Abstract
Background
The intersection of pain, addiction and mental health has not been adequately described. We describe the roles of these three conditions in a chronic pain patient population using opioid analgesics. Aims were to improve our understanding of this population as well as to explore ways of identifying different types of patients.
Methods
We conducted a retrospective cohort study in a large integrated group medical practice in Washington State with persons using opioids chronically (n=704). Patient classes were derived with latent class analysis using factors representing DSM-IV opioid abuse and dependence, opioid misuse, pain, anxiety and depression. Regression analyses explored the utility of automated and interview data to distinguish the empirically-derived patient groups. Results Three classes were identified: a Typical group, the substantial majority that had persistent, moderate mental health and pain symptoms; an Addictive Behaviors group with elevated mental health symptoms and opioid problems, but pain similar to the Typical class; and a Pain Dysfunction class with significantly higher pain interference as well as elevated mental health and opioid problems. Prescribed average daily dose of opioids was three times higher for those in the two atypical groups and was strongly associated with class membership after adjusting for other variables.
Conclusion
We describe three distinct types of patient classes as well as data elements that could help identify the two atypical types. Further research is needed to confirm these findings and determine the utility of this approach in other clinical settings.
Keywords: opioid misuse, opioid dependence, chronic pain, mental health, co-morbidity, latent class analysis, patient types
1. Introduction
Opioid addiction among chronic pain patients prescribed opioids is a vexing problem that is compounded by complex clinical presentations that often include mental health and persistent pain problems. Concerns about inappropriate opioid use have increased as the incidence and prevalence of long term opioid prescribing for non-cancer pain have increased (Boudreau et al., under review). Also concerning is evidence that rates of long term opioid use are significantly higher for those with mental health and substance abuse problems than those without (Sullivan et al., 2006). The intersection of pain, addiction and mental health, though increasingly recognized as important, has not been adequately described in a general patient population of chronic pain patients using opioids chronically. Neither conceptual frameworks nor appropriate integrated assessment approaches appear to exist that might guide research or practice in this area. As a result, clinicians are faced with evaluating multiple symptoms and behaviors without knowledge of any typical patterns of patient presentation in this setting.
Problematic opioid use patterns among patients with chronic pain have been described (Kouyanou et al, 1997; Jonasson et al. 1998; Chabal et al. 1997; Reid et al., 2002; Compton et al., 1998; Butler 2008, Adams et al., 2004, Wu et al., 2006) but these studies, while informative, are based on relatively small numbers of subjects, an average of 152 subjects with a range from 52 to 283, seen mostly in specialty pain management settings. Fleming et al. described the prevalence of substance use disorders in a large (n=801) population of patients taking opioids daily and being managed in primary care settings, reporting a low prevalence of opioid use disorders (4%) and a significant association between opioid use disorders and four opioid use behaviors: purposeful over-sedation, using for non-analgesic effect, non-sanctioned dose increases and feeling intoxicated (2007). Further empirical understanding of relationships among opioid misuse, mental health and pain issues is required.
Opioid addiction, mental health and pain symptoms and diagnoses often overlap. Chronic pain conditions are found in a substantial proportion of those presenting for addiction treatment. (Mertens et al., 2003; Rosenblum et al., 2003; Sproule et al., 1999). Substance use outcomes among drug treatment patients with pain conditions are mixed, with no differences for alcohol, heroin or cocaine use, but worse outcomes for marijuana, prescription opioids and sedative medicines (Trafton et al. 2004). Mental health symptoms are more common among those with chronic pain, those who use opioids regularly, and those with problematic opioid use. A general population survey sample found that those reporting regular use of prescribed opioids were much more likely to report a mental disorder after adjusting for socio-demographic and clinical characteristics (Sullivan et al., 2005). Findings of a multivariate analysis of the National Survey on Drug Use and Health indicated that those who reported non-medical use of opioids (4.5% of the U.S. population) were more likely to have panic, depressive or social phobic symptoms and that those who met opioid abuse/dependence criteria (0.6% of the U.S. population) had significantly higher odds of symptoms of panic and social phobic disorders (Becker et al., 2008). These studies point to the overlapping issues of pain, addiction and mental health that make identification of these co-morbid problems both complex and necessary for appropriate clinical care. These findings also suggest variables that might be of value to health plans or clinicians in identifying those with potentially problematic use of opioids.
To improve our understanding of these challenging clinical scenarios, information about pain, mental health and opioid use behaviors were integrated to categorize types of patients who are prescribed opioids chronically in a large integrated group practice (also known as a health maintenance organization). In addition, we sought to explore whether automated health plan data and simple screening tools could potentially be used to identify these different patient types.
2. Methods
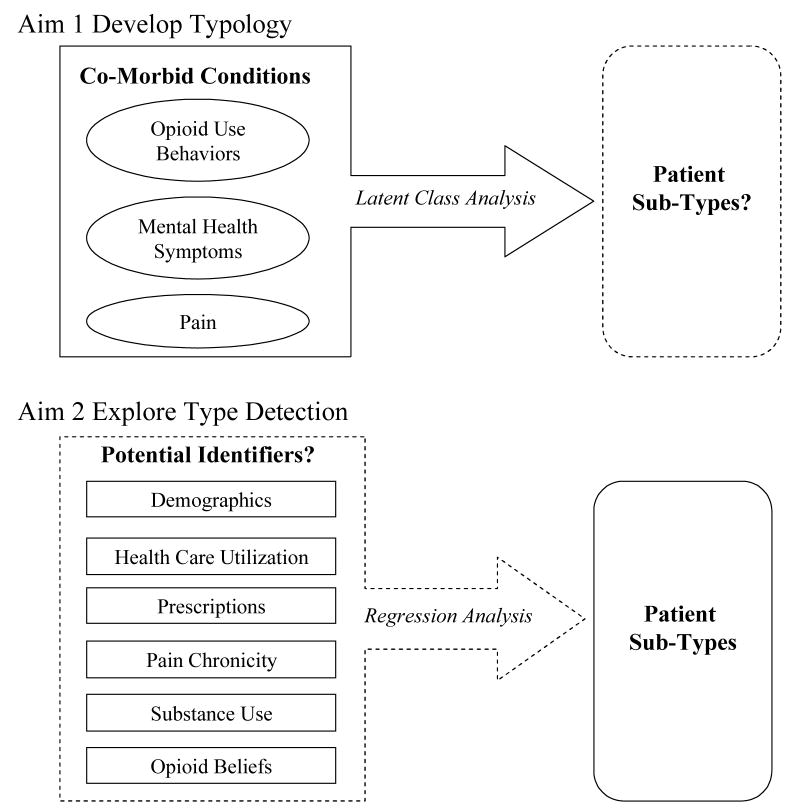
In order to meet the aims of the study we used a two step analytic approach: 1) a latent class analysis to determine the classes of patients and 2) regression analyses to explore the utility of automated and interview data elements for identifying patient classes (Figure 1).
