Abstract
Pulsed arterial spin labeling magnetic resonance imaging (MRI) was performed to investigate the local coupling between resting regional cerebral blood flow (rCBF) and BOLD (blood oxygen level dependent) signal changes in 22 normal human subjects during the administration of 0.25 MAC (minimum alveolar concentration) sevoflurane. Two states were compared with subjects at rest: anesthesia and no‐anesthesia. Regions of both significantly increased and decreased resting‐state rCBF were observed. Increases were limited primarily to subcortical structures and insula, whereas, decreases were observed primarily in neocortical regions. No significant change was found in global CBF (gCBF). By simultaneously measuring rCBF and BOLD, region‐specific anesthetic effects on the coupling between rCBF and BOLD were identified. Multiple comparisons of the agent‐induced rCBF and BOLD changes demonstrated significant (P < 0.05) spatial variability in rCBF–BOLD coupling. The slope of the linear regression line for AC, where rCBF was increased by sevoflurane, was markedly smaller than the slope for those ROIs where rCBF was decreased by sevoflurane, indicating a bigger change in BOLD per unit change in rCBF in regions where rCBF was increased by sevoflurane. These results suggest that it would be inaccurate to use a global quantitative model to describe coupling across all brain regions and in all anesthesia conditions. The observed spatial nonuniformity of rCBF and BOLD signal changes suggests that any interpretation of BOLD fMRI data in the presence of an anesthetic requires consideration of these insights. Hum Brain Mapp 2008. © 2007 Wiley‐Liss, Inc.
Keywords: spatial nonuniformity, pulsed arterial spin labeling, cerebral blood flow, BOLD, coupling, vascular effect, anesthesia, sevoflurane
INTRODUCTION
More than 25 million surgical procedures are performed under anesthesia each year in the United States alone. Although general anesthetics are widely used in the operating room and in human and animal research, the action of general anesthetics on brain physiology remains poorly understood. Imaging studies have offered one of the mostpromising approaches to exploring the relationship between anesthetics and neuronal activity. Investigators have postulated that regional cerebral blood flow (rCBF) is regulated by products of neuronal energy metabolism associated with neuronal activity. In theory, local feedback loops between rCBF and metabolism enable the brain to respond rapidly to the altered metabolic demands associated with changes in neuronal activity. Indeed, the intrinsic relationship between rCBF, vascular responses, metabolism, and neuronal activity—all phenomenon potentially linked to higher functions such as cognition and perception—forms the conceptual basis for in vivo functional PET and magnetic resonance imaging (MRI) [Kwong et al., 1992; Ogawa et al., 1992]. Imaging researchers have used these same imaging techniques to study human brain function in vivo in the presence of general anesthetics [Heinke and Schwarzbauer, 2001; Heinke et al., 2004; Kaisti et al., 2002, 2003; Langsjo et al., 2003; Schlunzen et al., 2004]. Our aim is to contribute to a growing body of functional magnetic resonance imaging (fMRI) literature that concerns the relationships between general anesthetics, vascular responses, CBF, oxidative metabolism, and neuronal activity.
An anesthetic alters brain physiology in a complicated fashion that remains poorly understood. It is known that most inhalational anesthetics are vasodilative, tending to increase rCBF in a dose related fashion, while most of the intravenous anesthetics are vasoconstrictive [Shapiro, 1986]. For the measures of rCBF or blood oxygen level dependent (BOLD) signal to serve as clear markers of regional neuronal activity, the changes observed in rCBF and BOLD must arise exclusively in response to changes in local neuronal activity. Thus investigators must find ways to dissociate purely vascular effects from neuronal activity driven changes.
A handful of studies have been carried out to quantify the functional component of task induced neuronal activity relative to different levels of baseline brain activity [Hyder et al., 2002; Shulman et al., 2001; Smith et al., 2002]. In these studies the baseline was altered as a function of anesthetic dose. Regional CBF and BOLD were measured, and a quantitative model was employed to assess the effect of an altered baseline on the task‐induced neuronal activity [Hyder et al., 2001]. Therefore, understanding the effects of an anesthetic on the resting rCBF is of utmost importance to investigations that use fMRI to model neurophysiological processes in the presence of an anesthestic agent.
The physiological changes introduced by different anesthetics have been studied extensively in animals. Using autoradiography on rats [Sokoloff et al., 1977], McCulloch et al. [McCulloch et al., 1982] investigated the effect of apomorphine on the relationship between local glucose utilization and CBF. They observed no significant change in the CBF‐metabolism coupling relationship after apomorphine administration, thus concluding that apomorphine acted directly upon tissue metabolic activity rather than on the vascular system. In a study focusing on the confounding effects of anesthesia on functional activation [Austin et al., 2005], however, a greater BOLD response was observed in rats during α‐chloralose anesthesia relative to halothane for a fixed stimulus, and the BOLD response to each drug varied with time in both location and magnitude. The authors suggested that the differential sensitivity of individual brain regions to the effects of anesthesia might bias the results of studies that depend on the coupling of vascular and metabolic components to functional activity. Understanding such bias is absolutely critical to proper interpretation of imaging data obtained when the subject is anesthetized.
To date, the hemodynamic effects of sevoflurane, an inhaled general anesthetic, have been investigated in several studies using PET [Kaisti et al., 2002, 2003; Schlunzen et al., 2004]. These studies have failed to resolve changes in baseline global CBF and rCBF in vivo. Furthermore, both global and local changes in CBF were unable to be dissociated from either changes in metabolism or direct changes in the local vasculature by the agent.
This work presents results from a carefully controlled fMRI study showing the spatial‐specificity of the drug‐induced incremental changes in resting state rCBF and the coupling of these changes to the drug‐induced changes in BOLD when the anesthetic was considered as a stimulus. The slopes of the fit lines of rCBF–BOLD coupling show that the observed change in BOLD per unit change in rCBF varies spatially in brain regions, indicating the spatial nonuniformity of the effects of sevoflurane. This finding is important to the interpretation of BOLD data in fMRI studies involving an anesthetic.
