Abstract
Objective
We have shown that acute treatment with candesartan in an experimental model of stroke resulted in vascular protection and improved outcomes at 24 hours post-stroke, but the mechanisms are unknown. We now examine effects of candesartan on proangiogenic factors and 7-day outcomes using the same treatment paradigm.
Methods
Male Wistar rats underwent 3 hours of middle cerebral artery occlusion (MCAO), followed by reperfusion. A single dose of candesartan 1mg/kg IV was given at reperfusion. Animals received neurobehavioral testing before MCAO, at 24 hours after MCAO, and at 7 days. BP was measured by telemetry. Animals sacrificed at 24 hours had tissue and spinal fluid (CSF) collected for matrix metalloproteinase (MMP) activity, vascular endothelial growth factor (VEGF) expression, and tube formation assay. Neurobehavioral testing included elevated-body swing test (EBST), Bederson, beam walk, and paw grasp. Cerebrovascular density was quantified using immunohistochemistry at 24 h and 7 days.
Results
MMP-2 activity and VEGF expression were higher (p=0.035, p=0.042, respectively) and CSF was significantly more proangiogenic (5X tube formation; p=0.002) in the candesartan group at 24 hours. Although no difference was seen in infarct size at 7 days, treatment improved Bederson scores (2.1 vs. 2.9, p=0.0083), EBST (22.9 vs. 39.4, p=0.021) and paw grasp (1.29 vs. 2.88, p=0.0001) at 7 days. Candesartan treatment resulted in increased vascular density in the striatum at 7 days (p=0.037).
Conclusion
Candesartan after reperfusion augments ischemia-induced angiogenic state and provides long-term benefits. The beneficial effects may involve vascular protection and enhancement of early angiogenic remodeling.
Keywords: stroke, vascular protection, blood pressure
Introduction
Manipulation of the renin-angiotensin system has emerged as an effective strategy to prevent stroke and other vascular events in patients at risk 1. It also appears that angiotensin II type 1 (AT1) receptor blockade may provide particularly robust protection of the cerebral vasculature 1,2. We have shown that delayed acute treatment with candesartan, in an experimental model of stroke in rats, resulted in both neurovascular protection and improved function at 24 hours post-stroke 3, which was beyond that of BP lowering 4 alone. Although the mechanisms and long term effects are unknown, it is possible that promotion of neovascularization with AT1 receptor blockade, as has been reported by others 5 may be involved, enhancing long-term functional recovery. We now examine the effects of a single dose of candesartan on the proangiogenic state after stroke and 7-day behavioral and histologic outcomes. We measured the activity of MMPs, expression of VEGF, and ability of spinal fluid (CSF) from treated animals to induce tube formation in cultured brain microvascular endothelial cells (BMECs). In addition we quantified vessel density at 7 days after stroke.
Material and Methods
The experimental protocol was approved by the Care of Experimental Animal Committee of the Medical College of Georgia/ Institutional Animal Care and Use Committee (IACUC) of the Veterans Affairs Medical Center. Forty adult male Wistar rats (Charles River Breeding Company, Wilmington, MA, USA), weighing between 270–300 grams, were divided randomly into saline and candesartan treatment groups.
Experimental cerebral ischemia
Temporary (3 hour) middle cerebral artery occlusion (MCAO) was achieved using the intraluminal suture model6 under isoflurane anesthesia, as we have previously reported 3,4. All animals were singly housed before and after surgery, with free access to food and water. At reperfusion, a single dose of candesartan 1mg/kg or saline control was given intravenously via a tail vein, at a volume of 1 mL/kg.
Blood Pressure Telemetry
For a subset of these animals (n=7), BP telemetry transmitters (Data Sciences International, St. Paul, MN, USA) were implanted according to the manufacturer’s specifications, as previously reported 3. BP measurements were obtained for 2 days before MCAO, and for 7 days afterwards.
MMP Zymography
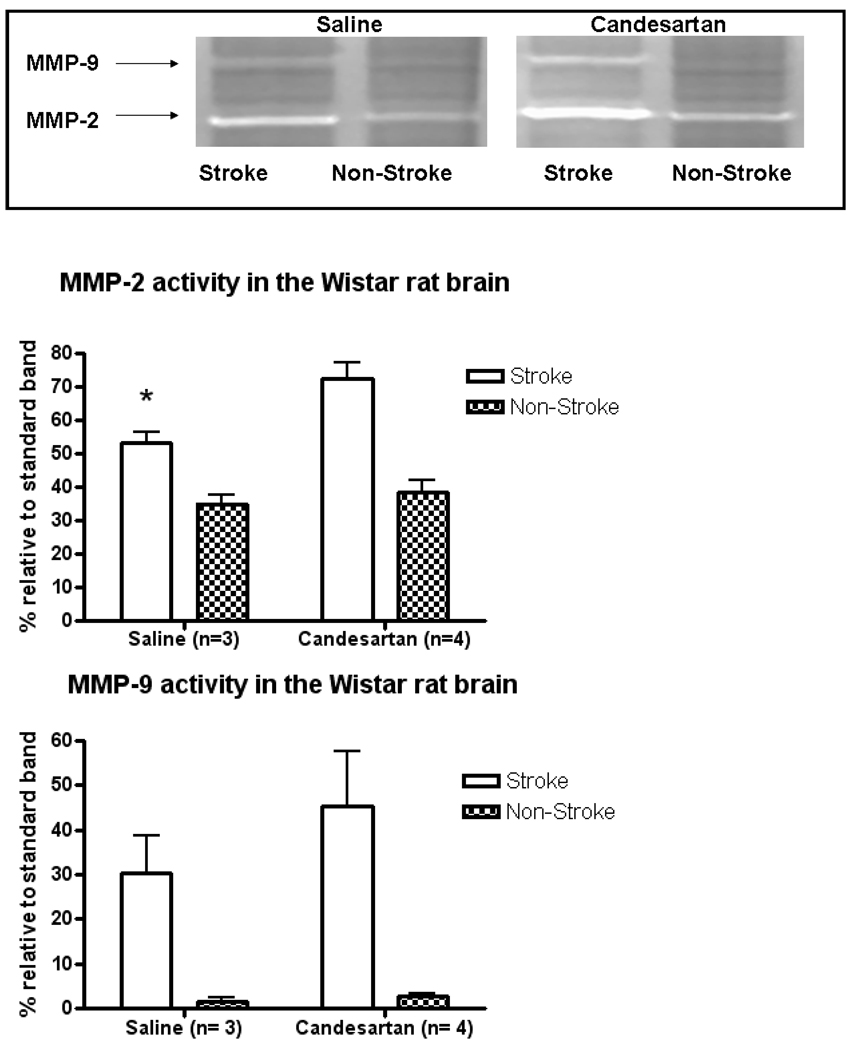
Animals (n=7) underwent MCAO and treatment with saline (n=3) or candesartan (n=4) and were sacrificed at 24 hours for quantification of MMP-2 and MMP-9 activity. The animals were anesthetized with a cocktail of ketamine (45 mg/kg) and xylazine (15 mg/kg) via intramuscular injection. The animals were then pericardially perfused with ice cold PBS, sacrificed and the brains were extracted. The ischemic and non-ischemic hemispheres were separated and stored at −80C until homogenization. The samples were homogenized in buffer as described previously 7. The gelatinolytic activity of the samples was assessed by densitometric analysis (Gel-Pro v 3.1, Media Cybernetics, Carlsbad, CA, USA) and compared to a standard band of recombinant protein.
