Abstract
High-fidelity genetically encoded bio-sensors that respond to changes in cellular environmental milieu in disease offer great potential in a range of patho- physiological settings. Here a unique hypoxia- regulated vector-based system with double oxygen–sensing transcriptional elements was developed for rapid and robust hypoxia-regulated gene expression in the heart. Hypoxia-responsive cis elements were used in tandem with a single proline-modified oxygen-dependent degradation (ODD) domain of hypoxia-inducible factor-1α to form a double oxygen–sensing vector system (DOSVS). In adult cardiac myocytes in vitro, the DOSVS demonstrated a low background expression not different from baseline control in normoxia, and with 100% efficiency, robust, 1,000-fold induction upon hypoxia. In the heart in vivo, hypoxic and ischemic challenges elicited rapid 700-fold induction in living animals, exceeding that obtained by a high-fidelity constitutive cytomegalovirus (CMV) viral promoter. DOSVS also showed high temporal resolution in the heart in response to cyclical bouts of hypoxia in vivo. We propose that DOSVS will be valuable for a range of applications, including bio-sensing and therapeutic gene expression in the heart and other organ systems that are confronted by chronic or episodic hypoxic/ischemic stresses in vivo.
INTRODUCTION
Gene-based therapeutic approaches offer a potential strategy to redress myocardial dysfunction for acquired and inherited cardiac diseases.1–6 Myocardial hypoxia is an important consequence of reduced blood flow to the myocardium.7 Efforts are on-going to design and test efficacious genetic “sensors” of hypoxia with the aim of inducing expression of potentially therapeutic genes in the heart.8 The ultimate goal of physiologically regulated gene expression in the heart includes silent transcription in normoxia and rapid, efficient, and robust induction in hypoxia and the ability for expression be turned “On” and “Off” in response to repeated bouts of hypoxia in vivo.9,10
Hypoxia-inducible factor-1 (HIF1) is a heterodimeric protein complex composed of HIF1α and HIF1β.11,12 In cells supplied with adequate oxygen, the HIF1α protein is rapidly degraded because of the presence of an oxygen-dependent degradation (ODD) domain in the protein.13 The ODD contains proline residues at positions 402 and 564 which are modified by oxygen-sensing hydroxylases (PHD1-3).13–15 When the ODD prolines are hydroxlyated in normoxia, this leads to an interaction of HIF with the von Hippel-Lindau tumor suppressor (pVHL), which targets HIF1α for rapid degradation by the ubiquitin pathway.14,16 Hypoxia inhibits proline hydroxylation, thus allowing HIF1α to accumulate in the cell.12,17 Hypoxia-responsive elements (HREs) are cis-regulatory DNA sequences that specifically bind HIF1 and permit transcriptional induction upon hypoxia exposure.17,18
In this study, we incorporated HRE concatamers together with and a proline-modified ODD to form a double oxygen–sensing system for gene transfer into adult cardiac myocytes and in whole heart in vivo. The double oxygen–sensing vector system (DOSVS) developed here consists of a sensor and effector tandem vector pair. The sensor element was designed with two or six HREs and the SV40 promoter driving expression of a fusion protein containing a GAL4 DNA-binding domain, a modified ODD domain containing only a single proline (codon 564), and a p65 activation domain. This sensor virus provides dual oxygen detection due to the presence of the HREs for transcriptional regulation and the modified ODD for posttranslational regulation. The effector DOSVS element has six GAL4-binding sites upstream of a minimal promoter and a reporter gene [enhanced green fluorescent protein (eGFP) or luciferase]. A GAL4-based system was first reported as a two-step transcriptional amplification for imaging reporter gene expression using weak promoters.19 Tang et al. recently reported the use of a double plasmid–based system for hypoxia-regulated gene expression in the h2c9 cell lines and by direct plasmid injection to the whole heart in vivo.20,21 Their results showed ∼2- to 17-fold induction by hypoxia in HEK and H9c2 cells in vitro with an undetermined level of hypoxia induction in the heart in vivo.20,22–24
Our main goal was to design and implement a new viral-based system to test the hypothesis that a proline-modified ODD DOSVS in tandem with concatemerized HREs would demonstrate a marked improvement in hypoxia-induced gene expression while retaining low background compared to currently available systems. We report in adult cardiac myocytes a robust ∼1,000-fold amplification of gene expression in hypoxia and nominal baseline expression in normoxia. In vivo cardiac gene transfer and imaging further demonstrated that this DOSVS produced ∼700-fold induction in hypoxia and in response to chronic left coronary artery ligation in vivo. In addition, real-time in vivo imaging revealed rapid amplification of cardiac gene expression in response to episodic hypoxic stimuli.
