Abstract
Atherosclerosis is a leading cause of mortality and morbidity in the western world. It has been recognized for over a century, and the understanding of its pathogenesis has undergone many changes. Pathophysiological studies have unravelled the interactions of molecular and cellular elements involved in atherogenesis. The focus has shifted to the novel risk factors as well as characteristics and stability of atherosclerotic plaque; the genetic predisposition has further broadened the pathogenetic mechanisms. This review focuses on the molecular mechanisms involved in the evolution of the atherosclerotic plaque that may pave the way for selecting optimal therapies and preventing plaque complications. Atherosclerosis is no longer a disease attributed mainly to the high lipid content of the body. New insight into the disease pathology has shown it to be a disease of much greater ramifications. Endothelial damage and reactive oxygen species (and other free radicals) have predominantly emerged as factors in virtually all pathways leading to the development of atherosclerosis due to hyperlipidemia, diabetes, hypertension or smoking. Novel risk factors such as hyperhomocysteinemia, infections and systemic lupus erythematosus have emerged. Atherosclerosis has come to be regarded as a chronic inflammatory disease with an autoimmune component. The genetic basis of the disease assumes significance as candidate genes are identified and gene therapy becomes a promising new addition to the existing, less substantial conventional therapies.
Keywords: Atherogenesis, Angiotensin, Cytokines, Growth factors, Oxidized low density lipoproteins, Plaque formation, Reactive oxygen species
A therosclerosis, a multifactorial disease involving the interplay of genetic and environmental factors, is the single largest cause of death and disability in the western world. Although the propensity for developing atherosclerosis is higher in men than in women, the incidence of atherosclerosis is on the rise in women as a result of dietary habits, smoking and mental stress. The disease tends to be more common in white than in black men (1).
Atherosclerosis derives its name from the Greek words ‘sclerosis’ meaning hardening and ‘athere’ meaning gruel (accumulation of lipid). The phenomenon is characterized by accumulation of cholesterol, infiltration of macrophages, proliferation of smooth muscle cells (SMC), accumulation of connective tissue components and formation of thrombus (2). Although many generalized or systemic factors predispose to its development, the disease preferentially affects certain regions of the circulatory system. The growth of the lesion is abluminal in early stages of the disease, and the progress may vary from total cessation in some cases to very rapid with intervening periods of relative quiescence (3). Atherosclerosis starts early in life, and some studies have shown that maternal hypercholesterolemia during pregnancy is associated with a marked increase in the formation of fatty streaks in the human fetus (4,5). The disease appears earliest in the aorta (during fetal life), while it appears in the coronary arteries in the second decade and in the cerebral arteries in the third decade. Some lesions regress while others become complicated. Focal development of the lesions is seen at predisposed sites such as the branch points, whereas the proximal parts and the curvatures of smaller vessels have a higher predilection (6,7).
Distinct clinical manifestations are seen depending on the type of vascular bed affected. Coronary lesions lead to myocardial ischemia or infarction. However, small lesions in the coronary circuit may lead to death when they occur along with arterial vasospasm. Vasospasm alone may lead to acute myocardial infarction in many cases. Similarly, transient ischemic attacks and stroke are seen in the cerebral circulation, whereas intermittent claudication occurs in the peripheral circulation. Infarction of the gut produces lesions in the splanchnic circulation, while renal artery lesions result in ischemia due to reduced renal perfusion and damage the renal parenchyma, leading to uremia and eventually renal failure. Kidneys are a frequent site of thromboembolic phenomena, while atherosclerosis of the renal artery is a common contributor to the development of hypertension, which in itself is a risk factor for atherosclerosis. Atherosclerosis reduces the perfusion of a tissue and, because of its chronic nature, collateral conduits develop over time. Thrombus formation or hemorrhage in an atherosclerotic plaque further reduces the lumen of the vessel. The thromboembolic phenomenon associated with atherosclerosis that occurs because of rupture of unstable plaques is responsible for the acute coronary syndromes and unstable angina (8).
CLASSIFICATION OF ATHEROSCLEROTIC LESIONS
Depending on the histological picture (9,10), the lesions are classified into six types. Type I contains atherogenic lipoproteins and infiltrates mononuclear leukocytes. The intima makes adaptive changes such as thickening. This is seen in most people at birth. Type II has layers of macrophages or foam cells with SMC infiltration from the media into the intima. The gross lesion is designated as a fatty streak and is unique to the disease. Type III is an intermediary stage between types II and IV, with scattered coarse lipid granules or particles that disrupt the integrity of the SMC. Type IV lesions are characterized by typical atheromas containing a large extracellular lipid core and the abluminally growing atherosclerotic lesion. Type V lesions have atheromas with large extracellular lipid cores and the developing fibrous caps. There is an increase in the collagen and (more often) SMC content. Type V lesions are further classified into the Vb and Vc subtypes. Vb are characterized by largely calcified lesions, whereas the Type Vc contain more fibrous connective tissue, little lipid and no calcium (10). Type VI lesions have ruptured atherosclerotic plaque with subsequent fissure formation or hematomas in the arterial lumen. As the thrombogenic lipid core comes into contact with the blood, thrombosis occurs due to platelet aggregation.
RISK FACTORS FOR ATHEROSCLEROSIS
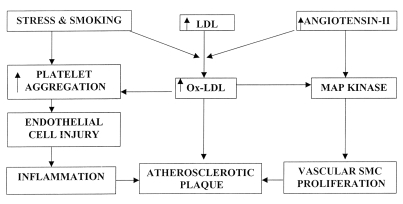
Hyperlipidemic states, diabetes mellitus, smoking and hypertension are some of the risk factors for atherosclerosis; however, any one of these alone is insufficient to produce an atherosclerotic lesion (Figure 1). Besides the traditional risk factors, hyperhomocysteinemia has been linked to coronary artery disease, cerebrovascular disease and peripheral vascular disease. Hyperhomocysteinemia refers to an increased concentration of the sulphur-containing amino acid homocysteine; it promotes atherogenesis by causing endothelial damage. Brief exposure of endothelial cells to homocysteine results in the formation of S-nitrosohomocysteine, a compound with antiplatelet and vasodilator properties. However, on prolonged exposure, the system becomes saturated, leading to decreased formation of reactive oxygen species (ROS) due to auto-oxidation of homocysteine, promoting lipid peroxidation, oxidation of low density lipoproteins (LDL) and generation of nuclear factor-κB (NF-κB), which has mitogenic effects on the vascular SMC (VSMC) (11,12). There is substantial evidence to suggest that fibrinogen (and fibrin) is involved in the formation and growth of atheroma. Fibrin binds thrombi, other coagulative factors and LDL. Its degradation products stimulate SMC migration and proliferation, and promote the uptake of lipids by macrophages. The importance of hyperfibrinogenemia as a risk factor for atherosclerosis is evident from an association between high fibrinogen and a number of other risk factors for ischemic heart disease. Several factors such as smoking, blood cholesterol, diabetes, hypertension, physical activity and arterial hypertension are seen to contribute to the genesis of atherosclerosis (13,14).
Figure 1).
Schematic design depicting the involvement of oxidized low density lipoprotein (oxLDL), injury of endothelial cells and proliferation of vascular smooth muscle cells (SMC) in the development of atherosclerotic plaque. MAP Mitogen-activated protein
GENETIC BASIS OF ATHEROSCLEROSIS
Genetic alterations adversely promote the development of atherosclerotic disease. Familial predilection is an acknowledged risk factor besides the other predisposing diseases such as polygenic disease and combined hyperlipidemia (which has an autosomal trait with high penetrance). In addition to the numerous individual mutations in distinct genes resulting in well defined phenotypes, allelic polymorphism or variations of normal genes without fully developed syndromes frequently occur in the general population and enhance the likelihood of cardiovascular disease. Genetic factors other than those involving lipid metabolism have also been implicated in atherogenesis. Genetic variations in coagulation factors and fibrinogen are responsible for increased thrombogenicity. Genetic alterations of the renin-angiotensin pathway and certain variants of endothelial nitric oxide (NO) synthase have also been associated with hypertension and coronary artery disease. Dissection of the genetic components of atherosclerosis by the forward approach or the candidate gene approach and positional cloning has helped develop the marker genes associated with atherosclerosis and provides an understanding of the pathogenetic mechanisms of the disease (1,15,16).
LIPID METABOLISM AND ITS ROLE IN ATHEROSCLEROSIS
Triglycerides, cholesterol and lipoproteins are implicated in the pathogenesis of coronary artery disease, especially atherosclerosis (9,10). Reduced concentrations of high density lipoprotein (HDL) and increased triglycerides have been shown to be responsible for the genesis of atherosclerotic lesions (17). The National Cholesterol Education Program guidelines consider less than 1 mmol/L of HDL to be the cut-off point below which patients are considered at risk of developing coronary artery disease. The Collaborative Heart Disease Study, conducted at two places in the United Kingdom, found triglyceride concentrations to be more predictive than total cholesterol in determining the risk for coronary artery disease (18). Both of the study groups conclusively reported that triglyceride concentrations greater than 2.25 mmol/L and LDL:HDL ratios greater than 5 were associated with a fivefold increase in the risk for cardiovascular events, especially in people with a metabolic syndrome, insulin resistance syndrome (characterized by a cluster of abnormalities such as hyperinsulinemia, insulin resistance and android fat distribution). Triglyceride concentrations of about 1.7 mmol/L would be considered by many to be the point beyond which risk for coronary artery disease begins. Triglyceride concentrations are commonly increased in diabetes mellitus, particularly the insulin-resistant type, noninsulin-dependent diabetes mellitus (NIDDM), and indicate an enhanced risk of coronary artery disease. Excess insulin promotes nonenzymatic glycation of the lipoproteins, which interact with cytokines and growth factors, and has a predominant role in the formation of atheroma (19).
HDL is a key element in atherosclerosis because of its role in reverse cholesterol transport. The protective effects of HDL are mediated by cell surface HDL receptors, which provide new avenues for the treatment of atherosclerotic cardiovascular diseases. SR-BI, a member of the CD36 protein super family, is a well defined HDL surface receptor that mediates selective HDL cholesterol uptake. Experimental evidence suggests that SR-BI gene transfer can alter the course of the atherogenic sequence by increasing plasma HDL. HDL also counteracts the proatherogenic activity of LDL by mobilizing cholesterol from the arterial intima and delivering it to the liver for excretion into the bile. Studies have found that HDL may function as an acceptor, transporter and inactivator of oxidized LDL (oxLDL) lipids. In addition, the nonlipid-related mechanisms attributed to HDL are (a) inhibition of monocyte adhesion and migration into the arterial intima; (b) stimulation of cell repair and proliferation; (c) preservation of endothelium-dependant vascular activity; (d) inhibition of growth factor-induced VSMC proliferation; and (e) prevention of thrombosis (20). Recent studies emphasize the importance of plasma cholesteryl ester transfer protein in lipoprotein metabolism. Cholesteryl ester transfer protein, a hydrophobic glycoprotein with a molecular weight of 70,000 to 74,000 Da, is synthesized in several organs of the body besides the heart and is induced during the differentiation of monocytes into macrophages. It is considered to be a major protein involved in reverse cholesterol transport, which regulates the plasma concentrations of HDL cholesterol and the size of HDL particles. Patients with cholesteryl ester transfer protein deficiency have been found to have marked hyperalphalipoproteinemia (21).