MATERIALS AND METHODS
Subjects and Anesthetic Administration
Twenty‐two healthy consenting subjects (American Society of Anesthesiologists (ASA), physical status class I) 19–30 years‐old were administered inhalational 0.25 MAC end‐tidal sevoflurane. Subjects on psychoactive drugs or any centrally acting medication were excluded. Subjects with a history of renal disease in the past and those with potentially difficult airway were also excluded. All patients fasted for 8 h before the study. After passing the physical and medical history examination subjects proceeded to the magnetic resonance research center where they were screened for ferromagnetic materials and then connected to standard ASA monitors. An intravenous (i.v.) infusion line was started with a 22G cannula for maintenance infusion (lactated ringer at 100 ml/h). Subjects were moved into the magnet with the monitors and i.v. in place. Anesthesia was induced and maintained with oxygen (5 l) and sevoflurane 0.5% (0.25 MAC) administered through a semiclosed circuit and facemask. The facemask was held in place with head straps. During anesthesia of 0.25 MAC sevoflurane subjects were able to talk and respond to questions. ECG, noninvasive blood pressure, oxygen saturation, end‐tidal carbon dioxide, and end‐tidal sevoflurane concentration were monitored continuously. The monitors used in this study are serviced and calibrated every 6 months, as recommended by the manufacturer, OHMEDA (Madison, WI).
Regional CBF and BOLD Measurements
Imaging was performed on a 3T whole‐body scanner Trio (Siemens Medical Systems, Erlangen, Germany) with a circularly polarized head coil. The subjects lay with their eyes open and focused on a white fixation (crosshair “+”) on a black screen. The scanning room was dark other than the light from the projection screen. Two anesthesia sessions were interleaved with three no‐anesthesia sessions. Clinical practice and our pilot studies indicated that the end‐tidal sevoflurane concentration reached a steady state ∼9 min after the induction of sevoflurane. On the basis of ECG readings, it took ∼9 min for the subjects to recover after the withdrawal of the agent. Acquisition of perfusion‐weighted images was started immediately after anesthesia induction (for the anesthesia sessions) or after withdrawal of sevoflurane (for the no‐anesthesia sessions). Each session lasted for 11 min, during which a total of 220 images were acquired. The subject was positioned once at the beginning of the experiment; head straps and pads were used to help control head motion during the experiment.
The modified STAR QUIPSS arterial spin labeling (ASL) MRI technique was used for simultaneous rCBF and BOLD measurement. This involved applying a train of thin‐slice saturation pulses at TI1 = 700 ms after the RF inversion pulse so as to control the bolus delivery and suppress the intravascular signal from large vessels [Luh et al., 1999]. A slab‐selective hypersecant inversion pulse was employed for ASL. A labeling radio frequency (RF) pulse was applied to a slab 20 mm inferior to the imaging slab. As a control, the same RF pulse was applied to a slab 20 mm superior to the imaging slab. Interleaved labeling and control images were acquired using a gradient echo‐planar imaging (EPI) sequence, followed by a recovery time, which allowed arterial blood to be refreshed.
The ASL acquisition parameters were: field of view = 256 × 256 mm2 matrix = 64 × 64; bandwidth = 2056 Hz/pixel; slice thickness = 8 mm; inter‐slice spacing = 2 mm. Ten anterior cingulate (AC)–anterior cingulate (PC) aligned slices were acquired from inferior to superior in an ascending order to cover most of the cortex. Acquisition of each slice took ∼54 ms. The time of repetition was TR = 3000 ms; the echo time was TE = 26 ms; the delay time TD within each TR was adjusted to maximum. During each EPI acquisition, fat was suppressed and phase‐correction was performed. A bipolar gradient of encoding velocity V enc = 20 mm/s was applied to the imaging slices for intravascular signal suppression (V enc = π/(γGτ 2) with γ being the gyromagnetic ratio, G the gradient amplitude, and 2τ the duration of the bipolar gradient.
To get the absolute values of regional CBF, 40 proton density weighted images were acquired with the same perfusion sequence, except for the following changes: TR = 10,000 ms; TD = 0 ms; and TI for the first slice was adjusted to the maximum. Mapping for the apparent longitudinal relaxation time T 1app was performed with an ultrafast Look‐Locker EPI T1 mapping sequence [Freeman et al., 1998].
Two additional images, one 3D and one 2D, were required for multisubject integration. First, a high‐resolution whole brain T 1‐weighted 3D image was acquired for each subject by means of MPRAGE (Magnetization Prepared Rapid Acquisition with Gradient‐Echo imaging), using the following settings: a slab of 160 slices with thickness 1 mm; TR = 1,500 ms; TI = 800 ms; TE = 2.83 ms; flip angle 15°; and one average. Next, a 2D T 1‐weighted image was acquired during each MR session using the same slice positions as the perfusion‐weighted images and the following additional settings: in‐plane resolution 1 × 1 mm2; TR = 300 ms; TE = 3.69 ms; flip angle 60°; and two averages.
Data Processing
Intrasubject motion correction, rCBF quantification, and BOLD quantification
The perfusion‐weighted and the proton‐density weighted images were motion‐corrected using the Statistical Parametric Mapping package (SPM99), via a 6‐parameter rigid‐body transformation. The mean image of the motion‐corrected proton density images was used for rCBF mapping. To reduce the potential session effect, the intensity of ASL images acquired during one session (anesthesia or no‐anesthesia) was adjusted voxel‐wise to the previous session in the following manner: (1) after the first 4 images for each session were discarded, the following 20 images (I pre) and the last 20 images (I cur) were averaged; (2) the voxel‐wise rescale factor was calculated as I pre/I cur; and (3) all the images in the current session were multiplied voxel‐wise by I pre/I cur. This intensity adjustment procedure was done for sessions two through five. Time series of the perfusion‐weighted images were then obtained by pair‐wise “surround” subtraction between interleaved label and control pairs [Aguirre et al., 2002; Wang et al., 2003; Wong et al., 1997] for each anesthesia or no‐anesthesia session. The BOLD weighted images were derived by adding each label/control pair then averaging within each condition.
