Abstract
BACKGROUND:
Patients surviving myocardial infarction (MI) are at a heightened risk for the development of congestive heart failure. This clinical syndrome has been associated with an antioxidant deficit and elevated oxidative stress in the myocardium. Effects of dietary vitamin E, a lipid-soluble antioxidant, on myocardial anti-oxidant enzyme activities, oxidative stress and hemodynamic function, were examined separately in the viable left ventricle (LV) and right ventricle (RV) of rats at 16 weeks post-MI.
METHODS AND RESULTS:
Animals were fed either a basal diet or a diet enriched with 1500 U of vitamin E/kg beginning two weeks before MI-inducing surgery and continued 16 weeks post-MI. In the MI animals on the basal diet, LV systolic pressure (LVSP) and RVSP were significantly depressed and LV end-diastolic pressure (LVEDP) and RVEDP were significantly elevated. These hemodynamic alterations were accompanied by clinical signs of heart failure including dyspnea, lethargy and cyanotic limbs. Supplementation of MI animals with dietary vitamin E resulted in complete normalization of RVSP and RVEDP. An increase in LVSP and a decrease in LVEDP was observed in the vitamin E-supplemented MI animals, although mild residual LV dysfunction remained. The myocardial enzymatic antioxidants catalase and glutathione peroxidase declined substantially in each of the ventricles of unsupplemented MI animals. Myocardial levels of vitamin E were reduced by 33% in the LV and no change was observed in the RV of the MI animals. Vitamin E-supplemented control animals and MI animals showed a significant increase in vitamin E levels in both ventricles. Myocardial oxidative stress, as assessed by lipid peroxidation and the ratio of reduced to oxidized glutathione, was significantly increased in each of the respective ventricles of untreated MI animals. Supplementation with dietary vitamin E resulted in a substantial increase in the myocardial activities of catalase and glutathione peroxidase in both the LV and RV. Furthermore, an increase in the ratio of reduced to oxidized glutathione concomitant with significantly less lipid peroxidation was also observed in each of the respective ventricles of MI animals supplemented with vitamin E. No overt clinical signs of heart failure were evident in these vitamin E-supplemented animals.
CONCLUSIONS:
An improved myocardial redox state and endogenous antioxidant reserve with vitamin E therapy, coupled with the modulation of the development of heart failure, lend strong support in favour of a pathophysiological role for increased oxidative stress in the pathogenesis of heart failure, at least in experimental animals. Association between an increase in oxidative stress and cardiac events in patients requires further examination.
Keywords: Heart failure, Myocardial infarction, Oxidative stress, Vitamin E
Chronic congestive heart failure (CHF) is a complex pathophysiological process resulting from diverse etiologies. However, ischemic heart disease and the sequelae of myocardial infarction (MI) remain, by far, the most frequent initiating cause of CHF (1). Between 15% and 25% of patients surviving MI ultimately develop chronic overt CHF in the subsequent five years (2), with New York Heart Association (NYHA) class IV patients having a one-year survival rate of less than 50% (3). Thus, CHF remains a daunting therapeutic challenge in view of continued mortality.
Experimental studies have demonstrated that a depressed antioxidant status and concomitant increase in oxidative stress may be intimately involved in mediating the pathogenesis of heart failure resulting from a variety of causes (4–6). Heart failure due to a variety of conditions has been shown to be accompanied by elevated oxidative stress, as indicated by reductions in the levels of both enzymatic and nonenzymatic antioxidants, a depressed redox state (reduced to oxidized glutathione [GSH:GSSG] ratio) and elevated lipid peroxidation (7–9). Limited clinical studies have demonstrated significant increases in oxidative stress in patients afflicted with coronary artery disease and resultant MI (10–12).
Antioxidant therapy involving vitamin E (alpha-tocopherol) has been shown to reduce oxidative stress and improve prognosis in surviving patients with MI (10,13). Furthermore, administration of 400 U to 800 U of vitamin E daily in patients with angiographically symptomatic coronary atherosclerosis substantially reduced the rate of nonfatal MI (13). However, assessment of the effects of vitamin E supplementation in high-risk patients on other cardiovascular events and complications did not yield any positive effects (14,15).
In a study (16) using the coronary artery ligation model of MI in rats, we previously reported that CHF subsequent to MI was associated with an antioxidant deficit and elevated oxidative stress, first in the left ventricle (LV) and then followed by the right ventricle (RV) in the more chronic stages. However, a cause and effect relationship between oxidative stress and CHF has yet to be established. Thus, in the present study, we examined the effects of long-term antioxidant therapy with dietary vitamin E on the development of CHF subsequent to MI.
METHODS
Experimental model
MI was induced as previously described in male Sprague-Dawley rats weighing 150±10 g via occlusion of the left coronary artery (17). Sham animals were treated in a similar fashion except that the suture around the left coronary artery was not tied. No mortality was observed in the sham control animals within 24 h of the procedure. The mortality among the coronary artery-ligated animals was 35% within the first 24 h.
This surgical procedure produced an infarct size ranging on average from 30% to 50% of the LV mass. Rats with infarcts compromising less than 20% of the LV mass (n=3) were excluded from further investigations. Following the surgeries, analgesia (buprenorphine, 0.01 mg/kg body weight to 0.05 mg/kg body weight) was administered subcutaneously every 12 h for up to 48 h postoperatively. Animals had access to their assigned food and water ad libitum.