RESULTS
In vitro analysis of DOSVS in adult cardiac myocytes
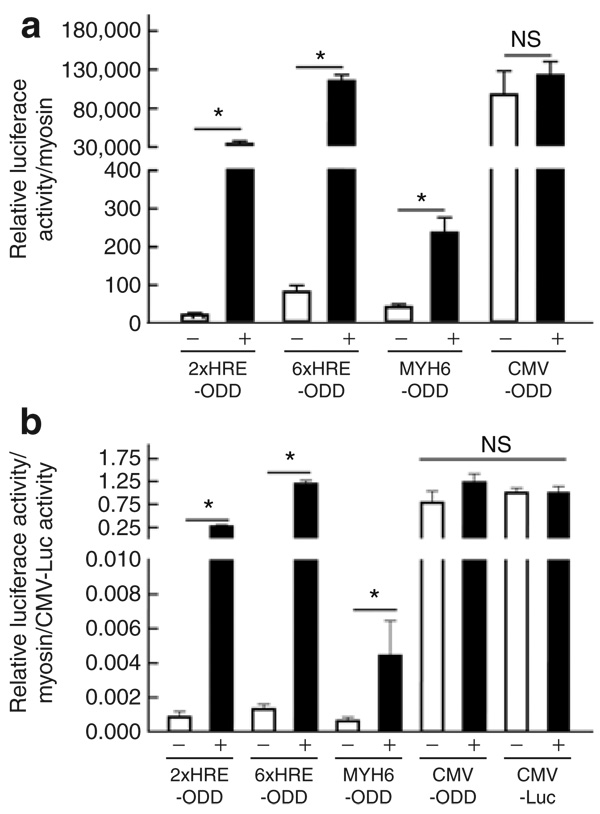
First, to determine the hypoxia-driven transcriptional amplification by DOSVS in adult cardiac myocytes, we transduced myocytes with one of four different sensor viruses containing the promoters 2xHRE-mp, 6xHRE-mp, Myh6, or cytomegalovirus (CMV) that were fused with GAL4-ODD-p65 (Figure 1). These myocytes were also transduced with the 6UAS-luciferase effector virus. We used the CMV-luciferase virus as a control/benchmark for maximal level of induction efficiency. After transduction, myocytes were divided into two groups. The first group was placed in a hypoxic chamber (0.5% O2, 5% CO2, and 94.5% N2) and a second group of control myocytes was kept in standard normoxic conditions (5% CO2, 20% O2, 75% N2), all in a humidified incubator at 37°C. After 48 hours, we assessed the transcriptional activity of each set of viruses by luciferase assay and normalized the data to myosin expression level (Figure 2a). The transcriptional activity for each set of DOSVS was also normalized to CMV-luciferase activity and determined in normoxia and hypoxia (Figure 2b). Data were summarized from three heart preparations, and each set of viruses was tested in triplicate (Figure 2a and b). Collectively these results demonstrate a robust, ∼1,000-fold, induction upon hypoxia exposure with little detected read-thru in normoxia. Regarding sensor promoter analysis in DOSVS, the two HRE-containing sensors induced marked luciferase reporter activity in hypoxia. Cardiac myocytes transduced with the 6xHRE-mp- GAL4-ODD-p65 sensor exhibited the highest ratio of activity driven by the 6UAS-luciferase effector and low activity in normoxia. By comparison, the ratio of murine Myh6 promoter activity was relatively low in rat myocytes upon hypoxia induction. The MyH6 sensor has a single oxygen–sensing element owing to the ODD downstream of MyH6; accordingly this sensor should have some activation in hypoxia in cardiac myocytes. This single oxygen–sensing capability, derived from the ODD element, accounts for the hypoxia induction that we observed, that, while induced (∼5–6 X induction), the level of induction was markedly less than experiments with the dual oxygen–sensing constructs, especially the 6xHRE–ODD element. The CMV GAL4-ODD-p65 sensor virus had high baseline activity in normoxia with no significant induction in hypoxia (Figure 2a and b).
Figure 1. Schematics of viral constructs for double oxygen–sensing vector system.
Six recombinant adenoviruses were generated for the double oxygen–sensing virus system. The four sensor vectors (top) have the GAL4-ODD-p65-coding sequence driven by different promoters: cytomegalovirus (CMV), Myh6, 2xHRE-mp, or 6xHRE-mp. Derivation of components: GAL4-yeast DNA-binding domain, ODD-oxygen-dependent degradation domain of hypoxia-inducible factor-1α, p65/RelA-the human pol II–activated domain from nuclear factor κB. The two effector vectors (bottom) consisted of six UAS-GAL4 binding sites upstream of a minimal viral promoter (E1BTATA) that was able to amplify the hypoxia signal by binding GAL4-ODD-p65 protein to activate transcription of enhanced green fluorescent protein (eGFP) or luciferase reporter genes. HRE, hypoxia-responsive element; LTR, left-terminal repeat; RTR, right-terminal repeat.
Figure 2. Hypoxia-mediated amplification of transcriptional activity in double oxygen–sensing vector system (DOSVS).
Rat cardiac myocytes were transduced with one of four different sensor viruses (Figure 1) containing unique promoters: 2xHRE-mp, 6xHRE-mp, Myh6, and cytomegalovirus (CMV) fused with GAL4-ODD-p65. The same myocytes were also transduced with the 6UAS-luciferase effector virus. The CMV-luciferase virus was used as a positive control of maximal adenoviral gene transfer efficiency. (a) At 48 hours after transduction, promoter activity of each pair of viruses was assessed by luciferase assay. (b) The transcriptional activity of each set of DOSVS was also normalized to CMV-luciferase activity. In each graph, “+” is hypoxic and “−” is normoxic. Values are mean ± SEM. HRE, hypoxia-responsive element; NS, not significant; ODD, oxygen-dependent degradation domain.
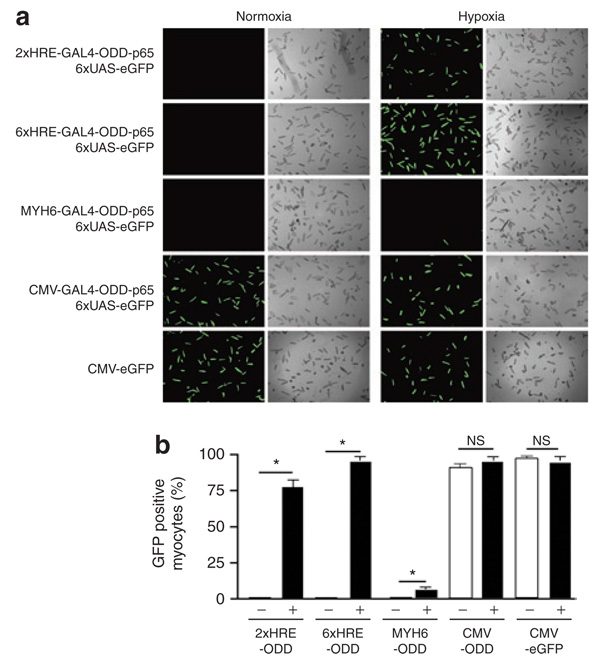
To determine the efficiency of DOSVS gene transfer, we also transduced myocytes with these same sensor viruses while using the 6UAS-eGFP effector virus as the reporter. We used the CMV-eGFP virus as a control for adenoviral transduction efficiency. In these experiments, hypoxia-induced eGFP fluorescence was apparent after 48 hours for 75 ± 5, 94 ± 5, and 95 ± 5% of the cells transduced with 2xHRE-mp, 6xHRE-mp, or CMV sensor viruses, respectively, and the number of eGFP positive myocytes was similar compared to cells transduced with the single CMV-eGFP control (95 ± 5%) (Figure 3a and b). Consistent with our findings of comparatively low promoter activity, the sensor vector driven by the Myh6 promoter resulted in a low frequency of eGFP fluorescence in transduced myocytes under hypoxic conditions. In normoxia, visible fluorescence was only evident in experimental myocytes transduced with the CMV-sensor virus (90 ± 5%) and myocytes with the single CMV-eGFP control virus (95 ± 5%) (Figure 3a and b).
Figure 3. Hypoxia-mediated amplification of enhanced green fluorescent protein (eGFP) expression by double oxygen–sensing vector system.