Intersubject integration
The last 36 ASL images (18 pairs) from each anesthesia or no‐anesthesia session were used to assess the steady‐state sevoflurane effect on normal human brain. We therefore had 108 ASL images for the no‐anesthesia condition and 72 ASL images for the anesthesia condition. The mean perfusion‐ and BOLD‐weighted images were calculated first, then the absolute rCBF was estimated from the mean perfusion‐weighted image for the anesthesia and no‐anesthesia conditions. The following parameters were used in rCBF quantification: a longitudinal relaxation time for arterial blood of T 1a = 1,490 ms; a tissue blood partition coefficient for water of λ = 0.9 ml/g; an RF labeling efficiency of α π = 0.95; and a postlabeling delay time for the first slice of TI = 1,400 ms. For each subject, maps of sevoflurane‐induced changes in rCBF or BOLD values, along with the respective t‐statistic, could be easily obtained by contrasting all measurements for the two conditions. A standard whole brain template (MNI‐1 mm) was used for subject spatial normalization of the individual data. Subject integration and registration were carried out using the BioimageSuite software package [bioimagesuite.org, Papademetris et al., 2004] for the maps of sevoflurane‐induced changes in the rCBF or BOLD values and t‐statistic. Two transformations were calculated and used in multiple subject integration: (1) an affine transformation was estimated by coregistering the 2D anatomical image to the high‐resolution 3D anatomical image, and this was then used to transform the individual maps of sevoflurane‐induced changes of rCBF or BOLD values, and respective t‐statistics, to the high‐resolution 3D anatomical space of that subject; (2) a nonlinear transformation was calculated by coregistering the high‐resolution 3D anatomical image to the brain template, which enabled wrapping of all the maps from step (1) to a common brain space. Trilinear interpolation was employed for image regridding. The mean, standard deviation and statistics were estimated in the common template space across subjects. The individual maps of sevoflurane‐induced changes in the rCBF or BOLD values and respective t‐statistics were first estimated and then transformed this way to the common reference brain space. Voxel‐wise contrasts between conditions were estimated in the common space across subjects using a t‐statistic to test the null hypothesis. Brain regions of interest were defined in the common reference space. The coupling between incremental changes in rCBF and BOLD induced by sevoflurane to that of the resting brain was assessed using analysis of covariance corrected for multiple comparisons [Hochberg and Tamhane, 1987].
RESULTS
After administration of 0.25 MAC sevoflurane to normal human subjects, steady‐state physiological parameters such as end‐tidal CO2, heart rate, and mean blood pressure, were not significantly altered relative to those during no‐anesthesia (see Table I). The drug‐induced change in the global CBF was not significant. However, significant increases and decreases in rCBF were observed in different regions of the brain. Regions with significant changes in rCBF were identified (P < 0.01, t‐test on pooled across subject data, uncorrected) and are displayed in Figure 1. A list of locations that had significantly increased or decreased rCBF is presented in Table II. These results indicate that increases in rCBF were limited primarily to subcortical structures and the insula, whereas decreases were observed primarily in neocortical regions.
Table I.
Mean and standard deviation of the physiological parameters monitored during awake and anesthesia
| Vitals | Awake | Sevoflurane | P |
|---|---|---|---|
| End‐tidal CO2, mm Hg | 36.1 ± 2.6 | 36.1 ± 3.1 | 0.92 |
| Heart rate, beats/min | 58.2 ± 4.7 | 58.3 ± 4.5 | 0.93 |
| Mean blood pressure, mm Hg | 87.3 ± 6.8 | 86.3 ± 6.5 | 0.44 |
Values were estimated across subjects. The significance, P, was calculated using a paired t‐test.
Figure 1.
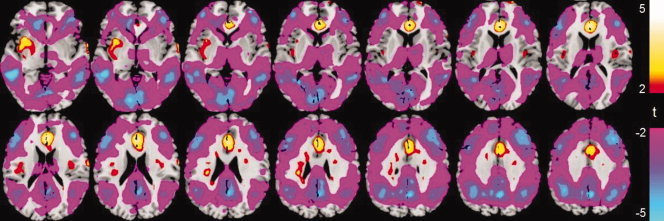
Maps of the t‐statistic show regional changes in resting rCBF induced by 0.25 MAC sevoflurane. The t‐test was performed, across all 22 subjects, on resting rCBF measured during conditions with and without sevoflurane.
Table II.
Typical locations with significantly increased or decreased rCBF induced by sevoflurane
| Brain region | Coordinates | rCBF% | t‐value |
|---|---|---|---|
| Anterior cingulate | −3, 26, 18 | 14.8 | 3.81 |
| R insula | 41, −13, 18 | 7.7 | 2.17 |
| L insula | −41, −13, 18 | 9.6 | 2.48 |
| R claustra | 28, 0, −2 | 14.7 | 2.98 |
| L claustra | −33, 0, −5 | 6.2 | 2.24 |
| R parahippocampal gyrus | 17, −29, −6 | 7.4 | 2.35 |
| L parahippocampal gyrus | −23, −21, −6 | 15.7 | 2.44 |
| R amydala | 26, −11, −12 | 33.7 | 3.01 |
| L amydala | −20, −10, −12 | 21.7 | 2.24 |
| R anterior cerebellum, culmen | 12, −35, −11 | 5.2 | 2.46 |
| L anterior cerebellum, culmen | −22, −41, −11 | 3.4 | 2.33 |
| R hippocampus | 29, −15, −14 | 13.8 | 2.29 |
| L hippocampus | −31, −22, −6 | 6.0 | 2.18 |
| Posterior cingulate | −2, −56, 8 | −21.5 | −6.75 |
| R superior temporal gyrus | 38, 46, 10 | −21.2 | −3.36 |
| L superior temporal gyrus | −42, 35, 7 | −23.0 | −3.56 |
| R middle temporal gyrus | 53, −42, 2 | −29.1 | −4.95 |
| L middle temporal gyrus | −54, 47, 7 | −19.9 | −3.11 |
| R inferior parietal lobule | 39, −47, 38 | −40.8 | −4.23 |
| L inferior parietal lobule | −38, −52, 38 | −31.97 | −3.15 |
| R middle frontal gyrus | 45, 27, 23 | −23.43 | −2.61 |
| L middle frontal gyrus | −35, 29, 33 | −23.85 | −3.14 |
These changes were evaluated in a composite map (i.e., changes in rCBF averaged voxel‐wise across subjects in a transformed Talairach template space).