Hemodynamic measurements
Animals were anesthetized 16 weeks post-MI with sodium pentobarbital (50 mg/kg intraperitoneally) and hemodynamic parameters (LV end-diastolic pressure [LVEDP] and LV systolic pressure [LVSP]) were measured as previously described (16). For recording of RV pressures, a miniature pressure transducer (Miller Instruments, USA) was inserted into the right jugular vein and then advanced into the RV. After hemodynamic recordings, animals were sacrificed and the heart and other organs were removed, frozen in liquid nitrogen and stored at −70°C until analyzed.
Vitamin E supplementation
Animals in the control and infarcted group were further divided into two subgroups. One subgroup received a basal commercial diet (PMI Feeds, USA) while the other received the same diet enriched with 1500 U of vitamin E/kg. Dietary supplementations were initiated two weeks before the surgeries and were continued until 16 weeks postsurgery.
Tissue weights
Wet to dry weight ratios were calculated for the lungs and liver as previously described (16).
Biochemical assays
Viable portions of the LV with septum were separated from the RV as previously described (16). The RV and LV were analyzed separately for antioxidants and oxidative stress. Before homogenization, hearts were placed in a Tris (0.2 mol/L)/KCl (0.16 mol/L) buffer, pH 7.4. The hearts were allowed to beat in buffer for a short period of time, allowing for perfusion of the myocardium and, thereby, minimizing the extent of contamination by blood-derived elements on antioxidant enzyme measurements.
Antioxidant studies
Glutathione peroxidase:
Glutathione peroxidase (GSHPx) activity was determined using a method previously described (18). Tissue was homogenized (1:10) in 75 mmol/L phosphate buffer, pH 7.0. Homogenate was centrifuged (Beckman J2-HS, Beckman, USA) at 18,000 g for 45 min and the supernatant was aspirated and assayed for total cytosolic GSHPx activity. GSHPx activity was assayed in a 3 mL cuvette containing 2.0 mL of 75 mmol/L phosphate buffer, pH 7.0. The following solutions were then added: 50 μL of 60 mmol/L glutathione, 100 μL of glutathione reductase solution (30 U/mL), 50 μL of 0.12 mol/L sodium azide, 100 μL of 15 mmol/L Na2 EDTA, 100 μL of 3.0 mmol/L NADPH and 100 μL of cytosolic fraction. The reaction was started by the addition of 100 μL of 7.5 mmol/L hydrogen peroxide and the conversion of NADPH to NADP was monitored by a continuous recording of the change in absorbency (Spectronic 601, Milton Roy, USA) at 340 nm at 1 min intervals for 5 min. GSHPx activity was expressed as nanomoles of NADPH oxidized to NADP per minute per milligram protein, using a molar extinction coefficient of 6.22×106 for NADPH at 350 nm.
Catalase:
Catalase activity was determined using a previously described method (19). Tissue was homogenized (1:10) in 50 mmol/L potassium phosphate buffer (pH 7.4) and the homogenate was centrifuged at 18,000 g for 45 min. Supernatant (50 μL) was added to a 3 mL cuvette which contained 2.95 mL of 19 mmol/L hydrogen peroxide in 50 mmol/L potassium phosphate buffer (pH 7.4). Changes in absorbency at 240 nm were continuously followed for 5 min. Catalase activity was expressed as Units/milligram protein.
Vitamin E:
Vitamin E (alpha-tocopherol) was measured in myocardial tissue and in the food using a modification of the extraction procedures and reverse-phase high-performance liquid chromatography detection method of Palace et al (20). Total run time was 30 min, with typical retention times of 10.7 min and 12.5 min for the internal standards tocopherol and tocopherol acetate, respectively.
Oxidative stress changes:
Oxidative stress changes evaluating thiobarbituric acid reacting substances (TBARS) as well as assessing glutathione (GSSG and GSH) in myocardium are described previously (16).
Proteins and statistical analysis
Proteins were determined using the method by Lowry et al (21). Data are expressed as the mean ± SEM. Group means were compared by one-way ANOVA, and ANOVA followed by Bonferroni’s test was used to identify differences between groups. Values of P<0.05 were considered statstically significant. In cases where Bonferroni’s test returned marginal significant differences (P<0.1) and the power of the test was P<0.8, type II errors were minimized by reanalyzing the groups using Student’s t test (P<0.05).
RESULTS
General observations
The rats were observed on a daily basis for their food intake, general behaviour and presence of any clinical signs of heart failure. No differences in food consumption were observed in the coronary artery-ligated animals supplemented with vitamin E compared with their respective sham controls. However, a 7% reduction in food consumed was observed in the untreated coronary artery-ligated animals compared with both their respective sham controls and vitamin E-treated, coronary artery-ligated animals. Unsupplemented, coronary artery-ligated rats exhibited lethargy, dyspnea, abdominal enlargement and cyanosis of peripheral extremities beginning approximately 12 weeks after the surgery. At autopsy, congested lungs and hepatomegaly were noted. In contrast, coronary artery-ligated animals supplemented with vitamin E did not display any of the above mentioned clinical signs. Sham animals receiving either diet also showed no clinical signs of dysfunction.