VEGF Quantification
VEGF was quantified by ELISA kit (RayBiotech, Norcross, GA, USA), performed on the same brain homogenate supernatant used for the zymography as described above. Briefly, antibody-coated wells were loaded with 100 µL of sample, incubated, washed, and incubated with secondary biotinylated antibody per manufacturer’s instructions. The secondary antibody binds a HRP-strepavidin conjugate, and TMB substrate is added. The resulting reaction is stopped and the spectrophotometric analysis is performed. Signal was read at 450 nm (BioTek, Winooski, VT, USA).
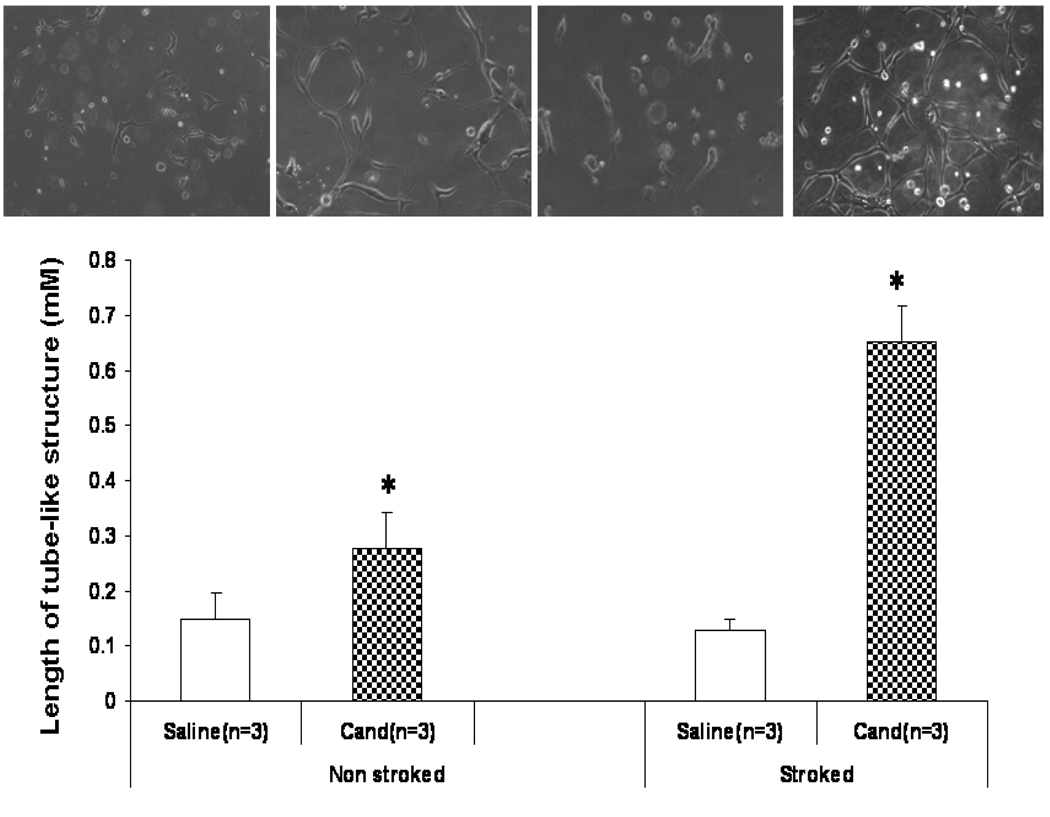
Tube formation assay
Growth factor-reduced Matrigel (BD Biosciences) was used according to the manufacturer’s protocol. Brain microvascular endothelial cells (BMECs) were counted and plated at 1×105 cells/ml. Once attached, cells were switched to serum free medium and treated with 50 µL of CSF from either non-stroked or stroked candesartan (n=3) or saline (n=3) treated animals and incubated for 24 hours. For inhibitor studies, 50 µl VEGF blocking antibody (1µg/ml) (R & D Systems, Minneapolis, MN, USA) was added 15 minutes prior to CSF treatment. Images of the tube-like structures were captured using a Zeiss Axiovert microscope. Analysis of the tube length was analyzed digitally using Meta Morph imaging system.
Neurobehavioral tests
Seventeen animals were given neurobehavioral testing before MCAO, at 24 hours after MCAO, and at 7 days. Tests that were used include elevated-body swing test (EBST), Bederson, beam walk, and paw grasp, performed in a blinded fashion 8,9.