To appreciate the spatial variability of resting state BOLD–rCBF coupling changes induced by sevoflurane, several regions with significant increases or decreases in rCBF were examined, including the AC, the PC, and bilateral inferior parietal lobule (IPL). It is assumed that sevoflurane‐induced changes in rCBF and BOLD could be observed within the linear regime of the BOLD–CBF coupling relationship [Hoge et al., 1999b]. Definition of these ROIs was subjected to Bonferroni correction for multiple voxel comparisons: the t‐statistics were first calculated on pooled subject data and then thresholded with P voxto obtain a corrected threshold of P = P vox × V roi < 0.05 (when V roi is the size of the ROI considered) [D'Esposito et al., 1999; Marcar et al., 2002; O'Craven et al., 1997; Siegle et al., 2006; Wilkinson et al., 2003].
Within an ROI, each voxel is associated with changes of both rCBF and BOLD after administration of sevoflurane (abbreviated ΔCBF and ΔBOLD). Figure 2 is a display of scatter plots that represent the ROIs that were inspected. The linear regression (LR) line for an ROI shows the dependence of ΔCBF on ΔBOLD within that ROI—in other words, it shows how large the change in BOLD is per unit change in rCBF. Representative time courses for one subject are shown in Figure 3. The LR lines show appreciable variability across the ROIs inspected. To further show the spatial nonuniformity of the rCBF–BOLD coupling relationship, comparisons of the parameters of the LR lines—slopes and intercepts—are given in Figure 4a,b with 95% confidence intervals.
Figure 2.
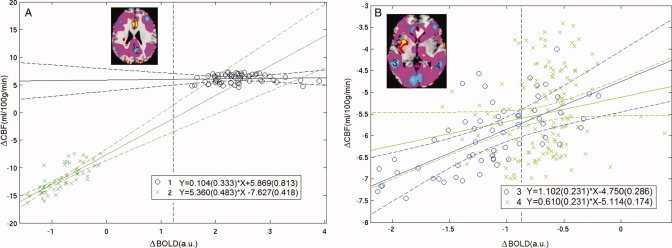
Regional CBF‐BOLD coupling was assessed in four brain regions: (A) AC (blue “o”) and posterior cingulate (green “x”); (B) Right IPL (blue “o”) and left IPL (green “x”). A composite map of rCBF changes represents changes in rCBF averaged voxel‐wise across subjects in the transformed common brain template space. These ROIs were defined on the composite map of rCBF changes induced by sevoflurane; Bonferroni correction was applied for multiple voxel comparisons (P < 0.05). Within an ROI, each voxel is associated with the changes both in rCBF and in BOLD stimulated by sevoflurane, i.e., ΔCBF and ΔBOLD. Scatter plots are presented for each of the four ROIs. The data is displayed as a collection of points, each representing the coordinated ΔCBF and ΔBOLD induced by the agent for one voxel of an ROI (abscissa ΔBOLD and ordinate ΔCBF). The LR line, regression equation and 95% confidence bounds are given for each ROI.
Figure 3.
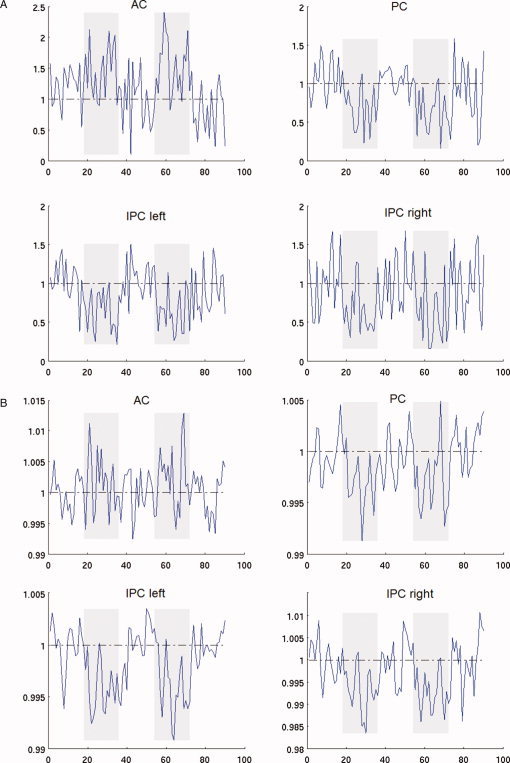
Display of (A) rCBF and (B) BOLD time courses of AC, posterior cingulate and bilateral IPL from one representative participant. Only data based on the last 36 ASL images (18 pairs) of each anesthesia or no‐anesthesia session were shown to demonstrate differences between the two steady‐states. The rCBF or BOLD time course of an ROI was normalized by dividing by the baseline rCBF or BOLD value, which was the value averaged in time for all no‐anesthesia sessions. Potential intersession (anesthesia or no‐anesthesia) effect was adjusted (see Data Processing for details). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 4.
