Abstract
Prepulse inhibition (PPI), whereby the startle eyeblink response is inhibited by a relatively weak non-startling stimulus preceding the powerful startle eliciting stimulus, is a measure of sensorimotor gating and has been shown to be deficient in schizophrenia patients. There is considerable interest in whether conventional and/or atypical antipsychotic medications can “normalize” PPI deficits in schizophrenia patients. 51 schizophrenia patients participated in a randomized, double-blind controlled trial on the effects of three commonly-prescribed antipsychotic medications (risperidone, olanzapine, or haloperidol) on PPI, startle habituation, and startle reactivity. Patients were tested at baseline, Week 4 and Week 8. Mixed model regression analyses revealed that olanzapine significantly improved PPI from Week 4 to Week 8, and that at Week 8 patients receiving olanzapine produced significantly greater PPI than those receiving risperidone, but not haloperidol. There were no effects of medication on startle habituation or startle reactivity. These results support the conclusion that olanzapine effectively increased PPI in schizophrenia patients, but that risperidone and haloperidol had no such effects. The results are discussed in terms of animal models, neural substrates, and treatment implications.
Keywords: prepulse inhibition, schizophrenia, olanzapine, risperidone, treatment
1. Introduction
Startle eyeblink modification, including prepulse inhibition (PPI) and habituation of the acoustic startle response has been extensively used, mainly to examine sensorimotor gating deficits in schizophrenia (Braff et al., 1978; Braff et al., 2001b; Hazlett et al., 1998; Kumari et al., 2002; Wynn et al., 2004). PPI usually refers to an auditory based paradigm in which a brief, non-startling stimulus (called a prepulse) shortly precedes a strong, sudden-onset startling stimulus, resulting in a reduction in the startle eyeblink compared to when the startling stimulus is presented alone (Graham, 1975). Depending on the interval between the prepulse and the pulse stimulus (termed the interstimulus (ISI) or lead interval), startle can either be inhibited (at ISIs < 500 ms) or facilitated (at ISIs > 1000 ms), with maximal PPI seen at approximately 60 to 120 ms (Blumenthal, 1999; Graham, 1975; Hsieh et al., 2006; Wynn et al., 2000).
Deficits in sensorimotor gating, including PPI deficits and sometimes startle habituation deficits, have been observed in schizophrenia patients. PPI deficits in schizophrenia have been reported in many laboratories in a typical automatic processing paradigm (Braff et al., 1978; Braff et al., 1992; Grillon et al., 1992; Hong et al., 2007; Kumari et al., 2000; Ludewig et al., 2002; Parwani et al., 2000), startle habituation deficits are sometimes seen in schizophrenia patients (e.g., Akdag et al., 2003; Braff et al., 1992; Ludewig et al., 2003; Meincke et al., 2004; Parwani et al., 2000)), though normal habituation has been reported as well (e.g., Braff et al., 2005; Kumari et al., 2004; Quednow et al., 2006) probably reflecting cohort or parametric effects. Other PPI deficits have been noted in patients’ ability to modulate PPI with attention to the prepulse (Dawson et al., 1993; Dawson et al., 2000; Hazlett et al., 1998; Hazlett et al., in press). There is an extensive literature on PPI, its neurobiology and its medication effects (e.g., Braff et al., 2001b; Geyer et al., 2001; Swerdlow et al., 2001).
Several between subject, non-prospective studies of the effects of antipsychotic medications on PPI in schizophrenia revealed that patients treated with atypical medications have higher levels of PPI compared to those treated with conventional medications (Kumari et al., 1999, 2000, 2002; Leumann et al., 2002; Oranje et al., 2002). However, some studies have failed to show that either atypical or conventional antipsychotic medications are lawfully associated with PPI in schizophrenia patients (Duncan et al., 2003). Only two studies have employed a randomized, controlled design, which permits stronger interpretation of findings.
One study (Mackeprang et al., 2002) randomized a group of young (approximately 27 years of age), never-medicated schizophrenia patients to either a conventional antipsychotic (zuclopenthixol) or an atypical antipsychotic (risperidone). Neither antipsychotic improved PPI relative to normal controls, and PPI between the two treatment groups was comparable.
In the second study (Quednow et al., 2006) 37 patients with schizophrenia were randomized to amisulpride (a pure dopamine D2/D3 receptor antagonist) or olanzapine and compared to normal control subjects at baseline, 4 weeks and 8 weeks. Both antipsychotic medications reversed PPI deficits, with both medication groups showing comparable PPI, though the interpretation of results was difficult because the reversal of PPI deficits could be attributed to habituation of PPI in controls across test sessions.
