Abstract
Batrachotoxins, including many congeners not previously described, were detected, and relative amounts were measured by using HPLC-mass spectrometry, in five species of New Guinean birds of the genus Pitohui as well as a species of a second toxic bird genus, Ifrita kowaldi. The alkaloids, identified in feathers and skin, were batrachotoxinin-A cis-crotonate (1), an allylically rearranged 16-acetate (2), which can form from 1 by sigmatropic rearrangement under basic conditions, batrachotoxinin-A and an isomer (3 and 3a, respectively), batrachotoxin (4), batrachotoxinin-A 3′-hydroxypentanoate (5), homobatrachotoxin (6), and mono- and dihydroxylated derivatives of homobatrachotoxin. The highest levels of batrachotoxins were generally present in the contour feathers of belly, breast, or legs in Pitohui dichrous, Pitohui kirhocephalus, and Ifrita kowaldi. Lesser amounts are found in head, back, tail, and wing feathers. Batrachotoxin (4) and homobatrachotoxin (6) were found only in feathers and not in skin. The levels of batrachotoxins varied widely for different populations of Pitohui and Ifrita, a result compatible with the hypothesis that these birds are sequestering toxins from a dietary source.
Homobatrachotoxin (6) was reported in 1992 to occur in the skins and feathers of three passerine bird species in the genus Pitohui (family Pachycephalidae) endemic to New Guinea and considered toxic by New Guineans (1). No defensive toxin had been previously isolated and identified from avian skin or feathers. Homobatrachotoxin is a member of a group of neurotoxic steroidal alkaloids, collectively called batrachotoxins, that stabilize the open form of voltage-gated sodium channels in nerve and muscle membranes (2). Previously, batrachotoxins were known only from the skins of five neotropical frog species in the genus Phyllobates (family Dendrobatidae). Batrachotoxins occur at high levels in three species of the true poison-dart frogs of western Colombia (Phyllobates terribilis, Phyllobates bicolor, and Phyllobates aurotaenia), while being present only at trace levels in the two Central American species (Phyllobates vittatus and Phyllobates lugubris) (3). In the frogs, batrachotoxin (4), homobatrachotoxin (6), and batrachotoxinin-A (BTX-A; 3) are the major alkaloids (for structures, see Fig. 1). Poison-dart frogs, when raised in captivity, lack batrachotoxins (4) and thus appear in the wild to obtain their skin alkaloids from some source, probably a dietary arthropod. Dendrobatid frogs, when raised in captivity, readily accumulate alkaloids, including BTX-A (3) from an alkaloid-containing diet, unchanged into their skin (5, 6), lending support to such a dietary hypothesis.
Figure 1.
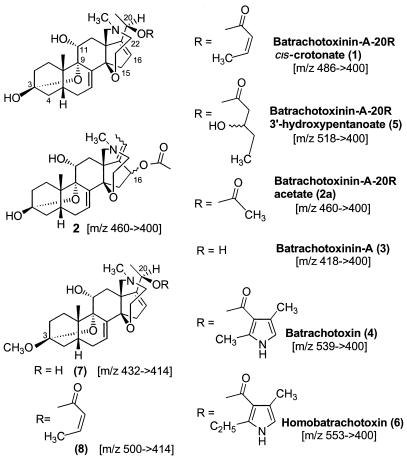
Batrachotoxins found in feathers and/or skins of New Guinean passerine birds. The protonated parent ion (m/z = [molecular ion + 1]) and major fragment ion in chemical ionization mass spectrometry (CIMS) is indicated in brackets for each structure.
Previous studies of Pitohui birds by using direct-probe mass spectrometry and TLC with a sensitive colorimetric reagent revealed only the presence of homobatrachotoxin (6) and, in some samples, traces of batrachotoxin (4) (1, 7). The colorimetric assay using the modified Ehrlich reagent detects only batrachotoxins containing the pyrrole moiety—i.e., 4 and 6. Pharmacological assays suggested, however, that some extracts were slightly more toxic than homobatrachotoxin levels alone could explain (7).
We have now examined feather and skin extracts of Pitohui birds by using HPLC-CIMS and have discovered several other batrachotoxin alkaloids (Fig. 1). Additionally, we examined skin and feathers from 11 specimens of an unrelated passerine species, Ifrita kowaldi, which New Guineans also report as toxic (8, 9). In some Ifrita specimens we detected high levels of 6, lesser levels of 4, as well as most of the other batrachotoxin alkaloids now found in the Pitohui birds.
The amounts and relative proportions of batrachotoxins varied widely among different populations of Pitohui and Ifrita, some specimens having none detectable. Such results are compatible with the hypothesis that these birds obtain batrachotoxin alkaloids from an environmental source and do not produce them de novo.