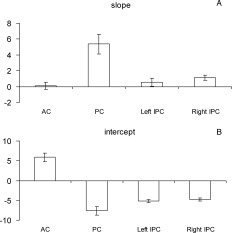
Comparisons of the parameters of the LR lines seen in Figure 2—(A) slopes and (B) intercepts—with 95% confidence intervals. The LR lines show appreciable variability across the ROIs inspected. Significant changes in slopes or intercepts of the LR lines between ROIs were observed, showing the spatial nonuniformity of the rCBF‐BOLD coupling relationship.
DISCUSSION
Using a noninvasive ASL technique on normal human subjects, we have shown that 0.25 MAC sevoflurane caused no significant change in global CBF relative to no‐anesthesia. At the same time, however, significant local CBF increases and decreases were observed following administration of 0.25 MAC sevoflurane. Increases in regional CBF were primarily limited to subcortical structures and the insula, while decreases were observed in neocortical regions. Although clinical observations indicate that memory and consciousness are particularly sensitive to anesthetics—they are ablated well below the concentrations needed to prevent movement—associating these functional ablations with cortical or subcortical regions where rCBF was significantly altered remains highly speculative. It is difficult to comment on changes in underlying neuronal activity based upon the observed changes in rCBF and BOLD for two reasons: (1) sevoflurane acts directly on vessels to cause vascular dilation and thereby alters rCBF; and (2) the BOLD signal is complex and depends upon several factors that impact levels of deoxygenated hemoglobin, such as oxygen supply (rCBF and saturation), oxygen consumption (cerebral metabolic rate of oxygen [CMRO2]), and vascular change (cerebral blood volume [CBV]). Nevertheless, since we measured rCBF and BOLD signals at same time, we were able to arrive at several new insights: (1) in general, rCBF–BOLD remains coupled when an anesthetic agent is used as a chemical stimulus; (2) this coupling relationship varies spatially across brain areas in a nonuniform fashion; (3) the slope of the LR line for AC, where rCBF was increased by sevoflurane, was markedly smaller than the slope for those ROIs where rCBF was decreased by sevoflurane, indicating a bigger change in BOLD per unit change in rCBF in regions where rCBF was increased by sevoflurane; and (4) the intercepts of all LR lines were significantly different from zero—the intercept of AC was positive and the intercept of all the other inspected ROIs was negative (see Fig. 4b).
The significant spatial variation in rCBF–BOLD coupling suggests that a single global model is unlikely to accurately describe coupling phenomenon within all brain regions in the presence of an anesthetic. It is not clear that the nonzero intercepts are of neurophysiological origin or due to the method used for analysis in this study, LR. Further study is needed to clarify these issues.
Regional Drug‐Induced Changes in rCBF
Although the nonuniformity of agent‐induced changes in rCBF was generally observed by previous researchers [Kaisti et al., 2002, 2003; Langsjo et al., 2003], the pattern of increases and decreases has not been consistent. Studies on the human brain from Kaisti's group showed that 0.75 MAC sevoflurane reduced the rCBF in all areas, and that both global and regional reductions in the absolute CBF were significant. Schlunzen et al., in a recent PET study [Schlunzen et al., 2004], investigated the effects of subanesthetic and anesthetic doses of sevoflurane on rCBF in healthy volunteers, showing (1) 0.2 MAC sevoflurane significantly increased the rCBF in the right AC gyrus and right lateral front‐orbital gyrus; (2) rCBF of insula was increased bilaterally, but not significantly; and (3) the region where rCBF was most decreased was in the cerebellum. Our 0.25 MAC sevoflurane study shows a similar pattern of rCBF increases and decreases during anesthesia—rCBF was increased significantly in bilateral AC and bilateral insula. Several other brain areas in our study demonstrated significant increases in rCBF, including the claustra, parahippocampal and hippocampal gyri, amydala, and anterior cerebellum. It has been reported in previous studies with other anesthetic agents that rCBF is reduced more profoundly in the neocortex than in the subcortex or midbrain. Werner et al. studied the effects of propofol on cerebral and spinal cord blood flow in rats compared with a N2O‐fentanyl control [Werner et al., 1993]. Propofol decreased cortical rCBF 60%, subcortical rCBF 40%, and midbrain 30%. Somewhat analogously, we observed significant rCBF decreases primarily in neocortical regions.
Regional CBF and Neuronal Activity
Although both significantly increased and decreased rCBF was observed in different regions, there has been little direct evidence to show that neural activity is accordingly increased where the rCBF is elevated during anesthesia. Less is known about the mechanism that underlies the increased rCBF in these regions. Isolated association of those brain regions with cognitive functions that are compromised by an agent might be an oversimplified interpretation of the complex action of the agent on the brain. Lenz et al. [Lenz et al., 1998] studied the relationship between absolute local CBF and the metabolic rate of glucose (refered to as static coupling) during sevoflurane and isoflurane anesthesia (1 MAC and 2 MAC) using autoradiographic methods. Local CBF and glucose utilization were measured in 40 brain regions. The results indicated that sevoflurane and isoflurane decreased glucose utilization but caused an increase in blood flow, thereby suggesting that the static coupling of CBF and glucose utilization changed with anesthetic dose. Another study of desflurane and isoflurane anesthesia (1 MAC and 2 MAC) by Lenz et al. [Lenz et al., 1999] showed similar results. As pointed out by Archer et al. [Archer and Pappius, 1999], rCBF and metabolic coupling conventionally refers to incremental changes in rCBF within a brain region in response to incremental changes in metabolism in the same region, while static coupling refers to the coupling between the absolute values of rCBF and metabolism across brain regions. Compared with the conventional coupling of incremental changes, static coupling of the absolute rCBF and glucose utilization underestimated the anesthetic effect on coupling with increasing levels of anesthesia, and in Lenz's studies, the incremental global measures showed that the increase in rCBF was not due to metabolic demand given that the glucose utilization actually decreased. Langsjo et al. employed PET to measure rCBF, CMRO2, and rCBV [Langsjo et al., 2003] in patients receiving subanesthetic doses of ketamine, an i.v. agent with a vasodilative effect. They showed only subtle and insignificant relative increases in CMRO2 in the insula, frontal, occipital, parietal, and AC cortices despite significant increases in rCBF; insignificant decreases in CMRO2 were found in the cerebellum; and there were no statistically significant absolute changes in rCMRO2 in brain regions studied. This data indicated that the regional oxygen extraction fraction was reduced throughout the brain due to rCBF increase as a result of vasodilation. It also suggested that the coupling between rCBF and CMRO2 was altered during anesthesia. Isoflurane and halothane, which are among the volatile anesthetics known to be vasodilative, have been used by Alkire et al. in normal human PET studies to investigate their effects on rCMRGl [Alkire et al., 1997, 1999]. Regional CMRGl was suppressed by both agents throughout all brain regions and there was no evidence of rCMRGl increases. For low dose sevoflurane anesthesia the coupling of the rCBF changes to metabolism remains unclear in the literature. In some studies [Kaisti et al., 2002, 2003; Schlunzen et al., 2004], the authors suggested that the changes in rCBF induced by an agent, whether increases or decreases, represented changes in metabolism and the underlying neuronal activity, although some data were interpreted based on an assumption of undisturbed CBF‐metabolic coupling.