Infarct Size Determination
Rats were deeply anesthetized with an 85% ketamine/ 15% xylazine combination and decapitated. For animals undergoing CSF collection, prior to decapitation, cerebral spinal fluid was collected (approx 150 µL) by inserting PE-20 tubing connected to a 26 guage needle into the foramen magnum. For evaluation of infarct at 7 days (n=10), rats were anesthetized and transcardially perfused with normal saline, followed by 4% paraformaldehyde (PFA). Brains were quickly removed and fixed in 4% PFA for 3 hours, then sliced into 2 mm coronal sections. These sections were further fixed in 4% PFA for 24 hours, and then transferred to 70% isopropyl alcohol. Hematoxylin and eosin (H&E) staining was performed on slide-mounted, paraffin-embedded 5µm thick sections taken from the 2 mm sections. Grossly visible infarction areas were imaged and quantified by image analysis software (Zeiss-KS300, Oberkochen, Germany) 10. Infarct areas were expressed as percentages of the contralateral hemisphere.
Cavitation Measurements
Cavitation was quantified as the difference in area between the hemispheres, expressed as a percentage of the contralateral hemisphere. These measurements were made on the H&E stained slices, representing the state of the brain volume at 7 days, at which time edema had subsided and collapse of brain volume into the ischemic region had occurred.
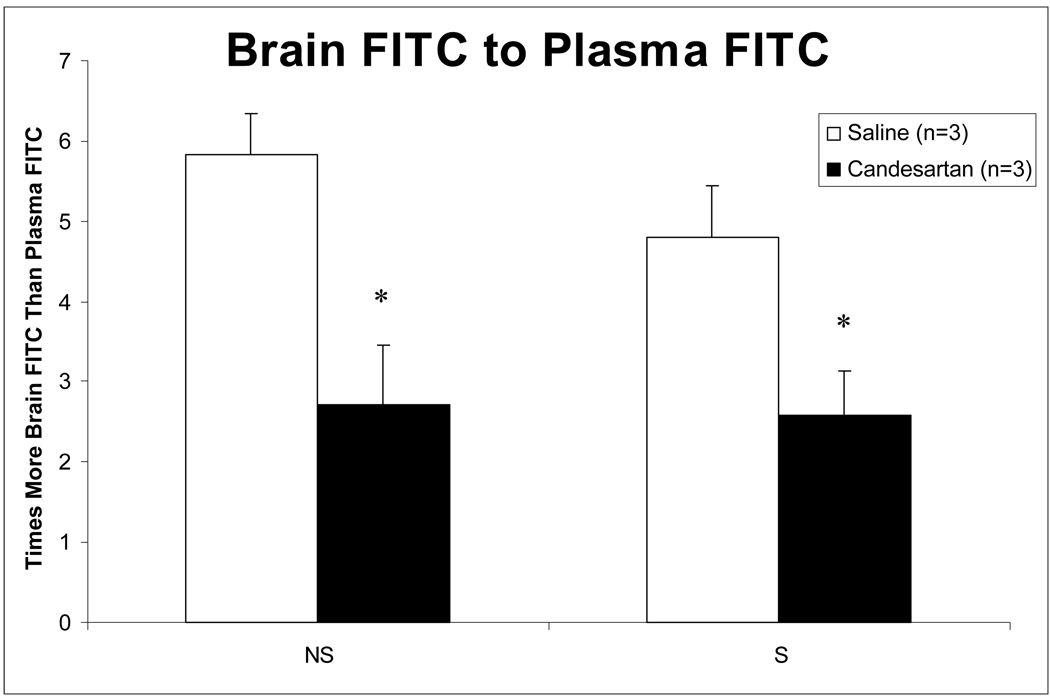
Vascular Permeability Assessment
A separate set of animals were subjected to 3h MCA0 and candesartan 1 mg/kg IV or saline was administered 5 min after onset of reperfusion. 450µl of 5mg albumin from bovine serum fluorescein isothiocyanate (FITC) conjugate diluted in 1 liter PBS was given one hour prior to sacrifice after 21 hours of reperfusion. At sacrifice blood was removed for plasma analysis. Animals were perfused with PBS and brains were removed, hemispheres separated, and each was frozen. Hemispheres were homogenized and normalized volumes of brain and plasma were plated in duplicate and fluorescence was measured. Permeability was expressed at the ratio of brain to plasma fluorescence.
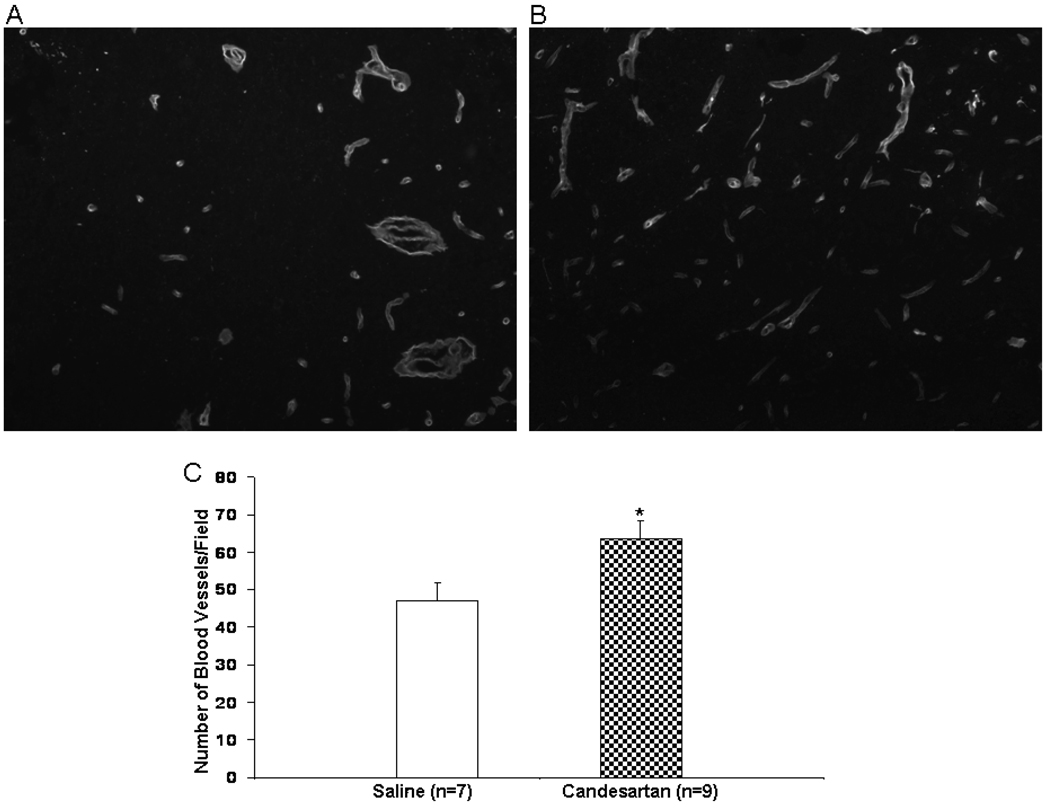
Endothelial Barrier Antigen (EBA) and Laminin Analysis
The immunohistochemical analyses were performed on additional slide-mounted, paraffin-embedded 5µm thick sections collected at 7 days as described above. Slides were deparaffinized, rehydrated in solution of 0.1% Triton-X-100 (Fisher Scientific), flooded with a solution of Proteinase K (20 µg/mL) and 0.05% Trypsin for antigen retrieval, and incubated for 30 minutes at 30° C. For EBA analysis, slides were rinsed 3 times with PBS and blocked with 2% normal calf serum solution (Sigma). A solution of primary mouse anti-EBA antibody (Sternberger Monoclonals SMI-71, Baltimore, MD) was flooded onto each slide and incubated at room temperature for 1 hour in a humidified chamber. Slides were washed 3 times in PBS and a solution of secondary antibody, biotinylated horse anti-mouse (Vector BA-2001 #C0804, Burlingame, CA) was applied and incubated at room temperature for 30 minutes in a humidified chamber. Slides were then washed 3 times with PBS, and incubated in a solution of the fluorescent streptavidin conjugate SA-Cy3 (Jackson #016-160-084, West Grove, PA) for 1 hour at room temperature in a humidified chamber. Slides were visualized by microscope (Zeiss-Observer.Z1, Oberkochen, Germany) using 20x objective, captured by camera (Zeiss Axiocam HRc) in the regions of interest (area of image in mm2) and densitometric measurements were made at 620 nm by Zeiss Axiovision 4.6.3.0 software. Regions of interest were defined as cingulate cortex, lateral cortex and striatum, in both hemispheres, with the cingulate cortex corresponding to the stroke penumbra, and the lateral cortex and striatum representing the core of the infarct.
For the analysis of laminin, slides were prepared as above through the blocking step, then washed and flooded with a solution of primary antibody (rabbit polyclonal – Novus Biologics, Littleton, Co) for 1 hour at room temperature in a humidified chamber. The slides were washed 3 times with PBS and flooded with a solution of biotinylated secondary antibody (Vector Labs #B1205, Burlingame, CA), and incubated for 30 minutes at room temperature in a humidified chamber. Slides were then washed and incubated as above. Images were captured as above using the same regions of interest. In a blinded manner, three images per area per animal were analyzed and the number of vascular profiles averaged.
Statistical Analyses
Differences between candesartan and saline were determined by Student’s t-test for average infarct size, edema, cavitation, MMP activity, VEGF expression, and tube formation. A Wilcoxon Rank Sum test was used to assess the differences on the post-perfusion values of the Bederson score. Differences from baseline behavior for paw grasp and beam walk were analyzed using t-tests for 24-h values and 7-d values. Since baseline values for both measures were 0 for all animals no adjustment for baseline values was needed. An adjusted p-value of 0.025 was used to determine significance to account for the multiple tests. Differences from baseline for EBST at 7-d were analyzed for group difference adjusted for baseline by analysis of covariance. These analyses were performed using SAS 8.2 (SAS Institute Inc., Cary, NC, USA).
Results
Blood Pressure
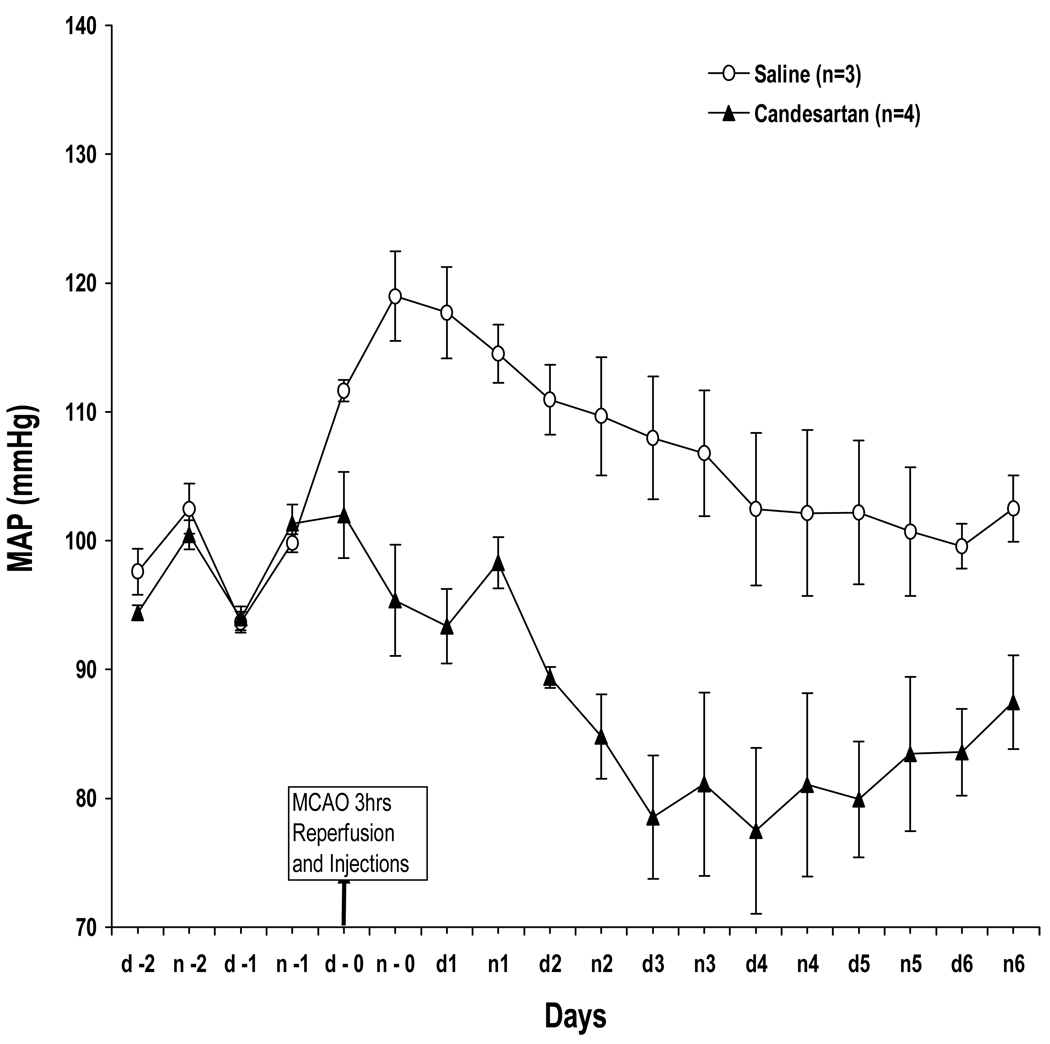
A single dose of candesartan 1mg/kg administered at reperfusion lowered BP for 4 days compared to saline controls (Figure 1). Baseline mean arterial pressure (MAP) was approximately 95 mmHg in both groups, and demonstrated normal circadian variation for the 48 hours prior to MCAO. Upon MCAO, MAP rose rapidly about 25 mmHg in both groups. At reperfusion, the candesartan-treated animals returned to baseline after approximately 4 hours (which agrees with published data regarding time to onset of hypotensive effect for candesartan 11) and remained low for the follow-up period.