Hemodynamic studies
Hemodynamic data at 16 weeks postsurgery are shown in Figure 1. There was a significant elevation in the LVEDP and a significant reduction in the LVSP in the untreated MI animals relative to their sham controls (Figure 1A). RVEDP was significantly elevated and RVSP was significantly reduced in these animals (Figure 1B). Vitamin E supplementation of MI animals resulted in the complete normalization of RVEDP and RVSP while an improvement was observed in the LVEDP and LVSP; however, LVEDP and LVSP in these animals still remained significantly different from their respective control values (Figure 1A).
Figure 1).
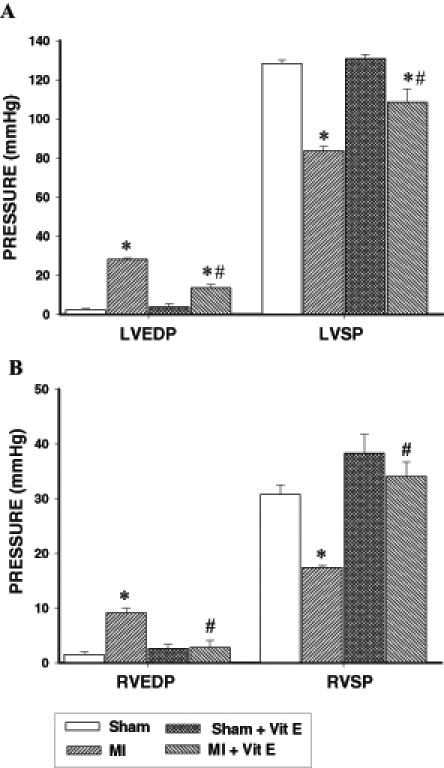
Effect of vitamin E supplementation on the (A) left ventricular end-diastolic pressure (LVEDP) and systolic pressure (LVSP), and (B) right ventricular end-diastolic pressure (RVEDP) and systolic pressure (RVSP) at 16 weeks after surgery in control and myocardial infarcted (MI) animals maintained on a basal or vitamin E (Vit E)-supplemented diet. Data are expressed as mean ± SEM for five rats. *Significantly different (P<0.05) from respective controls. #Significantly different (P<0.05) from corresponding ventricular tissue of unsupplemented MI hearts
Tissue weights
The ratios of wet to dry weight in both the lungs and liver were significantly higher in the untreated MI animals compared with their respective controls (Table 1). Vitamin E-supplemented coronary artery-ligated animals showed no gain in the wet to dry weight ratio for both the lungs and liver, such that these values were not statistically different from their respective controls (Table 1).
TABLE 1.
Lung and liver wet to dry weight ratio in rats at 16 weeks postmyocardial infarction with and without vitamin E supplementation
| Animal group | Lung | Liver |
|---|---|---|
| Basal diet | ||
| Control | 4.5±0.1 | 2.9±0.1 |
| MI | 7.1±0.8* | 4.5±0.5* |
| Vitamin E-supplemented diet | ||
| Control | 4.8±0.2 | 3.2±0.1 |
| MI | 5.1±0.3† | 3.2±0.1† |
Significantly different (P<0.05) from respective control group using ANOVA followed by Bonferroni’s test.
Significantly different (P<0.05) from unsupplemented myocardial infarction (MI) group using ANOVA followed by Bonferrroni’s test. Values are expressed as mean ± SEM for five rats
Myocardial antioxidant enzymes
Myocardial catalase and GSHPx activities were examined in the viable LV and RV separately at 16 weeks post-MI in the supplemented and unsupplemented infarcted animals and compared with their respective controls. Catalase activity in the viable LV (Figure 2A) of untreated infarcted animals was depressed by 66% while in the viable RV (Figure 2B), catalase activity was reduced by 42%. Supplementation with vitamin E resulted in a significant increase in catalase activities in both the viable LV and RV of infarcted animals. However, catalase activity in the LV of these vitamin E-supplemented infarcted animals remained significantly different from respective control values (Figure 2A). In contrast, no difference in catalase activity was observed in the RV of vitamin E-supplemented infarcted animals compared with respective controls (Figure 2B). A similar pattern of changes was seen in the RV and LV of treated and untreated infarcted animals with respect to GSHPx activity (Figure 3).
Figure 2).
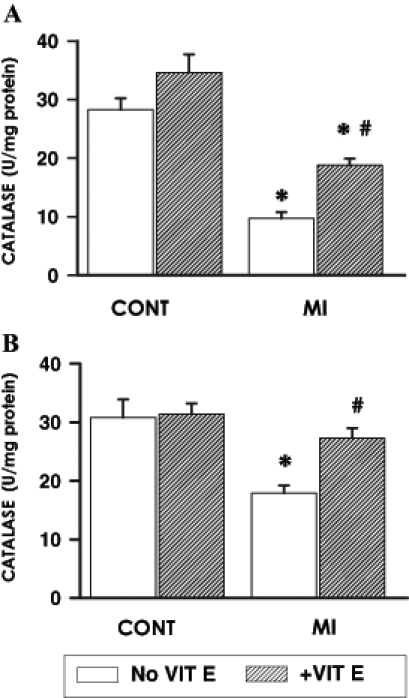
Myocardial catalase activity at 16 weeks after surgery in the viable (A) left ventricle and (B) right ventricle of control (CONT) and myocardial infarction (MI) animals maintained on a basal or vitamin E (VIT E)-supplemented diet. Data are expressed as mean ± SEM for five rats. *Significantly different (P<0.05) from respective controls; #Significantly different (P<0.05) from corresponding ventricular tissue of unsupplemented MI hearts
Figure 3).
