bilaterally to produce posterior rotation in the sagittal plane (truck extension) and to control forward rotation in the sagittal plane (trunk flexion). All four layers may act unilaterally to laterally rotate the lumbar spine in the frontal plane (side-bending) to the same side and to control lateral rotation in the frontal plane to the opposite side. All four layers of muscle may act also to stabilize the lumbar segments in all three planes.
While lumbar segmental rotation is limited by the position of the facets, the superficial, intermediate, and deep layers of the lumbar multifidus show a capacity for segmental rotation in the transverse plane. It appears that unilateral muscle action may rotate the vertebral body to the contralateral side (SP to ipsilateral side) if the vertebra below is fixed relative to moving vertebra above. If the vertebra above is fixed relative to the vertebra below, then unilateral muscle action may rotate the vertebral body below to the ipsilateral side (SP to the contralateral side). In addition to producing transverse trunk rotation, small side-to-side rotations in the transverse plane may produce adjustments in the articular space between facets. Because of the limited transverse motion at the lumbar facet joints, this type of side-to-side adjustment mechanism may minimize compressive impact and shear on the articular cartilage. Further, bilateral activity of the lumbar multifidus may stabilize the lumbar spine in the transverse plane, controlling the transverse position of the facets during trunk extension and flexion to reduce articular wear. This intersegmental multifunction role of the lumbar multifidus would also suggest a complex neuronal circuitry.
Conclusion
Rehabilitation of the lumbar spine commonly involves strengthening of the lumbar multifidus and other trunk muscles to increase spinal stability30–32. This study supports the clinical belief that the lumbar multifidus has a significant role in the control and stabilization of the lumbar spine in multiple planes of action, including rotatory movements as well as posterior rotation (extension) in the sagittal plane33. The multipennate arrangement of this muscle with fascicular inter-attachment supports the clinical premise that the multifidus is activated in a variety of positions and can potentially produce and mediate intersegmental mobility and provide proprioception34–36. It has been suggested that the superficial, intermediate, and deep layers of this muscle may have a role in controlling the transverse position of the facets to reduce articular wear as well as other varied functional roles, but further evidence is needed to support this concept35. Our findings support the potential for varied roles based on the variable attachments and division between the layers. The inter-attachment and pinnate nature of the fascicles in the second layer may enhance the fluidity of movement needed for intersegmental motion of the spine and allow for increased torque production in rotatory movements. Based on the morphological findings, considerations for rehabilitation include the use of varied planes of motion with motor control techniques to improve static and dynamic muscle action. Previously described exercises for the lumbar multifidus in quadruped with progression to training in functional positions and proprioceptive retraining may increase the overall muscle function34.
FIGURE 12.
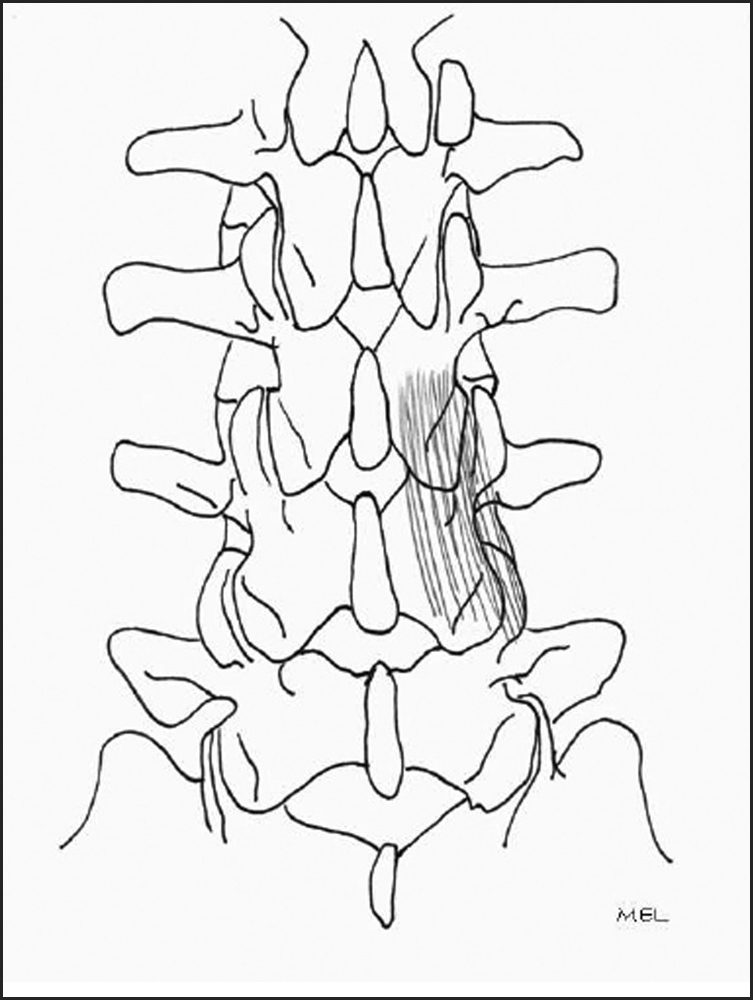
Fourth layer.