Figure 1.
BP telemetry. Continuous BP measurement from 2 days before to 7 days after MCAO. Treatment with a single dose of candesartan at reperfusion lowered BP for 4 days.
Effect of Treatment on Neurobehavioral Testing and Weight
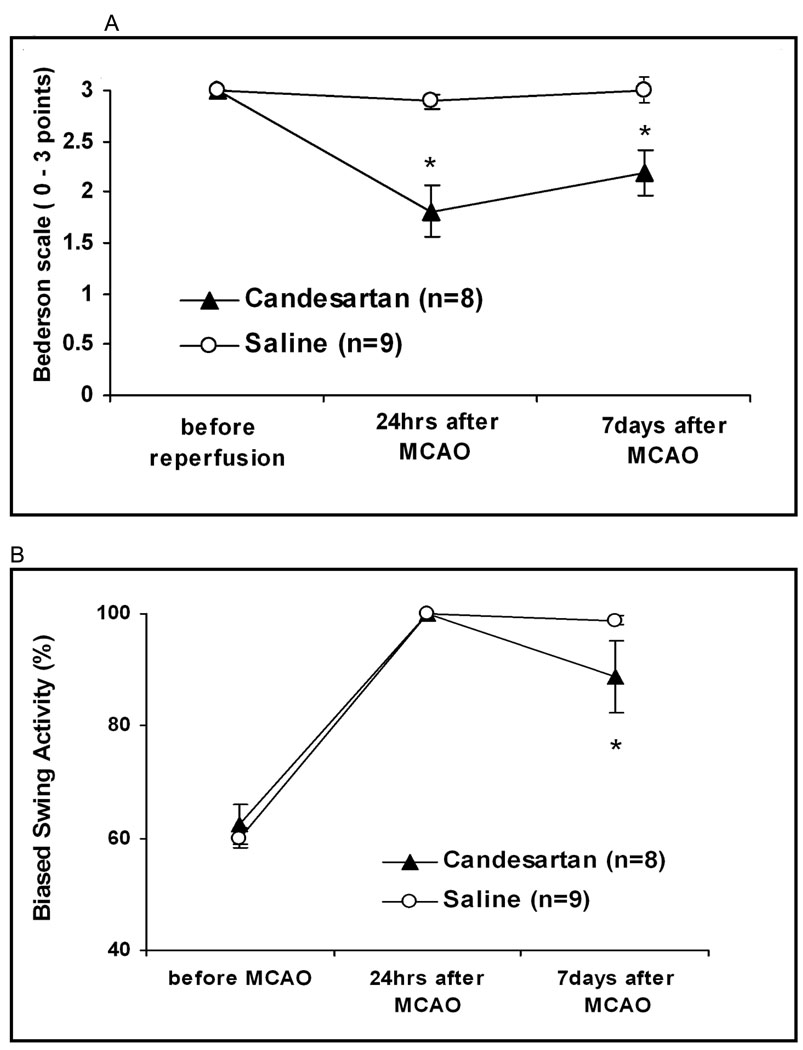
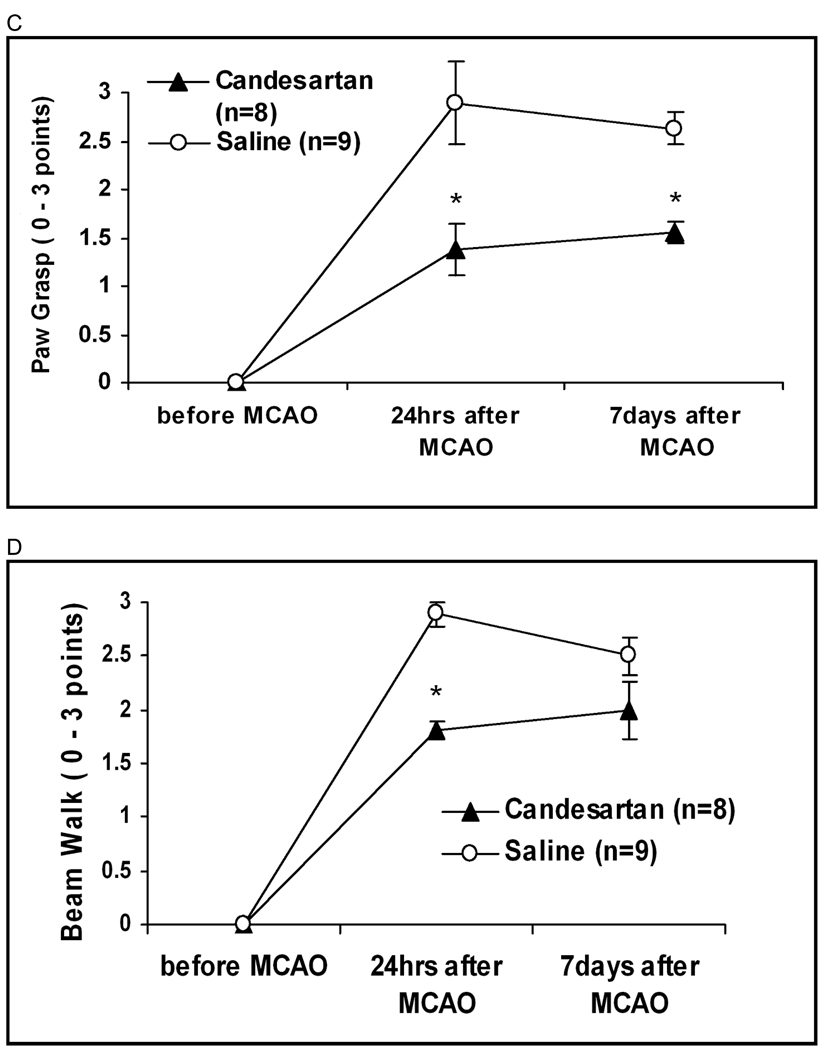
Treatment improved Bederson scores at all time points, including day 7 (2.1 vs. 2.9 points, p=0.0083). Treatment also improved EBST performance including day 7 (22.9 vs. 39.4 % biased left turns, p=0.021) and improved paw grasp including day 7 (1.29 vs. 2.88 points, p=0.0001). Beam walk was improved in the treatment group at 24 hours, but not at 7 days (Figure 2). Animals in the candesartan group had significantly higher weight at 7 days (82.8% vs. 63.1% of pre-operative weight, p=0.017).
Figure 2.
Neurobehavioral tests. Treatment improved Bederson scores (A) at all time points, including day 7 (2.1 vs. 2.9 p=0.0083). Treatment also improved EBST (B) at day 7 (22.9 vs. 39.4 p=0.021) and improved paw grasp (C) including day 7 1.29 vs. 2.88 p=0.0001). Beam walk (D) was improved in the treatment group at 24 hours, but not at day 7.