In the current study, we examined the effects of three antipsychotic medications on PPI, startle habituation and startle reactivity in schizophrenia patients in a randomized, double-blind study comparing olanzapine, risperidone and haloperidol. Patients with schizophrenia and schizoaffective disorder were assessed for PPI levels at baseline and at 4 and 8 weeks after treatment with the expectation that olanzapine and risperidone would have beneficial effects on PPI compared with haloperidol.
2. Methods
2.1 Participants and Inclusion Criteria
100 patients who met DSM-IV criteria for schizophrenia or schizoaffective disorder (both bipolar and depressive subtypes) were initially enrolled at the VA Greater Los Angeles Healthcare System, the VA Long Beach Healthcare System, and the San Diego VA Medical Center. Eight-one percent of the subject had a diagnosis of schizophrenia and 19% had a diagnosis of schizoaffective disorder. In addition to the psychophysiology measures, this study had a second component (social cognitive and neurocognitive performance measures) that will be presented separately. Patients had to be between the ages of 18–60 and competent to provide informed consent. Patients were excluded for mental retardation, identifiable neurological conditions, and alcohol and substance dependence in the last six months (urine toxicology screens were administered at each test session to ensure no subject was actively abusing substances or alcohol). Of the 100 patients enrolled, 90 received a baseline startle assessment. Nine were subsequently dropped from analysis due to equipment malfunction, 4 were dropped due to non-responsiveness (see criteria below), and 26 received only a baseline assessment. Thus, a total of 51 patients who received a baseline assessment and at least one follow up assessment were included in the analysis (Los Angeles: 19 at baseline, 19 at week 4, 9 at week 8; Long Beach: 27 at baseline, 25 at week 4, 23 at week 8; San Diego: 5 at baseline, 5 at week 4, 4 at week 8). See Table 1 for a description of patients randomized to each group and how many patients completed assessments at each week. There were no significant differences in baseline PPI or symptom ratings between non-completers (those receiving only a baseline PPI assessment) and completers (those receiving a baseline PPI assessment and at least one follow up PPI assessment).
Table 1.
Number of schizophrenia patients with prepulse inhibition data at each test assessment.
| Group | Baseline | Week 4 | Week 8 |
|---|---|---|---|
| Risperidone | 19 | 18 | 14 |
| Olanzapine | 21 | 21 | 16 |
| Haloperidol | 11 | 10 | 7 |
All procedures were approved by the Institutional Review Boards (IRB) at each site. All subjects were screened for the ability to provide informed consent and provided signed informed consent when they enrolled in the study.
2.2 Medication and Randomization
Patients were initially enrolled and tested at baseline on their current medication with no medication washout period. Patients receiving oral medication were given baseline assessments immediately. Those on depot medications were converted to an oral administration of the same medication and were given baseline assessments eight weeks after their last depot injection, while they were taking oral medications. Thirty patients were taking atypical antipsychotic medications at baseline, 15 were taking conventional antipsychotics, and 6 were not taking any antipsychotics at baseline (see Table 2 for a group breakdown of baseline medication; there was no significant difference between groups on type of antipsychotic medication at baseline).
Table 2.
Demographic and symptom ratings at baseline for all schizophrenia patients and for patients in each drug group.
| All Subjects | Risperidone | Olanzapine | Haloperidol | |
|---|---|---|---|---|
| N | 51 | 19 | 21 | 11 |
| Gender (M:F) | 43:8 | 15:4 | 17:4 | 11:0 |
| Ethnicity (C:AA)* | 46%:54% | 29%:71% | 65%:35% | 36%:64% |
| Baseline A/C/O** | 30/15/6 | 12/6/1 | 12/6/3 | 6/3/2 |
| PK/AD/MS*** | 8/19/7 | 1/8/2 | 4/8/4 | 3/3/1 |
| Age | 48.8 (7.5) | 46.8 (8.3) | 49.8 (7.2) | 50.3 (6.2) |
| Education | 12.6 (2.4) | 12.5 (1.6) | 12.2 (3.0) | 13.2 (2.4) |
| BPRS Positive | 2.5 (1.0) | 2.4 (1.0) | 2.3 (1.0) | 3.1 (.91) |
| BPRS Negative | 2.3 (.8) | 2.3 (.8) | 2.3 (.8) | 2.1 (.9) |
Percent: Caucasian (C), African American (AA);
Number of subjects on atypcial (A), conventional (C), or other (O) antipsychotic medication at baseline;
Number of subjects on antiparkinsonion (PK), antidepressant (AD), or mood stabilizer (MS) medications at baseline
Patients were randomized to receive either 4 mg of risperidone, 15 mg of olanzapine, or 8 mg of haloperidol. Two randomizations were used. One was a simple (1:1:1) randomization for the three medications. However, if patients had a history of adverse experiences, based on patient or clinician reports, with haloperidol, we used a two-way (1:1) randomization for risperidone and olanzapine only. Because of these two randomization methods, we had fewer subjects assigned to haloperidol than the other two medications. Thirty-three patients were assigned to the three-arm randomization (10 from Los Angeles, 22 from Long Beach, and 1 from San Diego) and 18 patients were assigned to the two-arm randomization (9 from Los Angeles, 5 from Long Beach, and 4 from San Diego. Random assignment of patients was stratified within each site and blocked in sets of 15 (for the three-way randomization) to keep groups relatively balanced throughout the study. During the first two weeks of the study, patients’ current medications were reduced and discontinued as their study medication was brought to full dose by day 14. Patients were allowed the following concomitant medications: valproate, SSRI antidepressants, antiparkisonian medications (including anticholinergics and amantadine), and lorazepam. However, benzodiazpenes were not allowed on the day of testing.