Interlaminar fibers.
REFERENCES
- 1.Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996;21:2763–2769. doi: 10.1097/00007632-199612010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Mattila M, Hurme M, Alaranta H, et al. The multifidus muscle in patients with lumbar disc herniation: A histochemical and morphometric analysis of intraoperative biopsies. Spine. 1986;11:732–738. doi: 10.1097/00007632-198609000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Gray H. Gray's Anatomy, 36th ed. London, UK: Warwick and Williams; 1980. [Google Scholar]
- 4.Cunningham DJ. Textbook of Anatomy, 12th ed. London, UK: Oxford University Press; 1981. [Google Scholar]
- 5.Morris JM, Benner G, Lucas DB. An electromyographic study of the intrinsic muscles of the back in man. J Anat. 1962;96:509–520. [PMC free article] [PubMed] [Google Scholar]
- 6.Morris JM, Lucas DB, Breslar B. Role of the trunk in stability of the spine. J Bone Joint Surg. 1962;43A:327–351. [Google Scholar]
- 7.Donisch EW, Basmajian JV. Electromyography of deep back muscles in man. Am J Anat. 1972;133:25–36. doi: 10.1002/aja.1001330103. [DOI] [PubMed] [Google Scholar]
- 8.Macintosh JE, Bogduk N. The biomechanics of the lumbar multifidus. Clin Biomech. 1986;1:205–213. doi: 10.1016/0268-0033(86)90147-6. [DOI] [PubMed] [Google Scholar]
- 9.Valencia FP, Munro RR. An electromyographic study of the lumbar multifidus in man. Electromyogr Clin Neurophysiol. 1985;25:205–221. [PubMed] [Google Scholar]
- 10.McGill S. Electromyographic activity of the abdominal and low back musculature during the generation of isometric and dynamic axial trunk torque: Implications for lumbar mechanics. J Orthop Res. 1991;9:91–103. doi: 10.1002/jor.1100090112. [DOI] [PubMed] [Google Scholar]
- 11.Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine. 2002;27:E29–E36. doi: 10.1097/00007632-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 12.Macintosh JE, Valencia F, Bogduk N, Munro RR. The morphology of the human lumbar multifidus. Clin Biomech. 1986;1:196–204. doi: 10.1016/0268-0033(86)90146-4. [DOI] [PubMed] [Google Scholar]
- 13.Jemmett RS, Macdonald DA, Agur AMR. Anatomical relationships between selected segmental muscles of the lumbar spine in the context of multi-planar segmental motion: A preliminary investigation. Man Ther. 2004;9:203–210. doi: 10.1016/j.math.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982:383–397. [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell WW, Vasconcelos O, Laine FJ. Focal atrophy of the multifidus muscle in lumbosacral radiculopathy. Muscle Nerve. 1998;21:1350–1353. doi: 10.1002/(sici)1097-4598(199810)21:10<1350::aid-mus21>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Indahl A, Kaigle A, Reikerås O, Holm S. Electromyographic response of the porcine multifidus musculature after nerve stimulation. Spine. 1995;20:2652–2658. doi: 10.1097/00007632-199512150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Wu PB, Date ES, Kingery WS. The lumbar multifidus muscle is polysegmentally innervated. Electromyogr Clin Neurophysiol. 2000;40:483–485. [PubMed] [Google Scholar]
