Abstract
A multitude of factors are involved in regulating the blood coagulation homeostatic processes in the body, which may ultimately lead to thromboemboli and thrombosis. The resolution of blood clots after healing is as important as clot formation at the site of a vascular lesion. This is accomplished by fibrinolytic drugs such as streptokinase (SK) and urokinase.
It must be noted that administration of SK may be accompanied by the lysis of blood clots in unwanted sites, and complications such as general lytic conditions, severe hemorrhaging, reduced serum fibrinogen and allergies can occur. Anti-SK antibodies neutralize the effects of SK. Studies on natural compounds and medicinal herbs with fewer side effects have been ongoing. In the present study, the fibrinolytic effect of Ginkgo biloba, an herb grown in Iran, was investigated.
A polyphenolic method was used to obtain Ginkgo extract from its leaves. The fibrinolytic effects of SK (positive control) were compared with those of Ginkgo extract using a fluorometry method.
In producing a labelled clot, fibrinogen was labelled with the fluorescent agent fluorescein isothiocyanate and precipitated in the presence of Ca2+. SK (100 U/mL to 1000 U/mL) and Ginkgo extract were added to labelled fibrin in a plasma environment at dilutions of 1, 1:10, 1:100 and 1:1000 (volume/volume). The fluorescence of the solution was measured between 15 min and 60 min later.
A linear relationship was observed between the fluorescence measured and SK concentrations ranging from 300 U/mL to 700 U/mL. Ginkgo extract displayed a remarkable effect in resolving the clot. As Ginkgo extract remained in the environment, fluorescence increased notably, showing a time-dependent relationship.
Overall, the results indicate that the effects of Ginkgo extract on the fibrinolytic system are similar to those of SK; hence, this herbal extract can be used as a complement to or a substitute for SK. Additionally, it is proposed that the effects of the active ingredients of Ginkgo extract should be studied in animals. Further studies are warranted for evaluating the possible side effects and toxicity of Ginkgo extract in human subjects.
Keywords: Fibrinolytic, Ginkgo, Streptokinase
Several homeostasis regulating factors can result in the formation of thrombi and subsequent emboli (1). Patients with congestive heart failure, arteriosclerosis and cancer, as well as pregnant women, are more prone to the formation of blood clots in abnormal locations (1). Prompt fibrinolytic therapy following coronary artery occlusion can minimize myocardial damage (2,3). Urokinase (UK) and streptokinase (SK) are among the oldest fibrinolytic agents. SK is a bacterial enzyme and UK is produced by the epithelial cells of renal tubules (1).
SK acts indirectly by initially forming a proteolytic complex with circulating plasminogen, which in turn activates other plasminogen molecules. Unlike SK, UK has inherent proteolytic properties and directly activates plasminogen (1). These drugs are not used in patients who have undergone surgery or those with a history of nervous lesions, gastrointestinal bleeding or hypertension (1).
SK is ineffective in individuals with antistreptococcal and antiprothrombin antibodies, and in patients who have had multiple SK injections (4,5). SK administration is followed by acute allergic reactions such as urticaria and serum sickness in some patients. Bleeding is the most notable complication of these drugs, with intracranial hemorrhaging posing a potentially fatal risk to the patient (1).
There is evidence that some natural products have fibrinolytic effects. Ginkgo has a vast array of therapeutic benefits including regulating blood flow to the heart and brain, and reducing ischemia. The antiarrhythmic effect of Ginkgo following myocardial infarction is mediated by ginkgolides. Furthermore, bilobalides are regarded as potent anti-ischemic agents (6). Additionally, the beneficial effects of Ginkgo in patients with cerebral problems of vascular and nonvascular origin (eg, deterioration of memory and thinking) have been observed in extensive clinical studies (6). During recent years, several case reports have been published that cast doubt on a causal relationship between hemorrhagic complications and the intake of Ginkgo preparations (7).
Some cases of spontaneous bleeding have been reported in patients treated with Ginkgo. This suggests that the reported bleeding events in patients receiving Ginkgo extract are not related to pharmacological properties of EGb761 (Ginkgo extract), and reports indicate no alteration in platelet function or coagulation (8); however, there are still some controversies about this function (9). The present study was conducted to investigate the fibrinolytic effects of Ginkgo.
METHODS
Extract preparation methods
Ginkgo leaves (Tolid Daru, Iran) were scientifically identified at Isfahan University of Medical Sciences. Fifty grams of Ginkgo powder was mixed with a water-ethanol solution (1:10) and kept at room temperature for 48 h. The mixture was then shaken for 2 h before being filtered. In the next stage, 70% ethanol was added to the residuum and the mixture was again processed as explained above. The volume of the resultant extract was reduced by one-third through vacuum distillation. The resulting solution was decanted a few times with chloroform to isolate lipids, terpenoids, chlorophyll and xanthophyll pigments. The remnant was completely dried in a vacuum distiller (10).