Effect of Treatment on Infarct Size and Cavitation
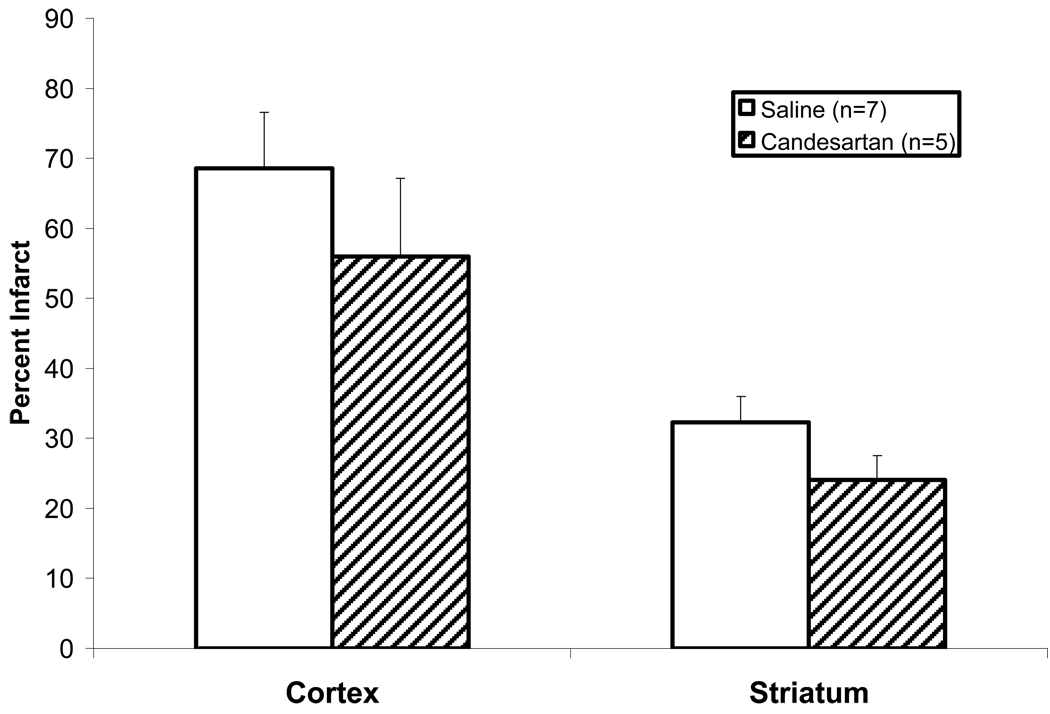
Infarct size was not significantly different between the 2 groups (neither cortex or stratum) at 7 days (but cavitation was decreased slightly in the treated animals at 7 days (4.3% vs. 6.6% p=0.040) suggesting some beneficial preservation of brain tissue integrity in the treatment group (Figure 3).
Figure 3.
Infarct Size at 7 Days. Infarct size was slightly lower in the cortex and striatum of the candesartan-treated animals but it was not significantly different at 7 days.
Effect of Treatment on MMP-2,9 activity, VEGF Expression, and Tube formation
MMP-2 activity was significantly increased in the stroke hemispheres of the treatment group (p=0.035) compared to stroke hemispheres of control animals. MMP-9 activity was not significantly different between the groups (Figure 4). VEGF expression was increased in the stroke hemispheres of the treatment group compared to controls (12.61 pg/mL vs. 5.61 pg/mL, p=0.042).
Figure 4.
MMP Zymography. MMP activity was measured by gelatin zymography on brain samples that were flash-frozen at −80°C at sacrifice (24h). Representative image, top.