- 18.Wu P, Kingery W, Frazier M, Date E. An electrophysiological demonstration of polysegmental innervation in the lumbar medial paraspinal muscles. Muscle Nerve. 1997;20:113–155. doi: 10.1002/(sici)1097-4598(199701)20:1<113::aid-mus18>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Lewin T, Moffett B, Viidik A. The morphology of the lumbar synovial intervertebral joints. Acta Morphol Neerl Scand. 1962;4:299–319. [PubMed] [Google Scholar]
- 20.Paris S. Anatomy as related to function and pain. Orthop Phys Ther Clin N Am. 1983;14:475–489. [PubMed] [Google Scholar]
- 21.Valencia F, Munro RR. Morphology of the lumbar multifidus in man. J Anat. 1984;139:196. [Google Scholar]
- 22.De Foa JL, Forrest W, Biedermann HJ. Muscle fibre direction of longissimus, iliocostalis and multifidus: Landmark-derived reference lines. J Anat. 1989;163:243–247. [PMC free article] [PubMed] [Google Scholar]
- 23.Porterfield J, DeRosa C. Mechanical Low Back Pain: Perspectives in Functional Anatomy. Philadelphia: W.B. Saunders Co.; 1991. [Google Scholar]
- 24.Biedermann HJ, De Foa JL, Forrest WJ. Muscle fibre directions of iliocostalis and multifidus: Male-female differences. J Anat. 1991;179:163–167. [PMC free article] [PubMed] [Google Scholar]
- 25.Shindo H. Anatomical study of the lumbar multifidus muscle and its innervation in human adults and fetuses. Nippon Ika Daigaku Zasshi. 1995;62:439–446. doi: 10.1272/jnms1923.62.439. [DOI] [PubMed] [Google Scholar]
- 26.Hollinshead W, Rosse C. Textbook of Anatomy, 5th ed. Baltimore: Lippincott Williams & Wilkins; 1997. [Google Scholar]
- 27.Loeb GE, Gans C. Electroymyography for Experimentalists. Chicago: The University of Chicago Press; 1986. [Google Scholar]
- 28.Loeb GE, Pratt CA, Chanaud CM, Richmond FJ. Distribution and innervation of short, interdigitated muscle fibers in parallel-fibered muscles of the cat hindlimb. J Morph. 1987;191:1–15. doi: 10.1002/jmor.1051910102. [DOI] [PubMed] [Google Scholar]
- 29.Heron M, Richmond F. In series fiber architecture in long human muscles. J Morph. 1993;216:35–45. doi: 10.1002/jmor.1052160106. [DOI] [PubMed] [Google Scholar]
- 30.Kaigle AM, Holm SH, Hansson TH. Experimental instability in the lumbar spine. Spine. 1995;20:421–430. doi: 10.1097/00007632-199502001-00004. [DOI] [PubMed] [Google Scholar]
- 31.Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord. 1992;5:383–389. doi: 10.1097/00002517-199212000-00001. discussion 397. [DOI] [PubMed] [Google Scholar]
- 32.Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord. 1992;5:390–397. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Richardson C, Jull G. Muscle control-pain control: What exercises would you prescribe? Man Ther. 1995;1:2–10. doi: 10.1054/math.1995.0243. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald DA, Moseley GL, Hodges PW. The lumbar multifidus: Does the evidence support clinical beliefs? Man Ther. 2006;11:254–263. doi: 10.1016/j.math.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine. 2002;27:29–36. doi: 10.1097/00007632-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 36.Hides JA, Jull GA, Richardson CA. Longterm effects of specific stabilizing exercises for first-episode low back pain. Spine. 2001;26:243–248. doi: 10.1097/00007632-200106010-00004. [DOI] [PubMed] [Google Scholar]