Lipoprotein lipase and hepatic lipase are members of a family of neutral lipases involved in lipoprotein metabolism. Lipoprotein lipase, the intravascular enzyme anchored onto the endothelial cells, is synthesized mainly in the heart and skeletal muscles; these tissues utilize fatty acids of adipose tissue and mammary glands for energy production. Lipoprotein lipase catalyzes the hydrolysis of various lipids on lipoprotein particles such as chylomicrons and very low density lipoprotein (VLDL) and turns them into smaller remnants that are rapidly cleared from the blood stream (22). On the other hand, hepatic lipase, synthesized and secreted by hepatocytes, affects HDL metabolism. Accumulation of β-VLDL seen on deficiency of hepatic lipase indicates that triglyceride-rich particles may be a substrate for hepatic lipase. Studies in hepatic lipase-deficient patients found hepatic lipase essential for LDL production and redistribution in the HDL fraction (22). These two lipases are important in the metabolism of triglyceride-rich proteins. Defects in these proteins have pathological relevance in causing atherosclerosis (lipoprotein lipase secreted by macrophages is involved in plaque formation). Therapy-associated changes in hepatic lipase alter LDL density, which favourably influences coronary artery disease progression (23). Postprandial lipemia also positively correlates with the progression of coronary artery disease because it involves an increase in triacylglycerol concentration (from dietary fats). Highly atherogenic chylomicrons, small dense LDL particles and reduced HDL concentrations are seen after a fatty meal, and these enhance thrombosis by activating coagulation factor VII and platelet activator inhibitor (24).
Retention of LDL in the vessel wall with subsequent oxidation is considered to be an important event in the early stages of an atherosclerotic lesion. OxLDL promotes the recruitment and retention of monocytes and lymphocytes (and conversion to macrophages) and increases the production of various growth factors and cytokines. The toxicity of oxLDL has been ascertained with cultured VSMC and fibroblasts. Fibroblast growth factor-1 (FGF-1) transfected mouse (NIH 3T3 cells) and FGF-1 transfected rabbit SMC were used to show that oxLDL induces the release of FGF-1 in a concentration-dependant manner; this effect was found to correlate well with the extent of oxidative modification of oxLDL (25). During oxidation of LDL, the apolipoproteins, cholesterol and the unsaturated fatty acids esterified in phospholipids or present in cholesterol esters are usually modified. ROS elicit in vivo and in vitro oxidative decomposition of omega-3 and omega-6 polyunsaturated fatty acids of membrane phospholipids, a process referred to as lipid peroxidation. The process involves the so-called beta-cleavage reaction of lipid hydroperoxides and eventually leads to the formation of aldehydic end products including malonyldialdehyde and 4-hydroxy-2,3-nonenal and other 4-hydroxy-2,3 alkenals. These lipid metabolites are proposed as putative and ultimately toxic messengers, which are potentially able to mediate oxidative stress injury at a molecular level. Their presence has been shown in the subendothelial space of human aortas (26). Lipid peroxidizing enzymes that oxidize polyenoic fatty acids to their corresponding hydroperoxy derivatives are of pathological importance in atherogenesis.
Much attention has been focused on the increased expression of 15-lipoxygenase (15-LOX) found in atherosclerotic lesions of animals and humans. In mammals, 15-LOX are expressed at high concentrations in reticulocytes, eosinophils and certain other cell types but not in peripheral monocytes or normal vessel wall. However, the expression of 15-LOX can be induced in human monocytes in the presence of interleukin (IL) -4 and IL-13. Transgenic mice with a knockout for the LOX gene have shown a reduced incidence of atherosclerosis. In vitro, LOX modifies LDL so as to facilitate its uptake by macrophages. It has been suggested that the overexpression of 15-LOX in macrophages and monocytes may be to counter the deleterious effects of hypercholesterolemia by increasing the cellular capability to metabolize LDL taken up by the cell. The data available suggest that overexpression of 15-LOX is to afford protection against atherosclerosis. Accordingly, it has been concluded that 15-LOX is a complex enzyme with proatherogenic and antiatherogenic effects (27).
LDL is formed in the early stages of atherosclerosis, while oxLDL is formed in the later stages (28). Although LDL binds to LDL receptor and oxLDL binds to scavenger receptor, the vascular effects of minimally modified LDL and oxLDL are similar. Both of these derivatives activate endothelial cells, SMC and monocytes. They also facilitate vasoconstriction, thrombosis and platelet aggregation associated with the activation of intracellular protein kinases and transcription factors such as NFκB or activator protein-1. Furthermore, the expression of cellular adhesion molecules on endothelial cells and monocytes is induced and the synthesis of monocyte chemoattractant protein-1 (MCP-1) –as well as of cytokines and growth factors, in particular platelet-derived growth factor (PDGF) and procoagulant factors such as tissue factor or plasminogen activator inhibitor-I – is stimulated in VSMC. OxLDL also induces a vasoconstrictor state by reducing the formation of the endothelium-derived vasodilators NO and prostaglandin while enhancing the production of the vasoconstrictor endothelin-1 (ET-1).
In the subendothelial space, the uptake of oxLDL by monocyte-derived macrophages reduces macrophage migration and leads to the formation of foam cells, the hallmark of atherosclerotic lesions. OxLDL is chemotactic for monocytes and T-cells. Furthermore, monocytes or macrophages present antigenic epitopes of oxLDL to B-cells, inducing the formation of antibodies to oxLDL and an immune reaction toward deposited oxLDL (28). Endothelial cells mediate the uptake of oxLDL by a recently cloned lectin-like oxLDL receptor-1, which is also involved in mediating endothelial phagocytosis of aged and apoptotic cells. The production of lectin-like oxLDL receptor-1 in endothelial cells is induced by tissue necrosis factor-alpha (TNF-α), ET-1, shear stress, tissue growth factor-beta (TGF-β) and angiotensin II, which accelerates foam cell formation by increased uptake in endothelial cells and macrophages (29).
ENDOTHELIUM: AN ENDOCRINE ORGAN
Vascular endothelium is a multifunctional endocrine organ located strategically between the blood and the vessel wall (30,31). It has a key role in determining vascular function and is intimately involved in atherosclerosis. It handles important regulatory functions that include regulation of vascular tone, formation of NO, prostacyclins and ETs, maintenance of the composition of subendothelial matrix, proliferation of SMC, coagulation, fibrinolysis, permeability of lipoproteins and plasma proteins, and adhesion and migration of blood cells. In 1980, Furchgott and Zawadzki (32) published data supporting the existence of an endothelium-derived product that caused vasorelaxation under the effect of acetylcholine. This endothelium-derived relaxing factor was later shown to be NO (31). Potent vasodilators such as prostacyclin and tissue-type plasminogen activator are also produced by the endothelium (33). Shear stress due to turbulent flow of the blood (34) and stretching of the blood vessels, as in the case of the vertebral arteries, predispose the endothelium to early development of atherosclerosis (35,36). Most damaging to the endothelium are the ROS produced by various risk factors of atherosclerosis such as cigarette smoking, stressful conditions, anaerobic metabolism and radiation. Initiation of oxidative stress through generation of ROS has been linked to diabetes mellitus and chronic uremia because these conditions are characterized by accumulation of ‘advanced glycation’ end products (AGEP) (37). The AGEP peptides perpetrate the vicious cycle of atherogenesis and contribute to atherosclerosis by activation of inflammatory cytokines and enhancement of apolipoprotein B modification, leading to increased uptake of LDL through the macrophage scavenger receptor (MSR) pathway.
Damaged or excessively activated endothelial cells secrete vasoconstrictor factors such as ET-1, as well as factors affecting the differentiation and growth of VSMC. These exert a chemotactic action on leukocytes and platelets, and induce the expression of specific surface adhesion molecules (selectins, integrins and the supergene family of immunoglobulins) that interact with ligands on the surface of leukocytes and platelets. Cellular adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and P selectin overexpressed on the surface of macrophages and endothelial cells are potential markers for atherosclerosis. These molecules mediate the adhesion of monocytes to the endothelial surface, followed by migration into the intima. ET-1 exerts a wide range of actions including vasoconstriction and mitogenic activity on SMC, and results in the formation and release of free radicals and inflammatory cytokines into the circulation (38). At sites of injury or inflammation, proinflammatory cytokines such as IL-1 and TNF-α promote leukocyte adhesion and activation. They also generate activators of neutrophils, such as granulocyte macrophage colony stimulating factor, plasminogen-activating factor and IL-8. These induce a marked activation of three subtypes of mitogen-activated protein kinase (MAPK) such as extracellular signal-regulated kinase (ERK), p38 MAPK and c-Jun N-terminal kinase/stress activated protein kinase for the transduction of messages generated by stressing agents as well as growth factors. The effects of granulocyte macrophage colony stimulating factor and plasminogen-activating factor synthesized by endothelial cells (cytokine activated) markedly potentiate neutrophil activation by increasing their adhesiveness (39). Activated neutrophils contribute to endothelial damage and injury by producing ROS. Pronounced antithrombotic activities and reduced expression of plasminogen-activating factors by the damaged endothelial cells perpetrate the inflammatory process (40–42).
ROLE OF NO IN ENDOTHELIAL FUNCTION
NO is a diatomic molecule that has cytoprotective and cytotoxic properties depending on its concentration. The homeostatic mechanisms of NO include vasodilation, inhibition of platelet adhesion and aggregation, reduction in leukocyte adherence, inhibition of SMC proliferation (43) and inhibition of LDL uptake (44). Impairment of endothelium-dependant vasodilation in hypercholesterolemia is largely caused by reduced bioavailability of NO. This molecule is synthesized by a family of enzymes called the NO synthases from the amino acid l-arginine through the l-arginine-NO pathway (45,46). Three isoforms of the enzyme exist. Two are named depending on the site of production of NO – neuronal and endothelial – whereas the third is inducible NO synthase, which is present in the monocytes and macrophages, SMC, microvascular endothelial cells, fibroblasts, cardiomyocytes, hepatocytes and megakaryocytes. The effects seen with NO result from its ability to undergo substitution, addition, redox and chain-terminating reactions, and its ability to bind with the heme-bound iron to form a nitrosylated adduct. The major site of action is the heme group of guanylyl cyclase enzyme, to which NO readily attaches (especially in the cardiovascular system) leading to its activation and production of cGMP in the cell. In the presence of ROS, however, NO combines with ROS to form peroxynitrite, a toxic metabolite of NO (47). Peroxynitrite has been implicated in the initiation of lipid peroxidation and is believed to have a role in lipoprotein oxidation (48). Recent studies have found that peroxynitrite has a dichotomous nature. Under in vivo conditions where thiol-containing agents such glutathione, albumin and cysteine are present, peroxynitrite is converted to nitrosothiols and related products that have antineutrophilic and cardioprotective properties (49). Being a free radical, it produces endothelial damage, thus acting as both an oxidant and an antioxidant (50).