Rat cardiac myocytes were transduced with identical sensor vectors as in Figure 1, containing unique promoters: 2xHRE-mp, 6xHRE-mp, Myh6, and cytomegalovirus (CMV) fused with GAL4- ODD-p65, and the 6UAS-eGFP effector virus was used as the reporter. (a) At 48 hours after transduction, we scored eGFP positive myocytes for each pair of viruses. (b) The percentage of GFP-positive myocytes within a cover slip was estimated by dividing GFP-positive by total myocytes. The CMV-eGFP virus served as a positive control for adenoviral transduction efficiency (a,b). In each graph, “+” is hypoxic and “−” is normoxic. Values are mean ± SEM. HRE, hypoxia-responsive element; NS, not significant; ODD, oxygen-dependent degradation domain.
DOSVS cardiac transfer in vivo
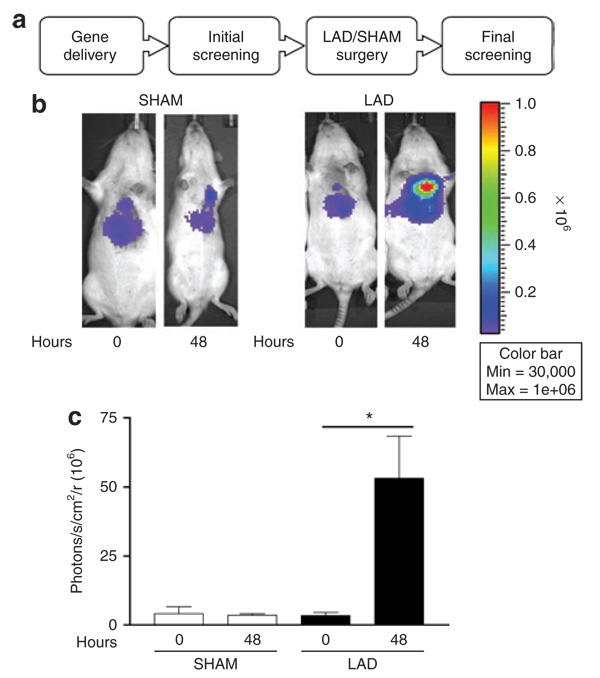
To determine whether DOSVS could be activated by low oxygen tension in vivo, we focused on the 6xHRE-mp- GAL4-ODD-p65 sensor and 6UAS-luciferase effector, as this tandem showed the greatest induction in hypoxia with minimal basal activity in normoxia in vitro. The DOSVS was delivered by ultrasound-guided intramyocardial injection directly to the heart of adult rats.25 This approach targets the left ventricular free wall and is not considered a global myocardial transduction modality. Nonetheless, single direct injection offers an effective approach to address in vivo responsiveness of DOSVS. We performed initial screening 24 hours later by using Xenogen IVIS 200 to assess noninvasively gene expression in the heart in vivo ( Figure 4a). In addition, a chronic left anterior descending artery ligation (LAD) protocol was used to create ischemia/low oxygen tension environment at the infarcted/peri-infarcted area.26 Sham (SHAM) operated animals were used as control group and underwent left thoracotomy but without arterial ligation. After 48 hours, the expression level was determined by Xenogene IVIS 200 (Figure 4a). Luciferase signals from the hearts of LAD of SHAM rats were quantified using the Living Image software (Figure 4b and c). Quantification of the luciferase signal of DOSVS showed marked induction of luciferase activity by applying LAD ligation and was significant at the 48 hour time point (Figure 4b and c).
Figure 4. Fidelity of double oxygen–sensing vector system (DOSVS) in an experimental model of myocardial infarction in vivo.
(a) The rats were imaged for in vivo luciferase expression using the Xenogen imaging system at 24 (initial screening) and 72 hours (final screening) after intramyocardial delivery of DOSVS. Four representative animals are shown at 24 and 72 hours after injection. (b) SHAM rats (n = 7) had the same surgical procedure but without left anterior descending artery (LAD) ligation. LAD rats (n = 11) were subjected to LAD surgery with 48 hours of recovery time. (c) Graph of imaging data showing the average luciferase expression of SHAM and LAD at time 0 of initial screening and at time 48 hours of final screening. LAD rats had significantly higher luciferase expression compared to SHAM animals; (P < 0.02). Values are mean ± SEM.
Responsive gene expression by episodic hypoxia stimulation in vivo
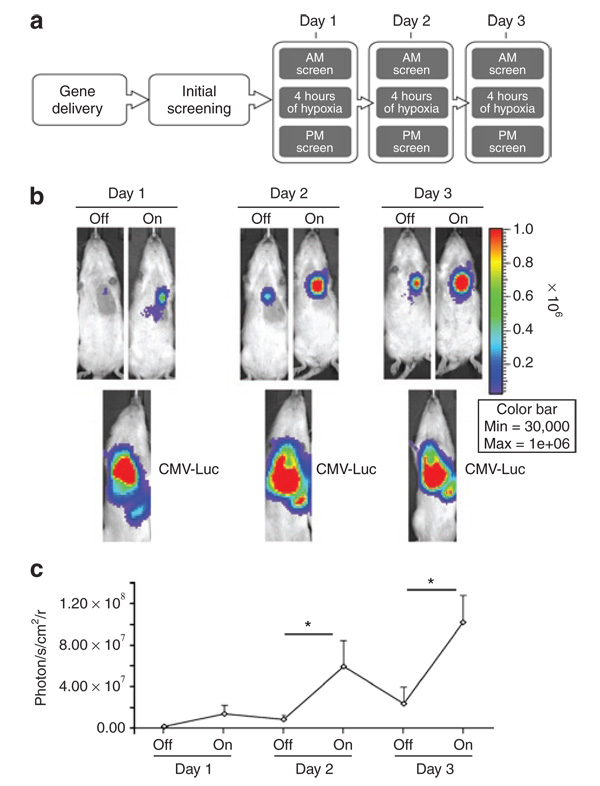
To examine the ability of the DOSVS to track and respond to episodic, short-term hypoxia stimuli, we exposed animals to multiple systemic hypoxic challenges (HC). The DOSVS was delivered as described earlier and initial screening was performed 24 hours later (Figure 5a). Episodic hypoxia was created by placing animals in 535-ml air tight flow-through chambers and subjecting them to gas mixture (7% O2, balance N2) at a flow rate of 5,284 ml/min for 4 hours).27,28 We used equal amounts of virus coding CMV-Luc as a control for adenoviral transduction efficiency. Resulting bioluminescent images and their quantification are presented in Figure 5b and c respectively. The luciferase activity measured after hypoxia was significantly higher compared to activity measured before hypoxia in animals subjected to 4 hours of hypoxia at days 2 and 3 (On/Off; P < 0.001). For control, some images were acquired before luciferin administration at evening sessions to exclude the accumulation of luciferase substrate at the subsequent morning session. We did not detect a bioluminescence signal without immediate prior luciferin administration. Results showed the DOSVS read-out activity was significant by switching hypoxia On and Off multiple times in living animals (Figure 5b and c).