Materials and Methods
Field Collection and Preparation of Samples.
Pitohui and Ifrita samples were collected from a variety of localities in Papua New Guinea (see footnote, Table 1). Ifrita is normally found at higher altitudes than Pitohui dichrous, so the two species were never collected at the same locality. Wet tissues were placed in glass vials with Teflon-lined lids in 95% (vol/vol) or 100% ethanol, and feathers were placed dry in Ziploc bags (S. C. Johnson & Son). Tissues were stored cold (4°C) whenever possible. Crude ethanol or methanol extracts were made from each sample. For feather extracts, approximately 30–60 mg of feathers was extracted with 2.0 ml of methanol in 3-ml vials with Teflon-lined caps by vigorously shaking for a few minutes. The methanol and two additional 2-ml methanol washes were withdrawn and concentrated under a stream of nitrogen to approximately 50 μl. A 10-μl aliquot corresponding to 6–12 mg of feathers was injected onto the HPLC column as described below. Skin samples, prepared in the field, were trimmed of feathers (some feather stubs remained) and were extracted with 95% ethyl alcohol. Typically samples representing about 50% of total skin from one bird were concentrated to 50 μl before analysis (data not shown).
Table 1.
Batrachotoxin profiles of Pitohui and Ifrita feathers
| Individual* | Feather type | Batrachotoxins†
|
|||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Pitohui cristatus: 2/4 | |||||||
| B-95 | All | +† | − | + | − | − | − |
| C-1/96 | All | − | − | + | − | − | − |
| Pitohui dichrous: 23/57 | |||||||
| A-7/91a | All | ++ | +++ | +++, ++‡ | − | ++ | − |
| A-9/91b | All | ++ | ++ | tr | + | +++ | +++ |
| D-9/93a | Head | − | ++ | ++ | tr | ++ | + |
| D-9/93b | Head | − | tr | + | − | + | − |
| Belly | − | tr | tr | + | ++ | − | |
| E-10/93a | All | − | − | − | − | − | − |
| E-10/93b | Head | +++ | + | + | − | tr | tr |
| Belly | +++ | + | + | tr | tr | + | |
| E-9/93c | Head | +++ | tr | tr | tr | tr | |
| Belly | +++ | +++ | +++, tr‡ | − | − | + | |
| Leg | +++ | +++ | +++ | − | − | − | |
| H-6/93 | All | + | − | + | − | − | − |
| M-1/94a–e | All | − | − | − | − | − | − |
| F-95 | All | ++ | tr | + | − | tr | − |
| A-11/99a | Back | + | + | + | − | +++ | ++ |
| Belly | ++ | ++ | ++ | − | +++ | +++ | |
| Leg | + | ++ | ++ | − | +++ | + | |
| A-11/99b | Back | ++ | ++ | ++ | − | +++ | ++ |
| Belly | ++ | ++ | ++ | − | +++ | ++ | |
| Pitohui ferrugineus: 3/4 | |||||||
| G-7/91; D-9/93; H-12/93 | All | − | − | − | − | − | − |
| Pitohui incertus: 5/5 | |||||||
| I-9/97a–e | All | − | − | − | − | − | − |
| Pitohui kirhocephalus: 13/23 | |||||||
| D-9/93 | Body | − | + | +++ | − | − | − |
| Leg | − | − | − | − | − | − | |
| J-11/93a | Head | − | − | − | − | ++ | − |
| Breast | − | tr | + | tr | +++ | − | |
| J-11/93b | Head | − | tr | tr | tr | ++ | − |
| Belly | − | tr | tr | + | +++ | tr | |
| F-95 | All | + | − | ++ | − | − | − |
| I-9/97a | All | ++ | − | + | − | ++ | − |
| I-9/97b | All | ++ | − | − | − | ++ | − |
| K-97a | All | − | − | − | + | +++ | ++ |
| K-97b–c | All | − | − | − | − | + | − |
| L-97 | All | tr | − | − | − | + | − |
| Pitohui nigrescens: 4/5 | |||||||
| E-10/93a | Back | tr | + | + | − | − | − |
| Belly | tr | tr | tr | − | − | − | |
| E-10/93b | Belly | − | − | tr | tr | + | − |
| Leg | − | − | − | − | − | − | |
| Ifrita kowaldi: 19/35 | |||||||
| M-2/94a | Head | + | − | − | − | − | − |
| Back | ++ | − | − | − | − | − | |
| Belly | +++ | − | − | − | − | + | |
| M-2/94b | All | + | tr | tr | − | − | tr |
| M-2/94c | All | +++ | tr | − | − | − | |
| M-2/94d | Head | + | − | − | − | − | + |
| Back | ++ | − | + | + | − | + | |
| Breast | +++ | +++ | +++ | ++ | tr? | +++ | |