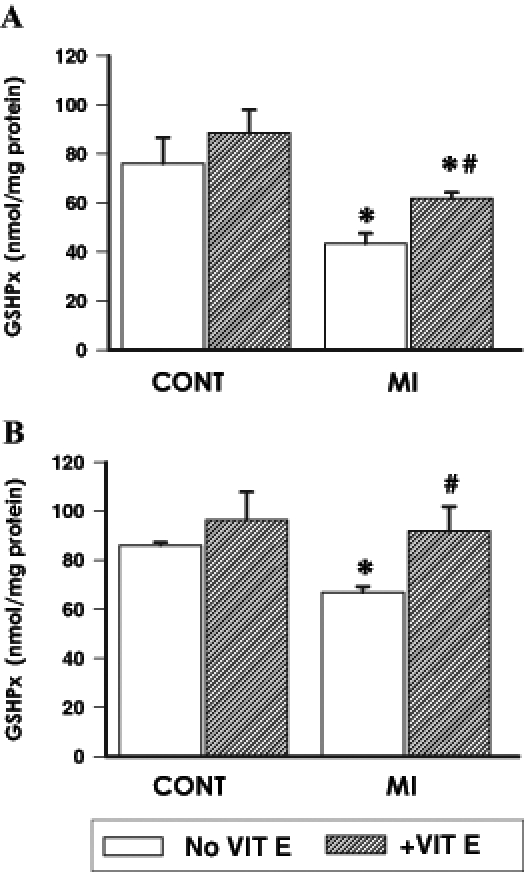
Myocardial glutathione peroxidase (GSHPx) activity at 16 weeks after surgery in the viable (A) left ventricle and (B) right ventricle of control (CONT) and myocardial infarction (MI) animals maintained on a basal or vitamin E (VIT E)-supplemented diet. Data are expressed as mean ± SEM for five rats. *Significantly different (P<0.05) from respective controls; #Significantly different (P<0.05) from corresponding ventricular tissue of unsupplemented MI hearts
Myocardial vitamin E content
Myocardial vitamin E content is shown in Table 2. Vitamin E content declined by 33% in the viable LV of unsupplemented infarcted animals relative to respective sham controls. In contrast, no change in vitamin E content was observed in the viable RV of infarcted animals compared with respective controls. Vitamin E supplementation resulted in a substantial increase in vitamin E content in the LV and RV of both sham controls and infarcted animals. The content of vitamin E in both the LV and RV of vitamin E-supplemented infarcted animals was not statistically different from that of vitamin E-supplemented sham controls.
TABLE 2.
Myocardial vitamin E levels at 16 weeks postmyocardial infarction with and without vitamin E supplementation
| Animal group |
Vitamin E levels (μg/g wet weight) |
|
|---|---|---|
| Left ventricle | Right ventricle | |
| Basal diet | ||
| Control | 65.40±2.61 | 53.50±3.55 |
| MI | 41.77±7.4* | 55.76±4.01 |
| Vitamin E-supplemented diet | ||
| Control | 109.40±8.60* | 84.21±7.98* |
| MI | 88.2±1.97† | 81.9±3.54† |
Significantly different (P<0.05) from respective control group using ANOVA followed by Bonferroni’s test;
Significantly different (P<0.05) from corresponding ventricular tissue of unsupplemented myocardial infarction (MI) hearts using ANOVA followed by Bonferroni’s test. Values are expressed as mean ± SEM for five ratss
Oxidative stress studies
Lipid peroxidation data are shown in Figure 4. TBARS in the LV (Figure 4A) of untreated infarcted animals were elevated by 90% compared with their respective control values while, in the RV (Figure 4B), an increase of 45% was observed. Myocardial TBARS in the RV of vitamin E-supplemented MI animals were no different from respective sham controls while, in contrast, myocardial TBARS in the LV of this supplemented group remained elevated compared with their respective sham controls. However, the magnitude of TBARS elevation in the LV was far less pronounced in the vitamin E-supplemented group compared with the unsupplemented group.
Figure 4).
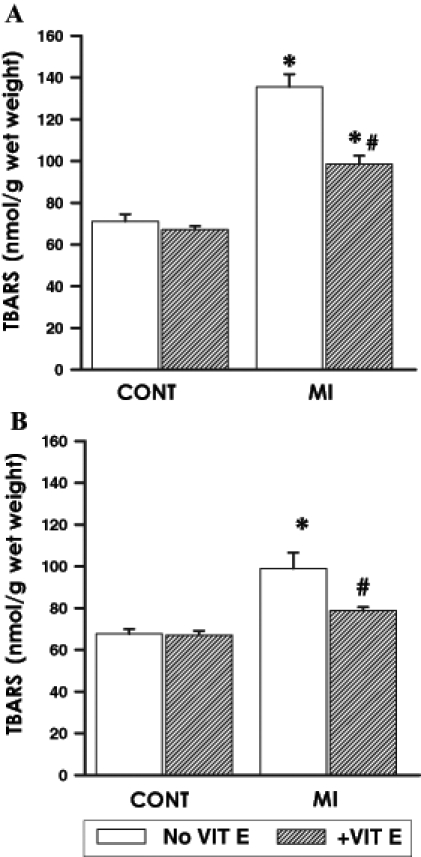
Lipid peroxidation as indicated by thiobarbituric acid reacting substances (TBARS) at 16 weeks after surgery in the viable (A) left ventricle and (B) right ventricle of control (CONT) and myocardial infarction (MI) animals maintained on a basal or vitamin E (VIT E)-supplemented diet. Data are expressed as mean ± SEM for five rats. *Significantly different (P<0.05) from respective controls. #Significantly different (P<0.05) from corresponding ventricular tissue of unsupplemented MI hearts
GSH and GSSG levels are also shown in Table 3. GSH levels in the LV of unsupplemented infarcted animals were reduced by 47% while, in the RV, a 20% reduction in GSH levels was observed. GSH levels in both the LV and RV in the vitamin E supplemented infarcted animals were near that of control values. GSSG content was significantly elevated in both the LV and RV of unsupplemented infarcted animals. Vitamin E supplementation resulted in maintaining the GSSG levels near control values.