NO-related mechanisms contributing to progression of the atherosclerotic disease process include impairment of membrane receptors in the arterial wall that interact with agonist physiological stimuli capable of generating NO, reduced concentrations or impaired utilization of l-arginine, reduction in the concentration or activity of inducible and endothelial NO synthases, and impaired release of NO from damaged atherosclerotic endothelium. Impaired NO diffusion from endothelium to VSMC enhances local degradation of NO by increased generation of free radicals or oxidative products and thus impairs the interaction of NO with guanylate cyclase and the consequent decrease in cGMP production (51,52).
ET-1 IN ATHEROSCLEROSIS
ET-1, a potent vasoconstrictor and mitogen for VSMC, was first isolated from porcine endothelial cells. ET-1 is a peptide with a short plasma half-life and is produced by three major cell types found in the atherosclerotic lesions: endothelial cells, VSMC and macrophages (53,54). The ET family consists of three distinct isopeptides: ET-1, ET-2 and ET-3. ET-1 (produced by endothelial cells) is the most abundant of the peptides in the cardiovascular system and its expression is stimulated by growth factors, cytokines, thrombin, TNF-α, TGF-β, IL-1, oxLDL and angiotensin II. Two ET receptor subtypes, ETA and ETB, have been cloned and characterized in mammalian tissue. Endothelial cells express only ETB but VSMC express both ETA and ETB; both these receptors are present in human myocardium and coronary arteries, though ETA receptors predominate. Stimulation of ETA and ETB receptors in the VSMC causes vasoconstriction. On the other hand, activation of ETB receptors located on endothelial cells evokes release of NO or prostacyclin, which results in vasodilation (45,55). However, the mitogenic and angiogenic effects of ET-1 are mediated through the ETB receptor, which requires functional NO synthase for its action (56). Besides atherosclerosis, enhanced ET-1 concentrations have been described in several cardiovascular pathological conditions such as myocardial infarction, congestive heart failure and septic shock. Findings in atherosclerotic lesions in apolipoprotein E-deficient mice suggest strong expression of ETB receptors in VSMC and macrophage foam cells. However, dual ETA/ETB receptor antagonists and not ETA receptor antagonist alone have been suggested to attenuate the progression of atherosclerosis at an advanced stage with VSMC proliferation. ET-1 increases intracellular calcium ions in macrophages, which causes an increase in the production of cytokines and superoxide anion in these cells (54). The initial capture of leukocytes, known as ‘tethering and rolling,’ within the microvessels is mediated by the selectins, including P selectin and E selectin, and perhaps by VCAM-1, and is reportedly caused by ET-1. ET-1 is a potent vasoconstrictor that increases not only leukocyte-endothelial interactions but also platelet-endothelial interactions, thereby potentially enhancing inflammation in the vasculature (57).
ANGIOTENSIN II, CHYMASE AND OXIDATIVE STRESS
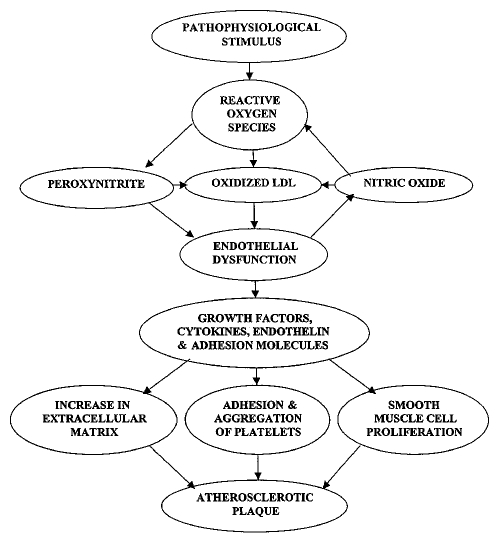
Angiotensin II is the main mediator of the renin-angiotensin-aldosterone system, which maintains the physiological salt and water balance, blood pressure and vascular tone in the body. It is believed to be involved in atherosclerosis (58), endothelial dysfunction (59) and ROS production (60). Angiotensin II promotes atherosclerosis by formation of ROS in macrophages, endothelial cells and VSMC by enhancing the activity of membrane-bound NADH/NADPH oxidase, which oxidizes LDL within the affected segment of the vessel, making it more atherogenic (61–64). Simultaneously, the enhanced expression of cytokines such as TNF-α, IL-1 and PDGF (65) further stimulates ROS production and VSMC proliferation (Figure 2). Angiotensin II exerts proatherogenic, proinflammatory and procoagulant effects on platelets and monocytes. Platelet aggregation is promoted by a decrease in the anticoagulant effect of NO and increased procoagulant plasminogen activating inhibitor-1. The effects of angiotensin II are mediated by angiotensin receptors (AT1, AT2 and AT4), the three high-affinity, tissue-specific and disease-specific receptors. AT1 seems to mediate most of the actions of angiotensin II (66), while AT2 mediates angiotensin II-induced apoptosis in endothelial cells and VSMC. VSMC also express high concentrations of AT1 as a result of high circulating concentrations of angiotensin II. The receptor specific to the endothelial cells is AT4, which is believed to be important in the expression of procoagulant molecules such as plasminogen-activating inhibitor-1. Angiotensin II stimulates SMC proliferation, autocrine growth factors and expression of proinflammatory enzymes such as phospholipase A2 and NADP/NADPH oxidase. It also induces gene transcription for proto-oncogenes, the common pathway for all these proatherogenic effects being ROS formation (67).
Figure 2).
Schematic diagram depicting the involvement of reactive oxygen species, endothelial dysfunction, growth factors, cytokines and adhesion molecules in the genesis of atherosclerosis. LDL Low density lipoprotein
Conventionally, angiotensin II is understood to be produced through a cascade involving renin, from the juxtaglomerular cells of the kidneys, acting on angiotensinogen, from the liver, to produce angiotensin I. Subsequently, angiotensin-converting enzyme (ACE), located at the luminal side of the endothelium, would cleave angiotensin I to angiotensin II. The discovery of ACE inhibiting drugs substantiated this. Angiotensin II receptor blockers provided better control under some circumstances, and this led to the discovery of the angiotensin II receptors and the so-called local renin-angiotensin system. In these local renin-angiotensin systems, renin and ACE are replaced by chymase and cathepsin-D/G. Angiotensin II is generated by other enzymes such as trypsin and kallikrein (68). Chymase is a serine protease that is released by the degranulation of mast cells. In the heart, Urata et al (69) showed that chymase replaces ACE for the conversion of angiotensin I to angiotensin II, and about 80% of angiotensin II comes from chymase compared with about 10% from ACE (69,70). More chymase is expressed in the ventricular tissue than in the atria (the seat of ACE expression in the heart). Other than the mast cells, it is secreted by the endothelial cells and the mesenchymal cells and is localized in the interstitium of the cardiac tissue (70). Chymase is not just localized to the heart and has been found in the alimentary tract tissue, uterus, skin, lungs, colon and spleen (71). It is highly specific for angiotensin II and probably has a much wider role in the effects seen due to angiotensin II but is probably not involved in blood pressure regulation. Chymase is known to have direct proatherogenic effects because it releases latent TGF-β binding protein from the matrix of cultured endothelial cells. TGF-β promotes recruitment and infiltration of inflammatory cells (72). It activates procollagenase to collagenase, indicating a role in matrix degradation and plaque instability (73,74). It also promotes the production of IL-1β, a proinflammatory cytokine. Chymase has a more direct role in atherogenesis because it degrades apolipoprotein B of LDL and apolipoprotein E of HDL (HDL3). Modified LDL is engulfed by the macrophages to form foam cells and is thus unable to perform its normal function of cholesterol clearance through reverse cholesterol transport (75). Chymases are believed to modulate endothelial cell function by activating them to produce more IL-6 and IL-8, thereby amplifying the inflammatory process in the vasculature and augmenting plaque formation (76). The other angiotensin II producer of significance, cathepsin, is a lysosomal enzyme that works best at an acidic pH and cleaves angiotensinogen to angiotensin I. Hence, if angiotensin I generating activity is monitored at a low pH, it would show cathepsin-generated angiotensin I and not renin. But evidence for an exact role for cathepsin in angiotensin II formation is lacking and it is even questionable whether it has a significant role in angiotensinogen cleavage in the cardiovascular system.
ROS IN ATHEROSCLEROSIS
ROS are generated by a variety of extracellular and intra-cellular mechanisms and are responsible for the oxidation of LDL. The extracellular production of ROS is mainly through the action of angiotensin II on the macrophages and endothelial cells; ROS production is also increased after the stimulation of endothelial cells by TNF-α and other cytokines. Intracellular ROS production in the VSMC is mainly through the membrane-bound NAD(P)H oxidases, xanthine oxidase and uncoupled NO synthase, which account for more than 90% of ROS production (60). Angiotensin II is a powerful inducer of NAD(P)H oxidase and can stimulate the production of ROS eightfold in the VSMC. ROS produced intracellularly in response to hormonal stimuli have been found to serve as second messengers (77,78). ROS appear to act as intracellular signalling molecules in vascular cells and mediate phenotypic changes in vascular endothelial and SMC with respect to growth, apoptosis and survival. The net balance between cell proliferation and apoptosis due to ROS production determines the extent of SMC growth response on stimulation with growth factors. A variety of cellular enzymes involved in arachidonic acid metabolism, microsomal cytochrome P-450, xanthine oxidoreductase and mitochondrial electron transport have been identified as potential sources of ROS; cytokines, physical forces and tissue hormones regulate the activity of these oxidases. On the other hand, increased vascular superoxide radical production in association with NO produced by endothelium enhances availability of the harmful peroxynitrite (79).
The formation of ROS in hypercholesterolemia is a result of an imbalance between their production and detoxification by antioxidant defence systems (80). A large amount of ROS is also produced by polymorphonuclear leukocytes. These species, though short lived, are highly reactive and are known to cause lipid peroxidation, DNA damage and protein changes (secondary to chemical reactions with aldehydes) (81). The potential targets of ROS in endothelial cells and SMC are ERKs, stress-activated protein kinases, Akt kinases and NF-κB. The ERKs, which belong to the MAPK family, are important mediators of cell proliferation. However, stress-activated protein kinases, which include c-Jun N-terminal kinases and p38 MAPK, have been implicated in apoptosis. It has been suggested that p38 MAPK is involved in the upregulation of ICAM-1 resulting in endothelial dysfunction. NF-κB has been associated with endothelial dysfunction and vascular inflammation, whereas Akt kinases are involved in antiapoptotic signalling and are regulated by ROS in the angiotensin II-stimulated SMC (25). Thus, ROS serve as important cellular signalling mechanisms that are responsible for the induction of vascular lesions and subsequent atherosclerosis.