| M-2/94e | Breast | +++ | ++ | − | − | − | − |
| All | +++ | − | + | − | − | + | |
| M-2/94f | All | tr | − | tr | − | − | − |
| M-2/94g | Head | +++ | +++ | + | − | − | + |
| Back | tr | + | + | − | − | + | |
| N-3/94a–d | Breast | − | − | − | − | − | − |
| Down | |||||||
| N-3/94e | All + skin | − | ++ | +++ | − | − | − |
| N-3/94f | All | − | − | − | − | − | − |
Representative samples are reported from total number of samples examined; for example, only two of four (2/4) feather samples of Pitohui cristatus are reported. Specimens are coded as follows: collection site from list below followed by month/yr and lowercase letters for individual birds. In some cases identical results from several birds are combined. Collection localities of specimens (latitude, longitude): A, Varirata National Park, Central Province, S 9° 9.43′, E 147° 21.9′; B, O'o-Pio, near Haia Village, Chimbu Province, S 6° 6.78′, E 145° 2.2′; C, Crater Mountain Research Station, Chimbu Province, S 6° 6.75′, E 145° 5.6′; D, Kau Wildlife Area, Baitabag Village, Madang Province, S 5° 5.11′, E 145° 47′; E, Nokopo Village, Huon Peninsula, Madang Province, S 5° 5.93′, E 146° 36.0′; F, So-obo, near Haia Village, Chimbu Province, S 6° 6.9′, E 145° 3.66′; G, Bonua Village, Bonua River, Central Province, S 10° 10.0′, E 149° 8.5′; H, Balbe Village, Madang Province, S 4° 4.94′, E 145° 36′; I, Ikamea on Fly River tributary of Elevala River, above Kiunga, Western Province, S 6° 07′, E 141° 33′; J, Ohu Village, Madang Province, S 5° 5.16′, E 145° 39′; K, Kakoro Field Station, Gulf Province, collected by staff of Honolulu Hawaii Zoo; S 7° 41′, E 146° 32′; L, Ilauru, Morobe Province Area, collected by staff of Honolulu Hawaii Zoo, S 7° 25′, E 146° 47′; M, Knjnyk, above Kaironk Village, Central Ranges, Madang Province, S 5° 10.1′, E 144° 29′; N, Bambu village, Finisterre Ranges, Huon Peninsula, S 5° 5.87′, E 146° 31.1′.
+++, Major, 20–100% total ion current (TIC); ++, minor, 5–20% TIC; +, very minor, 0.1–5% TIC; tr, trace, detectable only with ion profiling; −, not detectable.
Two isomers.
HPLC-MS Analysis.
A Hewlett Packard (HP) model 1100 liquid chromatograph with variable wavelength detector set at 260 nm (long-wavelength absorption maximum of the pyrrole moiety of 4 and 6) was interfaced with a Finnigan LCQ mass spectrometer operating in the atmospheric pressure chemical ionization (APCI) mode. Under these conditions, the batrachotoxin alkaloids yield a protonated molecular ion and only losses of neutral moieties are observed (e.g., H2O, HOAc, crotonic acid, or substituted pyrrolecarboxylic acids. An HP Zorbax column (SB-C18 column of 4.6 mm × 23 cm, 5-μm particle size) was used with a 0.5-ml/min flow rate of 90:10 A:B → 1:1 A:B over 30 min, where A = 1% HOAc in H2O and B = 1% HOAc in CH3CN. HPLC-grade solvents were used. Typically 10-μl injections were made from 300-μl Wheaton LVI vials. For measurement of deuterium (D) exchanges, solvent A was replaced by 1% HOAc in D2O (99.8% D).
Chemistry.
Crotonic anhydride (95%, “predominantly trans”) was from Aldrich. GC/electron impact mass spectrometry (EIMS) and vapor-phase GC/Fourier transform infrared (FTIR) spectra indicated two components, with the major one (93%) being the trans isomer. The diisopropylethylamine (97%) was also from Aldrich.
Results
Occurrence of Batrachotoxin Alkaloids in Pitohui and Ifrita.
The occurrence of batrachotoxin alkaloids in feathers and individual birds from representative populations and species is presented in Table 1; in some cases distribution of batrachotoxins in different feather types is also tabulated. A complete analysis of the presence of alkaloids in a total of over 150 feather or skin samples will be presented elsewhere.
Identification.