Figure 5. Dynamic temporal response of double oxygen–sensing vector system (DOSVS) during episodic hypoxic challenge in vivo.
(a) After intramyocardial delivery of DOSVS, luciferase expression was monitored in real time in living animals during multiple hypoxic challenge events. (b,c) The same amount CMV-Luc virus was used as a positive control for adenoviral transduction efficiency. DOSVS activity could be switched “On” and “Off” by subjecting the rats (n = 8) alternately between hypoxic or normoxic conditions. At days 2 and 3, the switch to hypoxia led to significant increases in bioluminescence (*P < 0.001). In each case, the basal level of bioluminescence of DOSVS in normoxia was lower then the preceding hypoxia event. By contrast, the level of expression driven by virus encoding CMV-Luc reporter which was high over all time points (lower panels) (b). Summary of increased bioluminescence by episodic hypoxia (c). Values are mean ± SEM. CMV, cytomegalovirus.
Analysis of bioluminescent signals of DOSVS as compared with the values of CMV promoter
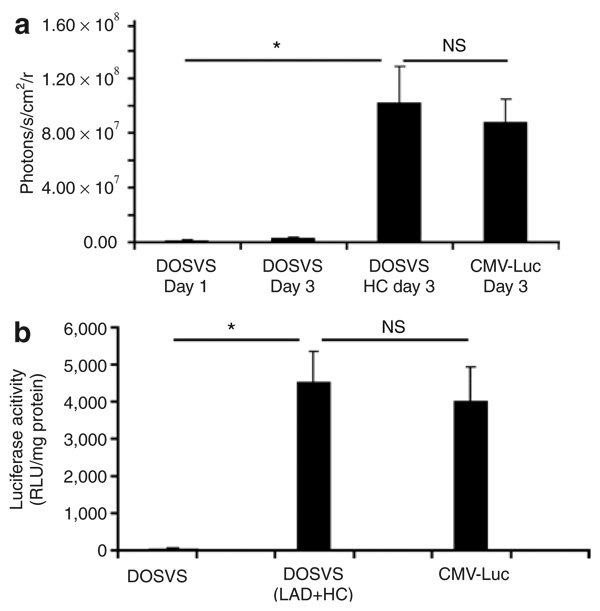
We further analyzed the luciferase activity in hearts from rats injected with CMV-Luc and compared this to rats with DOSVS subjected to three bouts of hypoxia (day 3). DOSVS reached levels of expression similar to that of the CMV driven reporter alone (Figure 6a). For controls, rat hearts injected with DOSVS kept a basal expression in normoxia (days 1–3) (Figure 6a).
Figure 6. In vivo and ex vivo analysis of luciferase expression of double oxygen–sensing vector system (DOSVS) compared to expression of CMV-Luc.
(a) Luciferase activity measured in rat hearts injected with CMV-Luc viruses was compared to values from hearts injected with DOSVS, with and without hypoxic challenge (HC). The induced group (N = 8) with DOSVS showed a significantly greater signal (P < 0.01) at day 3 as compared with preinduction levels at day 1 (N = 6). Also, the induced DOSVS group shows a significantly greater (P < 0.01) signal as compared with the uninduced group at day 3 (N = 6). The signal levels in the group induced with DOSVS and in the group with CMV-Luc were similar. Values are mean ± SEM. (b) Ex vivo luciferase signal was measured in isolated hearts using a luminometer. Data are presented as relative light units (RLU) per mg of protein and shown as mean ± SEM using three rats per group. Hearts from rats which underwent LAD surgery or HC treatment expressed at the highest level compared to hearts from rats injected with DOSVS without any treatment (P < 0.01). The luciferase signal from hearts injected with CMV-Luc virus was similar compared to signals from induced (LAD or HC) hearts with DOSVS. CMV, cytomegalovirus; NS, not significant.
To further validate noninvasive imaging of gene expression, we isolated hearts and performed a biochemical assay assessment of luciferase activity normalizing results per mg protein. Results showed a 700-fold hypoxia induction following hypoxia/ischemia challenge (LAD + HC data combined) with the maximal value comparable to that by CMV-Luc alone (Figure 6b).
DISCUSSION
This study demonstrates successful implementation of a unique dual oxygen–sensing hypoxia-responsive sensor/effector viral expression system (DOSVS) that robustly activates transcription in a (patho)physiologically relevant manner in adult cardiac myocytes in vitro and in the heart in vivo. The magnitude of induction by hypoxia/ischemia was 1,000-fold in vitro and 700-fold in vivo, representing an ∼100X enhancement over present systems tested in HEK and H9c2 cells.24 In addition, this system demonstrates minimal activation in normoxia and efficacy of repeated transgene switching “On” and “Off ” by cyclical bouts of (patho)physiological stimuli in living animals. This system will be valuable in cardiac and other cell/organ systems as a readout of local hypoxia/ischemic and for a multitude of therapeutic effector gene expression applications.
In vitro, the pairing of 6xHRE-mp-GAL4-ODD-p65 sensor with the 6UAS-luciferase effector induced gene expression in hypoxia 1,000-fold, with peak hypoxia-driven expression actually 25% higher than that of a nonhypoxia sensitive but robust CMV-luciferase system. Tandem oxygen sensing by multiple HREs in the promoter region of the sensor virus, together with the ODD as part of a chimeric protein, promotes synergistic interactions that allow the effector virus to maintain low baseline activity during normoxia while dramatically amplifying expression during hypoxia. The Myh6-GAL4-ODD-p65 system displayed low activity in normoxia, but only approximately tenfold induction during hypoxia. These effects may be explained, in part, by species specificity of the mouse promoter in the context of rat myocytes.29 For the CMV GAL4-ODD-p65 sensor virus, high baseline activity evident in normoxia could be explained by the strong nature of the CMV promoter and an inability to degrade all transcripts by the ubiquitin pathway.30 Overall, these results support the high fidelity of this dual oxygen–sensing system conferring marked hypoxia-induced gene expression in cardiac muscle.