2.3 Clinical Assessments
All patients met criteria for schizophrenia based on interviews with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID; First et al., 1997). Interviewers were trained to administer the SCID through the Diagnosis and Psychopathology Unit of the UCLA Clinical Research Center for the Study of Schizophrenia and demonstrated agreement between their ratings and the consensus ratings of the Center’s diagnosticians (minimum Kappa coefficient of .75). Symptom ratings were assessed at each testing session with the Brief Psychiatric Rating Scale (BPRS; Ventura et al., 1993) and the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1989).
2.4 Procedure
Participants were administered the same startle and prepulse inhibition assessment at three points: at baseline, at 4 weeks, and at 8 weeks. Before each assessment, participants had their hearing screened and were excluded if they could not detect 500, 1000 or 6000 Hz tones at 45 dB or less in either ear.
Subjects were seated in a comfortable chair during testing. Two 4-mm Ag/AgCl electrodes were positioned at the outer canthus and 1 mm medial to the outer canthus over the orbicularis oculi muscle of the eye. The intention was to record only from the right eye, but due to a procedural error, 14 patients had EMG measured from the left eye: 6 from the risperidone group, 5 from the olanzapine group, and 3 from the haloperidol group. Nearly all studies that have examined PPI and habituation of startle across both eyes have failed to identify main effects of left vs. right eye or higher order interactions with eye (Braff et al., 2001a; Swerdlow et al., 2006). The left eye assessments did not differ from right in amount of startle or prepulse inhibition, thus eye position was not considered further. Impedance was kept below 10 kΩ. A ground electrode was placed behind the right mastoid.
EMG was recorded using a San Diego Instruments computerized startle response system. EMG was bandpass filtered at 100–1000 Hz, and was rectified and digitized at 1000 Hz for 0–250 ms post-startle stimulus onset. Startle magnitude was defined as the difference between baseline to peak within a window of 20–100 ms following startle stimulus onset. Scoring of startle data was done blind to the participant’s group assignment.
The session began with a 5 minute acclimation period during which a 70 dB sound pressure level (SPL) continuous white noise background was presented; this background noise was present throughout the rest of the session. Startle stimuli were 115 dB SPL 40 ms white noise bursts. Two dB levels of the prepulse and two lead intervals were used to examine the effects of these parameter manipulations on PPI, not to examine treatment effects. Prepulse stimuli were 20 ms white noise bursts either 8 dB SPL or 16 dB SPL above background. Prepulses were presented at lead intervals of either 30 or 120 ms. Five startle-alone stimuli were presented in the first (Block 1) and last (Block 4) block. Blocks 2 and 3 contained a fixed pseudo-random order of startle alone and prepulse trials. Twelve startle alone and 6 of each of the four types of prepulse (i.e., 2 lead intervals and 2 dB levels of prepulse) were presented in blocks 2 and 3. Prepulse scores were averaged across blocks and used in the analysis. Intertrial intervals averaged 16 s (range 9–23 s).
A session was considered to be valid if subjects’ average startle amplitude in the second block was greater than 3.0 analog/digital units (ADUs). Sessions with an average startle amplitude in the second block less than 3.0 ADUs were not considered to be valid because a reliable eyeblink could not be measured. Each ADU unit is approximately 1.67 μV.
Prepulse inhibition was calculated with the following formula: 100-((prepulse amplitude/startle amplitude)*100), with positive scores indicating startle inhibition. Because we were not interested in the treatment effects across all parameter combinations, we simplified analyses and analyzed data separately for the 30- and 120-ms lead intervals that were 16 dB above background.
Two secondary measures were analyzed. Habituation of the average startle response across each block within and between each test session was analyzed. Startle reactivity, defined as the average startle amplitude in the first block, was also analyzed.