BTX-A (3), batrachotoxin (4), and homobatrachotoxin (6) were identified by HPLC-MS comparisons with authentic compounds isolated from dendrobatid frogs. An isomer (3a) of BTX-A (3) was detected that emerged with a slightly longer retention time. Batrachotoxins 1, 2, and 5 had not been detected before, and their structures were established as described in the following sections with various interconversions depicted in Fig. 2. A dehydro 3 (unknown position of third double bond) and mono- and dihydroxylated derivatives of 6 were commonly detected at trace to minor levels in feather extracts (M + H, m/z 569 → 400; 585 → 416; 585 → 400; data not shown). In addition, a number of other minor alkaloids, some undoubtedly artifacts, were characterized; their tentative structures will be presented elsewhere. However, the methyl ketal (8) of 1 was important in determining the crotonate stereochemistry of 1 and is discussed below. It is probably an artifact of storage of samples in methanol.
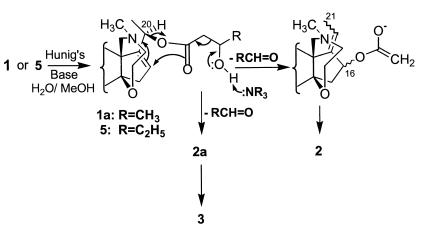
Figure 2.
Base-catalyzed rearrangements of batrachotoxins 1 and/or 5 to the acetates 2 and 2a.
Characterization of BTX-A cis-Crotonate (1).
Natural BTX-A (3) (40 μg in 5 μl of methanol) was converted to a C-20 crotonate with a 1:9 mixture of cis and trans-crotonic anhydride by treatment with excess reagent at room temperature for 18 hr. It had on HPLC-CIMS, a mass spectrum, and retention time on coinjection identical to a new and often major batrachotoxin analog found in feathers or skin of Pitohui or Ifrita species that did not absorb UV at 260 nm and had a CIMS showing a m/z 486 → 400 fragmentation. A synthetic 20S-epimer of BTX-A, dideuterated at C-22 (10), was crotonylated as above. The resulting 20S-crotonate coeluted on HPLC-MS with 1 as did the starting epimeric C-20 alcohols. The epimeric alcohol and ester pairs had virtually identical mass spectra outside of the effect of the two deuterium atoms and thus under our conditions, HPLC-CIMS is unable to distinguish C-20 epimers, either of the alcohols or crotonates. Since HPLC was also evidently not able to separate the 1:9 mixture of cis and trans crotonates expected from 3, we could not establish the crotonate stereochemistry of 1. In an attempt to prove the structure of another new batrachotoxin, namely the methyl ketal (8) of 1, we were able to observe a 1:9 ratio of HPLC peaks, evidently isomers, of identical chemical ionization mass spectra (m/z 500 → 414) resulting when an authentic sample of the methyl ketal (7) of 3 was crotonylated (18 hr, room temperature). The methyl ketal prepared from 1 (MeOH, p-toluenesulfonic acid, 4 hr) cochromatographed with the minor, leading peak of the mixture of crotonates resulting from 7, and consequently 1 has a cis-crotonate moiety. The ketal crotonates on mild acid hydrolysis (3 hr, 0.1 M HCl) gave a single peak on HPLC with m/z 486 → 400, proving that our HPLC conditions cannot separate the cis and trans crotonates of 3.
Characterization of a Rearranged Acetate (2) Formed from BTX-A cis-Crotonate (1).
The semisynthetic 20R-crotonate 1 at 70°C for 1 hr with methanol or 1:1 methanol/water gave little change. However when treated in 50 μl of methanol with 50 μl of a 1:10 suspension of diisopropylethylamine (Hunig's base) and water and heated 3 hr at 70°C in a sealed 300-μl vial, 1 yielded 3, 2, and an equivalent amount of unreacted 1. After 3 days at 70°C, the conversion of 1 to 2 and some 3 was complete. The synthetic dideuterated 20S-crotonate after such base treatment and heat provided a rearranged acetate, 2, that coeluted with 2 produced from the 20R-crotonate and had the same mass spectrum, except both protonated parent and the (MH+ − HOAc) fragment were increased by 2 atomic mass units. Thus again, HPLC is unable to separate what could possibly be C-16 epimers of the rearranged acetates, presuming a likely stereoselective rearrangement. The naturally occurring crotonate in mixtures of other alkaloids found in Pitohui feathers was likewise treated with Hunig's base and water at 70°C for several days and generated the rearranged acetate 2. Along with 2, small amounts of the BTX-A 20R-acetate (2a) were initially produced, which hydrolyzed in turn to 3.