EVOLUTION OF AN ATHEROMA
Role of proteoglycans
Altered permeability of the endothelium to the lipoproteins that transport lipids such as cholesterol and triglycerides leads to their binding to the extracellular matrix constituents called proteoglycans (chondroitin sulphate, keratin sulphate or heparin sulphate). Heparin sulphate proteoglycan has a strong affinity for lipoproteins and thus allows for their chemical modification (82). Lipid oxidation leads to the formation of hydroperoxides, oxysterols, lysophospholipids and aldehydic products of free fatty acids (83). Proteoglycans not only have a substantial role in atherosclerosis but also have an antiproliferative role with respect to the VSMC. Expression of the genes for apolipoprotein E and lipoprotein lipase increases the formation of proteoglycans in animal models, where atherosclerosis is found to develop much faster (84,85). The atherogenic molecules, especially oxLDL, positively modulate heparin sulphate proteoglycan.
Lipoprotein modification
Lipoproteins are modified by at least five different methods in the arterial wall; however, the oxidation carried out by the ROS and nonoxidative glycation in diabetes mellitus (mostly NIDDM) and chronic uremia is most significant. The oxidatively modified lipoproteins (oxLDL) are more atherogenic than the native LDL and lead to recruitment of the macrophages to the site of the lesion. The immune system identifies these modified lipoproteins as exogenous in nature, and the leukocytes infiltrate to clear these oxLDL from the intima of the artery. Thus, atherosclerosis is viewed as an autoimmune disease. Nonenzymatic glycation is seen mostly in diabetes mellitus (NIDDM), and the resultant AGEP have a multidirectional role in atherosclerosis. The AGEP not only interact with LDL, HDL and VLDL but also accelerate their oxidative modification and uptake by the macrophages to form foam cells. In addition, AGEP block the formation of NO (86).
The modified lipoproteins engulfed by macrophages result in the formation of foam cells. Subsequently the macrophages undergo apoptosis, leaving behind a lipid core in the subendothelial (intimal) region of the artery that progresses to the atherosclerotic plaque. Another type of modified LDL is the enzymatic nonoxidatively modified LDL (E-LDL), which has been found to activate the complement cascade and macrophages. It also causes selective induction of MCP-1, with no effects on the expression of IL-8 or monocyte inflammatory proteins-1 (alpha and beta). Further, E-LDL stimulates the expression of gp130, the signal-transducing chain of the IL-6 receptor (sIL-6R) family, and the subsequent secretion of IL-6. The mitogenic effects on SMC are invoked by E-LDL through two mechanisms. Foremost is an autocrine mitogenic circuit involving activation of PDGF and FGF-β. The second mechanism is through the upregulation of gp130, rendering VSMC sensitive to trans-signalling through the IL-6/sIL-6R activation pathway. Hence, this new modified LDL stimulates not only MCP-1 and IL-6 but also the proliferation of VSMC (87,88).
Role of inflammatory cells and adhesion molecules
Oxidatively modified lipoproteins promote the infiltration of T-lymphocytes, inflammatory mediators, hemoadhesive molecules and immunoregulatory molecules, and thus provide support to the hypothesis that atherosclerosis is an autoimmune disease (89–91). On the other hand, because endothelium is considered to be the stimulus for the migration of leukocytes, atherosclerosis is classified as an inflammatory disease (44). A more probable pathogenesis seems to be that the immune and inflammatory systems act in tandem and thus lead to the sequential development of the atherosclerotic lesion (44,92). However, atherosclerotic lesions found in patients with Takayasu’s arteritis strongly suggest inflammation as the important component, as does the presence of T-cells and leukocytes (62,63,93). Leukocyte migration is mediated as a consequence not only of the inflammatory response but also of accumulation and modification of the lipoproteins.
Oxidatively modified LDL induces the vascular wall cells to synthesize a chemoattractant cytokine, MCP-1 (94). Recruitment of the leukocytes to the site of the nascent lesion is also augmented by expression of the adhesion molecules VCAM-1, ICAM-1 and P selectin on the surface of the arterial endothelial cells (95,96). Cytokines (protein mediators of inflammation) such as IL-1 and TNF-α in turn induce or augment the expression the adhesion molecules (VCAM and ICAM) on the endothelial surface (97). Soluble forms of these leukocyte and endothelial cell adhesion molecules such as soluble ICAM-1, soluble E-selectin and soluble VCAM-1 have been shown to be present in high concentration in plasma in patients with peripheral ischemic arterial disease. Both soluble VCAM-1 and soluble E selectin serve as mediators of angiogenesis (98). The ICAMs, like an immunoglobulin-like adhesion molecule, are expressed by several cell types including leukocytes and endothelial cells. Their production can be induced by several cytokines such as TNF-α, IL-1 and interferon-gamma, and inhibited by glucocorticoids. These adhesion molecules allow cells to adhere to other cells in addition to binding other extracellular matrix molecules. Defective ICAM-1 expression is seen not only in atherosclerosis but also in a variety of diseases interfering with normal immune function. Inflammatory cytokines such as TNF-α, IL-1 and oxLDL, and increased shear stress contribute to increased ICAM-1 expression, a common finding in atherosclerotic plaques. Increased endothelial ICAM-1 expression contributes to fibrinogen deposition and monocyte attachment, followed by subendothelial migration, a crucial event in the development of atherosclerotic lesions (99).
Platelets also have an important role in the pathophysiology of atherosclerosis in that they induce the formation of MCP-1 and the expression of ICAM-1. MCP-1 belongs to the chemokine family and is induced significantly by IL-1 and is expressed in the activated endothelium; it has the ability to induce migration of leukocytes. The alpha-chemokines chemoattract neutrophils, whereas most beta-chemokines chemoattract monocytes and T-cells. One of the common functions of chemokines is the activation of integrin adhesion molecules, which control the binding of lymphocytes to other cells and to the stromal matrix, regulating lymphocyte activation and locomotion (100). Platelets prevent the differentiation of monocytes into macrophages, bring about a concomitant increase in the activity of secretory enzyme plasminogen-activating factor-acetylhydrolase and are believed to have a critical role in the formation of initial atherosclerotic lesion. Platelets reduce the atherogenic potential of monocytes and macrophages by inhibiting the differentiation process (101).
IL-1, a major cytokine, is involved in the activation of a transcription factor, NF-κB, which regulates the transcription of genes involved in the elaboration of MCP-1 and immunoglobulin-type adhesion molecules such as ICAM-1. This promotes monocyte chemotaxis, adhesion and transmigration into the intima of the vessel wall for the development of atherogenesis (62). It is pointed out that MCP-1 gene expression is increased by chlamydial bacteria through the activation of NF-κB (102). MCP-1 also initiates monocyte recruitment to the arterial wall and is believed to amplify the recruitment and formation of macrophages (45,103–105). Monocytes and macrophages express tissue factor, which leads to thrombin generation and contributes to their physiological and pathophysiological roles in wound repair. The regulation of the expression of tissue factor in monocytes in turn is controlled by the transcription factors NF-κB and activator protein-1 (106).
Foam cell formation
Once resident in the intima, the monocytes differentiate into macrophages and exhibit enhanced expression of scavenger receptors, which bind lipoproteins for endocytosis. In an effort to clear the lipids, these macrophages first attach the lipoproteins to the scavenger receptors, and they result in the formation foam cells. Lack of the LDL receptors responsible for endocytosis is seen in patients with familial hypercholesterolemia, where they have an abundance of arterial lesions and multiple xanthomata containing foam cell-rich lesions. MSR were discovered in studies of patients with familial hypercholesterolemia. Types II and I MSR are both integral membrane proteins containing a collagenase domain and elicit an extraordinarily wide range of ligand-binding capacity. These molecules are responsible for the accumulation of modified LDL during atherogenesis. Targeted disruption of the MSR gene results in the reduction of atherosclerotic lesions in apolipoprotein E-deficient animals. Macrophages from MSR-deficient mice exhibit a marked decrease in modified LDL uptake in vitro, whereas uptake from plasma remains normal, suggesting an alternative mechanism in the circulation. In addition, MSR knockout mice are more susceptible to L-monocytogenes and herpesvirus type 1 infection, indicating a role for MSR in host defence against various pathogens (107).
VSMC proliferation and modulation
Macrophages engulfing the modified lipoproteins produce cytokines and growth factors, which in turn cause further recruitment of the macrophages and VSMC to the site of the lesion. IL-1 and TNF-α stimulate the local production of PDGF and FGF, which have a pivotal role in plaque formation and complication. PDGF is secreted by the activated endothelial cells and causes migration of the SMC from the media to the intima. An important step in the migration and proliferation of SMC is believed to be the secretion of the matrix metalloproteinases (MMP), especially MMP9, which are responsible for the degradation of the internal elastic lamina in the cerebral arteries and abdominal aorta (108). Lipoprotein lipase secreted by the endothelium promotes SMC proliferation. This process involves a series of steps that include protein kinase-C activation and binding of lipoprotein lipase to the VSMC proteoglycans (62,63,109) and results in switching off the gene expressing the contractile proteins and switching on the genes for synthetic activity. This makes VSMC synthesize and produce extracellular matrix (110) and helps in the further progression toward a stable atherosclerotic plaque. TGF-β has been implicated in the production of collagen. TGF-β is also known to inhibit SMC proliferation along with interferon-gamma. Acting in tandem, all these factors lead to the formation of a fibrofatty lesion.
Neovascularization of adventitial vasa vasorum has been observed as part of the development of the atherosclerotic lesion. Angiogenesis is promoted by leptin, the product of the Ob-R gene. A high concentration of leptin within the vasa vasorum and plaque influences inflammatory neovascularization coupling with functional upregulation of vascular endothelial growth factor (VEGF). Activation of endothelial Ob-R (through lectin) generates a growth signal involving a tyrosine kinase-dependant intracellular pathway and promotes the angiogenic processes. There is evidence to suggest a possible involvement of oxLDL in the development of human atherosclerosis through VEGF induction in macrophages (111). SMC proliferation, thrombus and matrix organization involved in primary atherosclerotic lesions also have a role in restenotic lesions after balloon angioplasty and stenting (112).