Figure 1.
Diagram of study aims, Aim 1 to develop a patient typology, Aim 2 to identify patient types.
2.1. Study Design and Procedures
A retrospective cohort study was conducted among pain patients ages 21–79 with chronic opioid prescriptions enrolled continuously for at least 3 years in a large integrated group practice (more than 500,000 enrolled at the time of the study) in Washington State. The practice does not have any specialty pain clinics and analyses of automated data indicate that 86% of patients similar to those in the study receive opioid prescriptions from non-specialist providers. Administrative data were available for the 3 years preceding recruitment for the interview as well as time that elapsed between recruitment and interviewing. Patients with chronic opioid use in the 12 month period one year prior to the time of the interview were included. We planned for a minimum one year gap between chronic use and the interview in order to allow time for potentially problematic opioid use to develop. Chronic opioid use was defined as either: a) filling ten or more opioid prescriptions (excluding emergency room visits) during the 12 month period, or b) filling a prescription for at least a 120 day supply of opioids and six or more opioid prescriptions during the 12 month period. This definition was based on preliminary data that showed a majority of patients meeting these thresholds continued with chronic use in the subsequent year. Patients with cancer were excluded except for those with benign, non-melanoma skin cancers, because our focus was on non-cancer related chronic pain. Patients reporting no opioid use in the 90 days preceding the interview were excluded from these analyses.
Primary data were collected via a structured phone interview by interviewers at the Group Health Center for Health Studies’ Survey Research Program. General training was provided by the study investigators as well as supervisors at the Survey Research Program. Additionally, specialized training on administration of the Composite International Diagnostic Interview (CIDI) for DSM-IV opioid diagnoses was conducted by certified CIDI trainers who reviewed and approved tapes of practice interviews. The Survey Research Program also regularly monitored interviews as part of standard quality assurance protocols. Subjects received a $2 pre-incentive in the initial study mailing and $10 remuneration for completing the interview. Oral consent to access automated data and to conduct the phone interview were obtained. Permission to obtain automated data and conduct interviews was provided by the Group Health Cooperative human subjects review committee in June of 2006.
2.2 Study Instruments and Variables
2.2.1 Factor scores used for the development of patient types
A summary factor score was used for each of the seven factors of interest derived from confirmatory factor analyses (See the supplementary material with the online version of this article detailing the confirmatory factor analysis). Factor scores were selected because the items had different types of response categories and the goal was to develop a patient typology using the available data. Opioid use behaviors were summarized with four factor scores representing the areas of (1) Addictive Behaviors, (2) Addiction Concerns, (3)Pain Treatment Problems and (4) Opioid Abuse/Dependence. The derivation of these factors was reported elsewhere (Banta-Green et al., 2009), they were based upon items from DSM-IV (APA, 2004) opioid abuse and dependence and the Prescription Drug Use Questionnaire (PDUQ) (Compton et al., 1998). Common mental health symptoms were measured using the (5) Patient Health Questionnaire (PHQ) for anxiety symptoms (Spitzer et al., 1999) and the (6) two question version (PHQ-2) for depression symptoms (Kroenke et al., 2003). Pain intensity and pain interference were assessed using (7) The Graded Chronic Pain Scale (GCPS) (VonKorff et al., 1992). All of these items were collected with brief interview administered instruments.
2.2.2 Automated data based variables potentially associated with patient types
Several automated data elements considered were coded as binary: current smoker, any inpatient stay prior year, any emergency room/urgent care visit prior year. Health care utilization variables were included because they may be indicative of other sources of opioids and severity of health conditions. Diagnostic codes were used to create the following binary variables: any opioid abuse or dependence problems diagnosed in the prior three years, any non-opioid drug or alcohol abuse or dependence problems diagnosed in the prior three years, any anxiety disorder related visit in the prior year, and any depression related visit in the past year. Age and gender were obtained from the enrollment database. A continuous variable for age was used.
Electronic pharmacy data were also utilized. Previous research in the same setting indicates that enrollees receive approximately 97% of their prescriptions from integrated group practice pharmacies (Saunders et al., 2005 and Boudreau et al., 2004). A binary variable indicating any prescriptions for benzodiazepines in the prior year was created. Opioid types used in the prior year was a categorical variable based upon Drug Enforcement Administration scheduling and duration of action: schedule III, schedule II short-acting and schedule II long-acting; these categories are related to prescribing rules and may relate to clinical decision making. Average prescribed daily opioid dose was derived from pharmacy data for the year prior to the interview. The total annual morphine equivalent dose was obtained by multiplying the strength of each dose by the number of units prescribed and converting to milligrams morphine equivalent as described previously (Von Korff et al., 2008). The total annual dose was divided by the sum of the total days supply recorded in the pharmacy database. The resultant average prescribed daily opioid dose should be considered to be the maximum intended daily dose.
2.2.3 Interview based variables potentially associated with patient types
Simple survey measures were obtained from the phone interview. Substance involvement scores were assessed with the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) utilizing the substance specific binary, cut point indicating need for at least a brief intervention as detailed in the instrument scoring section (World Health Organization, 2008). The specific substances include alcohol, marijuana, cocaine, methamphetamine, prescription type stimulants, sedatives, prescription type opioids and heroin. The original ASSIST category of opioids was separated into heroin and prescription type opioids and amphetamine type stimulants was separated into methamphetamine and prescription type stimulants. Race, employment and marital status were also obtained during the interview.
Interview questions documenting the number of years since first chronic pain occurrence and two opioid belief questions were summarized with binary variables. Opioid beliefs were explored because beliefs about medications have been shown to be related to opioid misuse (Schieffer et al. 2005). Believing more pain relief from opioids was possible was a derived variable that was based upon differences in responses to two interview questions developed by the authors “How much pain relief do you receive after taking your opiate medicine?” and “How much pain relief do you think is possible from opiate medicine?”, both of which utilized a five point Likert scale response. A belief that opioids work best for pain was based upon the question “…which treatment has been the most important in helping to control your type of pain” which was a follow up question to a series of questions documenting all of the types of medications, interventions and treatments the patient had ever obtained for their pain. Onset of initial chronic pain condition more or less than ten years prior was based upon the response to “What year did you first have any pain condition that lasted for 3 or more months in a row?”.