BMECs cultured in reduced growth factor Matrigel and incubated with CSF from candesartan-treated stroked animals exhibited 5X increased tube formation (p=0.002) at 24 hours, compared to the saline group. Even without prior stroke, candesartan facilitated tube formation (Figure 5). Administration of a VEGF blocking antibody prior to addition of the CSF significantly reduced the tube formation (data not shown).
Figure 5.
Tube Formation Assay. Wistar animals treated with either candesartan 1 mg/kg or saline had CSF collected prior to sacrifice at 24 hours after MCAO or no stroke. There were 3 animals per group. CSF (50 µL) was added to reduced growth factor Matrigel wells with human BMECs. Candesartan-treated animals demonstrated significantly more tube-like formation after 24 hours of incubation and the effect was blocked by using a VEGF neutralizing antibody (not shown). The largest effect was seen in the CSF from animals treated with candesartan after MCAO.
Effect of Treatment on Vascular Permeability at 24 hours
Despite the increase in VEGF and the resulting proangiogenic state, vascular permeability, as measured by the ratio of brain to plasma fluoresence after administration of FITC-labelled albumin, was significantly decreased in both hemispheres of the candesartan-treated animals (Figure 6; * = p<0.05). This confirms the uncoupling of the permeability and proangiogenic effects of VEGF by candesartan in this model.
Figure 6.
Vascular permeability at 24 hours after MCAO. Candesartan-treated animals had a significant decrease in vascular permeability at 24 hours, as measured by the brain to plasma FITC ratio (mean ± SEM), in both the stroke (S) and nonstroke (NS) hemispheres. * = p< 0.05
Effect of Treatment on EBA and Laminin Staining
In both groups, EBA and laminin staining were decreased in the ischemic hemisphere at 7 days and there was no difference between the treatments in any of the three areas for EBA and for the lateral cortex and cingulate cortex in the laminin staining. In the candesartan treated animals, however, laminin staining was significantly increased in the striatum at 7 days, compared to saline treated animals, indicating an increase in vascular density (Figure 7).
Figure 7.
Laminin staining in the saline (A) and candesartan (B)-treated animals. Shown are representative images from the ischemic hemisphere. Candesartan animals had significantly more vascular profiles per field in the ischemic hemisphere (C) (p=0.037)
Discussion
A single dose of candesartan after reperfusion provided benefits at 7 days as measured by a variety of neurobehavioral tests. It also led to better recovery as measured by weight gain. This suggests that the status immediately after reperfusion is an important determinant of the ultimate damage due to the stroke. The concurrent elevation in two markers of ECM turnover and a proangiogenic state at 24 hours after stroke suggests the intriguing possibility that the observed beneficial effects of candesartan in our model may involve enhancement of early angiogenesis and remodeling, rather than resulting merely from BP lowering. We have previously shown that BP lowering alone can be neurovascular protective4, but we were unable to demonstrate improved functional outcome as a result. Interestingly, although elevated BP has been implicated in increasing MMP activity12, the elevation of MMP-2 activity seen in this experiment occurred in the context of normal and even slightly sub-normal blood pressures, suggesting that the MMP-2 activity was independent of candesartan’s BP lowering effect. The mechanism by which candesartan might lead to increased MMP-2 activity and VEGF expression is unknown at this time, but it seems probable that it is mediated by one of its pleiotropic effects (e.g. antioxidant effect or AT2 agonism) 13.
It is becoming clear that it is the timing of MMP and VEGF expression that determines whether their actions will be positive (promoting angiogenesis and remodeling), or negative (promoting edema and hemorrhage) 14. Specifically, in the experiments of Zhao et al, early inhibition of MMP-9 (within 24 hours) proved beneficial, whereas later inhibition was deleterious. In contrast, in our experiment, early elevation of MMP-2 and VEGF was associated with improved outcomes. It is possible that by blunting the typical acute hypertensive response following ischemic stroke, we allowed remodeling to get underway earlier (within 24 hours) with a decreased risk of hemorrhage and edema. Additionally, it has been shown in mice that AT1 blockade can uncouple VEGF’s proangiogenic function from its permeability-inducing effects 15, reducing the hazards of VEGF elevation seen in our experiments. Even in control animals with no stroke, we were able to demonstrate a proangiogenic effect in the central nervous system.
Although the focus of our research is on the vasculoprotective effects of angiotensin antagonism, it is probable that the vascular and neuronal effects are inextricably linked and are mutually responsible for the recovery benefits seen in our model. In our experiment, there was increased vascularization in the striatum in the candesartan – treated animals at 7 days but no increase in BBB staining or reduction in infarct size. This suggests that the benefits seen may be due to an enhancement of recovery due to increased neovascularization. Others have reported a transient and modest increase in early angiogenesis in the striatum after ischemia and reperfusion (6 days after stroke) associated with recovery 16. It is possible that candesartan augments this effect, leading to sustained benefits in neurologic outcome.
The neurobehavioral benefit from candesartan treatment did not correspond to infarct size reductions in our experiment. Many animal models of stroke have reported little or no correlation between the observed neurobehavioral benefits and the morphological measures of stroke severity 17, 18. In this experiment, the dose of candesartan lowered BP below the baseline levels. Some experimental evidence suggests that over-aggressive lowering of BP with ARBs can worsen infarct area 17. Given the blood pressures achieved, it would be expected that conditions were optimal for infarct extension. It is possible that this in fact occurred and is responsible for the lack of difference seen in infarct size at 7 days.
It is unknown whether a less aggressive dose would confer the same benefits, and research is ongoing to address this issue. Non-hypotensive doses of ARBs have demonstrated benefits in a murine stroke model 19. Clinically, a more conservative approach is prudent; in the ACCESS clinical trial, blood pressure lowering with candesartan was targeted to achieve a 10%–15% reduction over the first 24 hours post-stroke 2 and this resulted in improved long term outcomes.
The use of candesartan as an acute stroke therapy has many exciting possible advantages. First, it is already an established medication approved for use in humans. Second, it can be administered without waiting for the stroke phenotype to be determined, as there is little risk of exacerbating hemorrhagic strokes. The additional attribute of possibly enhancing early angiogenesis and remodeling make it a particularly promising intervention.