ATHEROSCLEROTIC PLAQUE CHARACTERISTICS
The vasculature is dynamic in nature and adapts appropriately in response to different stimuli. Compensatory enlargement of the artery (affected by atherosclerosis) to maintain a desired amount of blood flow illustrates this dynamic nature (113). Peripheral arteries dilate at the site of the lesion, and the amount of compensatory enlargement is proportional to plaque thickness (114). The growth of the plaque in the initial period is abluminal in nature, and this results in a failure to identify the acute coronary syndrome at an early stage by various angiographic methods. It should be noted that the stability of an atherosclerotic lesion or plaque depends on the balance between inflammatory and reparative processes. Migration and proliferation of the VSMC and their subsequent phenotypic modulation under the effect of cytokines, metalloproteinases, growth factors and matrix proteins are indicative of this dynamic balance. Phenotypic change in the SMC results in the formation of a fibrous cap (4,110,115). A thick fibrous cap confers stability on the plaque by reducing the circumferential tensile stress and prevents contact between the lipid-rich necrotic core and the blood. The lipid core in the plaque is thrombogenic in nature. While a thick cap also confers structural stability to the plaque, a thin cap experiences a tensile stress and is predisposed to rupture. A circumferential stress is not the only reason for rupture; the plaque constituents may predispose some other parts to rupture as well (116). With the passage of time, SMC lose their reparative character, thereby increasing the likelihood of plaque rupture (117,118). Various factors in the environment of the affected SMC predispose them to early apoptosis (119).
MECHANISMS OF PLAQUE RUPTURE
Inflammatory cells erode the fibrous cap by various mechanisms, and the activation of MMP has been identified as the most significant factor in this regard. MMPs are a group of proteolytic enzymes that are secreted by activated macrophages and endothelial cells (or inflammatory cells). These propeptides are activated by other proteolytic enzymes such as plasmin (120). Chymase also activates procollagenase to collagenase (MMP). The main function or MMPs is in wound healing and repairs by catabolism of the basement membranes and extracellular matrix to allow for the migration of cells. They have an established role in the degenerative chronic diseases due to their catabolic effect. In aneurysms of the abdominal aorta and the cerebrovascular circulation, these are believed to eat away the internal elastic lamina and weaken the artery. Although MMPs favour the migration and proliferation of SMC, they predispose the artery to the formation of aneurysms. In the fibrous cap, stromelysins activate other members of the family, degrade a broad spectrum of peptides that include proteoglycans, type IV collagen, fibronectin and laminin, and destroy the matrix proteins of the cap, and thus increase the likelihood of rupture. Various hormones, cytokines, proto-oncogenes, steroids and growth factors regulate the expression of MMPs. Along with fibrin(ogen)-related antigen, MMPs have been identified in atherosclerotic lesions (121).
Apart from the MMPs, it has also been shown that inflammatory cytokines such as interferon-gamma, which is produced by the T-cells, inhibit the synthesis of collagen by the SMC in combination with the inflammatory molecules IL-1β and TNF-α. A lack of the type-1 procollagen at the sites of plaque rupture suggests that interferon-gamma inhibits matrix synthesis. Erosion of the plaque disposes it to platelet aggregation and intravascular thrombosis, which, being a rich source of SMC mitogens, would lead to a fresh wave of SMC proliferation and repair. The underlying thrombogenic lipid-rich necrotic core is exposed to the circulating platelets. The occurrence of an acute coronary syndrome would thus depend on the balance between prothrombotic and antithrombotic factors.
ANGIOGENESIS AND ATHEROSCLEROSIS
A mitogenic factor (VEGF) that induces the development and formation of new blood vessels was isolated in 1989. It was found to influence tumour growth, wound healing, diabetic retinopathy and collateral formation in ischemic tissues (122). VEGF belongs to the heparin-binding family of growth factors, which include PDGF, FGF and epidermal growth factor (123). This growth factor induces migration and proliferation of endothelial cells, enhances vascular permeability and modulates thrombogenicity. VEGF expression in cultured cells, which include SMCs, macrophages and endothelial cells, is controlled by growth factors and cytokines. VEGF expression is upregulated by various factors in atherosclerotic lesions including hypoxia, TGF-β, angiotensin II, basic FGF and IL-1.
Angiogenesis is also stimulated by FGF, which acts directly on vascular cells and stimulates endothelial cell growth. FGF-1 also has an important role in atherogenesis. Experiments on animals have shown that these growth factors stimulate re-endothelialization, mediate proliferation of SMCs and induce adventitial angiogenesis. FGF-1 is found in macrophages, SMC and endothelial cells, the principal cell type in atherosclerosis with FGF-1 expression (26). In an environment of atherosclerotic lesion, oxLDL-induced FGF-1 release may be among the mediators of endothelial and SMC proliferation. VSMC are a source or target of a large number of growth factors and proinflammatory cytokines. TGF-β is a multifunctional growth factor peptide, which is reported to be present in the milieu of the fibroinflammatory process, promotes cell growth and differentiation, modulates extracellular matrix synthesis and has a role as an immunoregulatory molecule. Evaluation of the in vitro effects of TGF-β on gene expression in cultured rat VSMC suggests that TGF-β1 is produced in the vascular wall during atherogenesis and thus has a pathophysiological role in the autocrine control of IL-1 actions (124). Angiogenesis of the atherosclerotic intima has a critical role in the progression of atherogenesis as well as in the occurrence of its complications, such as intimal hemorrhage, plaque rupture, formation of occlusive thrombus and development of acute coronary syndrome.
The angiogenic processes involve various factors, especially growth factors and cytokines. A pleiotropic cytokine hepatocyte growth factor, which is involved in tissue protection and repair of the endothelium, has been identified in atherosclerotic lesions. A relation between hepatocyte growth factor and the severity of carotid atherosclerosis has been identified. There is evidence to indicate that proinflammatory cytokines IL-1α, IL-1β and TNF-α induce hepatocyte growth factor synthesis (26,125). Defensins, constituting a family of cationic peptides possessing antimicrobial activity, have also been found in the atherosclerotic vessels associated with intimal SMC. Defensins are released in the circulation by activated polymorphonuclear neutrophils, and they inhibit fibrinolysis, thus promoting thrombus formation. These peptides have been reported to be chemotactic for monocytes and T-lymphocytes, stimulate proliferation of fibroblasts and alter intercellular permeability. Furthermore, it has been suggested that defensins may modulate plasmin-mediated effects such as matrix turnover and intimal proliferation (126).
INFECTIOUS AGENTS IN ATHEROSCLEROSIS
Both viral and bacterial infectious agents have been implicated in the evolution of atherosclerosis; the common offending agents are the Chlamydia sp, Helicobacter pylori and cytomegalovirus (127). Seroepidemological studies have shown the presence of Chlamydia pneumoniae (the causative agent of persistent respiratory infections) in the atherosclerotic lesion (128). The first observation of the disease associated with chlamydia came from the seroepidemiological studies of Saikku and colleagues in 1988 (129,130). Subsequent studies involving polymerase chain reaction, electron microscopy, immunocytochemistry and cell culture have reaffirmed the link. An association has been found between smoking and chlamydia (smoking is believed to promote the harbouring of the organism leukocytes) (131). Chlamydial organisms influence the activity of monocytes and uptake of lipoproteins, being resident in the monocytes. Chlamydial lipopolysaccharides and heat shock protein-60 released by the organism induce the macrophages to change to foam cells (132). This leads to the new modality of treating atherosclerosis with antibiotics.
ATHEROSCLEROSIS AND SYSTEMIC LUPUS ERYTHEMATOSUS
Systemic lupus erythematosus (SLE), a multifaceted disease affecting the kidneys, joints and the skin, is associated with atherosclerosis. It has been found to increase the rate of progression of the atherosclerotic lesion and has been accepted as a risk factor for acute coronary syndrome (133–135). Overall, there seems to be a fivefold increase in the incidence of acute coronary syndrome associated with SLE. In case of juvenile-onset lupus nephritis or frank SLE, ultrasound evidence suggests the occurrence of premature atherosclerotic disease (136). The unravelling of the correlation between SLE and acute coronary syndrome has further consolidated the opinion that the latter is an autoimmune inflammatory disease. OxLDL seems to trigger the immune reaction leading to the formation of antibodies that cross-react with the antiphospholipid antibodies found in SLE (137,138). Apart from this, corticosteroids associated with the treatment of SLE are responsible for causing a hyperlipidemic state in the patient. Lysophosphatidylcholine, a peroxide derivative formed during LDL oxidation, has been found to result in a humoral response, and concentrations of antibodies to lysophosphatidylcholine of the immunoglobulin G type were lower in SLE patients than in controls. This led to the hypothesis that these may be ‘consumed’ into the oxLDL-containing immune complexes that are formed during atherosclerosis. Therefore, patients with SLE exhibit a humoral autoimmune response toward the antigenic candidates incriminated in the progression of atherosclerosis (41,42). Formation of lysophospholipid by oxidation or phospholipase A2 activation in the cell membranes from phosphatidylcholine suggests that phospholipase A2 and oxLDL have an enhanced role in SLE associated with atherosclerosis (139).
SUMMARY AND CONCLUSIONS
Atherosclerosis is a complex disease involving the arterial part of the vasculature. It is a fibroproliferative inflammatory process that proceeds through a series of pathological events involving the cardiovascular system, the inflammatory and immune systems, lipid and cholesterol handling mechanisms and blood clotting mechanisms. The disease is modulated by genetic and environmental factors with respect to the end point presentation of the atherosclerotic plaque. Newer diagnostic techniques and analytical methods have made it possible to define and target the disease early in its development at the molecular and genetic levels. Pathological phenomena triggered by the vascular endothelium involve the participation of key cells such as endothelial cells, T-lymphocytes, monocytes and macrophages, VSMC and platelets. Myriad mediators including vasodilators such as NO and prostacyclins, vaso-constrictors such as ETs and angiotensin, cytokines such as IL-1 and TNF-α, and growth factors such as PDGF, FGF and epidermal growth factor contribute to the development and progression of atherosclerosis apart from the cell adhesion molecules such as the integrin, ICAM and VCAM superfamilies and the selectin family.
The atherosclerotic plaque evolves sequentially with an insult to the endothelium (endothelium dysfunction) resulting in the deposition of lipids. Subsequent modification and uptake of lipids by various cells, macrophages and VSMC form foam cells and eventually the characteristic plaque. The clinical manifestations of the disease depend on the site of the plaque and the occurrence of the thromboembolic phenomena as determined by the plaque integrity.
Acknowledgments
The work described in this article was supported by a grant from the Canadian Institutes of Health Research (CIHR). NSD holds a CIHR/Pharmaceutical Research and Development Chair in Cardiovascular Research supported by Merck Frosst Canada Ltd. SM was a visiting scientist from CU Shah College of Pharmacy, SNDT Women’s College, Mumbai, India.