2.3 Analyses
2.3.1 Determining the patient typology
Latent class analysis (LCA) (Hagenaars & McCutcheon, 2002) was used to determine classification of cases into discrete classes based on the regression factor scores representing each of the seven factors of interest. This method allows for the assignment of individuals to distinct patient types based on posterior probabilities for class membership according to a particular response pattern on the measured factors. The Mplus software was used for this procedure. The analysis was conducted for a range of 2 to 5 classes. The final determination of the number of classes was based on the Lo-Mendell-Rubin likelihood ratio test (LMR-LRT) for mixture distributions (Lo et al., 2001), a measure of entropy (Ramaswamy et al., 1993), the average latent class probabilities for most likely latent class membership and logical description of the classes selected. Entropy is a summary measure ranging from 0 to 1 and is closely related to the average estimated conditional probability of each subject being classified into the groups derived. Values closer to one indicate clear classification across all classes, since entropy will decrease when the probabilities associated with each class are not near 0 or 1; values above 0.80 indicate a good classification result. Although the Bayesian Information Criterion (BIC) is widely used as an index to guide the decision on the number of classes in mixture modeling, the BIC indices obtained in this study were not useful; they steadily decreased in value as more groups were added. For 2 to 5 classes, the BIC values are 5569.73, 5416.24, 5319.23 and 5254.70, respectively. Given this situation, more subjective criteria based on knowledge of the domain of interest and the purpose of the analysis was used to identify the best model as recommended by Nagin (2005).
Given the latent classes selected, t-tests were then used for multiple comparisons to assess whether the classes differed on each of the regression factor scores using the SAS software (SAS Institute, 2001). The magnitude of these differences was measured with Cohen’s (1988) effect sizes (differences in means divided by total standard deviation). Bi-variate statistics, t-tests and ANOVA, were used to test for differences in prevalences and means across classes for the 40 individual variables that made up the seven factors.
2.3.2 Regression analyses of data elements associated with patient types
Multivariate multinomial regression was used to explore the associations between selected automated and interview variables with each of the classes identified with the LCA. Variables were included in the multinomial regression if: 1) prior research indicated the variable was significantly associated with any of the domains of interest, or 2) bi-variate, unadjusted statistics indicated the distribution of the variable differed significantly (univariate p-value < 0.20 for the omnibus test) across patient types (Table 1). Use of the 0.20 level of significance as a screening criterion is based on the work by Mickey and Greenland (1989). All variables of interest were not included in the final model since considerable confounding (Miettinen, 1976) and suppressor effects (Tzelgov & Henik, 1991) may be present in the data, and this approach may lead to model over fitting and numerically unstable estimates (Hosmer & Lemeshow, 1989).
Table 1.
Descriptive Statistics for Opioid Problem, Pain and Mental Health Symptom Variables
| Factor | Most Likely Class | |||
|---|---|---|---|---|
| Item, paraphrased | Typical (n = 580) | Addictive Behaviors (n=82) | Pain Dysfunction (n=42) | p-value omnibus test |
| Addictive Behaviors (yes/no) | % | % | % | |
| Ever bought opioids on the street | 1 | 7 | 5 | <0.001 |
| Doctor ever refused Rx due to abuse concern | 5 | 17 | 10 | <0.001 |
| You have been treated for any AOD problem | 12 | 23 | 14 | 0.022 |
| You had any AOD problem | 17 | 32 | 19 | 0.009 |
| Ever borrowed opioid medicines | 9 | 20 | 10 | 0.018 |
| Used for other symptoms e.g. sleep, anxiety | 12 | 17 | 14 | 0.366 |
| Requested early refill | 19 | 29 | 31 | 0.029 |
| Used alcohol for pain | 6 | 5 | 7 | 0.845 |
|
| ||||
| Addictive Concerns (yes/no) | % | % | % | |
| You think you might be addicted to opioids | 1 | 74 | 90 | <0.001 |
| Family concerned about your being addicted to opioids | 0 | 29 | 55 | <0.001 |
| Doctor said you were addicted to opioids | 0 | 26 | 52 | <0.001 |
| Lost opioid meds and needed replaced | 13 | 18 | 31 | 0.005 |
|
| ||||
| Pain Treatment Problems (yes/no) | % | % | % | |
| Angry or mistrustful of doctor | 14 | 30 | 24 | <0.001 |
| Pain been inadequately treated past 90 days | 30 | 33 | 29 | 0.858 |
| Increase amount opioids used past 90 days | 22 | 24 | 10 | 0.133 |
| Doctor ever refused Rx opioids due to abuse concern | 5 | 17 | 10 | <0.001 |
|
| ||||
| Opioid Abuse and Dependence (yes/no) | % | % | % | |
| Lifetime problems with family/friends, work, cops | 3 | 15 | 12 | <0.001 |
| Withdrawal symptoms | 34 | 79 | 62 | <0.001 |
| Ever used opioids larger amounts, longer time | 4 | 18 | 17 | <0.001 |
| Spent a lot of time using/getting opioids | 4 | 21 | 10 | <0.001 |
| Use despite psych/med consequences | 7 | 21 | 17 | <0.001 |
| Wanted to stop or cut down | 49 | 85 | 64 | <0.001 |
| Reduced important activities to get or use | 2 | 7 | 5 | 0.007 |
| Lifetime interference opioids work, job, home | 8 | 22 | 19 | <0.001 |
| Need more opioids to get same effect | 34 | 63 | 55 | <0.001 |
|
| ||||
| Pain Intensity and Interference (0–10 scale) | mean (s.d.) | mean (s.d.) | mean (s.d.) | |
| How much pain interfered with ability to work | 5.2 (2.9) | 5.2 (2.7) | 6.6 (2.1) | 0.185 |
| How much pain interfered with social/family activities | 5.5 (3.0) | 5.5 (3.0) | 7.2 (2.6) | 0.571 |
| How much pain interfered with daily activities | 5.3 (2.6) | 5.0 (2.6) | 6.5 (2.2) | 0.571 |