The 20R- or 20S-acetates (2a), produced by several hours exposure of 3 or the deuterium-labeled 20S-epimer to acetic anhydride at room temperature, also could not be separated on the HPLC column. These BTX-A acetates are distinguished from the rearranged acetates by parent and fragment ions of equivalent intensity: i.e., m/z 460 (or 462) ≈ m/z 400 (or 402), unlike the rearranged acetate 2, which exhibits a longer retention time and m/z 460 ≪ m/z 400.
Characterization of BTX-A 3′-Hydroxypentanoate (5).
Another alkaloid (5), found in a number of Pitohui feather extracts but absent from Ifrita extracts, exhibited a fragmentation (m/z 518 → 400) on CIMS corresponding to a loss from the protonated parent of a hydroxypentanoic acid moiety. The assigned structure is based on biosynthetic considerations and is supported by the following observations: (i) no change was detected on heating 1 with methanol for 2 hr at 70°C, thus ruling out an addition of methanol to the crotonyl group to produce an isomeric 3′-methoxycrotonate artifact, and (ii) on deuterium exchange, three exchangeable hydrogens were seen with 5. On heating 5 for 18 hr at 70°C with Hunig's base and water (1:10), 5 was converted to a mixture of 2 and 3 along with unreacted 5.
Discussion
Batrachotoxin-Containing New Guinean Birds: Comparisons of Pitohui and Ifrita.
The genus Pitohui (family Pachycephalidae) comprises six species, five of which had been previously assayed for toxicity both in mice and with radioligand-binding assays (1, 7). All five species surveyed contained detectable levels of batrachotoxins. Two species, P. dichrous and P. kirhocephalus, were significantly more toxic than others. Furthermore, only these two species contained toxin levels that are easily detected in the field, where we occasionally experienced sneezing and upper respiratory irritation when handling them, as described by others (8, 11, 12). Because batrachotoxins are nonvolatile, sloughed-off skin dander or feather bits may carry such toxins through the air, which can then be inhaled. Down feathers are known to release bits of dust or powder, and down-like portions of contour feathers contain high concentrations of batrachotoxins.
The hooded pitohui (P. dichrous) and the variable pitohui (P. kirhocephalus) were found the most likely to contain high levels of toxins, although in some populations/individuals, toxins were absent or present only in trace amounts (see Table 1). Samples of the crested pitohui (P. cristatus) and the black pitohui (P. nigrescens) had traces but only in some specimens, whereas all samples currently tested of the rusty pitohui (P. ferrugineus) and the white-bellied pitohui (P. incertus) were devoid of toxins. An earlier sample of skin from P. ferrugineus was toxic (1) suggesting the presence of batrachotoxins. Passerine species thought to be related to the Pitohui, namely Eulacestoma nigropectus, Pachycephala schlegelii, and Rhagologus leucostigma, had no detectable batrachotoxins, although one of two specimens of another related species, Colluricincla megarhyncha, did have traces of 3 (data not shown).
Pitohui incertus, the white-bellied pitohui, had previously not been sampled for toxins. Its range encompasses a limited area of southern New Guinean lowland swamp forests centered near the Fly River (13). Little is known of this species, but it appears to be a close relative of the rusty pitohui. It apparently feeds on fruit and insects, much like P. ferrugineus and P. kirhocephalus, which share its habitat and join it in mixed-species flocks (13). Unlike the variable pitohui, P. kirhocephalus, which was also collected at the same locality (the Elevala River, a tributary of the Fly River above Kiunga), P. incertus contained no detectable batrachotoxins. Because many pitohui species have variable toxin levels and profiles depending upon season or locality, we do not know whether P. incertus has toxins at other times of the year or other localities. Local hunters do not consider P. incertus as poisonous.
Ifrita kowaldi, or the blue-capped ifrita, is recognized by New Guinean villagers as being toxic or bitter (8, 14). In Kaironk and Simbai Villages (Madang Province), the Ifrita is called “Slek-Yakt,” which literally means “bitter bird” (8), and Simbai villagers claim that eating an Ifrita will cause a burning sensation in buccal tissue similar to but stronger than tasting hot chili peppers. They also claim that breathing deeply from plumage of Ifrita induces coughing or allergy-like reactions. They claim the toxins do not cause vomiting, but that the taste is irritating enough so that few hunters eat them. These descriptions are similar to those attached to the Pitohui (8, 11).
Ifrita is restricted to the New Guinean highlands, generally elevations exceeding 2,000 m above sea level, and live in mossy, moist montane forest. They behave much like nuthatches, gleaning insects and worms from moss, tree trunks, and major branches in the forest midstory. They often move in groups of up to six and are not commonly seen alone. Above Kaironk, where the Ifrita were highly toxic, the habitat is dominated by oak/Garcinia forests and at higher altitudes by Nothofagus forests. Geographic ranges of the Ifrita and the highly toxic P. dichrous and P. kirhocephalus overlap little, if at all; Ifrita occurs at much higher altitudes. Any diet overlap would likely consist of insect groups found throughout altitudinal extremes of New Guinea.