TABLE 3.
Myocardial reduced glutathione (GSH) and oxidized glutathione (GSSG) content in rat at 16 weeks postmyocardial infarction with and without vitamin E supplementation
| Animal group |
GSH (nmol/g tissue weight) |
GSSG (nmol/g tissue weight) |
GSH:GSSG |
|||
|---|---|---|---|---|---|---|
| LV | RV | LV | RV | LV | RV | |
| Basal diet | ||||||
| Control | 70.0±2.6 | 70.7±1.8 | 6.0±0.7 | 6.3±1.1 | 11.7 | 11.2 |
| MI | 37.4±3.4* | 59.6±3.0† | 30.5±2.2* | 16.3±2.2* | 1.2* | 4.0* |
| Vitamin E-supplemented diet | ||||||
| Control | 71.1±2.6 | 68.5±3.0 | 5.9±0.4 | 5.9±0.3 | 12.1 | 11.6 |
| MI | 65.3±5.0‡ | 65.5±1.6 | 8.0±1.1‡ | 5.9±0.7‡ | 8.2‡ | 11.1‡ |
Significantly different (P<0.05) from respective control group using ANOVA followed by Bonferroni’s test;
Significantly different (P<0.05) from respective control group using Student’s t test;
Significantly different (P<0.05) from unsupplemented myocardial infarction (MI) group using ANOVA followed by Bonferroni’s test. Values are expressed as mean ± SEM for five rats. LV Left ventricle; RV Right ventricle
The ratio of GSH to GSSG was also analyzed and these data are shown in Table 3. Baseline values for this ratio in the LV and RV of controls were not different from each other. A significant depression in the GSH:GSSG ratio was observed in both the LV and RV of unsupplemented infarcted animals. However, the severity of reduction in this ratio was lesser in the RV. Supplementation with vitamin E maintained the GSH:GSSG ratio in both the LV and RV near that of respective controls.
DISCUSSION
Vitamin E supplementation and attenuation of CHF
At 16 weeks post-MI, unsupplemented rats showed several signs of CHF including elevated LVEDP and RVEDP and depressed LVSP and RVSP. Pulmonary edema, hepatomegaly, dyspnea, ascites, lethargy and cyanosis of peripheral limbs were also observed. Supplementation with dietary vitamin E blunted these LV and RV hemodynamic changes subsequent to coronary artery ligation. The fact that lung and liver congestion were absent in these animals indicated that the residual LV functional abnormalities were not severe enough to exert any influence on these tissues. In addition, no other clinical signs of CHF were present in the supplemented animals.
Maintenance of normal RV function with vitamin E supplementation in the presence of coronary artery ligation and left ventricular MI. It has been reported that there is a high frequency (40%) of RV dysfunction accompanying inferior left ventricular MI (22). Some clinical studies have shown that patients with acute inferior MI and RV involvement have a poor short-term prognosis than patients in whom RV function is unaffected (22–24). Poor outcome in this subset of patients is related primarily to the development of low cardiac output shock (25,26). Furthermore, the presence of postinfarction RV dysfunction is associated with a higher incidence of major cardiac complications (23).
Oxidative stress and cardiac dysfunction
Increased oxidative stress has been reported in a variety of experimental models of CHF such as pressure overload in guinea pigs (8), doxorubicin-induced cardiomyopathy in rats (4) and cardiomyopathy in hamsters (9). Clinically, it has been shown that patients with ischemic heart disease have elevated levels of plasma lipid peroxides relative to controls (27), and an increase in lipid peroxidation, as measured by breath pentane content, has also been shown to occur in patients with acute MI (28) and CHF (29). In patients suffering from CHF, there is a progressive increase in plasma TBARS and worsening along with NYHA functional class (30). Although these studies suggest that increased oxidative stress may contribute to the development of CHF, these findings are largely correlative and do not examine a cause and effect relationship. In our model of CHF, poor cardiac function in each of the respective ventricles was accompanied by significant depressions in catalase and GSHPx activities along with a concomitant increase in TBARS. The ratio of GSH:GSSG was also substantially reduced and vitamin E content declined in the LV. However, dietary supplementation of coronary artery-ligated animals with vitamin E reduced oxidative stress as indicated by an increase in the GSH:GSSG ratio and a decrease in TBARS. An improved hemodynamic function in each of the ventricles of the vitamin E supplemented animals may suggest a causal role for oxidative stress in the pathogenesis of heart failure.
Therapeutic effects of vitamin E in patients
A recent surge in patient studies has provided evidence for and against the clinical value of vitamin E supplementation in the prevention of cardiovascular complications in patients who are at an increased risk for such events.