| Rate your pain right now | 4.5 (2.5) | 4.6 (2.3) | 5.4 (2.2) | 0.350 |
| Intensity of usual pain | 5.4 (2.0) | 5.2 (1.9) | 5.8 (1.9) | 0.031 |
| Intensity of worst pain | 8.5 (1.5) | 8.6 (1.4) | 9.0 (1.2) | 0.223 |
|
| ||||
| Anxiety (1–4 scale not at all-nearly daily ) | mean (s.d.) | mean (s.d.) | mean (s.d.) | |
| Nervous, anxious, on edge, or worrying a lot | 1.9 (1.0) | 2.3 (1.1) | 2.2 (1.1) | 0.007 |
| Getting tired very easily past 4 wks | 2.5 (1.1) | 2.4 (1.1) | 2.6 (1.2) | 0.967 |
| Trouble concentrating on things | 1.6 (0.9) | 2.0 (1.2) | 1.7 (1.1) | 0.050 |
| Trouble falling asleep or staying asleep | 2.4 (1.2) | 2.4 (1.3) | 2.5 (1.3) | 0.167 |
| Becoming easily annoyed or irritable | 1.6 (0.8) | 1.9 (0.9) | 2.0 (1.1) | 0.004 |
| Muscle tension, aches, or soreness | 2.6 (1.1) | 2.8 (1.2) | 2.7 (1.2) | 0.574 |
| Feeling restless so that it is hard to sit still | 1.6 (0.9) | 2.0 (1.1) | 1.8 (0.9) | 0.003 |
|
| ||||
| Depression (1–4 scale not at all-nearly daily ) | mean (s.d.) | mean (s.d.) | mean (s.d.) | |
| Little interest or pleasure in doing things | 0.7 (0.9) | 1.0 (1.0) | 1.1 (1.2) | 0.004 |
| Feeling down, depressed, or hopeless | 0.6 (0.9) | 0.9 (1.0) | 0.9 (1.0) | 0.034 |
Next, variables were included in a stepwise method of selection for the multivariate multinomial regression model. The 0.20 alpha level for inclusion into the final prediction model was based on the work by Bendel and Afifi (1977) and Costanza and Afifi (1979). Stepwise methods were appropriate given that: the variables were selected based on their theoretical relevance; the variables were basically independent, revealing low correlations ranging from −0.19 to 0.31, with an average correlation of 0.03; and this was an exploratory analysis The SAS software was used for both the univariate and multivariate multinomial regression analysis. Three models were run to explore the relationship of different data elements with class membership: 1) automated data elements, 2) interview data and 3) automated data and interview data.
3. Results
3.1 Respondent characteristics
A total of 778 interviews were conducted, for a response rate of 57% among eligible patients. The three most common reasons for being ineligible, among those determined to likely be eligible via automated data, were being physically or mentally unable to participate in the phone interview, having a non-working phone number, and using opioid medicines for reasons other than pain. Among those who met eligibility criteria, a lack of participation in the study was most commonly due to active refusal. Reasons for refusal were not documented. Non-respondents were significantly more likely to be younger (p<0.001), male (p<0.05) and have a higher opioid dose (p<0.001). All analyses were conducted with the 704 of the 778 respondents who reported use of opioids in the prior 90 days. The majority of subjects were non-Hispanic white (89%), females (62%), with an average age of 55. A majority also graduated high school and were married or partnered. A total of 40% were currently employed with substantial proportions unable to work (25%) or not in the paid work force (30%), most of whom were retired. The average total days supply of opioids was 349 days (standard deviation = 241) in the prior 365 days. The average daily dose of opioids in the past year was 50mg (S.D. 64) morphine equivalent dose per day. A minority had screened positive for anxiety (15%) or depression (21%) based on current symptoms assessed with the PHQ. Opioid dependence was present among 13% of subjects with another 8% diagnosed with opioid abuse without concomitant dependence per DSM-IV criteria. A majority (59%) indicated high pain interference per the GCPS.
3.2 Latent class analysis for determining class membership
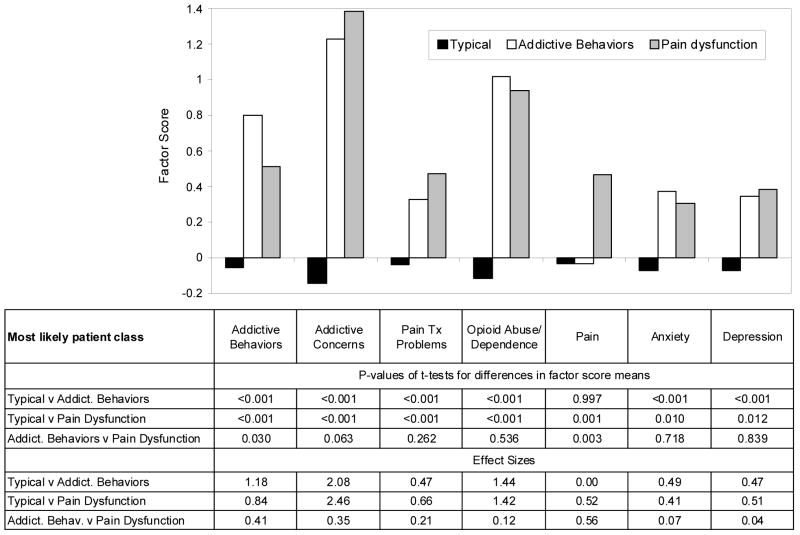
A three class model was determined to be most appropriate. The proportion in each class and the average latent class probability for each class are described below. A graphical presentation of the three classes, the pattern of factor scores on each of the seven factors and statistical tests of the differences between groups are presented in Figure 2.
Figure 2.
Factor scores by most likely class: Typical (n=580), Addictive Behaviors (n=82) and Pain Dysfunction (n=42), statistical tests of differences in factor scores by most likely class and effect sizes of differences in factor scores by most likely class.
3.2.1 Number of classes/patient types
Either 3 or 4 classes were appropriate per results of the LMR-LRT test (2 x loglikelihood difference, df = 8): 3 vs. 2 classes LMR-LRT = 208.50, p-value=0.005, entropy 3 classes =0.979 compared with 4 vs. 3 classes LMR-LRT= 133.84, p-value=0.005, entropy 4 classes 0.986. For 5 vs. 4 classes, the LMR-LRT=60.710, p-value= 0.702 with an entropy of 0.944 for 5 classes. Three classes were selected based on logical description of the classifications and the small difference in entropy values for each model. Average latent class probabilities were high, between 94.9% and 99.9%, suggesting that the model assigned subjects’ most likely class membership with a high degree of certainty. (Intuitively, one can be more confident in a model where subjects are assigned to a particular class with a probability close to 100% than if the class probabilities are close to 50%.) Class labels were based upon the patterns of factor scores, difference in factor scores as indicated by t-tests (Figure 2) and the content of the items which constituted the factors. The three classes were labeled: Typical (82%, n= 580), Addictive Behaviors (12%, n=82) and Pain Dysfunction (6%, n=42); the latter two classes are also referred to as atypical because they represent a minority of patients in this study and to contrast them with the single, large typical group. The four class solution which was rejected divided the Pain Dysfunction group into two very small groups which were difficult to clearly describe.