Labelling fibrinogen with a fluorescent agent
Fifty millilitres of water were added to a vial containing 1 g human fibrinogen solution. A 2% fibrinogen solution was labelled by adding 2.35 mg fluorescine isothiocyanate (FITC) in an alkaline environment (pH=8.5). The solution was incubated at 4°C and, later, the fibrinogen-FITC complex was purified by column chromatography containing G100 cephadex (Pharmacia, Sweden) (11,12).
Method of preparing a labelled clot
Ten microlitres of 1 M CaCl2 stock solution was added to 0.5 mL plasma and 25 μL fibrinogen-FITC complex solution. The mixture was incubated for 80 min at 37°C and centrifuged at 4000 rpm for 15 min until the clot was deposited. The supernatant was discarded and the remainder was washed by adding 0.1 M phosphate buffer. Two millilitres of plasma was added to the solution to create an environment suitable for fibrinolysis (13).
Before adding the substance to be treated by fibrinolytic agent, the fluorescence was measured in the environment which contained 2 mL plasma and labelled clot as a negative control. SK solutions ranging in concentration from 100 U/mL to 1000 U/mL were prepared.
Dried extract of Ginkgo was dissolved in 5 mL DMSO and reserved as a stock. Alcohol solutions of Ginkgo were then prepared at dilutions (volume/volume) of 1, 1:10, 1:100 and 1:1000, and 100 μL of each dilution was added to the plasma environment. After 15 min, 30 min and 60 min of exposure to SK or Ginkgo extract, 10 μL samples were taken from the plasma environment and increased to 1 mL by adding phosphate buffer. Fluorescence of the samples was measured by a spectrofluorometer (Perkins-Elmer, Germany). Every test was conducted three times and the mean ± SD was calculated.
RESULTS
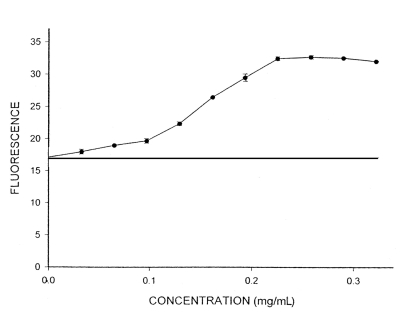
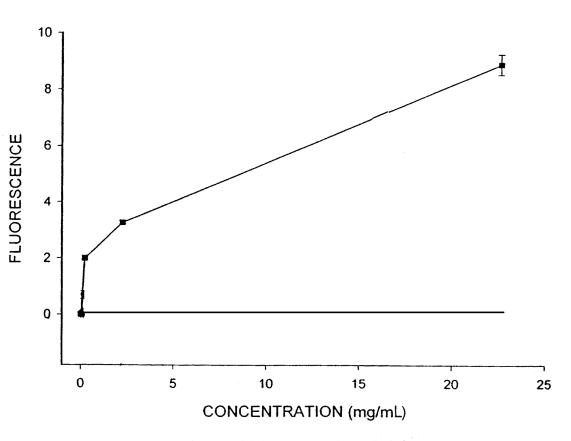
In evaluating the fibrinolytic effects of SK, the data showed a linear relationship between fluorescence and SK concentrations ranging from 300 U/mL to 700 U/mL. Despite the direct relationship between concentration and effect (Figure 1), there was no relationship between time and SK effect; fluorescence did not change significantly after 1 min, 15 min, 30 min and 60 min (P>0.05). Therefore, the reported results are only those obtained after 60 min. Adding Ginkgo extract to a clot in a plasma environment significantly increased fluorescence, indicating Ginkgo’s fibrinolytic effect. A direct relationship was observed between the concentration of Ginkgo and fluorescence (Figure 2). Additionally, clot solubility increased notably over time, showing a relationship between fluorescence and time.
Figure 1).
Changes in fluorescence at different concentrations of streptokinase
Figure 2).
Changes in fluorescence at different concentrations of Ginkgo extract
DISCUSSION
The effect of Ginkgo in dissolving clots in unwanted locations was investigated. Studies have shown that thromboemboli may decrease blood flow to organs (heart, brain, etc) and cause irreparable damage and even death (1).
Although fibrinolysis is part of the normal homeostasis system which starts immediately following clot formation with secretion of tissue-type plasminogen activator and pro-UK by endothelial cells, reopening of the vessel may take between seven and 10 days (1).
Many studies (6,14,15) have investigated the effect of herbal agents on cardiovascular diseases. There are several drugs with specific therapeutic effects in conditions such as atherosclerosis and homeostatic disorders, especially thromboemboli. Studies from around the world (6,14) have demonstrated the potent antiplatelet properties of Ginkgo, which inhibit platelet aggregation and thrombin activity.