Substantial decreases in plasma vitamin E levels with reperfusion after percutaneous transluminal coronary angioplasty in patients with acute MI have also been reported (31). A similar reduction in vitamin E levels after reperfusion during coronary artery bypass graft surgery has also been shown to occur in the plasma as well as the myocardium (32). In patients with angiographically symptomatic coronary atherosclerosis, oral administration of 400 U to 800 U of vitamin E daily resulted in a substantial reduction in the rate of nonfatal MI (13), although no effects on cardiovascular death were observed. Moreover, preoperative oral administration of vitamin E, alone or in combination with vitamin C, for five days before coronary bypass prevented the reductions in blood vitamin E levels associated with revascularization (33). Previous research has demonstrated an inverse correlation between antioxidant vitamin intake and incidence of acute MI. Daily administration of vitamin E in combination with vitamins A, C and beta-carotene in patients with suspected acute MI resulted in a significant decline in total cardiac end points, including average infarct size, total cardiac deaths and nonfatal acute MI (10).
Clinical studies examining the effects of vitamin E in patients with CHF have been sparse. Plasma levels of malondialdehyde have been shown to progressively increase with the advancing severity of NYHA functional class (30,34), while the levels of the plasma enzymatic antioxidants superoxide dismutase, catalase and GSHPx have all been shown to progressively decline (34). Daily vitamin E (400 mg) for four weeks substantially reduced the plasma levels of malondialdehyde and, in addition, produced a significant elevation in plasma enzymatic antioxidants (34). Normalization of these oxidative indexes was accompanied by a markedly improved response in these vitamin E-supplemented patients. Epidemiological studies have indicated that death from cardiovascular disease is inversely proportional to the plasma levels of vitamin E (35). Furthermore, evidence of an association between a high intake of vitamin E and a subsequent lower risk of coronary artery disease has also been reported in both men and women (36,37).
On the other hand, in the Heart Outcomes Prevention Evaluation (HOPE) trial, vitamin E supplementation for 4.5 years in high-risk patients showed no beneficial effects in terms of decreasing the incidence of cardiovascular events (14). In the HOPE-The Ongoing Outcomes (HOPE-TOO) trial, a further follow-up for seven years did not reveal any positive effects of vitamin E supplementation (15). Individual variations in nutritional status as well as stages of the cardiovascular condition which may have not been controlled in a similar fashion may explain the conflicting results between these two clinical trials (14,15) and the other clinical studies (34,35). A positive effect in an experimental study, such as ours, is most likely due to vitamin E supplementation as a prophylactic treatment (loading the animals with vitamin E before the coronary ligation). In these two clinical studies (14,15) the supplementation was initiated after the clinical diagnosis. Although vitamin E supplementation in the HOPE-TOO study increased the plasma vitamin E levels by approximately 64%, the oxidative stress levels were not analyzed. In this regard, an increase in oxidative stress has been reported in doxorubicin-induced cardiomyopathy despite a 100% increase in vitamin E levels (38). Furthermore, antioxidant therapy with probucol had a beneficial effect in this model (4).
Mechanism of protection afforded by vitamin E
Although the present study does not directly address the mechanism by which vitamin E modulates the development of CHF, the fact that vitamin E-supplemented infarcted rats had reduced oxidative stress and an improved antioxidant reserve concomitant with enhanced hemodynamic functioning suggests that the protection afforded by vitamin E may be attributable, in part, to its antioxidant properties. Vitamin E is a lipid-soluble, naturally occurring antioxidant and its presence in biological membranes is thought to represent the major defense system against peroxidation of lipid components. Vitamin E acts to stabilize biological membranes by interrupting the chain of free radical reactions as well as by reducing lipid peroxidation (39). A decline in myocardial vitamin E levels, as observed in the present study, may compromise antioxidant protection, leaving the myocardium vulnerable to free radical injury. Because vitamin E supplementation produced an improvement in myocardial enzymatic antioxidants in the present study, its beneficial effect may be due to a combination of its direct antioxidant properties as well as exerting a sparing effect on the remaining myocardial enzymatic antioxidants.
A protective mechanism of vitamin E independent of its direct free radical scavenging effect may also exist. Vitamin E has been shown to decrease platelet adhesion and aggregation (40), promote the inhibition of vitamin K-dependent clotting factors (41) and inhibit the production of nitric oxide (42). In animals, vitamin E supplementation has been shown to reduce the susceptibility of low-density lipoproteins to oxidation (43), thereby exerting antiatherogenic properties. Vitamin E is also thought to have a nonoxidant effect in suppressing atherosclerosis by inhibiting protein kinase C activity that is associated with stimulating smooth muscle cell proliferation by low-density lipoproteins (44–46). Recently, vitamin E has been shown to have antiarrhythmic properties, preventing lethal ventricular arrhythmias in dogs subjected to MI (47). These operative mechanisms of action may explain why the use of vitamin E in primary prevention of coronary artery disease has been successful (36–37).
CONCLUSIONS
Beneficial effects of vitamin E in modulating the development and severity of heart failure subsequent to MI have been demonstrated in animal studies. These findings in experimental animals support a causal role for oxidative stress in mediating the pathogenesis of heart failure. However, different clinical studies have yielded conflicting information. A study of the oxidative stress end points simultaneously with a study of the cardiac end points will more conclusively shed light on any strong association between oxidative stress and cardiac events.