3.2.2 Differences between patient types
Significant differences between the Typical class and the Addictive Behaviors class on all 7 factors except for Pain were found (see first three rows of data in Figure 2). Individuals in the Typical and Pain Dysfunction classes differed statistically on all 7 factors. The Addictive Behaviors and Pain Dysfunction classes differed significantly only on the Addictive Behaviors (p=0.030) and Pain factors (p=0.003), with Addictive Concerns higher, but not significantly, among the Pain Dysfunction group (p=0.063). Values of 0.20 indicate a low effect size, 0.50 moderate, and ≥0.70 large effect sizes. With this sample, effect sizes for differences between the Addictive Behaviors and Pain Dysfunction classes compared to the Typical class were moderate to very large in value (see bottom three rows of Figure 2). Effect sizes comparing the Addictive Behaviors and Pain Dysfunction classes were low to moderate.
Prevalences or means for the individual variables that constituted the seven factors are presented in Table 1. For instance, patients’ self-report of addiction concerns (a PDUQ item) was reported by 1% of those most likely in the Typical class, compared to 74% and 90% for the Addictive Behaviors and Pain Dysfunction classes respectively. This compares to the DSM-IV item ever wanted to “stop or cut down” opioids endorsed by 49% of the Typical class and 85% and 64% for the Addictive Behaviors and Pain Dysfunction classes respectively.
3.3 Associations between automated and interview data and patient class
The unadjusted relationship between automated and interview variables with the most likely patient class are presented in Table 2. Comparing the most likely classes, several variables met the p<0.20 criteria used for variable selection, including: gender, age, employment, smoker, opioid or non-opioid problems diagnosed in the past 3 years, potential alcohol or sedative problem, any benzodiazepine use in the past year, anxiety or mood disorder visits, any past year inpatient or urgent care/ER visit, years since chronic pain onset, and believing opioids worked best for their pain. Average prescribed daily opioid dose in year three was significantly different across classes, with the Typical group averaging a dose only one third the level of the two atypical groups. Opioid type, a related variable in that those on long acting opioids tend to have a higher dose, had a similar pattern with the Typical group having a much higher proportion of Schedule III opioids, which are short acting by definition.
Table 2.
Unadjusted associations between automated and interview data and most likely patient class
| Most Likely Latent Class | Data Source | |||||
|---|---|---|---|---|---|---|
| Typical (n = 580) | Addictive Behaviors (n=82) | Pain Dysfunction (n=42) | p-value omnibus test | Automated | Interview | |
| Male | 35% | 51% | 45% | 0.014 | x | |
| Age mean (s.d.) | 55.9 (10.6) | 52.24 (10.6) | 53.8 (10.3) | 0.010 | x | |
| Education, more than high school | 75% | 68% | 78% | 0.338 | x | |
| Married or living as married | 67% | 70% | 76% | 0.410 | x | |
| Caucasian | 90% | 90% | 95% | 0.581 | x | |
| Employement Status | 0.052* | x | ||||
| Employed | 42% | 41% | 17% | |||
| Unemployed | 3% | 1% | 2% | |||
| Not in paid work force | 29% | 27% | 37% | |||
| Unable to work | 23% | 31% | 41% | |||
| Other | 3% | 0% | 2% | |||
| Current Smoker | 24% | 32% | 32% | 0.160 | x | |
| Opioid problems diagnosed past 3 yrs | 3% | 10% | 10% | 0.002 | x | |
| Non-opioid drug problems diagnosed past 3 yrs | 6% | 11% | 19% | 0.003 | x | |
| Alcohol potential problem | 6% | 11% | 2% | 0.150 | x | |
| Sedative potential problem | 4% | 12% | 10% | 0.004 | x | |
| Marijuana potential problem | 8% | 11% | 5% | 0.472 | x | |
| Heroin, cocaine and/or methamphetamine potential problem | 3% | 6% | 2% | 0.248 | x | |
| Benzodiazepines any prescribed past year | 32% | 46% | 40% | 0.026 | x | |
| Anxiety visit past year | 16% | 23% | 29% | 0.039 | x | |
| Mood disorder visit past year | 27% | 41% | 26% | 0.020 | x | |
| Inpatient stay any past year | 14% | 22% | 19% | 0.131 | x | |
| Ugent care/E.R. visit any past year | 18% | 24% | 33% | 0.021 | x | |
| Years since first chronic pain >10 | 56% | 44% | 56% | 0.150 | x | |
| Believe opiates work best for pain | 54% | 63% | 76% | 0.009 | x | |
| Believe more pain relief possible w/ opiates | 35% | 32% | 40% | 0.653 | x | |
| Avg. prescribed daily opioid dose in mg past year mean (s.d.) | 43.0 (84.2) | 131.9 (195.6) | 127.8 (129.6) | <0.001 | x | |
| Opioid type used during past year | <0.001* | x | ||||
| Schedule III only | 31% | 15% | 10% | |||
| Schedule II- any short acting no long acting | 37% | 22% | 19% | |||
| Schedule II- any long acting | 32% | 63% | 71% | |||
df=8
df=4
The results of the stepwise multivariate multinomial regression model are presented in Table 3 with variables listed in order of the greatest statistical significance within each model. Opioid dose in the prior year was much higher for the Addictive Behaviors group and the Pain Dysfunction group compared to the Typical group for the automated-data-only-model and the combined-data-source-model, adjusting for other variables in the model; the distribution of average prescribed daily opioid dose was transformed with a log10 transformation which yielded an approximately normal distribution for use in multivariate analyses.
Table 3.