In vivo, the DOSVS of the paired 6xHRE-mp-GAL4-ODD-p65 sensor with the 6UAS-luciferase effector induced gene expression in whole heart of living animals subjected to hypoxic stimulation by 700-fold. We recorded a similar level of bioluminescence induction in the rat model of myocardial ischemia monitored by Xenogen imaging system in real time. The fidelity of DOSVS was further validated by the chronic LAD ligation experiments and biochemical analysis of gene expression. Recently, Safran and colleagues developed a mouse line that expresses the ODD domain of HIF1α fused to the common firefly luciferase gene, under the control of a promoter that ensures organism-wide expression.31 Surprisingly, in vivo bioluminescent imaging of ROSA26 ODD-Luc/+ mice breathing in a low-oxygen environment (8%) or subjected with FG4383—a PHD inhibitor—showed modest induction only in the kidney area. They suggested that the kidney is an important oxygen sensor in the body, but their finding might also be interpreted as a high bioavailability of luciferin in kidneys before secretion.31 In contrast, we observed a profound induction of bioluminescence signal comparable to the maximal level driven by CMV promoter in the heart of animals subjected to hypoxia or LAD. We noticed the increasing of bioluminscence signals in the heart area over time following episodic hypoxia that might be explained by adaptation of animals to hypoxia. The mechanism of this apparent conditioning effect, perhaps due to HIF1α itself, is not a part of this study but will be interesting to investigate further in future works.
Our finding that this viral-based DOSVS generated a robust hypoxia-regulated response significantly expands upon earlier studies assessing the potential of an oxygen-dependent two-step transcriptional activation system.19 One recent study used lentiviral vectors for obtaining high levels of tissue-specific gene expression from a weak promoter.32 The efficiency of this lentivirus system, its cell-type specificity, and persistence of gene expression were evaluated in cell culture and in living mice carrying prostate tumor xenografts.32,33 Similarly, a DNA-bearing system based on GAL4/UAS with a cardiac-specific rat mlC-2v promoter has been tested in H9c2 cell lines and in mouse heart using direct plasmid delivery.20,21 These reports showed a 2- to 17-fold hypoxia induction and report high transcription in normoxia (200- to 400-fold) relative to single-vector systems alone.20 Interestingly, this study did not show hypoxia-mediated amplification for HRE-based constructs, and this may be explained by the use of only a single HRE element proximal to either SV40 minimal or mlC-2v promoters.21 Many groups have reported that a tandem repeat of HREs enhances the transcription of reporter genes under hypoxic conditions.34 We have shown here a dose-dependent hypoxic induction that is related to the number of HREs in the sensor virus. The 6xHRE yielded about a threefold increase in hypoxia-regulated activity compared to the expression level driven by the 2xHRE sensor. Previous reports suggested that only the ODD acts as a regulator of transcriptional silencing during normoxia.21 In our experiments, linking ODD as part of a chimeric protein driven by the strong viral CMV promoter resulted in high background in normoxia with no induction in hypoxia, thus demonstrating the inability of ODD alone to regulate transcription as a single oxygen sensor in this system.
There are several major differences between the present viral DOSVS approach and previous DNA plasmid-bearing ODD-based systems for hypoxia-regulated cardiac gene expression.21,35 First, we used a weak SV40 minimal promoter with concatermerized HREs in our sensor virus which gave a 1,000-fold increase in activity during hypoxic induction compared to ∼2- to 17-fold seen previously.21 This might be explained by previous use of the moderately active mlC-2v promoter which showed a distinctly elevated baseline activity in normoxia21 compared to the present DOSVS system. In addition, we also modified the HIF ODD to encompass amino acids 530–603, thus including proline 564 and eliminating proline 402. Recent results suggest that hydroxylation of proline 564 appears to be more important than proline 402 during normoxia in directing efficient HIF1α degradation.36 Oxygen tension–dependent sensitivity of proline hydroxylation also appears different between prolines 564 and 402 (refs. 36,37). Under experimental conditions of modest to severe hypoxia, proline 564 hydroxylation status appears to be the most sensitive, whereas in high oxygen to mild hypoxia 402 hydroxylation appears to be the most sensitive.36,38 We speculate that, in cardiac myocytes or in whole heart, retention of proline 564 is important and accounts at least in part for our observed low background in normoxia and robust activation in hypoxia.
In conclusion, these results establish a highly effective hypoxia sensor system featuring dual oxygen sensing for physiologically relevant induction during hypoxia. Results show robust transgene expression in the heart exceeding that of current systems by ∼×100. In future studies it will be interesting to engineer these elements into rAAV vectors that demonstrate marked cardiac tropism via systemic delivery in vivo.39 Candidate genes, including those encoding sarcomeric and Ca2+ handling proteins shown important for heart function in ischemic/hypoxia,40 will be of interest to test in future work. Another potential application for this DOSVS comes in the form of on-line monitoring of the extent and magnitude of hypertrophic cardiomyopathy– mediated episodic hypoxia as well as other animal models which commonly manifest ischemia with impaired microvascular density and reduced myocardial perfusion flow.41,42 This system could also be extended to other cell/organ systems as a bio-sensor of hypoxia for a range of other disease applications including cancer.
MATERIALS AND METHODS
Recombinant adenoviral vectors for DOSVS
We generated six recombinant adenoviral vectors for use in this study (Figure 1). CMV-luciferase was purchased from The University of Michigan Viral Core. pSG424 was kindly provided by Dr. O. MacDougald (University of Michigan, Ann Arbor, MI). The Myh6-murine alpha-MHC 5.5-kilobase promoter was a generous gift from Dr. J. Robbins (Children’s Hospital, Cincinnati, OH).