2.5 Statistical Analysis
Fifty-one patients who had one valid baseline session and at least one other valid test session at either week 4 or 8 were included in the analysis. The analyses of PPI and startle reactivity were mixed effects regressions with medication included as a between-groups effect with three levels, and repeated measures at weeks 4 and 8. The analysis specified an unstructured covariance matrix and assumed data were missing at random. When site was included as a random effect, the variance component was estimated as zero. Dependent variables were PPI and startle reactivity. Baseline measures were included as covariates in all analyses. Follow-up pairwise t-tests were performed across time both between and within groups. A change score created by subtracting startle amplitude in block 1 from the start amplitude in block 4 was used to examine habituation. A drug by time mixed effects analysis, using baseline habituation as a covariate, was used to analyze habituation of the startle response. For PPI only, a separate analysis was performed to determine whether changes in symptoms may have affected the results. The positive and negative symptom summary scores from the BPRS were entered into the analysis as covariates.
3. Results
3.1 Demographic Data and Clinical Symptom Ratings
Demographic data and clinical symptoms can be seen in Table 2. As can be seen, the schizophrenia patients were predominantly male and consisted of a large proportion of African Americans (54%). The olanzapine group tended to have more Caucasians than African Americans (Chi-square = 5.20, p < .08). However, there was no difference in PPI between ethnic groups at baseline (Cohen’s d = .10, p > .7) therefore ethnicity was not considered further in the analyses. There were no differences between groups on age, education, or symptom ratings at baseline. Symptom ratings at baseline did not correlate with prepulse inhibition at baseline and were not included as a covariate in analyses.
3.2 Prepulse Inhibition
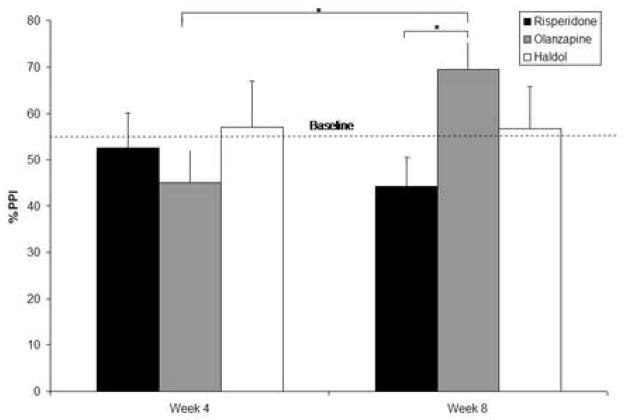
There were no significant differences in baseline PPI assessed with the 30-ms lead interval among the three treatment groups (F (2,50) = 0.5, p < . 60), with means (standard deviations) of 38.4% (26.3%) for risperidone, 31.3% (30.4%) for olanzapine, and 27.4% (33.8%) for haloperidol. There were no significant differences in baseline PPI assessed with the 120-ms lead interval among the three treatment groups (F (2,50) = 2.0, p < . 15), with means (standard deviations) of 62.4% (20.3%) for risperidone, 54.9% (24.7%) for olanzapine, and 38.8% (51.9%) for haloperidol. Least squares adjusted means for PPI assessed with the 120-ms lead interval for each group for weeks 4 and 8 are shown in Figure 1. A horizontal line indicates the the average baseline PPI across the three groups. A 2 (Week 4, Week 8) × 3 (group: risperidone, olanzapine, haloperidol) mixed effects regression, using baseline as a covariate, was used to analyze PPI, separately for the 30- and 120-ms lead interval conditions. Analysis of PPI assessed with a 30-ms lead interval revealed no significant main effect of drug (F (2, 76) = .58, p < .57), test session (F (1, 76) = .09, p < .76), or drug X test session interaction (F (2, 76) = .77, p < .47). Analysis of PPI assessed with a 120-ms lead interval revealed no effect of drug (F (2, 76) = .75, p < .50) or of test session (F (1, 76) = 1.01, p < .40), but did reveal a significant drug X test session interaction (F (2, 76) = 4.81, p < .02). Follow-up t-tests on the least square means revealed significantly greater improvement in PPI at week 8 (69.48%) vs. week 4 (45.18%) for patients receiving olanzapine (t (76) = 3.27, p < .01) and significantly greater improvement in PPI for patients receiving olanzapine (69.48%) vs. patients receiving risperidone (44.25%) at week 8 (t (76) = 2.92, p < .01). There were no changes in the main results when entering the symptom ratings as covariates.
Figure 1.
Least square means (adjusted for PPI at baseline) of prepulse inhibition at week 4 and week 8 for each group. Average PPI at baseline for all three groups is presented for reference as a dashed line (see Results for baseline PPI values for each group separately). * p < .05
3.3 Habituation
A 2 (Week 4, Week 8) × 3 (group: risperidone, olanzapine, risperidone) mixed effects regression, using baseline as a covariate, was used to analyze startle habituation. Analysis of the change in amplitude from block 1 to block 4 revealed no significant main effects of test session or medication group and no significant interaction between the two (F’s < 2.02, p’s > .14). Unadjusted means and standard deviations of pulse-alone startle amplitudes for each test occasion for each group can be seen in Table 3.