The present study showed that the fibrinolytic effects of Ginkgo are comparable with those of SK and may be of high therapeutic value. These effects may be accounted for by the presence of flavonoids such as ginkgolides and bilobalides in Ginkgo.
Our study also showed that increasing the concentration of SK does not have a notable effect on the lysis of blood clots, which may be due to a lack of additional plasminogen in the environment (after being used up by SK). In other words, plasminogen has been saturated by SK so that increased enzyme concentration does not have a direct relationship with increased fluorescence. This is also the case in enzymatic reactions (ie, the rate of reaction levels off when the enzyme is saturated by substrate); however, this was not true of total Ginkgo extract (ie, clot lysis increased with concentration and time, suggesting that Ginkgo extract dissolves the clot via a mechanism different than that of SK and plasminogen). Further studies are required to understand this mechanism.
We propose that Ginkgo extract be considered as a complement to or a substitute for SK; however, its side effects and toxicity first need to be extensively studied in animal models.
Acknowledgments
Supported by Isfahan Cardiovascular Research Center.
REFERENCES
- 1.Braunwald F, Kasper H, Longo J, Harrison S. Principles of Internal Medicine. 15th edn. New York: The McGraw-Hill Companies; 2001. pp. 751–63. [Google Scholar]
- 2.Lee R, Foerster J, Luken J, Paraskeras F, Greer JA, Rodgers GM. Wintrobe’s Clinical Hematology. 10th edn. Vol 2. London: Williams and Willkins; 1999. pp. 1744–91. [Google Scholar]
- 3.Stiene-Martin EA, Lotspeich CA, Koepke JA. Clinical Hematology, Principles, Procedures, Correlations. 2nd edn. Philadelphia: Lippincott Company; 1998. pp. 612–49. [Google Scholar]
- 4.Buchalter MB, Suntharalingam G, Jennings I, et al. Streptokinase resistance: When might streptokinase administration be ineffective? Br Heart J. 1992;68:449–53. doi: 10.1136/hrt.68.11.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puurunen M, Manttari M, Manninen V, Palosuo T, Vaarala O. Antibodies to prothrombin crossreact with plasminogen in patients developing myocardial infarction. Br J Haematol. 1998;100:374–9. doi: 10.1046/j.1365-2141.1998.00562.x. [DOI] [PubMed] [Google Scholar]
- 6.Mills S. Principles and Practice of Phytotherapy. 1st edn. Edinburgh: Churchill Livingstone; 2000. pp. 31–33.pp. 262pp. 267pp. 300pp. 396pp. 403pp. 465pp. 418 [Google Scholar]
- 7.Kohler S, Funk P, Kieser M. Influence of a 7-day treatment with Ginkgo biloba special extract Egb761 on bleeding time and coagulation: A randomized, placebo controlled, double-blind study in healthy volunteers. Blood Coagul Fibrinolysis. 2004;15:303–9. doi: 10.1097/00001721-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bal Dit Sollier C, Caplain H, Drouet L. No alteration in platelet function or coagulation induced by EGb761 in a controlled study. Clin Lab Haematol. 2003;25:251–3. doi: 10.1046/j.1365-2257.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 9.Pizzorno JE, Murray MT. Textbook of Natural Medicine. 2nd edn. London: Churchill Livingstone; 1999. pp. 567–69.pp. 577pp. 619–23.pp. 629–32.pp. 679–692.pp. 817–855. [Google Scholar]
- 10.Markham KR. Techniques of Flavonoids Identification. London: Academic Press; 1982. p. 16. [Google Scholar]
- 11.Sakharov DV, Rijken DC. Superficial accumulation of plasminogen during plasma clot lysis. Circulation. 1995;92:1883–90. doi: 10.1161/01.cir.92.7.1883. [DOI] [PubMed] [Google Scholar]
- 12.Sakharov DV, Nagelkerke JF, Rijken DC. Rearrangements of the fibrin network and spatial distribution of fibrinolytic components during plasma clot lysis. Study with confocal microscopy. J Biol Chem. 1996;271:2133–8. doi: 10.1074/jbc.271.4.2133. [DOI] [PubMed] [Google Scholar]
- 13.Sakharov DV, Rijken DC. The effect of flow on lysis of plasma clots in a plasma environment. Thromb Haemost. 2000;83:469–74. [PubMed] [Google Scholar]
- 14.Teng CM, Ko FN, Wang JP, et al. Antihaemostatic and antithrombotic effect of some antiplatelet agents isolated from Chinese herbs. J Pharm Pharmacol. 1991;43:667–9. doi: 10.1111/j.2042-7158.1991.tb03561.x. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal M. Therapeutic Guide to Herbal Medicine. 1st edn. Boston: Integrative Medicine; 1998. pp. 166–7.pp. 446 [Google Scholar]