Acknowledgments
This work was supported by the American Heart Association - SE affiliate (SCF), NIH-NINDS-RO1NS044216-01 (SCF) and VA Merit Review (SCF), NIH-NINDS-RO3-054688(AE), American Heart Association EIA (AE), American Heart Association SDG (ABE)
Footnotes
Conflict of Interest Disclosures:
Anna Kozak, MS – nothing to disclose
Adviye Ergul, MD, PhD – nothing to disclose
Azza El-Remessy, PhD – nothing to disclose
Maribeth H. Johnson, MS – nothing to disclose
Livia S. Machado, BPharm – nothing to disclose
Hazem F. Elewa, RPh – nothing to disclose
Mohammed Abdelsaid, MS – nothing to disclose
Daniel Wiley, BS – nothing to disclose
Susan Fagan, PharmD – Speaker: Boehringer Ingelheim and Pfizer; Consultant: Pfizer; Grant funding: Pfizer
Contributor Information
Anna Kozak, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, Veterans Administration Medical Center, Augusta, Georgia.
Adviye Ergul, Department of Physiology, Medical College of Georgia, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy.
Azza B. El-Remessy, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, Veterans Administration Medical Center, Augusta, Georgia.
Maribeth H. Johnson, Department of Biostatistics, Medical College of Georgia.
Livia S. Machado, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, Veterans Administration Medical Center, Augusta, Georgia.
Hazem F. Elewa, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, Veterans Administration Medical Center, Augusta, Georgia.
Mohammed Abdelsaid, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, Veterans Administration Medical Center, Augusta, Georgia.
Daniel C. Wiley, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, Veterans Administration Medical Center, Augusta, Georgia.
Susan C. Fagan, Program in Clinical and Experimental Therapeutics, University of Georgia College of Pharmacy, Veterans Administration Medical Center, Augusta, Georgia.
References
- 1.Reboldi G, Angeli F, Cavallini C, Gentile G, Mancia G, Verdecchia P. Comparison between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on the risk of myocardial infarction, stroke and death: A meta-analysis. J Hypertens. 2008;26:1282–1289. doi: 10.1097/HJH.0b013e328306ebe2. [DOI] [PubMed] [Google Scholar]
- 2.Schrader J, Luders S, Kulschewski A, Berger J, Zidek W, Treib J, Einhaupl K, Diener HC, Dominiak P. The access study: Evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke. 2003;34:1699–1703. doi: 10.1161/01.STR.0000075777.18006.89. [DOI] [PubMed] [Google Scholar]
- 3.Fagan SC, Kozak A, Hill WD, Pollock DM, Xu L, Johnson MH, Ergul A, Hess DC. Hypertension after experimental cerebral ischemia: Candesartan provides neurovascular protection. J Hypertens. 2006;24:535–539. doi: 10.1097/01.hjh.0000209990.41304.43. [DOI] [PubMed] [Google Scholar]
- 4.Elewa HF, Kozak A, Johnson MH, Ergul A, Fagan SC. Blood pressure lowering after experimental cerebral ischemia provides neurovascular protection. J Hypertens. 2007;25:855–859. doi: 10.1097/HJH.0b013e3280149708. [DOI] [PubMed] [Google Scholar]
- 5.Li JM, Mogi M, Iwanami J, Min LJ, Tsukuda K, Sakata A, Fujita T, Iwai M, Horiuchi M. Temporary pretreatment with the angiotensin ii type 1 receptor blocker, valsartan, prevents ischemic brain damage through an increase in capillary density. Stroke. 2008;39:2029–2036. doi: 10.1161/STROKEAHA.107.503458. [DOI] [PubMed] [Google Scholar]
- 6.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlongan CV, Sanberg PR. Elevated body swing test: A new behavioral parameter for rats with 6-hydroxydopamine-induced hemiparkinsonism. J Neurosci. 1995;15:5372–5378. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 10.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 11.Gohlke P, Von Kugelgen S, Jurgensen T, Kox T, Rascher W, Culman J, Unger T. Effects of orally applied candesartan cilexetil on central responses to angiotensin ii in conscious rats. J Hypertens. 2002;20:909–918. doi: 10.1097/00004872-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Derosa G, D'Angelo A, Ciccarelli L, Piccinni MN, Pricolo F, Salvadeo S, Montagna L, Gravina A, Ferrari I, Galli S, Paniga S, Tinelli C, Cicero AF. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension. Endothelium. 2006;13:227–231. doi: 10.1080/10623320600780942. [DOI] [PubMed] [Google Scholar]
- 13.Koh KK, Ahn JY, Han SH, Kim DS, Jin DK, Kim HS, Shin MS, Ahn TH, Choi IS, Shin EK. Pleiotropic effects of angiotensin ii receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003;42:905–910. doi: 10.1016/s0735-1097(03)00846-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 15.Sano H, Hosokawa K, Kidoya H, Takakura N. Negative regulation of vegf-induced vascular leakage by blockade of angiotensin ii type 1 receptor. Arterioscler Thromb Vasc Biol. 2006;26:2673–2680. doi: 10.1161/01.ATV.0000245821.77155.c3. [DOI] [PubMed] [Google Scholar]
- 16.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 17.Brdon J, Kaiser S, Hagemann F, Zhao Y, Culman J, Gohlke P. Comparison between early and delayed systemic treatment with candesartan of rats after ischaemic stroke. J Hypertens. 2007;25:187–196. doi: 10.1097/01.hjh.0000254376.80864.d3. [DOI] [PubMed] [Google Scholar]
- 18.Durukan A, Tatlisumak T. Acute ischemic stroke: Overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Iwai M, Liu HW, Chen R, Ide A, Okamoto S, Hata R, Sakanaka M, Shiuchi T, Horiuchi M. Possible inhibition of focal cerebral ischemia by angiotensin ii type 2 receptor stimulation. Circulation. 2004;110:843–848. doi: 10.1161/01.CIR.0000138848.58269.80. [DOI] [PubMed] [Google Scholar]