Table 3.
Mean (SD) startle reactivity (in A/D units) for each group across four blocks of startle-alone pulses.
| Block 1 | Block 2 | Block 3 | Block 4 | |
|---|---|---|---|---|
|
Risperidone |
||||
| Baseline | 84.9 (65.4) | 52.4 (55.0) | 35.7 (39.9) | 31.7 (41.1) |
| Week 4 | 68.2 (57.0) | 37.4 (37.1) | 26.5 (32.0) | 27.5 (28.0) |
| Week 8 | 69.5 (69.9) | 39.5 (42.2) | 31.3 (41.7) | 25.3 (33.9) |
|
Olanzapine |
||||
| Baseline | 61.5 (76.6) | 35.9 (59.0) | 27.4 (51.4) | 26.0 (61.6) |
| Week 4 | 50.9 (59.6) | 29.9 (50.5) | 23.9 (43.1) | 21.3 (34.6) |
| Week 8 | 43.5 (64.7) | 31.0 (55.1) | 21.2 (40.9) | 21.7 (50.2) |
|
Haloperidol |
||||
| Baseline | 67.6 (54.6) | 39.8 (39.0) | 23.1 (24.7) | 26.4 (27.2) |
| Week 4 | 82.8 (95.0) | 56.8 (101.5) | 40.6 (77.5) | 27.2 (38.1) |
| Week 8 | 55.8 (61.1) | 28.9 (31.7) | 22.8 (24.0) | 22.9 (23.4) |
3.4 Startle Reactivity
Startle reactivity, defined as the mean startle amplitude in block 1 (see Table 3 for unadjusted means) was analyzed with a 2 (Week 4, Week 8) × 3 (group: risperidone, olanzapine, risperidone) mixed effects regression, using baseline as a covariate. There were no significant main effects of test session or medication group nor was the interaction between the two significant (F’s < 1.4, p’s > .25).
4. Discussion
The present study found that schizophrenia patients receiving olanzapine showed a significant increase in PPI over time (the other two groups did not), and greater PPI compared to patients receiving risperidone after 8 weeks of treatment. There were no effects of medication or time, despite equivalent baseline scores, on either startle habituation or startle reactivity.
These findings are mainly consistent with prior studies examining effects of antipsychotic medication on PPI in schizophrenia patients. Several uncontrolled studies have reported improvement in PPI with atypical antipsychotic medications, showing superiority of atypical vs. conventional medication in reversing PPI deficits or showing atypicals normalizing PPI compared to normal controls. However, to date only two of these studies utilized a randomized design. Quednow et al. (2006), utilizing a longitudinal, double-blind design, showed that both amisulpride and olanzapine improved PPI in schizophrenia patients, with no difference in PPI between the two medication groups, providing support that D2/D3 antagonists reverse schizophrenia-related PPI deficits. However, the “improvement” in PPI was seen only in comparison to normal controls, whose PPI habituated across test sessions. For the schizophrenia patients, there was very little change in PPI in either medication group. However, Mackeprang et al. (2002), utilizing a randomized but not double-blind design, found no differences in PPI in patients randomized to either conventional or atypical antipsychotics, with both groups showing lower PPI compared to normal controls.
Our results are also consistent with findings in animal models of disrupted PPI. These animal studies show that both conventional and atypical antipsychotic medications reverse apomorphine-induced deficits in PPI (Swerdlow et al., 1994). In contrast, olanzapine (but not risperidone or haloperidol) is highly effective at reversing NMDA antagonist-induced PPI deficits, mostly in rodent models (Bakshi & Geyer, 1995; Duncan et al., 2000; Geyer et al., 2001). In general, rodent model studies show that NMDA antagonist-induced PPI deficits are selectively reversed by second- vs. first-generation antipsychotic medications (Bubenikova et al., 2005; Geyer & Ellenbroek, 2003; Geyer et al., 2001). The results from the current study are consistent with this animal literature in NMDA models. However, the specific mechanisms for such differences in PPI effects remain unknown. Also, despite the moderate to high level of homology between rodents and humans, key differences make inferences based on animal model studies of the NMDA system challenging. For example, the glutamate antagonist ketamine has been observed to increase PPI in normal human subjects, which is opposite from what would be predicted based on the animal literature (for discussion see Braff et al., 2001b).
The study merits a few caveats: First, the study did not include healthy controls, so we cannot compare PPI in the patients at baseline to controls, and do not know if antipsychotic medication restored PPI to normal levels. However, it appears that the schizophrenia patients are within the range of “normal” PPI by week 8, based on the reported means from normal controls in previous reports (e.g., Braff et al., 2001a; Quednow et al., 2006; Swerdlow et al., in press). Second, there were fewer subjects in the haloperidol group, and few who completed testing, compared to the other two randomization groups, potentially limiting the conclusions about the effectiveness of haloperidol in improving PPI. Future studies will need to ensure that adequate numbers of subjects are included in each group.