Comparison of Pitohui and Ifrita Birds.
The genus Ifrita consists of a single species, I. kowaldi. The taxonomic position of the genus is enigmatic. Ifrita has been placed in the family Orthonychidae (15, 16), the subfamily Cinclosomatinae (family Corvidae) (17), or the family Muscicapidae (18), but no molecular phylogenetic studies, such as those of Sibley and Ahlquist (19), exist. DNA sequences (J.P.D. and R. Fleischer, unpublished data) suggest Ifrita and Pitohui are not closely related, but additional taxa will be necessary to determine true taxonomic affinities.
Ifrita is insectivorous (8), although I. S. Majnep has speculated (personal communication) that they may incidentally ingest mosses while foraging. Majnep departs from Bulmer's assertion on the diet of the Ifrita, insisting that these birds are strictly insectivorous and do not eat, as alleged by Bulmer (9), the bitter berries of Pittosporum pullifolium Burkhill, which are found in the Schrader Mountains area. Analysis of stomach contents of Ifrita have evidently not been published. Preliminary results by us indicate only insect parts in the stomachs.
The Pitohui, which are much larger than Ifrita, feed on large insects, fruit, and possibly small vertebrates. The body masses of Pitohui vary from 65 g (P. dichrous) to over 100 g (P. kirhocephalus), whereas Ifrita body mass is approximately 30 g.
In Ifrita, we find approximately the same spectrum of batrachotoxins as in Pitohui, and furthermore in some populations we find the same wide variance in the proportions and total amounts of these alkaloids as found in P. dichrous and P. kirhocephalus (see Table 1). One Ifrita population (Finisterre Mountains, Huon Peninsula) lacked alkaloids in five specimens, while the sixth had both 2 and 3. On the other hand, five specimens of a second population found only 240 km away (Schrader Mountains) and sampled just 2 weeks earlier, all contained batrachotoxins. A single individual (Table 1, M-2/94d) from the latter site had toxin concentrations at least 10 times greater than any of the other four specimens collected at that site. It should be noted that all five specimens (Table 1, M-1/94a–e) of the hooded pitohui (P. dichrous) collected the same year at lower elevations in the foothills of the Schrader Mountains had no detectable batrachotoxins. The environments of the sites in the Schrader and Finisterre Mountains at which Ifrita were collected were very similar in daily temperatures and ranges; both are mossy cloud forests. However, the Finisterre Mountains are on the Huon Peninsula and have an insular flora and fauna because of the fact that Huon originated as an oceanic island arc that collided with mainland New Guinea as recently as two million years ago (20). Thus the Ifrita diet likely will differ significantly between the sites.
Toxin Occurrence in Feathers and Skins: Variation Among Populations and Individuals.
Different populations of the same species, either Pitohui or Ifrita, varied widely in toxin profiles and overall toxin concentrations (Table 1). Only in P. dichrous or P. kirhocephalus do we find 5, often as a major alkaloid in the former; it was absent from all samples of Ifrita examined. Toxin-bearing specimens of Pitohui had little or no 4, whereas it appeared commonly along with 6 in Ifrita. Alkaloids 1-3 were shown to be present in freshly extracted skin of voucher specimens of Ifrita kowaldi, supporting the view that 2 and 3 are not merely artifacts of storage in alcohol, although we found considerably more 2 and 3 in field-prepared, aged extracts from the same source.
Quantification of the Ifrita feather extract with the highest level of alkaloids (Table 1, M-2/94d) indicated 5.3 μg of 1, 0.29 μg of 4, and 3.4 μg of 6 per mg of breast down. Comparison with amounts in the Colombian poison-dart frog Phyllobates aurotaenia would suggest that a bird may carry a total amount of 4 and 6 (ca, 0.3 mg) gonewhat greater than in one such frog, but severalfold less than that of the very toxic Colombian poison-dart frog Phyllobates terribilis (3). It should be noted that levels of batrachotoxin in that Ifrita bird were 10-fold higher than in other birds from the same site, and much higher than previously estimated for Pitohui birds (1). In the bird the toxin is concentrated in the belly, breast and leg feathers, but more or less uniformly throughout each feather, whereas in the frog the toxins are highly concentrated in cutaneous granular glands, which are most dense on the dorsal areas. The alkaloids 1 and 5 may prove to be unique to New Guinean birds and were not detected on reanalysis by HPLC-CIMS of various HPLC fractions of frog skin extracts (unpublished data with T. Tokuyama). Significant amounts of 2 were detected in an HPLC fraction of an alkaloid extract from the poison-dart frog Phyllobates terribilis. It is possible that 1, 5, or both were initially present in that extract and degraded to 2 on prolonged storage.