REFERENCES
- 1.Hegele RA. The genetic basis of atherosclerosis. Int J Clin Lab Res. 1997;27:2–13. doi: 10.1007/BF02827237. [DOI] [PubMed] [Google Scholar]
- 2.Turunen MP, Hiltunen MO, Yla-Herttuala S. Gene therapy for angiogenesis, restenosis and related diseases. Exp Gerontol. 1999;34:567–74. doi: 10.1016/s0531-5565(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Schoenbeck U, Mach F, Selwyn AP, Ganz P. Current concepts in cardiovascular pathology: the role of LDL cholesterol in plaque rupture and stabilization. Am J Med. 1998;104:14S–8S. doi: 10.1016/s0002-9343(98)00041-2. [DOI] [PubMed] [Google Scholar]
- 4.Palinski W, Napoli C. Pathophysiological events during pregnancy influence the development of atherosclerosis in humans. Trends Cardiovasc Med. 1999;9:205–14. doi: 10.1016/s1050-1738(00)00022-0. [DOI] [PubMed] [Google Scholar]
- 5.Napoli C, D’Armiento FP, Corso G, et al. Occurrence of the same peroxidative compounds in low density lipoprotein and in atherosclerotic lesions from a homozygous familial hypercholesterolemic patient: a case report. Int J Cardiol. 1997;62:77–85. doi: 10.1016/s0167-5273(97)00180-0. [DOI] [PubMed] [Google Scholar]
- 6.Smedby O, Johansson J, Molgaard J, Olsson AG, Walldius G, Erikson U. Predilection of atherosclerosis for the inner curvature in the femoral artery. A digitized angiography study. Arterioscler Thromb Vasc Biol. 1995;15:912–7. doi: 10.1161/01.atv.15.7.912. [DOI] [PubMed] [Google Scholar]
- 7.Smedby O. Geometric risk factors for atherosclerosis in the aortic bifurcation: a digitized angiography study. Ann Biomed Eng. 1996;24:481–8. doi: 10.1007/BF02648110. [DOI] [PubMed] [Google Scholar]
- 8.Davies MG, Fulton GJ, Hagen PO. Clinical biology of nitric oxide. Br J Surg. 1995;82:1598–610. doi: 10.1002/bjs.1800821206. [DOI] [PubMed] [Google Scholar]
- 9.Stary HC, Chandler AB, Glagov S, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–78. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 10.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 11.van Guldener C, Robinson K. Homocysteine and renal disease. Semin Thromb Hemost. 2000;26:313–24. doi: 10.1055/s-2000-8407. [DOI] [PubMed] [Google Scholar]
- 12.Zappacosta B, Mordente A, Persichilli S, Giardina B, De Sole P. Effect of homocysteine on polymorphonuclear leukocyte activity and luminol-dependent chemiluminescence. Luminescence. 2000;15:257–60. doi: 10.1002/1522-7243(200007/08)15:4<257::AID-BIO594>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Novo S, Failla G, Liquori M, et al. Vascular damage in arterial hypertension: its noninvasive assessment. Cardiologia. 1991;36:323–37. [PubMed] [Google Scholar]
- 14.Novo S, Avellone G, Di Garbo V, et al. Prevalence of risk factors in patients with peripheral arterial disease. A clinical and epidemiological evaluation. Int Angiol. 1992;11:218–29. [PubMed] [Google Scholar]
- 15.Hegele RA. Candidate genes, small effects, and the prediction of atherosclerosis. Crit Rev Clin Lab Sci. 1997;34:343–67. doi: 10.3109/10408369708998097. [DOI] [PubMed] [Google Scholar]
- 16.Kadar A, Glasz T. Development of atherosclerosis and plaque biology. Cardiovasc Surg. 2001;9:109–21. doi: 10.1016/s0967-2109(00)00097-1. [DOI] [PubMed] [Google Scholar]
- 17.Miller NE. Associations of high-density lipoprotein subclasses and apolipoproteins with ischemic heart disease and coronary atherosclerosis. Am Heart J. 1987;113:589–97. doi: 10.1016/0002-8703(87)90638-7. [DOI] [PubMed] [Google Scholar]
- 18.Chanu B. Hypertriglyceridemia: danger for the arteries. Presse Med. 1999;28:2011–7. [PubMed] [Google Scholar]
- 19.Abate N. Obesity and cardiovascular disease. Pathogenetic role of the metabolic syndrome and therapeutic implications. J Diabetes Complications. 2000;14:154–74. doi: 10.1016/s1056-8727(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 20.Acton SL, Kozarsky KF, Rigotti A. The HDL receptor SR-BI: a new therapeutic target for atherosclerosis? Mol Med Today. 1999;5:518–24. doi: 10.1016/s1357-4310(99)01600-7. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita S, Hirano K, Sakai N, Matsuzawa Y. Molecular biology and pathophysiological aspects of plasma cholesteryl ester transfer protein. Biochim Biophys Acta. 2000;1529:257–75. doi: 10.1016/s1388-1981(00)00164-5. [DOI] [PubMed] [Google Scholar]
- 22.Beisiegel U. New aspects on the role of plasma lipases in lipoprotein catabolism and atherosclerosis. Atherosclerosis. 1996;124:1–8. doi: 10.1016/0021-9150(95)05792-7. [DOI] [PubMed] [Google Scholar]
- 23.Zambon A, Hokanson JE, Brown BG, Brunzell JD. Evidence for a new pathophysiological mechanism for coronary artery disease regression: hepatic lipase-mediated changes in LDL density. Circulation. 1999;99:1959–64. doi: 10.1161/01.cir.99.15.1959. [DOI] [PubMed] [Google Scholar]
- 24.Bradley WA, Gianturco SH. Triglyceride-rich lipoproteins and atherosclerosis: pathophysiological considerations. J Intern Med Suppl. 1994;736:33–9. [PubMed] [Google Scholar]
- 25.Irani K. Oxidant signaling in vascular cell growth, death and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–83. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- 26.Ananyeva NM, Tjurmin AV, Berliner JA, et al. Oxidized LDL mediates the release of fibroblast growth factor-1. Arterioscler Thromb Vasc Biol. 1997;17:445–53. doi: 10.1161/01.atv.17.3.445. [DOI] [PubMed] [Google Scholar]
- 27.Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal. 1999;1:255–84. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn H, Chan L. The role of 15-lipoxygenase in atherogenesis: pro- and antiatherogenic actions. Curr Opin Lipidol. 1997;8:111–7. doi: 10.1097/00041433-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Sellmayer A, Hrboticky N, Weber PC. Lipids in vascular function. Lipids. 1999;34(Suppl):S13–8. doi: 10.1007/BF02562222. [DOI] [PubMed] [Google Scholar]
- 30.Furchgott RF. The 1989 Ulf von Euler lecture. Studies on endothelium-dependent vasodilation and the endothelium-derived relaxing factor. Acta Physiol Scand. 1990;139:257–70. doi: 10.1111/j.1748-1716.1990.tb08923.x. [DOI] [PubMed] [Google Scholar]
- 31.Pearson JD. Normal endothelial cell function. Lupus. 2000;9:183–8. doi: 10.1191/096120300678828299. [DOI] [PubMed] [Google Scholar]
- 32.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 33.Celermajer DS. Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol. 1997;30:325–33. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 34.Smedby O, Nilsson S, Bergstrand L. Development of femoral atherosclerosis in relation to flow disturbances. J Biomech. 1996;29:543–7. doi: 10.1016/0021-9290(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 35.Ravensbergen J, Krijger JK, Verdaasdonk AL, Hillen B, Hoogstraten HW. The influence of the blunting of the apex on the flow in a vertebro-basilar junction model. J Biomech Eng. 1997;119:195–205. doi: 10.1115/1.2796080. [DOI] [PubMed] [Google Scholar]
- 36.Ravensbergen J, Ravensbergen JW, Krijger JK, Hillen B, Hoogstraten HW. Localizing role of hemodynamics in atherosclerosis in several human vertebrobasilar junction geometries. Arterioscler Thromb Vasc Biol. 1998;18:708–16. doi: 10.1161/01.atv.18.5.708. [DOI] [PubMed] [Google Scholar]
- 37.Schwedler S, Schinzel R, Vaith P, Wanner C. Inflammation and advanced glycation end products in uremia: simple coexistence, potentiation or causal relationship? Kidney Int. 2001;59(Suppl 78):S32–6. doi: 10.1046/j.1523-1755.2001.59780032.x. [DOI] [PubMed] [Google Scholar]
- 38.Adamopoulos S, Parissis J, Kroupis C, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22:791–7. doi: 10.1053/euhj.2000.2285. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi T, Hato F, Yamane T, et al. Activation of human neutrophil by cytokine-activated endothelial cells. Circ Res. 2001;88:422–9. doi: 10.1161/01.res.88.4.422. [DOI] [PubMed] [Google Scholar]
- 40.Haller H. Endothelial function. General considerations. Drugs. 1997;53(Suppl 1):1–10. doi: 10.2165/00003495-199700531-00003. [DOI] [PubMed] [Google Scholar]
- 41.George J, Harats D, Gilburd B, Levy Y, Langevitz P, Shoenfeld Y. Atherosclerosis-related markers in systemic lupus erythematosus patients: the role of humoral immunity in enhanced atherogenesis. Lupus. 1999;8:220–6. doi: 10.1191/096120399678847597. [DOI] [PubMed] [Google Scholar]
- 42.Shoenfeld Y, Harats D, George J. Heat shock protein 60/65, b2-glycoprotein I and oxidized LDL as players in murine atherosclerosis. J Autoimmun. 2000;15:199–202. doi: 10.1006/jaut.2000.0393. [DOI] [PubMed] [Google Scholar]
- 43.Cooke JP, Tsao PS. Is NO an endogenous antiatherogenic molecule? Arterioscler Thromb. 1994;14:653–5. doi: 10.1161/01.atv.14.5.653. [DOI] [PubMed] [Google Scholar]
- 44.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 45.Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis. 1995;38:87–104. doi: 10.1016/s0033-0620(05)80001-5. [DOI] [PubMed] [Google Scholar]
- 46.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med. 1999;92:164–9. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mugge A, Forstermann U, Lichtlen PR. Endothelial functions in cardiovascular diseases. Z Kardiol. 1989;78:147–60. [PubMed] [Google Scholar]
- 48.Carrizo PH, Dubin M, Stoppani AO. [Physiopathologic effects of nitric oxide and their relationship with oxidative stress] Medicina (B Aires) 1998;58:367–73. [PubMed] [Google Scholar]
- 49.Ronson RS, Nakamura M, Vinten-Johansen J. The cardiovascular effects and implications of peroxynitrite. Cardiovasc Res. 1999;44:47–59. doi: 10.1016/s0008-6363(99)00184-4. [DOI] [PubMed] [Google Scholar]