Third, smoking was not controlled in this study (and almost all similar studies). It has been demonstrated that nicotine administration briefly enhances PPI in normal controls (Duncan et al., 2001; Kumari et al., 1996, 1997; Postma et al., 2006). Furthermore, it has been demonstrated that schizophrenia patients who smoked immediately prior to a PPI session (< 5 minutes) produced greater PPI compared to patients who did not smoke within an hour of testing (Kumari et al., 2001). Although smoking might have influenced the results, it is probable that randomization would have equalized this factor across groups. Future longitudinal studies should more rigorously control for smoking (e.g., Swerdlow et al., 2006). Fourth, menstural cycle phase (i.e., follicular vs. luteal phase) was not measured in women who were menstruating. It has been shown that women in the luteal phase, but not the follicular phase, show lower PPI than men (Jovanovic et al., 2004; Swerdlow et al., 1997). It is possible that women who were menstruating and in the luteal phase may have shown lower PPI thus affecting the results of the current study. Future studies will need to control for menstrual cycle phase in women.
Fifth, the olanzapine group showed a trend to have a relatively larger proportion of Caucasians compared to the other two groups. While no study to our knowledge has examined differences in PPI or startle between Caucasians and African Americans, it is possible that racial differences exist. Importantly, there were no differences in PPI (or startle amplitude) between African American and Caucasian schizophrenia patients at baseline, consistent with the observation of non-significant racial effects in PPI for normal Caucasians and Asian Americans (Swerdlow et al., 2005). In addition, ongoing studies of PPI as one endophenotype in the Consortium on the Genetics of Schizophrenia (COGS) should ultimately clarify these racial results (Braff et al., 2007; Turetsky et al., 2007). Another consideration is that the study used fixed doses of antipsychotic medication. The doses of medications used in the current study were chosen to approximate modal doses for Veterans Administration Health Services patients at the time of study initiation. It is possible that different doses of risperidone or haloperidol might have resulted in improvements in PPI, particularly since it has been suggested that “effective” doses of conventional medications may also enhance PPI (Weike et al., 2000). Further controlled studies should examine the effects of dose response curves of these medications on PPI in schizophrenia patients. A final caveat is the relatively short duration of the current study. It is possible that significant PPI improvements could be seen with risperidone or haloperidol given a longer study period.
In conclusion, this study found that olanzapine significantly improved PPI in schizophrenia patients, expanding upon past findings from schizophrenia patients and rodent models delineating the effects and mechanism of action of antipsychotic medications on PPI. This is the first longitudinal randomized, double-blind controlled trial of three commonly prescribed antipsychotic medications that examine PPI in schizophrenia patients. The broader implications of medication-induced changes in PPI are not known, but may have functional and endophenotypic/genetic implications based on a recent report of an association between higher levels of PPI and higher global functioning (Swerdlow et al., 2006). In addition, understanding pharmacogenetic factors that influence PPI response to medications may be complementary to information being gained about the genetic architecture underlying PPI (Braff et al., 2007).
Acknowledgments
Support for this study came from a Veterans Affairs Merit Grant, the Department of Veterans Affairs VISN-22 Mental Illness Research Education Clinical Center (MIRECC), NIMH Translational Study Grant MH042228 (DLB) and an investigator-initiated grant from Janssen, FP. Medications for this study were provided by Janssen, FP and Eli Lilly.
The authors thank Scott Fish, Bi Hong Deng, Ewa Witt, Ayala Ofek, Danielle Goldstein, Anahita Gheytanchi, and Jennifer Churg for their help with data collection. The authors also wish to thank Mark Geyer, Ph.D. for comments on earlier drafts of the manuscript.
Role of Funding Source
The funding sources had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
The authors disclose consulting over the last two years to any company that makes an antipsychotic medication. Dr. Braff has consulted for Pfizer, Inc and Jannsen (the latter consultation being without compensation). Dr. Green has consulted for Eli Lilly, Bristol Meyers Squib, Otsuka, and Pfizer, Inc. Dr. Light has consulted for AstraZeneca and Pfizer, Inc. Dr. Marder has consulted for Bristol Meyers Squibb, Otsuka, Pfizer, Inc., Solvay, and Wyeth. Dr. Reist has consulted for Johnson and Johnson.