The belly or breast feathers of three Ifrita specimens from the Schrader Mountains were richest in 1 and 6, and one specimen had, in addition, substantial amounts of 2, 3, and 4. Detailed analysis of skin of Ifrita by using tape to remove successive layers indicated that 4 and 6 were not present in even the surface layer of skin, where 1 and 2 were the most abundant alkaloids along with a trace of 3. Lower levels of these three alkaloids were present in lower layers of skin. Details of these analyses and those of Pitohui skin will be presented elsewhere. It appears likely that the reported occurrence of homobatrachotoxin (6) in Pitohui skin (1) may have been in error because of incomplete removal of downy feathers from the skin. This is difficult in the field, and hence most skin samples contained small amounts of attached feather stubs.
No batrachotoxin-related alkaloids have yet been detected in stomach contents of any of the Pitohui or Ifrita specimens examined, nor have any alkaloids yet been detected in dietary items of Pitohui examined so far, which include many species of ants, termites, millipedes, earthworms, grasshoppers, and some fruits (data not shown). Whatever factors are responsible for the production or possible sequestration of these unusual alkaloids is not yet known, although the wide variability of toxins in specimens of the same species, even from the same population, strongly suggests environmental rather than genetic factors. Toxin levels do not appear to be related to the sex of the bird (7). A comparison of a juvenile and adult sample of combined feathers of P. kirhocephalus from the same site indicated that toxins were virtually absent from the juvenile (data not shown).
Alkaloid Chemistry.
The relative base stability of the rearranged C-16 acetate (2) may be due to steric hindrance offered by the N-methylhomomorpholine ring and perhaps the C-21 methyl. The chemical ionization mass spectrum shows very facile loss of HOAc—i.e., a very weak parent at m/z 460 (2%), probably because of favorable orientation for elimination (allylic and pseudoaxial). The crotonate (1) under base catalysis can add hydroxide (to give 1a (Fig. 2) and cleave directly by a retro-aldol process to the 20R acetate (2a) or undergo a concerted [3.3] sigmatropic rearrangement to afford the rearranged C-16 acetate (2) of likely pseudoaxial conformation. The rearranged acetate (2) is also found in freshly extracted skin, so it may be a naturally occurring substance. The hydroxypentanoate (5), found only in Pitohui specimens, does rearrange, as does 1a, by undergoing a base-catalyzed retro-aldol cleavage to propionaldehyde and 2 (Fig. 2).
The second isomer of BTX-A (3a) exhibits a much weaker loss of water and may be a nonallylic alcohol: e.g., Δ15–16. It has now been detected in skins of the poison-dart frog Phyllobates aurotaenia (unpublished data with T. Tokuyama). It is generally assumed that the facile loss of water from 3 seen under CIMS conditions is a result of the C-20 hydroxyl leaving with a proton and not from a C-3 or C-11 hydroxyl cleavage. Supporting this view is the observed facile loss of water from the 3-methyl ketal of BTX-A (7) and no water losses from the various C-20 acylated batrachotoxins.
The cis-crotonate (1) of BTX-A (m/z 486 → 400) may well be an intermediate in the biosynthesis of batrachotoxin (4). The hydroxypentanoate 5 and/or lesser amounts of a homolog of 1 (m/z 500 → 400) were also detected in some extracts and might be precursors of homobatrachotoxin (6).
Ecological Importance.
The fact that natives of New Guinea recognize the bright plumage of both Pitohui and Ifrita and avoid them as food because of their toxins suggests that other predators may also. Both Ifrita and Pitohui build nests in the forest understory roughly 2–3 m from the ground. Breast or belly contour feathers contained the highest toxin concentrations and might rub off onto eggs or be deposited in the nest to provide protection from predators such as snakes, rodents or other birds; particularly any predator that might normally eat an egg whole. Batrachotoxins found in Pitohui or Ifrita feathers may repel or kill lice or other parasites (21).