DOSVS sensor vectors
HRE sequences were generated according to a method described previously43 and subcloned into the HhoI and SalI sites of pSP72 (Promega). Concatamers of HRE (2 and 6) were generated by PCR of pSP72 (2,6 HRE) vectors using primers 5′-GCG AGC TCA CAT ACG ATT TAG GTG AC-3′ and 5′-ACG CTA GCT CCT CTA GAG TCG AAA GAG-3′ (containing embedded SacI and NheI sequences). Digestion with SacI and NheI released the HREs which were inserted into the pGL-3 promoter plasmid at SacI and NheI sites. We subcloned ODD, a 222-base pair DNA fragment corresponding to amino acids 530–603 (nucleotides 1616–1837) from pCEP4/HIF1α U22431 (American Type Culture Collection), by PCR using primers 5′- AAG AAT TCA GGC CAC CAT GGA ATT CAA GTT GGA ATT A-3′ and 5′-GCG AGC TCC TGG AAT ACT GTA AC-5′ (containing embedded EcoRI and SacI sequences). The amplification product was digested with EcoRI and SacI and inserted into pSG424-GAL4 at the EcoRI and SacI sites. The p65/RelA-coding domain was created by PCR from pSWITCH (Invitrogen) at position 1,674–2,483 (encoding amino acids 283–551) using primers 5′-AAG AGC TCA TGG AAT TCC AGT ACC TGC C-3′ and 5′-AAG AGC TCT TAG GAG CTG ATC TGA CTC-3′ (containing embedded SacI sequence). The PCR product was digested with SacI and inserted into pSG424 as the terminal part of the GAL4-ODD fusion protein. Ad-pDC312 (2 or 6xHRE) constructs were digested with SacI and BamHI to release Luc gene and re-ligated to the circular form. We subcloned GAL4-ODD-p65-PA by PCR using primers 5′-CAT-AAG CTT CCT GAA AGA TGA AGC TAC-3′ and 5′-ATG GAT CCC GGC GTA GAG GAT CGA TCC AG-3′ (containing embedded sequences). The PCR product was digested with corresponding enzymes and inserted into Ad-pDC312 at HindIII and BamHI sites. We also subcloned GAL4-ODD-p65 by PCR using primers 5′-AAA GCT AGC CCT GAA AGA TGA AGC TAC-3′ and 5′-AAT GGA TCC TCT AGA GCT CGA ATT CCC CGG G-3′ (containing embedded NheI and BamHI sequences). The PCR product was digested with corresponding enzymes and inserted into Ad-pDC315-CMV at NheI and BamHI sites. The Myh6 promoter was inserted into Ad-pDC312 at SalI sites. We then subcloned GAL4-ODD-p65-PA by PCR using primers 5′-AAA GGA TCC CCT GAA AGA TGA AGC TAC-3′ and 5′-ATA GCT AGC GGC GTA GAG GAT CGA TCC AG-3′ (containing embedded HindIII and NheI sequences). The PCR product was digested with the corresponding enzyme and inserted into Ad-pDC312-Myh6 at HindIII and NheI sites. The four sensor vector sequences were verified by automated DNA sequencing (University of Michigan, Sequencing Core).
DOSVS effector vectors
The Ad-pDC316-CMV-eGFP construct, eGFP, was subcloned into the HindIII–SacI sites by digesting pSP72-eGFP with HindIII and SacI. To generate Ad-pDC312-6UAS-eGFP, eGFP was first subcloned into the HindIII–BamHI sites of pGENE/V5-His A (Invitrogen) from pSP72-eGFP. We then sublconed 6UAS-E1BTATA-eGPF-PA by PCR using primers 5′-AAT AGA TCT CGG GCT CTT ACG CGG GTC GAA-3′ and 5′-AAT AGA TCT GCC ATA GAG CCC ACC GCA TCC C-3′ (containing embedded BglII sequences) into Ad- pDC312 at the BglII site. To generate vectors containing the luciferase reporter gene, the luciferase cDNA was excised from the pGL-3 promoter (Promega) and subcloned into the HindIII and XbaI sites of pGENE/V5-His A (Invitrogen). The 6UAS-E1BTATA-luciferase-PA was subcloned into Ad-pDC312 by PCR using the same primers which were used for subcloning eGFP.
Adult cardiac myocyte isolation, primary culture, and gene transfer
These methods have been previously described in detail.44 The multiplicity of infection ranged from 50 to 750 plaque-forming units (plaque-forming units/rod-shaped myocyte) to optimize gene transfer efficiency for each vector tested. One hour later, 2 ml of M199 medium containing P/S was added to each coverslip. The medium was replaced with fresh M199 containing P/S 24 hours after gene transfer.
Adult rat myocytes culture and hypoxia treatment
Primary culture of rat myocytes was performed in M199. Hypoxic treatment (0.5% O2, 5% CO2 and 94.5% N2) was carried out in C-Chamber from BioSpherix where oxygen levels were monitored by the oxygen gas controller Pro-Ox (BioSpherix, –Redfield, NY). This system works by closed-loop control and is connected to an oxygen sensor, which monitors the oxygen concentration within the host chamber.
Myocardial injection of adenovirus
Studies were performed in compliance with the guide for care by the Unit for Laboratory Animal Medicine, and protocols were approved by the University Committee on the Use and Care of Animals. Sprague Dawley rats (Harlan, Houston, TX) weighing 150–200 g were used for all experiments. Rats were anesthetized with isofluorane. Adenoviruses were delivered by ultrasound (VisualSonic)-guided intramyocardial injection directly to the left ventricle using a 30 3/4-gauge needle with 100 µl of injected volume. Animals were injected with 1 × 1010 plaque-forming units of DOSVS or CMV-Luc.
Rat model of ligation of the left anterior descending artery
Twenty-four hours after DOSVS injection, animals underwent wither sham operation (pericardiectomy only) or proximal left anterior descending artery (LAD) ligation. After induction, endotracheal intubation with a 16-gauge angiocatheter was performed. Rats were supported by a small animal ventilator (Kent Scientific pressure controlled ventilator, Litchfield, CT) and maintained with isoflurane (Hospira, Lake Forest, IL) for anesthesia. A left thoracotomy was performed to expose the anterior surface of the heart. The proximal LAD was identified and a 7-0 prolene suture placed around the artery and surrounding myocardium. The landmarks used for the ligation placement included the line (1–2mm distal) between the left border of the pulmonary conus and the right border of the left atrial appendage.26 Ischemia was confirmed with ST-segment elevation on electrocardiogram. The pneumothorax was evacuated using saline. The ribs were reapproximated and incision closed in two layers. Postoperative care was continued using blood oxygen monitoring (Mouse Ox Starr Life Sciences, Allen Park, PA) until extubation and full recovery from anesthesia.
In vivo imaging of luciferase activity
In vivo imaging was performed using IVIS Imaging System 200 Series (Xenogen, Alameda, CA) at the Center of Molecular Imaging, University of Michigan. Rats were anesthetized with isofluorane and injected intraperitoneally with 150 mg/kg of luciferin.45 Ten minutes after the luciferin injection, rats were imaged for 15 seconds. Photons emitted from heart region were quantified using Living Image software (Xenogen). In vivo luciferase activity is expressed as photons/second/cm2.