Independent associations between automated and interview data and most likely patient class using multivariate multinomial stepwise regression
| Classes Compared | Addict. Behaviors v Typical | Pain Dysfunction v Typical | Addict. Behaviors v Pain Dysfunction |
|---|---|---|---|
| Reference Group | Typical | Pain dysfunction | |
| O.R. (p-value) | O.R. (p-value) | O.R. (p-value) | |
| Automated Data Only | |||
| Avg prescribed daily dose opioids(log10) * | 3.53 (<.001) | 6.79 (<.001) | 0.54 (0.080) |
| Age | 0.97 (0.006) | 0.99 (0.544) | 0.98 (0.238) |
| Male | 1.95 (0.010) | 1.18 (0.574) | 1.60 (0.238) |
| Mood disorder visit past year | 1.53 (0.118) | 0.57 (0.174) | 2.67 (0.031) |
| Non-opioid drug problem diagnosis | 1.21 (0.660) | 2.77 (0.041) | 0.44 (0.157) |
| Survey Data Only | |||
| Sedative potential problem | 3.39 (0.003) | 2.43 (0.123) | 1.40 (0.596) |
| Believe opiates work best for pain | 1.34 (0.239) | 2.76 (0.009) | 0.49 (0.106) |
| Years since first chronic pain >10 | 0.64 (0.067) | 1.01 (0.969) | 0.63 (0.241) |
| Automated and Survey Data Combined | |||
| Avg prescribed daily dose opioids(log10) * | 3.67 (<.001) | 6.18 (<.001) | 0.59 (0.138) |
| Sedative potential problem | 3.23 (0.009) | 2.82 (0.095) | 1.15 (0.831) |
| Age | 0.97 (0.009) | 0.98 (0.353) | 0.98 (0.396) |
| Male | 1.85 (0.021) | 1.30 (0.458) | 1.42 (0.392) |
| Mood disorder visit past year | 1.47 (0.168) | 0.50 (0.104) | 2.93 (0.022) |
| Non-opioid drug problem diagnoses | 1.12 (0.807) | 2.75 (0.044) | 0.41 (0.126) |
| Alcohol potential problem | 1.98 (0.117) | 0.48 (0.489) | 4.11 (0.194) |
log10(average prescribed daily dose of opioid medications in mg) past year
The Addictive Behaviors group was significantly younger and more likely to be male than the Typical group in the model based upon automated data as well as the model incorporating both data sources. Potential prescription sedative problems, per the ASSIST screening tool, was statistically much more likely among the Addictive Behaviors group compared to the Typical group in both models, but not for the Pain Dysfunction group. In the interview data only model the Pain Dysfunction group was much more likely to believe that opiates worked best for their pain compared to other treatments, however this variable was not statistically significant in the final model which incorporated automated data elements. Having a non-opioid drug problem diagnosed in the automated data records was more likely in the Pain Dysfunction group than the Typical group, though the p-value was only barely significant at 0.044 and there was no association with the Addictive Behaviors group. In both models using automated data, the Addictive Behaviors group was much more likely to have had a visit related to a mood disorder in the proceeding year than the Pain Dysfunction group with no other significant differences between the two non-Typical groups.
4. Discussion
We sought to comprehensively describe the population of pain patients prescribed opioids chronically in a general medical population served by an integrated group practice. Latent class analysis was used to identify response patterns across seven factors to identify the class structure that best fit the data and was of value clinically. Three classes were identified: 1) a Typical group, the great majority, which had moderate levels of pain and mental health symptoms, but very low levels of addictive behaviors or concerns, and two atypical groups, 2) an Addictive Behaviors group with elevated mental health symptoms and opioid problems, notably addictive behaviors, but pain similar to Typical pain patients and 3) a Pain Dysfunction group with significantly higher pain as well as elevated mental health and opioid problem indicators. The categorization scheme we propose is novel in that it directly incorporates important, common co-morbidities and it divides patients with potentially problematic opioid use into Pain Dysfunction and Addictive Behaviors groups. Automated and interview data elements independently associated with potentially problematic opioid were found that may help to identify these atypical pain patients on opioids.
4.1. Patient Typologies
Incorporating co-morbidities into the patient typology allowed for a closer fit to the complex clinical picture that is common for chronic pain patients prescribed opioids. Patients identified as belonging to the Addictive Behaviors or Pain Dysfunction groups likely require additional treatment intervention; fortunately, such interventions have some common treatment approaches, e.g. cognitive behavioral therapy in combination with appropriate and carefully monitored medications (Compton, 2008). A primary care based intervention study with chronic pain patients at risk for substance abuse found that a substantial proportion could adhere to opioid treatment agreements (Wiedemer et al., 2007). Whether or not these more complicated atypical patients can be managed in a usual primary care setting, their identification is a high priority.
Several authors have advocated for better understanding of nonmedical use of opioids in different settings and contexts (Compton and Volkow, 2006; Fisher and Rehm 2008; Zacny and Lichtor 2008). While our findings are preliminary and are inadequate to definitively distinguish the two atypical groups, addictive behaviors and pain dysfunction, they suggest that the critical issues behind these symptoms and behavioral patterns may be different. In particular, the continuation of high pain levels along with some addictive behaviors among those most likely to be in the Pain Dysfunction class may correspond to the “pseudo-addiction” problem noted by some pain specialists (Weissman and Haddox, 1989; Lusher et al., 2006), that is, behaviors motivated by inadequate pain control that may appear similar to addiction, but remit when pain is adequately managed. Our findings provide empirical support for the construct of pseudo-addiction, though given the complexity of these patients and their problems as well as their higher average dose, this does not imply that a simple increase in opioid dose would solve all opioid use, nor other, problems. These findings are also of value in that they describe the characteristics of the more typical pain patient, who has some persistent pain and low level mental health issues, but no opioid use problems.
4.2. Automated and Interview Data Elements Associated with Patient Types
In an exploratory regression analysis of variables associated with most likely patient type, we found that higher opioid dose was strongly associated with being in both the Addictive Behaviors and Pain Dysfunction groups after adjusting for other factors. Unadjusted analyses indicate that the two atypical groups have an average prescribed daily dose three times that of Typical patients. Haller (2008) reported that among a pain clinic population with DSM abuse or dependence diagnoses, a high dose sub-group had greater addiction severity, but equivalent pain, functionality, opioid adherence and other drug use outcomes after a structured treatment intervention. We cannot discern the temporal order of problematic use and opioid dose level due to the generally long lengths of time opioids were used and the lack of historical or temporal data on problematic opioid use. Unadjusted analyses also indicated that the use of long acting schedule II opioids was much more common among the atypical groups, approximately twice the proportion as the Typical class, however this is likely due to the strong correlation with opioid dose as opioid type was not significant in the adjusted regression models which included opioid dose. The role of opioid dose in opioid use problems remains unclear, but these findings support efforts to augment patient assessment, monitoring and treatment for patients who are prescribed higher opioid doses.