Regional CBF, CBV, BOLD, and fMRI
Inferencing neuronal activity and metabolism based on the MR‐measured vascular changes (e.g., CBF and CBV) and BOLD is the goal of quantitative fMRI. The technique has brought about tremendous enthusiasm in recent years, and has been one of the most active areas in MRI research [Hoge et al., 1999a, b; Hyder et al., 2001; Kim, 1995; Kim et al., 1999]. Given that the BOLD signal is an epi‐phenomenon—a result of the complex interplay between neurophysiological processes involving oxygen supply, oxygen consumption, and the vascular system—interpreting quantitative fMRI has turned out to be a very formidable task, especially in those cases when a drug is involved [Austin et al., 2005]. For example, in most quantitative fMRI practices, a constant rCBF–CBV coupling relationship is assumed for different conditions and brain regions. However, the validity of this assumption is to be verified, particularly when a drug is involved. Under these circumstances, both the drug and external sensory stimuli are presented to the central nervous system simultaneously, muddying the interpretation of task‐induced neuronal activity and metabolism. Although we still have not been able to make any decisive statement about the spatial nonuniformity in terms of oxidative metabolism and neuronal activity affected by sevoflurane, the work in our study is an important first step to attacking this challenging problem. We have not only demonstrated how an anesthetic agent might affect rCBF and rCBF–BOLD coupling, but we have also provided reasons for neuroscientists to be especially cautious when interpreting BOLD or rCBF fMRI data.
Some Methodological Considerations
Physiological and physical limits on the number of slices for each RF labeling make whole brain coverage difficult [Campbell and Beaulieu, 2006; Frank et al., 1997; Yongbi et al., 1999]. In a recent study [Donahue et al., 2006], single slice PASL MRI measurements were performed as a function of in‐plane spatial resolution and postlabeling delay, and their results indicated that when using postlabeling delays shorter than 1,500 ms, higher MRI gray matter flow values may be observed owing to signal contamination from the remaining arterial blood water label. For delays above 1,500 ms, regional PASL‐based CBF values from frontal gray matter and occipital gray matter were comparable with PET‐based measurements which can be obtained by using spatial resolutions comparable with PET (5–7.5 mm in‐plane). At high resolution (2.5 × 2.5 × 3 mm3), compared with that at low resolution (7.5 × 7.5 × 3 mm3), gray matter CBF values were found to increase by 10–20%, as a consequence attributed to reduction in partial volume effects. They concluded that the recent availability of MRI field strengths of 3.0 T and higher will facilitate the use of MRI‐based CBF measurements in the clinic. To cover most of the cortical regions in current study, the slice thickness was set to 8 mm, with 2‐mm gap between slices. A calculation based on the voxel sizes shows that an 8–16% underestimation for rCBF is possible. Beside some disadvantages like low signal‐to‐noise ratio [Detre et al., 1992], contamination from the intravascular signal [Ye et al., 1997], variability of the arterial transit time [Gonzalez‐At et al., 2000; Yang et al., 2000; Zhou and van Zijl, 1999], and physiological and physical limits on the number of slices for each RF labeling, recently Woolrich et al. [Woolrich et al., 2006] modeled the double‐echo ASL process using a general linear model and a Bayesian statistical inference model. They compared the results of these two models. Their data indicate that the fractional change in BOLD obtained by averaging tag/control pairs from a single‐echo ASL experiment could be underestimated by up to 25%. This underestimation is caused by the components of static magnetization from the labeled or unlabeled blood. Since in this study we focus on the spatial variability of rCBF–BOLD coupling, the bias in rCBF or BOLD measurement will unlikely affect our observation if it is uniform within brain regions. Further studies are required to clarify these issues.
CONCLUSIONS
After administration of 0.25 MAC sevoflurane, regions of both significantly increased and decreased rCBF were identified. Increases in rCBF were primarily limited to subcortical structures and insula, decreases were observed in neocortical regions. No significant change in global CBF was observed. Results from our simultaneous rCBF‐BOLD MRI have shown that in the normal human brain there are significant region‐specific variations in the coupling between rCBF and BOLD. For a proper interpretation of fMRI data in terms of metabolism and neuronal activity, the vascular and neuronal effects of anesthetic must be dissociated in future studies examining function in the presence of an anesthetic agent.