- 50.Patel RP, Levonen A, Crawford JH, Darley-Usmar VM. Mechanisms of the pro- and anti-oxidant actions of nitric oxide in atherosclerosis. Cardiovasc Res. 2000;47:465–74. doi: 10.1016/s0008-6363(00)00086-9. [DOI] [PubMed] [Google Scholar]
- 51.Napoli C, Ignarro LJ. Nitric oxide and atherosclerosis. Nitric Oxide. 2001;5:88–97. doi: 10.1006/niox.2001.0337. [DOI] [PubMed] [Google Scholar]
- 52.Malek AM, Izumo S, Alper SL. Modulation by pathophysiological stimuli of the shear stress-induced up-regulation of endothelial nitric oxide synthase expression in endothelial cells. Neurosurgery. 1999;45:334–44. doi: 10.1097/00006123-199908000-00028. [DOI] [PubMed] [Google Scholar]
- 53.Dashwood MR, Timm M, Muddle JR, et al. Regional variations in endothelin-1 and its receptor subtypes in human coronary vasculature: pathophysiological implications in coronary disease. Endothelium. 1998;6:61–70. doi: 10.3109/10623329809053405. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi T, Miyauchi T, Iwasa S, et al. Corresponding distributions of increased endothelin-B receptor expression and increased endothelin-1 expression in the aorta of apolipoprotein E-deficient mice with advanced atherosclerosis. Pathol Int. 2000;50:929–36. doi: 10.1046/j.1440-1827.2000.01152.x. [DOI] [PubMed] [Google Scholar]
- 55.Pernow J, Wang QD. Endothelin in myocardial ischaemia and reperfusion. Cardiovasc Res. 1997;33:518–26. doi: 10.1016/s0008-6363(96)00265-9. [DOI] [PubMed] [Google Scholar]
- 56.Goligorsky MS, Budzikowski AS, Tsukahara H, Noiri E. Co-operation between endothelin and nitric oxide in promoting endothelial cell migration and angiogenesis. Clin Exp Pharmacol Physiol. 1999;26:269–71. doi: 10.1046/j.1440-1681.1999.03029.x. [DOI] [PubMed] [Google Scholar]
- 57.Sanz MJ, Johnston B, Issekutz A, Kubes P. Endothelin-1 causes P-selectin-dependent leukocyte rolling and adhesion within rat mesenteric microvessels. Am J Physiol. 1999;277:H1823–30. doi: 10.1152/ajpheart.1999.277.5.H1823. [DOI] [PubMed] [Google Scholar]
- 58.Arakawa K, Urata H. Hypothesis regarding the pathophysiological role of alternative pathways of angiotensin II formation in atherosclerosis. Hypertension. 2000;36:638–41. doi: 10.1161/01.hyp.36.4.638. [DOI] [PubMed] [Google Scholar]
- 59.Keidar S, Attias J, Smith J, Breslow JL, Hayek T. The angiotensin-II receptor antagonist, losartan, inhibits LDL lipid peroxidation and atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 1997;236:622–5. doi: 10.1006/bbrc.1997.6844. [DOI] [PubMed] [Google Scholar]
- 60.Rajagopolan S, Kurz S, Munzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okura Y, Brink M, Itabe H, Scheidegger KJ, Kalangos A, Delafontaine P. Oxidized low-density lipoprotein is associated with apoptosis of vascular smooth muscle cells in human atherosclerotic plaques. Circulation. 2000;102:2680–6. doi: 10.1161/01.cir.102.22.2680. [DOI] [PubMed] [Google Scholar]
- 62.Gawaz M, Brand K, Dickfeld T, et al. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis. 2000;148:75–85. doi: 10.1016/s0021-9150(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 63.Mamputu JC, Desfaits AC, Renier G. Lipoprotein lipase enhances human monocyte adhesion to aortic endothelial cells. J Lipid Res. 1997;38:1722–9. [PubMed] [Google Scholar]
- 64.Wolf G. The role of oxidized low-density lipoprotein in the activation of peroxisome proliferator-activated receptor gamma: implications for atherosclerosis. Nutr Rev. 1999;57:88–91. doi: 10.1111/j.1753-4887.1999.tb06929.x. [DOI] [PubMed] [Google Scholar]
- 65.Wolf G. Free radical production and angiotensin. Curr Hypertens Rep. 2000;2:167–73. doi: 10.1007/s11906-000-0078-z. [DOI] [PubMed] [Google Scholar]
- 66.Strehlow K, Wassmann S, Bohm M, Nickenig G. Angiotensin AT1 receptor over-expression in hypercholesterolaemia. Ann Med. 2000;32:386–9. doi: 10.3109/07853890008995944. [DOI] [PubMed] [Google Scholar]
- 67.Haendeler J, Ishida M, Hunyady L, Berk BC. The third cytoplasmic loop of the angiotensin II type 1 receptor exerts differential effects on extracellular signal-regulated kinase (ERK1/ERK2) and apoptosis via Ras- and Rap1-dependent pathways. Circ Res. 2000;86:729–36. doi: 10.1161/01.res.86.7.729. [DOI] [PubMed] [Google Scholar]
- 68.Arakawa K. Serine protease angiotensin II systems. J Hypertens. 1996;14:S3–7. [PubMed] [Google Scholar]
- 69.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–57. [PubMed] [Google Scholar]
- 70.Urata H, Hoffmann S, Ganten D. Tissue angiotensin II system in the human heart. Eur Heart J. 1994;15(Suppl D):68–78. doi: 10.1093/eurheartj/15.suppl_d.68. [DOI] [PubMed] [Google Scholar]
- 71.Urata H, Strobel F, Ganten D. Widespread tissue distribution of human chymase. J Hypertens. 1994;12(Suppl):S17–22. [PubMed] [Google Scholar]
- 72.Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995;270:4678–96. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- 73.Saarinen J, Kalkkinen N, Welgus HG, Kovanen PT. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem. 1994;269:18134–40. [PubMed] [Google Scholar]
- 74.Kovanen PT. Chymase-containing mast cells in human arterial intima: implications for atherosclerotic disease. Heart Vessels. 1997;12(Suppl):125–7. [PubMed] [Google Scholar]
- 75.Lee M, Lindstedt LK, Kovanen PT. Mast cell-mediated inhibition of reverse cholesterol transport. Arterioscler Thromb. 1992;12:1329–35. doi: 10.1161/01.atv.12.11.1329. [DOI] [PubMed] [Google Scholar]
- 76.Jehle AB, Li Y, Stechschulte AC, Stechschulte DJ, Dileepan KN. Endotoxin and mast cell granule proteases synergistically activate human coronary artery endothelial cells to generate interleukin-6 and interleukin-8. J Interferon Cytokine Res. 2000;20:361–8. doi: 10.1089/107999000312298. [DOI] [PubMed] [Google Scholar]
- 77.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–83. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 78.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91:21–7. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 79.Zalba G, Beaumont J, San Jose G, Fortuno A, Fortuno MA, Diez J. Vascular oxidant stress: molecular mechanisms and patho-physiological implications. J Physiol Biochem. 2000;56:57–64. doi: 10.1007/BF03179777. [DOI] [PubMed] [Google Scholar]
- 80.Landmesser U, Hornig B, Drexler H. Endothelial dysfunction in hypercholesterolemia: mechanisms, pathophysiological importance, and therapeutic interventions. Semin Thromb Hemost. 2000;26:529–37. doi: 10.1055/s-2000-13209. [DOI] [PubMed] [Google Scholar]
- 81.Tooke JE. Possible pathophysiological mechanisms for diabetic angiopathy in type 2 diabetes. J Diabetes Complications. 2000;14:197–200. doi: 10.1016/s1056-8727(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 82.Morawietz H, Rueckschloss U, Niemann B, et al. Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation. 1999;100:899–902. doi: 10.1161/01.cir.100.9.899. [DOI] [PubMed] [Google Scholar]
- 83.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–71. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 84.Paka L, Kako Y, Obunike JC, Pillarisetti S. Apolipoprotein E containing high density lipoprotein stimulates endothelial production of heparin sulfate rich in biologically active heparin-like domains. A potential mechanism for the anti-atherogenic actions of vascular apolipoprotein. J Biol Chem. 1999;274:4816–23. doi: 10.1074/jbc.274.8.4816. [DOI] [PubMed] [Google Scholar]
- 85.Pillarisetti S. Lipoprotein modulation of subendothelial heparan sulfate proteoglycans (perlecan) and atherogenicity. Trends Cardiovasc Med. 2000;10:60–5. doi: 10.1016/s1050-1738(00)00048-7. [DOI] [PubMed] [Google Scholar]
- 86.Kropiewnicka EH, Paczek L. [The importance of advanced glycosylation end products in the creation and progression of atherosclerosis in non-insulin-dependent diabetes mellitus] Przegl Lek. 2000;57:283–7. [PubMed] [Google Scholar]
- 87.Klouche M, May AE, Hemmes M, et al. Enzymatically modified, nonoxidized LDL induces selective adhesion and transmigration of monocytes and T-lymphocytes through human endothelial cell monolayers. Arterioscler Thromb Vasc Biol. 1999;19:784–93. doi: 10.1161/01.atv.19.3.784. [DOI] [PubMed] [Google Scholar]
- 88.Klouche M, Rose-John S, Schmiedt W, Bhakdi S. Enzymatically degraded, nonoxidized LDL induces human vascular smooth muscle cell activation, foam cell transformation, and proliferation. Circulation. 2000;101:1799–805. doi: 10.1161/01.cir.101.15.1799. [DOI] [PubMed] [Google Scholar]
- 89.Nagornev VA, Iakovleva OA, Mal’tseva SV. [Atherogenesis as a reflection of immune inflammation in the vascular wall] Vestn Ross Akad Med Nauk. 2000;(10):45–50. [PubMed] [Google Scholar]
- 90.Wick G, Perschinka H, Xu Q. Autoimmunity and atherosclerosis. Am Heart J. 1999;138:S444–9. doi: 10.1016/s0002-8703(99)70272-3. [DOI] [PubMed] [Google Scholar]
- 91.Wick G. Atherosclerosis: an autoimmune disease due to an immune reaction against heat-shock protein 60. Herz. 2000;25:87–90. doi: 10.1007/pl00001957. [DOI] [PubMed] [Google Scholar]
- 92.Hansson GK. Inflammation and immune response in atherosclerosis. Curr Atheroscler Rep. 1999;1:150–5. doi: 10.1007/s11883-999-0011-0. [DOI] [PubMed] [Google Scholar]
- 93.Numano F, Kishi Y, Tanaka A, Ohkawara M, Kakuta T, Kobayashi Y. Inflammation and atherosclerosis. Atherosclerotic lesions in Takayasu arteritis. Ann NY Acad Sci. 2000;902:65–76. doi: 10.1111/j.1749-6632.2000.tb06301.x. [DOI] [PubMed] [Google Scholar]
- 94.Romano M, Diomede L, Sironi M, et al. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab Invest. 2000;80:1095–100. doi: 10.1038/labinvest.3780115. [DOI] [PubMed] [Google Scholar]