Acknowledgments
Drs Hill and Palace were supported by fellowships from the Canadian Institutes of Health Research and the Manitoba Health Research Council, respectively. Dr Khaper was supported by a studentship from the Heart and Stroke Foundation of Canada.
REFERENCES
- 1.Anversa P, Olivetti G, Meggs LG, Sonnenblick EH, Capasso JM. Cardiac anatomy and ventricular loading after myocardial infarction. Circulation. 1993;87:VII22–7. [Google Scholar]
- 2.Francis GS, McDonald KM, Cohn JN. Neurohumoral activation in preclinical heart failure. Remodeling and the potential for intervention. Circulation. 1993;87:IV90–6. [PubMed] [Google Scholar]
- 3.Ferguson DW. Sympathetic mechanisms in heart failure. Pathophysiological and pharmacological implications. Circulation. 1993;87:VII68–75. [Google Scholar]
- 4.Siveski-Iliskovic N, Kaul N, Singal PK. Probucol promotes endogenous antioxidants and provides protection against adriamycin-induced cardiomyopathy. Circulation. 1994;89:2829–35. doi: 10.1161/01.cir.89.6.2829. [DOI] [PubMed] [Google Scholar]
- 5.Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol. 1996;28:506–14. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 6.Giugliano D, Ceriello A, Paolisso G. Diabetes mellitus, hypertension, and cardiovascular disease: Which role for oxidative stress? Metabolism. 1995;44:363–8. doi: 10.1016/0026-0495(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 7.Gupta M, Singal PK. Higher antioxidant capacity during a chronic stable heart hypertrophy. Circ Res. 1989;64:398–406. doi: 10.1161/01.res.64.2.398. [DOI] [PubMed] [Google Scholar]
- 8.Dhalla AK, Singal PK. Antioxidant changes in hypertrophied and failing guinea pig hearts. Am J Physiol. 1994;266:H1280–5. doi: 10.1152/ajpheart.1994.266.4.H1280. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi A, Yamashita T, Kaneko M, Nishiyama T, Hayashi H, Yamazaki N. Effects of verapamil on experimental cardiomyopathy in the Bio 14.6 Syrian hamster. J Am Coll Cardiol. 1987;10:1128–38. doi: 10.1016/s0735-1097(87)80356-x. [DOI] [PubMed] [Google Scholar]
- 10.Singh RB, Niaz MA, Rastogi SS, Rastogi S. Usefulness of antioxidant vitamins in suspected acute myocardial infarction (the Indian experiment of infarct survival-3) Am J Cardiol. 1996;77:232–6. doi: 10.1016/s0002-9149(97)89384-8. [DOI] [PubMed] [Google Scholar]
- 11.McMurray J, Mclay J, Chopra M, Bridges A, Belch JJ. Evidence for enhanced free radical activity in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol. 1990;65:1261–2. doi: 10.1016/0002-9149(90)90985-a. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Velez CR, Garcia-Castineiras S, Mendoza-Ramos E, Hernandez-Lopez E. Increased malonidaldehyde in peripheral blood of patients with congestive heart failure. Am Heart J. 1996;131:146–52. doi: 10.1016/s0002-8703(96)90063-0. [DOI] [PubMed] [Google Scholar]
- 13.Stephens NG, Parsons A, Schofield PM, et al. Randomised controlled trail of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–6. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–60. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 15.Lonn E, Bosch J, Yusuf S, et al. HOPE and HOPE-TOO Trial Investigators Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 16.Hill MF, Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation. 1997;96:2414–20. doi: 10.1161/01.cir.96.7.2414. [DOI] [PubMed] [Google Scholar]
- 17.Hill MF, Singal PK. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol. 1996;148:291–300. [PMC free article] [PubMed] [Google Scholar]
- 18.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterixation of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 19.Clairborne A. Catalase activity. In: Greenwald RA, editor. Handbook of Methods for Oxygen Radical Research. Boca Raton, Florida: CRC Press; 1985. pp. 283–4. [Google Scholar]
- 20.Palace VP, Brown SB. HPLC determination of tocopherol, retinol, dehydroretinol and retinyl palmitate in tissues of lake char (Salvelinus namaycush) exposed to coplanar 3,3′,4,4′,5-pentachlorobiphenyl. Environ Toxicol Chem. 1994;13:473–6. [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 22.Shah PK, Maddahi J, Berman DS, Pichler M, Swan HJ. Scintigraphically detected predominant right ventricular dysfunction in acute myocardial infarction: Clinical and hemodynamic correlates and implications for therapy and prognosis. J Am Coll Cardiol. 1985;6:1264–72. doi: 10.1016/s0735-1097(85)80212-6. [DOI] [PubMed] [Google Scholar]
- 23.Bueno H, Lopez-Palop R, Bermejo J, Lopez-Sendon JL, Delcan JL. In-hospital outcome of elderly patients with acute inferior myocardial infarction and right ventricular involvement. Circulation. 1997;96:436–41. doi: 10.1161/01.cir.96.2.436. [DOI] [PubMed] [Google Scholar]