REFERENCES
- Aguirre GK,Detre JA,Zarahn E,Alsop DC ( 2002): Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage 15: 488–500. [DOI] [PubMed] [Google Scholar]
- Alkire MT,Haier RJ,Shah NK,Anderson CT ( 1997): Positron emission tomography study of regional cerebral metabolism in humans during isoflurane anesthesia. Anesthesiology 86: 549–557. [DOI] [PubMed] [Google Scholar]
- Alkire MT,Pomfrett CJ,Haier RJ,Gianzero MV,Chan CM,Jacobsen BP,Fallon JH ( 1999): Functional brain imaging during anesthesia in humans: Effects of halothane on global and regional cerebral glucose metabolism. Anesthesiology 90: 701–709. [DOI] [PubMed] [Google Scholar]
- Archer DP,Pappius HM ( 1999): Coupling of local cerebral blood flow to local cerebral glucose utilization during isoflurane and sevoflurane anesthesia. Anesthesiology 91: 889–891. [DOI] [PubMed] [Google Scholar]
- Austin VC,Blamire AM,Allers KA,Sharp T,Styles P,Matthews PM,Sibson NR ( 2005): Confounding effects of anesthesia on functional activation in rodent brain: A study of halothane and alpha‐chloralose anesthesia. Neuroimage 24: 92–100. [DOI] [PubMed] [Google Scholar]
- Campbell AM,Beaulieu C ( 2006): Comparison of multislice and single‐slice acquisitions for pulsed arterial spin labeling measurements of cerebral perfusion. Magn Reson Imag 24: 869–876. [DOI] [PubMed] [Google Scholar]
- D'Esposito M,Postle BR,Jonides J,Smith EE ( 1999): The neural substrate and temporal dynamics of interference effects in working memory as revealed by event‐related functional MRI. Proc Natl Acad Sci USA 96: 7514–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA,Leigh JS,Williams DS,Koretsky AP ( 1992): Perfusion imaging. Magn Reson Med 23: 37–45. [DOI] [PubMed] [Google Scholar]
- Donahue MJ,Lu H,Jones CK,Pekar JJ,van Zijl PC ( 2006): An account of the discrepancy between MRI and PET cerebral blood flow measures. A high‐field MRI investigation. NMR Biomed 19: 1043–1054. [DOI] [PubMed] [Google Scholar]
- Frank LR,Wong EC,Buxton RB ( 1997): Slice profile effects in adiabatic inversion: Application to multislice perfusion imaging. Magn Reson Med 38: 558–564. [DOI] [PubMed] [Google Scholar]
- Freeman AJ,Gowland PA,Mansfield P ( 1998): Optimization of the ultrafast Look‐Locker echo‐planar imaging T1 mapping sequence. Magn Reson Imag 16: 765–772. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐At JB,Alsop DC,Detre JA ( 2000): Cerebral perfusion and arterial transit time changes during task activation determined with continuous arterial spin labeling. Magn Reson Med 43: 739–746. [DOI] [PubMed] [Google Scholar]
- Heinke W,Schwarzbauer C ( 2001): Subanesthetic isoflurane affects task‐induced brain activation in a highly specific manner: A functional magnetic resonance imaging study. Anesthesiology 94: 973–981. [DOI] [PubMed] [Google Scholar]
- Heinke W,Fiebach CJ,Schwarzbauer C,Meyer M,Olthoff D,Alter K ( 2004): Sequential effects of propofol on functional brain activation induced by auditory language processing: An event‐related functional magnetic resonance imaging study. Br J Anaesth 92: 641–650. [DOI] [PubMed] [Google Scholar]
- Hochberg Y,Tamhane AC ( 1987): Multiple Comparison Procedures,Vol. 22 New York: Wiley; 450 p. [Google Scholar]
- Hoge RD,Atkinson J,Gill B,Crelier GR,Marrett S,Pike GB. ( 1999a): Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: The deoxyhemoglobin dilution model. Magn Reson Med 42: 849–863. [DOI] [PubMed] [Google Scholar]
- Hoge RD,Atkinson J,Gill B,Crelier GR,Marrett S,Pike GB. ( 1999b): Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA 96: 9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F,Kida I,Behar KL,Kennan RP,Maciejewski PK,Rothman DL ( 2001): Quantitative functional imaging of the brain: Towards mapping neuronal activity by BOLD fMRI. NMR Biomed 14: 413–431. [DOI] [PubMed] [Google Scholar]
- Hyder F,Rothman DL,Shulman RG ( 2002): Total neuroenergetics support localized brain activity: Implications for the interpretation of fMRI. Proc Natl Acad Sci USA 99: 10771–10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisti KK,Metsahonkala L,Teras M,Oikonen V,Aalto S,Jaaskelainen S,Hinkka S,Scheinin H ( 2002): Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology 96: 1358–1370. [DOI] [PubMed] [Google Scholar]
- Kaisti KK,Langsjo JW,Aalto S,Oikonen V,Sipila H,Teras M,Hinkka S,Metsahonkala L,Scheinin H ( 2003): Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 99: 603–613. [DOI] [PubMed] [Google Scholar]
- Kim SG ( 1995): Quantification of relative cerebral blood flow change by flow‐sensitive alternating inversion recovery (FAIR) technique: Application to functional mapping. Magn Reson Med 34: 293–301. [DOI] [PubMed] [Google Scholar]
- Kim SG,Rostrup E,Larsson HB,Ogawa S,Paulson OB ( 1999): Determination of relative CMRO2 from CBF and BOLD changes: Significant increase of oxygen consumption rate during visual stimulation. Magn Reson Med 41: 1152–1161. [DOI] [PubMed] [Google Scholar]
- Kwong KK,Belliveau JW,Chesler DA,Goldberg IE,Weisskoff RM,Poncelet BP,Kennedy DN,Hoppel BE,Cohen MS,Turner R,Cheng HM,Brady TJ and Rosen BR ( 1992): Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 89: 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsjo JW,Kaisti KK,Aalto S,Hinkka S,Aantaa R,Oikonen V,Sipila H,Kurki T,Silvanto M,Scheinin H ( 2003): Effects of subanesthetic doses of ketamine on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 99: 614–623. [DOI] [PubMed] [Google Scholar]