Acknowledgments
We thank Dr. Takashi Tokuyama (retired, Osaka City Univ., Osaka, Japan) for HPLC fractions from an extract of the poison-dart frog Phyllobates terribilis and a purified sample of the 3-O-methyl ketal of BTX-A (7), Dr. H. Wehrli (Eidgenössische Technische Hochschule, Zurich, Switzerland) for synthetic 22-dideutero-20S-BTX-A, Dr. Yoshihiro Ito (National Heart, Blood, and Lung Institute, National Institutes of Health) for a generous gift of D2O and Andy Mack for feather and tissue samples from O'o Pio and So'obo. For assistance in Papua New Guinea, we thank I. S. Majnep and Kaironk Village; F. Bonaccorso and I. Bigilale at the Papua New Guinea National Museum and Art Gallery; the Christensen Research Institute; A. Mack and the Crater Mountain Wildlife Management Area; Variata National Park, the Papua New Guinea Office of Environment and Conservation, and the people of Bambu Village. Funding for field work was provided by a National Geographic Grant and the Hinds Fund of the Univ. of Chicago. J.P.D. was supported by Wm. Rainey Harper and Graduate Assistance in Areas of National Need Ecology Fellowships from the Univ. of Chicago and a Smithsonian Institution postdoctoral fellowship through the National Zoological Park.
Abbreviations
- CIMS
chemical ionization mass spectrometry
- BTX-A
batrachotoxinin-A
Footnotes
See commentary on page 12948.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.200346897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.200346897
References
- 1.Dumbacher J P, Beehler B M, Spande T F, Garraffo H M, Daly J W. Science. 1992;258:799–801. doi: 10.1126/science.1439786. [DOI] [PubMed] [Google Scholar]
- 2.Albuquerque E X, Daly J W, Witkop B. Science. 1971;172:995–1002. doi: 10.1126/science.172.3987.995. [DOI] [PubMed] [Google Scholar]
- 3.Myers C W, Daly J W, Malkin B. Bull Am Mus Nat Hist. 1978;161:307–366. [Google Scholar]
- 4.Daly J W, Myers C W, Warnick J E, Albuquerque E X. Science. 1980;208:1383–1385. doi: 10.1126/science.6246586. [DOI] [PubMed] [Google Scholar]
- 5.Daly J W, Garraffo H M, Spande T F, Jaramillo C, Rand A S. J Chem Ecol. 1994;20:943–955. doi: 10.1007/BF02059589. [DOI] [PubMed] [Google Scholar]
- 6.Daly J W, Secunda S I, Garraffo H M, Spande T F, Wisnieski A, Cover J F., Jr Toxicon. 1994;32:657–663. doi: 10.1016/0041-0101(94)90335-2. [DOI] [PubMed] [Google Scholar]
- 7.Dumbacher J P. Ph.D. dissertation. Chicago: Univ. of Chicago; 1997. [Google Scholar]
- 8.Majnep I S, Bulmer R. Birds of My Kalam Country. Auckland, New Zealand: Auckland Univ. Press; 1977. [Google Scholar]
- 9.Bulmer R N H. Man. 1964. , article no. 183, pp. 147–150. [Google Scholar]
- 10.Imhof R, Gössinger E, Graf W, Berner-Fenz L, Berner H, Schaufelberger R, Wehrli H. Helv Chim Acta. 1973;56:139–162. doi: 10.1002/hlca.19730560107. [DOI] [PubMed] [Google Scholar]
- 11.Kocher-Schmid C. Of People and Plants: A Botanical Ethnography of Nokopo Village, Madang and Morobe Provinces, Papua New Guinea. in Kommission bei Wepf und Co., Basel, Switzerland: Ethnologisches Seminar der Universität und Museum für Völkerkunde; 1991. [Google Scholar]
- 12.Kocher-Schmid C. Muruk. 1993;6:1–15. [Google Scholar]
- 13.Diamond J M, Raga M N. Emu. 1978;78:49–53. [Google Scholar]
- 14.Gorlich J. Science in New Guinea. 1995;21:41–42. [Google Scholar]
- 15.Rand A L, Gilliard E T. Handbook of New Guinea Birds. London: Weidenfeld and Nicolson; 1967. [Google Scholar]
- 16.Beehler B M, Pratt T K, Zimmerman D A. Birds of New Guinea. Princeton, NJ: Princeton Univ. Press; 1986. [Google Scholar]
- 17.Sibley C G, Monroe B L., Jr . Distribution and Taxonomy of Birds of the World. New Haven, CT: Yale Univ. Press; 1990. [Google Scholar]
- 18.Weldon P J, Rappole J H. J Chem Ecol. 1997;23:2609–2633. [Google Scholar]
- 19.Sibley C G, Ahlquist J E. Phylogeny and Classification of Birds. New Haven, CT: Yale Univ. Press; 1990. [Google Scholar]
- 20.DeBoer A J. Tijdschrift voor Entomologie. 1995;138:169–244. [Google Scholar]
- 21.Dumbacher J P. Auk. 1999;116:957–963. [Google Scholar]