Luciferase reporter assay
Myocytes were washed in phosphate-buffered saline for 5 minutes at room temperature, and then lysed by adding 50 µl passive lysis buffer from Promega. Selected hearts were removed and sonicated in 500 µl of phosphate-buffered saline containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). A volume of 20 µl of heart lysate was diluted twice and placed into Costar 96-well flat bottom plates. The emitted luminescence was then measured in the VICTOR Light Luminescence Counter (Perkin Elmer Life Sciences) using Luciferase Assay system (Promega). The corrected luciferase activity for myocytes was defined as the ratio of the values of firefly relative luciferase activity to the values of Coomassie-stained myosin protein band quantified using commercially available software (Quantiscan).46 Each set of experiments was repeated three times for N = 6 for both normoxic and hypoxic conditions. For heart tissue, luciferase activity was defined at the ratio of the values of relative luciferase activity per of mg protein. Protein concentration was estimated using BCA Protein Assay Kit (Pierce).
Statistical analysis
Each experiment was performed at least three times and representative data are shown. Data in bar graphs are given as mean values ± SEM. Two group comparisons were by Students t-test, P < 0.05 (*). One-way analysis of variance was used for multiple comparisons with Student Newman Keuls post-hoc analysis (On/Off hypoxia bout data).
ACKNOWLEDGMENTS
We thank David Pinsky, Diane Bouis, and Hui Liao for assistance and access with their hypoxia station and Steven Whitesall for help with hypoxic-conditioning experiments. We also thank Eric Devaney and Jackie Cale for helpful comments on an earlier version of this manuscript and Jennifer Davis for assistance with the figures. This work was supported by an National Institutes of Health grant to J.M.M. and a postdoctoral fellowship grant from The American Heart Association to Ekaterina Fomicheva (0620089Z).
REFERENCES
- 1.Yenari MA, Sapolsky RM. Gene therapy in neurological disease. Methods Mol Med. 2005;104:75–88. doi: 10.1385/1-59259-836-6:075. [DOI] [PubMed] [Google Scholar]
- 2.Woo SL, Skarlatos SI, Joyce MM, Croxton TL, Qasba P. Critical resources for gene therapy in Heart, Lung, and Blood Diseases Working Group. Mol Ther. 2006;13:641–643. doi: 10.1016/j.ymthe.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Williams ML, Koch WJ. Viral-based myocardial gene therapy approaches to alter cardiac function. Annu Rev Physiol. 2004;66:49–75. doi: 10.1146/annurev.physiol.66.032102.141555. [DOI] [PubMed] [Google Scholar]
- 4.Pachori AS, Melo LG, Dzau VJ. Gene therapy: role in myocardial protection. Handb Exp Pharmacol. 2006:335–350. doi: 10.1007/3-540-36028-x_11. [DOI] [PubMed] [Google Scholar]
- 5.Hermonat PL, Mehta JL. Potential of gene therapy for myocardial ischemia. Curr Opin Cardiol. 2004;19:517–523. doi: 10.1097/01.hco.0000136452.01448.5b. [DOI] [PubMed] [Google Scholar]
- 6.Hata JA, Williams ML, Koch WJ. Genetic manipulation of myocardial beta-adrenergic receptor activation and desensitization. J Mol Cell Cardiol. 2004;37:11–21. doi: 10.1016/j.yjmcc.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Corno AF, Boone Y, Mallabiabarrena I, Augstburger M, Tozzi P, Ferrari E, et al. Myocardial and pulmonary effects of aqueous oxygen with acute hypoxia. Ann Thorac Surg. 2004;78:956–960. doi: 10.1016/j.athoracsur.2004.03.104. discussion 956–960. [DOI] [PubMed] [Google Scholar]
- 8.Boast K, Binley K, Iqball S, Price T, Spearman H, Kingsman S, et al. Characterization of physiologically regulated vectors for the treatment of ischemic disease. Hum Gene Ther. 1999;10:2197–2208. doi: 10.1089/10430349950017185. [DOI] [PubMed] [Google Scholar]
- 9.Goverdhana S, Puntel M, Xiong W, Zirger JM, Barcia C, Curtin JF, et al. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol Ther. 2005;12:189–211. doi: 10.1016/j.ymthe.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binley K, Kan O, White J, Naylor S. Exploiting the hypoxia response. Curr Opin Mol Ther. 2003;5:650–656. [PubMed] [Google Scholar]
- 11.Wenger RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203:1253–1263. doi: 10.1242/jeb.203.8.1253. [DOI] [PubMed] [Google Scholar]
- 12.Erbel PJ, Card PB, Karakuzu O, Bruick RK, Gardner KH. Structural basis for PAS domain heterodimerization in the basic helix--loop--helix-PAS transcription factor hypoxia-inducible factor. Proc Natl Acad Sci USA. 2003;100:15504–15509. doi: 10.1073/pnas.2533374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krek W. VHL takes HIF's breath away. Nat Cell Biol. 2000;2:E121–E123. doi: 10.1038/35017129. [DOI] [PubMed] [Google Scholar]
- 15.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 16.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 17.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 18.Bruick RK, McKnight SL. Transcription. Oxygen sensing gets a second wind. Science. 2002;295:807–808. doi: 10.1126/science.1069825. [DOI] [PubMed] [Google Scholar]
- 19.Iyer M, Wu L, Carey M, Wang Y, Smallwood A, Gambhir SS. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci USA. 2001;98:14595–14600. doi: 10.1073/pnas.251551098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y, Jackson M, Qian K, Phillips MI. Hypoxia inducible double plasmid system for myocardial ischemia gene therapy. Hypertension. 2002;39:695–698. doi: 10.1161/hy0202.103784. [DOI] [PubMed] [Google Scholar]
- 21.Tang Y. Gene therapy for myocardial ischemia using the hypoxia-inducible double plasmid system. Methods Mol Med. 2005;112:37–47. doi: 10.1385/1-59259-879-x:037. [DOI] [PubMed] [Google Scholar]
- 22.Phillips MI, Tang Y, Schmidt-Ott K, Qian K, Kagiyama S. Vigilant vector: heart-specific promoter in an adeno-associated virus vector for cardioprotection. Hypertension. 2002;39:651–655. doi: 10.1161/hy0202.103472. [DOI] [PubMed] [Google Scholar]