Potentially problematic use of prescription type sedatives, being younger and male were all significantly more likely to be present in the Addictive Behaviors group compared to the Typical group, adjusting for other variables. A similar finding regarding misuse of other prescription medications was reported among those with opioid abuse/dependence in the National Survey of Drug Use and Health (Becker et al. 2008). Similar age and gender relationships have been reported to be associated with opioid misuse in several studies (Turk et al., 2008; Ives et al., 2006; Fleming et al., 2007). There is also evidence that younger patients, defined as 50 or younger, tend to have higher doses and escalate to those higher doses more quickly than older patients, those age 60 and older (Buntin-Mushock et al., 2005). Despite these associations between demographic characteristics and addictive behaviors the absolute risk is diminished by the fact that chronic pain patients are generally older and female (Tsang, et al., 2008).
There were no significant differences in past year mood disorder visits for either of the atypical groups compared to the Typical group, though the Addictive Behaviors group did have significantly higher odds of such visits compared to the Pain Dysfunction group. It is important to note the difference between receiving care in the prior year for a mood disorder, most often depression, and having current symptoms of depression as was utilized in developing the class types. While there were no differences in the level of current depression symptoms between the two atypical groups, it appears that those with Addictive Behaviors may be more likely to have received treatment for a mood disorder. The utility of each these variables as possible risk factors and/or markers requires further study.
4.3. Limitations
There were several limitations to our study. Screening for addiction remains problematic and instruments need to be improved. Though we used a modified version of the PDUQ, an instrument which has also been used by others, no single instrument stands out as ideal (Turk et al., 2008 Passik et al., 2008). We believe that the content of the items from the PDUQ that we utilized are generally important and were adequate for our aim of determining the broad types of opioid users in a general medical population. Refinement of addiction screening instruments for specialty pain clinics and general practice is still needed.
The integrated group practice from which data were obtained has a population similar to that of Washington State as a whole (Saunders et al., 2005). However, the findings are limited to those able to remain enrolled in an integrated group practice for three years and thus who may have fairly stable lives or employment. Those suspected of opioid misuse may have been discontinued from opioids and therefore not met eligibility criteria for the study. It is possible that a greater proportion of patients in the two atypical classes would be found in less stable populations. It is also possible that a different patient typology would be detected in other populations. At the time of the study neither the integrated group practice generally or the pharmacy in particular had any protocols or guidelines in place regarding chronic pain management or opioid dosing.
Our non-response rate was relatively high; the interviewers reported that this was a particularly challenging study population to recruit and interview. However, the response rate is similar to that of other studies with populations that have co-morbid, chronic health conditions (Katon et al., 2004). Non-response can potentially impact both the representativeness of the subjects who participated as well as bias estimates. Non-responders were more likely to be younger, male, and have a higher opioid dose in the past year. These variables were each associated with higher odds of being in one or both of the atypical classes in the regression analyses, suggesting that these patients were under-represented in this study. However, our aim was not to estimate the prevalence of various opioid use problems and our results should not be interpreted with this as a goal. Second, because we were able to statistically adjust for these variables associated with non-response in multivariate regression analyses, bias is partially addressed in the odds ratios reported.
The exploratory multivariate analyses did not account for the fact that likely class membership is based upon a probability with an associated error component. In order to utilize stable classes/patient typologies in each of the three regression models, we conducted LCA in Mplus and then used the resultant class memberships for regression analyses in SAS, an approach used by others (Monga et al, 2006). We believe that our approach is satisfactory given the high entropy values, high probabilities of class membership, our emphasis on findings that were highly statistically significant and our framing of findings as hypothesis generating. Our aim with this regression modeling is exploratory, with results used to refine future research.
4.4. Conclusions
Integrating addiction issues, pain and mental health yielded a new typology of patients that may support a more comprehensive and clinically meaningful approach for screening, assessment and intervention. We strongly believe more research is needed to determine whether a similar patient typology is found in other patient populations, including those seeking addiction treatment, less stable general medical populations, and those obtaining services in specialty pain clinics. Higher opioid dose in particular is a relatively easily obtained marker for patients who could benefit from further assessment of potential medical, mental health and opioid use problems.
Supplementary Material
Footnotes
Additional details about the factor analyses used in this report is available as supplementary material with the online version of this article at doi: xxxxxx/j.drugalcdep. xxxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams LL, Gatchel R, Robinson RC, Polatin P, Gajraj N, Deschner M, Noe C. Development of a self-report screening instrument for assessing potential opioid medication misuse in chronic pain patients. J Pain Symptom Manage. 2004;27:440–459. doi: 10.1016/j.jpainsymman.2003.10.009. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Institute. Diagnostic and statistical manual of mental disorder. 4. American Psychiatric Institute; Washington D.C: 1994. [Google Scholar]
- Banta-Green C, Merrill J, Doyle S, Boudreau D, Calsyn D. Measurement of Opioid Problems Among Chronic Pain Patients in a General Medical Population. Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2009.03.022. Under editorial review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W, Sullivan L, Tetrault J, Desai R, Fiellin D. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: Psychiatric, medical and substance use correlates . Drug Alcohol Depend. 2008;94:38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Bendel RB, Afifi AA. Comparison of stopping rules in forward regression. J Am Stat Assoc. 1977;72:46–53. [Google Scholar]
- Boudreau DM, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, Campbell C, Merrill JO, Silverberg M, Banta-Green C, Weisner C. Trends in De-facto Long-term Opioid Therapy for Chronic Non-Cancer Pain. Pharmacoepidemiol Drug Saf. 2009 doi: 10.1002/pds.1833. Under editorial review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau DM, Doescher MP, Jackson JE, Fishman PA, Saver BG. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38:1317–1318. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- Buntin-Mushock C, Phillip L, Moriyama K, Palmer PP. Age-Dependent Opioid Escalation in Chronic Pain Patients. Anesth Analg. 2005;100:1740–1745. doi: 10.1213/01.ANE.0000152191.29311.9B. [DOI] [PubMed] [Google Scholar]
- Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R) J Pain. 2008;9:360–372. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabal C, Erjavec MK, Jacobson L, Mariano A, Chaney E. Prescription opiate abuse in chronic pain patients: clinical criteria, incidence, and predictors. Clin J Pain. 1997;13:150–5. doi: 10.1097/00002508-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Cohen C. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Compton P. Should opioid abusers be discharged from opioid-analgesic therapy? Pain Medicine. 2008;9:383–390. doi: 10.1111/j.1526-4637.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355–63. doi: 10.1016/s0885-3924(98)00110-9. [DOI] [PubMed] [Google Scholar]
- Compton W, Volkow N. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Costanza MC, Afifi AA. Comparison of stopping rules in forward stepwise discriminant analysis. J Am Stat Assoc. 1979;74:777–785. [Google Scholar]
- Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance use disorders in a primary care samples receiving daily opioid therapy. J Pain. 2007;8:573–582. doi: 10.1016/j.jpain.2007.02.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Rehm J. Nonmedical use of prescription opioids: Furthering a meaningful research agenda. J Pain. 2008;9:490–493. doi: 10.1016/j.jpain.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Hagenaars JA, McCutcheon A. Applied Latent Class Analysis. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- Haller DL. Opioid analgesic abuse in patients with chronic non-cancer pain: Project Pain. Presented at the College on Problems of Drug Dependence. 2008 June;18:2008. [Google Scholar]
- Hosmer DW, Lemeshow S. Applied logistic regression. John Wiley and Sons; New York: 1989. [Google Scholar]
- Ives T, Chelminski P, Hammett-Stabler C, Malone R, Perhac JS, Potisek N, Shilliday B, DeWalt D, Pignone M. Predictors of Opioid Misuse in Patients with Chronic Pain: A Prospective Cohort Study. BMC Health Serv Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries NO. A note on ‘Testing the number of components in a normal mixture’. Biometrika. 2003;90:991–994. [Google Scholar]
- Jonasson U, Jonasson B, Wickstrom L, Andersson E, Saldeen T. Analgesic use disorders among orthopedic and chronic pain patients at a rehabilitation clinic. Subst Use Misuse. 1998;33:1375–85. doi: 10.3109/10826089809062222. [DOI] [PubMed] [Google Scholar]
- Jöreskog KG, Sörbom D. LISREL V: Analysis of linear structural relationships by the method of maximum likelihood. National Educational Resources; Chicago: 1981. [Google Scholar]
- Katon WJ, Lin EHB, Russo J, Von Korff M, Ciechanowski P, Simon G, Ludman E, Bush T, Young B. Cardiac Risk Factors in Patients with Diabetes Mellitus and Major Depression. J Gen Intern Med. 2004;19:1192–1199. doi: 10.1111/j.1525-1497.2004.30405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyanou K, Pither CE, Wessely S. Medication misuse, abuse and dependence in chronic pain patients. J Psychosom Res. 1997;43:497–504. doi: 10.1016/s0022-3999(97)00171-2. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- Lusher J, Elander J, Bevan D, Telfer P, Burton B. Analgesic addiction and pseudoaddiction in painful chronic illness. Clin J Pain. 2006;22:316–24. doi: 10.1097/01.ajp.0000176360.94644.41. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Lu YW, Parthasarathy S, Moore C, Weisner CM. Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: comparison with matched controls. Arch Intern Med. 2003;163:2511–2517. doi: 10.1001/archinte.163.20.2511. [DOI] [PubMed] [Google Scholar]
- Mickey J, Greenland S. A study of the impact of confounder-selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- Miettinen OS. Stratification by multivariate confounder score. Am J Epidemiol. 1976;104:609–620. doi: 10.1093/oxfordjournals.aje.a112339. [DOI] [PubMed] [Google Scholar]
- Monga N, Rehm J, Fischer B, Brissette S, Bruneau J, El-Guebaly N, Noel L, Tyndall M, Wild C, Leri F, Allu JSF, Bahl S. Using latent class analysis (LCA) to analyze patterns of drug use in a population of illegal opioid users. Drug Alcohol Depend. 2006;88:1–8. doi: 10.1016/j.drugalcdep.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-based Modeling of Development. Cambridge MA: Harvard University Press; 2005. [Google Scholar]
- Passik S, Kirsh KL, Casper D. Addiction-related assessment tools and pain management: Instruments for screening, treatment planning and monitoring compliance. Pain Med. 2008;9(Suppl 2):S145–S166. [Google Scholar]
- Ramaswamy V, Desarbo WS, Reibstein DJ, Robinson WT. An empirical pooling approach for estimating marketing mix elasticities with PIMS data. Mark Sci. 1993;12:103–124. [Google Scholar]
- Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O'Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17:173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA. 2003;289:2370–2378. doi: 10.1001/jama.289.18.2370. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT Software: Reference (Version 8.1) Author; Cary, NC: 2001. [Google Scholar]
- Saunders K, Davis R, Stergachis A. Group Health Cooperative. In: Strom B, editor. Pharmacoepidemiology. John Wiley and Sons; West Sussex, England: 2005. pp. 223–239. [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD, The PHQ primary care study. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Sproule BA, Busto UE, Somer G, Romach MK, Sellers EM. Characteristics of dependent and nondependent regular users of codeine. J Clin Psychopharmacol. 1999;19:367–372. doi: 10.1097/00004714-199908000-00014. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Edlund MJ, Zhang L, Unutzer J, Wells KB. Association Between Mental Health Disorders, Problem Drug Use, and Regular Prescription Opioid Use . Arch Intern Med. 2006;116:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Edlund MJ, Steffick D, Unutzer J. Regular use of prescribed opioids: Association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K. Treatment needs associated with pain in substance use disorder patients: implications for concurrent treatment. Drug Alcohol Depend. 2004;73:23–31. doi: 10.1016/j.drugalcdep.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–91. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: A systematic review and literature synthesis. Clin J Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- Tzelgov J, Henik A. Suppression situations in psychological research: Definitions, implications, and applications. Psychol Bull. 1991;109:524–536. [Google Scholar]
- Von Korff M, Saunders K, Ray GT, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg M, Banta-Green C, Weisner C. De-facto long-term opioids therapy for non-cancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Korff M, Ormel J, Keefe F, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–149. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573–584. doi: 10.1111/j.1526-4637.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- Weissman DE, Haddox JD. Opioid pseudoaddiction- an iatrogenic syndrome. Pain. 1989;36:363–366. doi: 10.1016/0304-3959(89)90097-3. [DOI] [PubMed] [Google Scholar]
- World Health Organization. [March 18, 2008 Accessed];The effectiveness of a brief intervention for illicit drugs linked to the alcohol, smoking and substance involvement screening test (ASSIST) in primary health care settings: a technical report of phase III findings of the WHO ASSIST randomized controlled trial. 2008 at: http://www.who.int/entity/substance_abuse/activities/assist_technicalreport_phase3_final.pdf.
- Wu SM, Compton P, Bolus R, Schieffer B, Pham Q, Baria A, Van Vort W, Davis F, Shekelle P, Naliboff BD. The addiction behaviors checklist: Validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manage. 2006;32:342–351. doi: 10.1016/j.jpainsymman.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor SA. Nonmedical use of prescription opioids: motive and ubiquity issues. J Pain. 2008;9:473–486. doi: 10.1016/j.jpain.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.