Contributors
JKW, MFG, JS, GL, CW, CR, SE, SRM and DLB assisted with data collection. JS and GL provided quality assurance of the EMG data. JM assisted with data analysis. JKW, MFG and DLB drafted the manuscript and all authors provided comments and feedback on the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Akdag SJ, Nestor PG, O’Donnell BF, Niznikiewicz MA, Shenton ME, McCarley RW. The startle reflex in schizophrenia: Habituation and personality correlates. Schizophrenia Research. 2003;64:165–173. doi: 10.1016/s0920-9964(03)00059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (sans): Conceptual and theoretical foundations. British Journal of Psychiatry Suppl. 1989;7:49–58. [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Antagonism of phencyclidine-induced deficits in prepulse inhibition by the putative atypical antipsychotic olanzapine. Psychopharmacology. 1995;122:198–201. doi: 10.1007/BF02246096. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD. Short lead interval startle modification. In: Dawson ME, Schell AM, Böhmelt AH, editors. Startle modification: Implications for neuroscience, cognitive science, and clinical science. New York: Cambridge University Press; 1999. pp. 51–71. [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: An overview of the use of endophenotypes in order to understand a complex disorder. Schizophrenia Bulletin. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, et al. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophrenia Research. 2001a;49:173–180. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001b;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Archives of General Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA, Ellwanger J, Sprock J, Swerdlow NR. Female schizophrenia patients have prepulse inhibition deficits. Biological Psychiatry. 2005;57:817–820. doi: 10.1016/j.biopsych.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Bubenikova V, Votava M, Horacek J, Palenicek T, Dockery C. The effect of zotepine, risperidone, clozapine and olanzapine on mk-801-disrupted sensorimotor gating. Pharmacology, Biochemistry and Behavior. 2005;80:591–596. doi: 10.1016/j.pbb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Hazlett EA, Filion DL, Nuechterlein KH, Schell AM. Attention and schizophrenia: Impaired modulation of the startle reflex. Journal of Abnormal Psychology. 1993;102:633–641. doi: 10.1037//0021-843x.102.4.633. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Hazlett EA, Nuechterlein KH, Filion DL. On the clinical and cognitive meaning of impaired sensorimotor gating in schizophrenia. Psychiatry Research. 2000;96:187–197. doi: 10.1016/s0165-1781(00)00208-0. [DOI] [PubMed] [Google Scholar]
- Duncan EJ, Madonick SH, Chakravorty S, Parwani A, Szilagyi S, Efferen TR, et al. Effects of smoking on acoustic startle and prepulse inhibition in humans. Psychopharmacology. 2001;156:266–272. doi: 10.1007/s002130100719. [DOI] [PubMed] [Google Scholar]
- Duncan EJ, Szilagyi S, Efferen TR, Schwartz MP, Parwani A, Chakravorty S, et al. Effect of treatment status on prepulse inhibition of acoustic startle in schizophrenia. Psychopharmacology. 2003;167(63–71) doi: 10.1007/s00213-002-1372-z. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA. Comparison of the effects of clozapine, risperidone, and olanzapine on ketamine-induced alteration in regional brain metabolism. The Journal of Pharmacology and Experimental Therapeutics. 2000;293:8–14. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbons M, Williams JBW. The structured clinical interview for dsm-iv axis i disorders-patient edition. New York: Biometrics Research; 1997. [Google Scholar]
- Geyer MA, Ellenbroek BA. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Progress in Neuropsychopharmacology and Biological Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Graham FK. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Charney DS, Krystal J, Braff D. Startle gating deficits occur across prepulse intensities in schizophrenic patients. Biological Psychiatry. 1992;32:939–943. doi: 10.1016/0006-3223(92)90183-z. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Haznedar MM, Singer MB, Germans MK, Schnur DB, et al. Prefrontal cortex glucose metabolism and startle eyeblink modification abnormalities in unmedicated schizophrenia patients. Psychophysiology. 1998;35:186–198. [PubMed] [Google Scholar]
- Hazlett EA, Romero MJ, Haznedar MM, New AS, Goldstein KE, Newmark RE, et al. Deficient attentional modulation of startle eyeblink is associated with symptom severity in the schizophrenia spectrum. Schizophrenia Research. doi: 10.1016/j.schres.2007.03.012. (in press) [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Wonodi I, Adami H, Buchanan RW, Thaker GK. Independent domains of inhibitory gating in schizophrenia and the effect of stimulus interval. American Journal of Psychiatry. 2007;164:61–65. doi: 10.1176/ajp.2007.164.1.61. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Swerdlow NR, Braff DL. Effects of background and prepulse characteristics on prepulse inhibition and facilitation: Implications for neuropsychiatric research. Biological Psychiatry. 2006;59:555–559. doi: 10.1016/j.biopsych.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Szilagyi S, Chakravorty S, Fiallos AM, Lewison BJ, Parwani A, et al. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology. 2004;41:401–406. doi: 10.1111/1469-8986.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Sharma T. Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophrenia Research. 2004;69:219–235. doi: 10.1016/j.schres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Kumari V, Checkley SA, Gray JA. Effect of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacology. 1996;128:54–60. doi: 10.1007/s002130050109. [DOI] [PubMed] [Google Scholar]