- 23.Tang YL, Tang Y, Zhang YC, Agarwal A, Kasahara H, Qian K, et al. A hypoxia-inducible vigilant vector system for activating therapeutic genes in ischemia. Gene Ther. 2005;12:1163–1170. doi: 10.1038/sj.gt.3302513. [DOI] [PubMed] [Google Scholar]
- 24.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Protection from ischemic heart injury by a vigilant heme oxygenase-1 plasmid system. Hypertension. 2004;43:746–751. doi: 10.1161/01.HYP.0000120152.27263.87. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Porcel M, Gheysens O, Chen IY, Wu JC, Gambhir SS. Image-guided cardiac cell delivery using high-resolution small-animal ultrasound. Mol Ther. 2005;12:1142–1147. doi: 10.1016/j.ymthe.2005.07.532. [DOI] [PubMed] [Google Scholar]
- 26.Samsamshariat SA, Samsamshariat ZA, Movahed MR. A novel method for safe and accurate left anterior descending coronary artery ligation for research in rats. Cardiovasc Revasc Med. 2005;6:121–123. doi: 10.1016/j.carrev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Rising CL, D'Alecy LG. Hypoxia-induced increases in hypoxic tolerance augmented by beta-hydroxybutyrate in mice. Stroke. 1989;20:1219–1225. doi: 10.1161/01.str.20.9.1219. [DOI] [PubMed] [Google Scholar]
- 28.Zwemer CF, Song MY, Carello KA, D'Alecy LG. Strain differences in response to acute hypoxia: CD-1 versus C57BL/6J mice. J Appl Physiol. 2007;102:286–293. doi: 10.1152/japplphysiol.00536.2006. [DOI] [PubMed] [Google Scholar]
- 29.Gupta MP, Gupta M, Zak R. An E-box/M-CAT hybrid motif and cognate binding protein(s) regulate the basal muscle-specific and cAMP-inducible expression of the rat cardiac alpha-myosin heavy chain gene. J Biol Chem. 1994;269:29677–29687. [PubMed] [Google Scholar]
- 30.Dong X, Liu J, Zheng H, Glasford JW, Huang W, Chen QH, et al. In situ dynamically monitoring the proteolytic function of the ubiquitin-proteasome system in cultured cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1417–H1425. doi: 10.1152/ajpheart.01233.2003. [DOI] [PubMed] [Google Scholar]
- 31.Safran M, Kim WY, O'Connell F, Flippin L, Gunzler V, Horner JW, et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci USA. 2006;103:105–110. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iyer M, Salazar FB, Lewis X, Zhang L, Carey M, Wu L, et al. Noninvasive imaging of enhanced prostate-specific gene expression using a two-step transcriptional amplification-based lentivirus vector. Mol Ther. 2004;10:545–552. doi: 10.1016/j.ymthe.2004.06.118. [DOI] [PubMed] [Google Scholar]
- 33.Ray S, Paulmurugan R, Hildebrandt I, Iyer M, Wu L, Carey M, et al. Novel bidirectional vector strategy for amplification of therapeutic and reporter gene expression. Hum Gene Ther. 2004;15:681–690. doi: 10.1089/1043034041361271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada H, Kizaka-Kondoh S, Itasaka S, Shibuya K, Morinibu A, Shinomiya K, et al. The combination of hypoxia-response enhancers and an oxygen-dependent proteolytic motif enables real-time imaging of absolute HIF-1 activity in tumor xenografts. Biochem Biophys Res Commun. 2007;360:791–796. doi: 10.1016/j.bbrc.2007.06.149. [DOI] [PubMed] [Google Scholar]
- 35.Tang Y, Schmitt-Ott K, Qian K, Kagiyama S, Phillips MI. Vigilant vectors: adeno-associated virus with a biosensor to switch on amplified therapeutic genes in specific tissues in life-threatening diseases. Methods. 2002;28:259–266. doi: 10.1016/s1046-2023(02)00231-1. [DOI] [PubMed] [Google Scholar]
- 36.Chan DA, Sutphin PD, Yen SE, Giaccia AJ. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2005;25:6415–6426. doi: 10.1128/MCB.25.15.6415-6426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koivunen P, Hirsila M, Kivirikko KI, Myllyharju J. The length of peptide substrates has a marked effect on hydroxylation by the hypoxia-inducible factor prolyl 4-hydroxylases. J Biol Chem. 2006;281:28712–28720. doi: 10.1074/jbc.M604628200. [DOI] [PubMed] [Google Scholar]
- 38.D'Angelo G, Duplan E, Boyer N, Vigne P, Frelin C. Hypoxia up-regulates prolyl hydroxylase activity: a feedback mechanism that limits HIF-1 responses during reoxygenation. J Biol Chem. 2003;278:38183–38187. doi: 10.1074/jbc.M302244200. [DOI] [PubMed] [Google Scholar]
- 39.Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol Ther. 2007;15:1086–1092. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- 40.Day SM, Westfall MV, Fomicheva EV, Hoyer K, Yasuda S, La Cross NC, et al. Histidine button engineered into cardiac troponin I protects the ischemic and failing heart. Nat Med. 2006;12:181–189. doi: 10.1038/nm1346. [DOI] [PubMed] [Google Scholar]
- 41.Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, et al. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 2007;115:2418–2425. doi: 10.1161/CIRCULATIONAHA.106.657023. [DOI] [PubMed] [Google Scholar]
- 42.Friehs I, Barillas R, Vasilyev NV, Roy N, McGowan FX, del Nido PJ. Vascular endothelial growth factor prevents apoptosis and preserves contractile function in hypertrophied infant heart. Circulation. 2006;114:I290–I295. doi: 10.1161/CIRCULATIONAHA.105.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Post DE, Van Meir EG. Generation of bidirectional hypoxia/HIF-responsive expression vectors to target gene expression to hypoxic cells. Gene Ther. 2001;8:1801–1807. doi: 10.1038/sj.gt.3301605. [DOI] [PubMed] [Google Scholar]
- 44.Westfall MV, Rust EM, Albayya F, Metzger JM. Adenovirus-mediated myofilament gene transfer into adult cardiac myocytes. Methods Cell Biol. 1997;52:307–322. [PubMed] [Google Scholar]
- 45.Contag CH, Spilman SD, Contag PR, Oshiro M, Eames B, Dennery P, et al. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol. 1997;66:523–531. doi: 10.1111/j.1751-1097.1997.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 46.Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, et al. Efficient transduction of skeletal muscle using vectors based on adenoassociated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]