- 95.Munro JM. Endothelial-leukocyte adhesive interactions in inflammatory diseases. Eur Heart J. 1993;14(Suppl K):72–7. [PubMed] [Google Scholar]
- 96.Munro JM, Briscoe DM, Tedder TF. Differential regulation of leucocyte L-selectin (CD62L) expression in normal lymphoid and inflamed extralymphoid tissues. J Clin Pathol. 1996;49:721–7. doi: 10.1136/jcp.49.9.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mach F. The role of chemokines in atherosclerosis. Curr Atheroscler Rep. 2001;3:243–51. doi: 10.1007/s11883-001-0067-y. [DOI] [PubMed] [Google Scholar]
- 98.Blann A, Kumar P, Krupinski J, McCollum C, Beevers DG, Lip GY. Soluble intercellular adhesion molecule-1, E-selectin, vascular cell adhesion molecule-1 and von Willebrand factor in stroke. Blood Coagul Fibrinolysis. 1999;10:277–84. doi: 10.1097/00001721-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 99.van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 100.Howard OM, Ben Baruch A, Oppenheim JJ. Chemokines: progress toward identifying molecular targets for therapeutic agents. Trends Biotechnol. 1996;14:46–51. doi: 10.1016/0167-7799(96)80920-6. [DOI] [PubMed] [Google Scholar]
- 101.Pennewitz A, Finkelberg L, Krause S, Losche W. Platelets inhibit the activity of platelet-activating factor acetylhydrolase in monocyte-derived macrophages. Thromb Res. 1997;86:427–30. doi: 10.1016/s0049-3848(97)00089-3. [DOI] [PubMed] [Google Scholar]
- 102.Molestina RE, Miller RD, Lentsch AB, Ramirez JA, Summersgill JT. Requirement for NF-kappaB in transcriptional activation of monocyte chemotactic protein 1 by Chlamydia pneumoniae in human endothelial cells. Infect Immun. 2000;68:4282–8. doi: 10.1128/iai.68.7.4282-4288.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kowala MC, Recce R, Beyer S, Gu C, Valentine M. Characterization of atherosclerosis in LDL receptor knockout mice: macrophage accumulation correlates with rapid and sustained expression of aortic MCP-1/JE. Atherosclerosis. 2000;149:323–30. doi: 10.1016/s0021-9150(99)00342-1. [DOI] [PubMed] [Google Scholar]
- 104.Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–25. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 105.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 106.Osterud B. Tissue factor expression by monocytes: regulation and pathophysiological roles. Blood Coagul Fibrinolysis. 1998;9(Suppl 1):S9–14. [PubMed] [Google Scholar]
- 107.Suzuki H, Kurihara Y, Takeya M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–6. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 108.Palombo D, Maione M, Cifiello BI, Udini M, Maggio D, Lupo M. Matrix metalloproteinases. Their role in degenerative chronic diseases of abdominal aorta. J Cardiovasc Surg. 1999;40:257–60. [PubMed] [Google Scholar]
- 109.Mamputu JC, Levesque L, Renier G. Proliferative effect of lipoprotein lipase on human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:2212–9. doi: 10.1161/01.atv.20.10.2212. [DOI] [PubMed] [Google Scholar]
- 110.Shanahan CM, Weissberg PL. Smooth muscle cell heterogeneity: patterns of gene expression in vascular smooth muscle cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1998;18:333–8. doi: 10.1161/01.atv.18.3.333. [DOI] [PubMed] [Google Scholar]
- 111.Kang SM, Kwon HM, Hong BK, et al. Expression of leptin receptor (Ob-R) in human atherosclerotic lesions: potential role in intimal neovascularization. Yonsei Med J. 2000;41:68–75. doi: 10.3349/ymj.2000.41.1.68. [DOI] [PubMed] [Google Scholar]
- 112.Glover C, O’Brien ER. Pathophysiological insights from studies of retrieved coronary atherectomy tissue. Semin Interv Cardiol. 2000;5:167–73. doi: 10.1053/siic.2000.0136. [DOI] [PubMed] [Google Scholar]
- 113.Nava E, Noll G, Luscher TF. Nitric oxide in cardiovascular diseases. Ann Med. 1995;27:343–51. doi: 10.3109/07853899509002587. [DOI] [PubMed] [Google Scholar]
- 114.Labropoulos N, Zarge J, Mansour MA, Kang SS, Baker WH. Compensatory arterial enlargement is a common pathobiologic response in early atherosclerosis. Am J Surg. 1998;176:140–3. doi: 10.1016/s0002-9610(98)00135-4. [DOI] [PubMed] [Google Scholar]
- 115.Libby P, Aikawa M, Schonbeck U. Cholesterol and atherosclerosis. Biochim Biophys Acta. 2000;1529:299–309. doi: 10.1016/s1388-1981(00)00161-x. [DOI] [PubMed] [Google Scholar]
- 116.Cheng GC, Loree HM, Kamm RD, Fishbein MC, Lee RT. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation. 1993;87:1179–87. doi: 10.1161/01.cir.87.4.1179. [DOI] [PubMed] [Google Scholar]
- 117.Libby P, Aikawa M. New insights into plaque stabilisation by lipid lowering. Drugs. 1998;56(Suppl 1):9–13. doi: 10.2165/00003495-199856001-00002. [DOI] [PubMed] [Google Scholar]
- 118.Libby P. Multiple mechanisms of thrombosis complicating atherosclerotic plaques. Clin Cardiol. 2000;23(Suppl 6):1–7. doi: 10.1002/clc.4960231103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bennett MR, Macdonald K, Chan SW, Boyle JJ, Weissberg PL. Cooperative interactions between RB and p53 regulate cell proliferation, cell senescence, and apoptosis in human vascular smooth muscle cells from atherosclerotic plaques. Circ Res. 1998;82:704–12. doi: 10.1161/01.res.82.6.704. [DOI] [PubMed] [Google Scholar]
- 120.Zanger D, Yang BK, Ardans J, et al. Divergent effects of hormone therapy on serum markers of inflammation in postmenopausal women with coronary artery disease on appropriate medical management. J Am Coll Cardiol. 2000;36:1797–802. doi: 10.1016/s0735-1097(00)00952-9. [DOI] [PubMed] [Google Scholar]
- 121.Bini A, Mann KG, Kudryk BJ, Schoen FJ. Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1852–61. doi: 10.1161/01.atv.19.8.1852. [DOI] [PubMed] [Google Scholar]
- 122.Inoue M, Itoh H, Tanaka T, et al. Oxidized LDL regulates vascular endothelial growth factor expression in human macrophages and endothelial cells through activation of peroxisome proliferator-activated receptor-gamma. Arterioscler Thromb Vasc Biol. 2001;21:560–6. doi: 10.1161/01.atv.21.4.560. [DOI] [PubMed] [Google Scholar]
- 123.Stephan CC, Brock TA. Vascular endothelial growth factor, a multifunctional polypeptide. P R Health Sci J. 1996;15:169–78. [PubMed] [Google Scholar]
- 124.Di Febbo C, Baccante G, Reale M, et al. Transforming growth factor beta1 induces IL-1 receptor antagonist production and gene expression in rat vascular smooth muscle cells. Atherosclerosis. 1998;136:377–82. doi: 10.1016/s0021-9150(97)00240-2. [DOI] [PubMed] [Google Scholar]
- 125.Malatino LS, Mallamaci F, Benedetto FA, et al. Hepatocyte growth factor predicts survival and relates to inflammation and intima media thickness in end-stage renal disease. Am J Kidney Dis. 2000;36:945–52. doi: 10.1053/ajkd.2000.19087. [DOI] [PubMed] [Google Scholar]
- 126.Barnathan ES, Raghunath PN, Tomaszewski JE, Ganz T, Cines DB, Higazi A. Immunohistochemical localization of defensin in human coronary vessels. Am J Pathol. 1997;150:1009–20. [PMC free article] [PubMed] [Google Scholar]
- 127.Noll G. Pathogenesis of atherosclerosis: a possible relation to infection. Atherosclerosis. 1998;140(Suppl 1):S3–9. doi: 10.1016/s0021-9150(98)00113-0. [DOI] [PubMed] [Google Scholar]
- 128.Farsak B, Yildirir A, Akyon Y, et al. Detection of Chlamydia pneumoniae and Helicobacter pylori DNA in human atherosclerotic plaques by PCR. J Clin Microbiol. 2000;38:4408–11. doi: 10.1128/jcm.38.12.4408-4411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saikku P. Chlamydia pneumoniae and atherosclerosis: an update. Scand J Infect Dis Suppl. 1997;104:53–6. [PubMed] [Google Scholar]
- 130.Saikku P. Epidemiology of Chlamydia pneumoniae in atherosclerosis. Am Heart J. 1999;138:S500–3. doi: 10.1016/s0002-8703(99)70285-1. [DOI] [PubMed] [Google Scholar]
- 131.Berger M, Schroder B, Daeschlein G, et al. Chlamydia pneumoniae DNA in non-coronary atherosclerotic plaques and circulating leukocytes. J Lab Clin Med. 2000;136:194–200. doi: 10.1067/mlc.2000.108941. [DOI] [PubMed] [Google Scholar]
- 132.Laurila A, Bloigu A, Nayha S, Hassi J, Leinonen M, Saikku P. Chronic Chlamydia pneumoniae infection is associated with a serum lipid profile known to be a risk factor for atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:2910–3. doi: 10.1161/01.atv.17.11.2910. [DOI] [PubMed] [Google Scholar]
- 133.Bruce IN, Gladman DD, Urowitz MB. Premature atherosclerosis in systemic lupus erythematosus. Rheum Dis Clin North Am. 2000;26:257–78. doi: 10.1016/s0889-857x(05)70138-1. [DOI] [PubMed] [Google Scholar]
- 134.Urowitz M, Gladman D, Bruce I. Atherosclerosis and systemic lupus erythematosus. Curr Rheumatol Rep. 2000;2:19–23. doi: 10.1007/s11926-996-0064-9. [DOI] [PubMed] [Google Scholar]
- 135.Urowitz MB, Gladman DD. Accelerated atheroma in lupus: background. Lupus. 2000;9:161–5. doi: 10.1191/096120300678828271. [DOI] [PubMed] [Google Scholar]
- 136.Falaschi F, Ravelli A, Martignoni A, et al. Nephrotic-range proteinuria, the major risk factor for early atherosclerosis in juvenile-onset systemic lupus erythematosus. Arthritis Rheum. 2000;43:1405–9. doi: 10.1002/1529-0131(200006)43:6<1405::AID-ANR26>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 137.Vaarala O. Antiphospholipid antibodies and atherosclerosis. Lupus. 1996;5:442–7. doi: 10.1177/096120339600500522. [DOI] [PubMed] [Google Scholar]
- 138.Vaarala O. Antibodies to oxidised LDL. Lupus. 2000;9:202–5. doi: 10.1191/096120300678828280. [DOI] [PubMed] [Google Scholar]
- 139.Wu R, Svenungsson E, Gunnarsson I, et al. Antibodies against lysophosphatidylcholine and oxidized LDL in patients with SLE. Lupus. 1999;8:142–50. doi: 10.1191/096120399678847434. [DOI] [PubMed] [Google Scholar]