- Lenz C,Rebel A,van Ackern K,Kuschinsky W,Waschke KF ( 1998): Local cerebral blood flow, local cerebral glucose utilization, and flow‐metabolism coupling during sevoflurane versus isoflurane anesthesia in rats. Anesthesiology 89: 1480–1488. [DOI] [PubMed] [Google Scholar]
- Lenz C,Frietsch T,Futterer C,Rebel A,van Ackern K,Kuschinsky W,Waschke KF ( 1999): Local coupling of cerebral blood flow to cerebral glucose metabolism during inhalational anesthesia in rats: Desflurane versus isoflurane. Anesthesiology 91: 1720–1723. [DOI] [PubMed] [Google Scholar]
- Luh WM,Wong EC,Bandettini PA,Hyde JS ( 1999): QUIPSS II with thin‐slice TI1 periodic saturation: A method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med 41: 1246–1254. [DOI] [PubMed] [Google Scholar]
- Marcar VL,Girard F,Rinkel Y,Schneider JF,Martin E ( 2002): Inaudible functional MRI using a truly mute gradient echo sequence. Neuroradiology 44: 893–899. [DOI] [PubMed] [Google Scholar]
- McCulloch J,Kelly PA,Ford I ( 1982): Effect of apomorphine on the relationship between local cerebral glucose utilization and local cerebral blood flow (with an appendix on its statistical analysis). J Cereb Blood Flow Metab 2: 487–499. [DOI] [PubMed] [Google Scholar]
- O'Craven KM,Rosen BR,Kwong KK,Treisman A,Savoy RL ( 1997): Voluntary attention modulates fMRI activity in human MT‐MST. Neuron 18: 591–598. [DOI] [PubMed] [Google Scholar]
- Ogawa S,Tank DW,Menon R,Ellermann JM,Kim SG,Merkle H,Ugurbil K ( 1992): Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA 89: 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papademetris X,Jackowski AP,Schultz RT,Staib LH,Duncan JS ( 2004): Integrated intensity and point‐feature nonrigid registration. Medical Image Computing and Computer‐Assisted Intervention—Miccai 2004,Part 1, Proceedings 3216, Saint‐Malo, France. pp 763–770. [DOI] [PMC free article] [PubMed]
- Schlunzen L,Vafaee MS,Cold GE,Rasmussen M,Nielsen JF,Gjedde A (2004): Effects of subanaesthetic and anaesthetic doses of sevoflurane on regional cerebral blood flow in healthy volunteers. A positron emission tomographic study. Acta Anaesthesiol Scand 48: 1268–1276. [DOI] [PubMed] [Google Scholar]
- Shapiro HM ( 1986): Anesthetic effects upon cerebral blood flow, cerebral metabolism, electroencephalogram, and evoked potentials In: Miller RD, editor. Anesthesia. New York: Churchill Livingstone. [Google Scholar]
- Shulman RG,Hyder F,Rothman DL ( 2001): Cerebral energetics and the glycogen shunt: Neurochemical basis of functional imaging. Proc Natl Acad Sci USA 98: 6417–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ,Carter CS,Thase ME ( 2006): Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatr 163: 735–738. [DOI] [PubMed] [Google Scholar]
- Smith AJ,Blumenfeld H,Behar KL,Rothman DL,Shulman RG,Hyder F ( 2002): Cerebral energetics and spiking frequency: The neurophysiological basis of fMRI. Proc Natl Acad Sci USA 99: 10765–10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L,Reivich M,Kennedy C,Des Rosiers MH,Patlak CS,Pettigrew KD,Sakurada O,Shinohara M ( 1977): The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28: 897–916. [DOI] [PubMed] [Google Scholar]
- Wang J,Aguirre GK,Kimberg DY,Roc AC,Li L,Detre JA ( 2003): Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med 49: 796–802. [DOI] [PubMed] [Google Scholar]
- Werner C,Hoffman WE,Kochs E,Schulte am Esch J,Albrecht RF ( 1993): The effects of propofol on cerebral and spinal cord blood flow in rats. Anesth Analg 76: 971–975. [DOI] [PubMed] [Google Scholar]
- Wilkinson ID,Romanowski CA,Jellinek DA,Morris J,Griffiths PD ( 2003): Motor functional MRI for pre‐operative and intraoperative neurosurgical guidance. Br J Radiol 76: 98–103. [DOI] [PubMed] [Google Scholar]
- Wong EC,Buxton RB,Frank LR ( 1997): Implementation of quantitative perfusion imaging techniques for functional brain mapping using pulsed arterial spin labeling. NMR Biomed 10: 237–249. [DOI] [PubMed] [Google Scholar]
- Woolrich MW,Chiarelli P,Gallichan D,Perthen J,Liu TT ( 2006): Bayesian inference of hemodynamic changes in functional arterial spin labeling data. Magn Reson Med 56: 891–906. [DOI] [PubMed] [Google Scholar]
- Yang Y,Engelien W,Xu S,Gu H,Silbersweig DA,Stern E ( 2000): Transit time, trailing time, and cerebral blood flow during brain activation: Measurement using multislice, pulsed spin‐labeling perfusion imaging. Magn Reson Med 44: 680–685. [DOI] [PubMed] [Google Scholar]
- Ye FQ,Mattay VS,Jezzard P,Frank JA,Weinberger DR,McLaughlin AC ( 1997): Correction for vascular artifacts in cerebral blood flow values measured by using arterial spin tagging techniques. Magn Reson Med 37: 226–235. [DOI] [PubMed] [Google Scholar]
- Yongbi MN,Yang Y,Frank JA,Duyn JH ( 1999): Multislice perfusion imaging in human brain using the C‐FOCI inversion pulse: Comparison with hyperbolic secant. Magn Reson Med 42: 1098–1105. [DOI] [PubMed] [Google Scholar]
- Zhou J,van Zijl PC ( 1999): Effect of transit times on quantification of cerebral blood flow by the FAIR T(1)‐difference approach. Magn Reson Med 42: 890–894. [DOI] [PubMed] [Google Scholar]