- Kumari V, Cotter PA, Checkley SA. Effect of acute subcutaneous nicotine on prepulse inhibition of the acoustic startle reflex in healthy male non-smokers. Psychopharmacology. 1997;132:389–395. doi: 10.1007/s002130050360. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Mathew VM, Sharma T. Prepulse inhibition of the startle response in men with schizophrenia: Effects of age of onset of illness, symptoms, and medication. Archives of General Psychiatry. 2000;57:609–614. doi: 10.1001/archpsyc.57.6.609. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. American Journal of Psychiatry. 1999;156(7):1046–1051. doi: 10.1176/ajp.156.7.1046. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Influence of cigarette smoking on prepulse inhibition of the acoustic startle response in schizophrenia. Human Psychopharmacology Clinical and Experimental. 2001;16:321–326. doi: 10.1002/hup.286. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Prepulse inhibition of the startle response in risperidone-treated patients: Comparison with typical antipsychotics. Schizophrenia Research. 2002;55:139–146. doi: 10.1016/s0920-9964(01)00276-6. [DOI] [PubMed] [Google Scholar]
- Leumann L, Feldon J, Vollenweider FX, Ludewig K. Effects of typical and atypical antipsychotics on prepulse inhibition and latent inhibition in chronic schizophrenia. Biological Psychiatry. 2002;52:729–739. doi: 10.1016/s0006-3223(02)01344-6. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Etzensberger M, Vollenweider FX. Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia. Schizophrenia Research. 2002;55:129–137. doi: 10.1016/s0920-9964(01)00198-0. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biological Psychiatry. 2003;54:121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naïve schizophrenic patients. Biological Psychiatry. 2002;52:863–873. doi: 10.1016/s0006-3223(02)01409-9. [DOI] [PubMed] [Google Scholar]
- Meincke U, Light GA, Geyer MA, Braff DL, Gouzoulis-Mayfrank E. Sensitization and habituation of the acoustics startle reflex in patients with schizophrenia. Psychiatry Research. 2004;126:51–61. doi: 10.1016/j.psychres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Oranje B, van Oel CJ, Gispen-de Wiede CC, Verbaten MN, Kahn RS. Effects of typical and atypical antipsychotics on the prepulse inhibition of the startle reflex in patients with schizophrenia. Journal of Clinical Psychopharmacology. 2002;22:359–365. doi: 10.1097/00004714-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, et al. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biological Psychiatry. 2000;47(7):662–669. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Postma P, Gray JA, Sharma T, Geyer M, Mehrotra R, Das M, et al. A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology. 2006;184:589–599. doi: 10.1007/s00213-006-0307-5. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Wagner M, Westheide J, Becmann K, Bliesener N, Maier W, et al. Sensorimotor gating and habituation of the starte response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biological Psychiatry. 2006;59:536–545. doi: 10.1016/j.biopsych.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Archives of General Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: Current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Hartman PL, Auerbach PP. Changes in sensorimotor inhibition across the menstrual cycle: Implications for neuorpsychiatric disorders. Biological Psychiatry. 1997;41:452–460. doi: 10.1016/S0006-3223(96)00065-0. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: Relationship to medications, symptoms, neurocognition and level of function. Archives of General Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Sprock J, Light GA, Cadenhead K, Calkins ME, Dobie DJ, et al. Multi-site studies of acoustic startle and prepulse inhibition in humans: Initial experience and methodological considerations based on studies by the consortium on the genetics of schizophrenia. Schizophrenia Research. doi: 10.1016/j.schres.2007.01.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Talledo JA, Braff DL. Startle modulation in caucasian-americans and asian-americans: A prelude to genetic/endophenotypic studies across the ‘pacific rim’. Psychiatric Genetics. 2005;15:61–65. doi: 10.1097/00041444-200503000-00010. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: The viability of selected candidate measures. Schizophrenia Bulletin. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RL, Green MF, Shaner A. Brief psychiatric rating scale (bprs) International Journal of Methods in Psychiatric Research. 1993;3:227–243. [Google Scholar]
- Weike AI, Bauer U, Hamm AO. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biological Psychiatry. 2000;47(1):61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Dawson ME, Schell AM. Discrete and continuous prepulses have differential effects on startle prepulse inhibition and skin conductance orienting. Psychophysiology. 2000;37:224–230. [PubMed] [Google Scholar]
- Wynn JK, Dawson ME, Schell AM, McGee M, Salveson D, Green MF. Prepulse facilitation and prepulse inhibition in schizophrenia patients and their unaffected siblings. Biological Psychiatry. 2004;55(5):518–523. doi: 10.1016/j.biopsych.2003.10.018. [DOI] [PubMed] [Google Scholar]