- 24.Zehender M, Kasper W, Kauder E, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med. 1993;328:981–8. doi: 10.1056/NEJM199304083281401. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd EA, Gersh BJ, Kennelly BM. Hemodynamic spectrum of “dominant” right ventricular infarction in 19 patients. Am J Cardiol. 1981;48:1016–22. doi: 10.1016/0002-9149(81)90314-3. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein JA, Vlahakes GJ, Verrier ED, et al. The role of right ventricular systolic dysfunction and elevated intrapericardial pressure in the genesis of low cardiac output in experimental right ventricular infarction. Circulation. 1982;65:513–22. doi: 10.1161/01.cir.65.3.513. [DOI] [PubMed] [Google Scholar]
- 27.Stringer MD, Gorog PG, Freeman A, Kakkar VV. Lipid peroxides and atherosclerosis. BMJ. 1989;298:281–4. doi: 10.1136/bmj.298.6669.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weitz Z, Birnbaum AJ, Sobotka PA, Zarling EJ, Skosey JL. High breath pentane concentrations during acute myocardial infarction. Lancet. 1991;337:933–5. doi: 10.1016/0140-6736(91)91569-g. [DOI] [PubMed] [Google Scholar]
- 29.Sobotka PA, Brottman MD, Weitz Z, Birnbaum AJ, Skosey JL, Zarling EJ. Elevated breath pentane in heart failure reduced by free radical scavenger. Free Radical Biol Med. 1993;14:643–7. doi: 10.1016/0891-5849(93)90145-k. [DOI] [PubMed] [Google Scholar]
- 30.Charney RH, Levy DK, Kalman J, et al. Free radical activity increases with NYHA class in congestive heart failure. J Am Coll Cardiol. 1997;29(Suppl):102A. (Abst) [Google Scholar]
- 31.Lafont A, Marwick TH, Chisolm GM, Van Lente F, Vaska KJ, Whitlow PL. Decreased free radical scavengers with reperfusion after coronary angioplasty in patients with acute myocardial infarction. Am Heart J. 1996;131:219–23. doi: 10.1016/s0002-8703(96)90344-0. [DOI] [PubMed] [Google Scholar]
- 32.Weisel RD, Mickle DA, Finkle CD, et al. Myocardial free-radical injury after cardioplegia. Circulation. 1989;80:III14–8. [PubMed] [Google Scholar]
- 33.Ferreira RF, Milei J, Llesuy S, et al. Antioxidant action of vitamins A and E in patients submitted to coronary artery bypass surgery. Vasc Surg. 1991;25:191–5. [Google Scholar]
- 34.Ghatak A, Brar MJ, Agarwal A, et al. Oxy free radical system in heart failure and therapeutic role of oral vitamin E. Int J Cardiol. 1996;57:119–27. doi: 10.1016/s0167-5273(96)02787-8. [DOI] [PubMed] [Google Scholar]
- 35.Gey KF, Brubacher GB, Stahelin HB. Plasma levels of antioxidant vitamins in relations to ischemic heart disease and cancer. Am J Clin Nutr. 1987;45:1368–77. doi: 10.1093/ajcn/45.5.1368. [DOI] [PubMed] [Google Scholar]
- 36.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993;328:1450–6. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 37.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993;328:1444–9. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 38.Danelisen I, Palace V, Lou H, Singal PK. Maintenance of myocardial levels of vitamin A in heart failure due to adriamycin. J Mol Cell Cardiol. 2002;34:789–95. doi: 10.1006/jmcc.2002.2015. [DOI] [PubMed] [Google Scholar]
- 39.Packer L. Interactions among antioxidants in health and disease: Vitamin E and its redox cycle. Proc Soc Exp Biol Med. 1992;200:271–6. doi: 10.3181/00379727-200-43433. [DOI] [PubMed] [Google Scholar]
- 40.Steiner M. Influence of vitamin E on platelet function in humans. J Am Coll Nutr. 1991;10:466–73. doi: 10.1080/07315724.1991.10718173. [DOI] [PubMed] [Google Scholar]
- 41.Dowd P, Zheng ZB. On the mechanism of the anticlotting action of vitamin E quinone. Proc Natl Acad Sci USA. 1995;92:8171–5. doi: 10.1073/pnas.92.18.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boulanger CM, Tanner FC, Bea ML, Hahn AW, Werner A, Luscher TF. Oxidized low density lipoproteins induce mRNA expression and release of endothelin from human and porcine endothelium. Circ Res. 1992;70:1191–7. doi: 10.1161/01.res.70.6.1191. [DOI] [PubMed] [Google Scholar]
- 43.Jialal I, Grundy SM. Effect of dietary supplementation with alpha-tocopherol on the oxidative modification of low density lipoprotein. J Lipid Res. 1992;33:889–906. [PubMed] [Google Scholar]
- 44.Ozer NK, Boscoboinik D, Azzi A. New roles of low density lipoproteins and vitamin E in the pathogenesis of atherosclerosis. Biochem Mol Biol Int. 1995;35:117–24. [PubMed] [Google Scholar]
- 45.Stauble B, Boscoboinik D, Tasinato A, Azzi A. Modulation of activator protein-1 (AP-1) transcription factor and protein kinase C by hydrogen peroxide and D-alpha-tocopherol in vascular smooth muscle cells. Eur J Biochem. 1994;226:393–402. doi: 10.1111/j.1432-1033.1994.tb20064.x. [DOI] [PubMed] [Google Scholar]
- 46.Chatelain E, Boscoboinik DO, Bartoli GM, et al. Inhibition of smooth muscle cell proliferation and protein kinase C activity by tocopherols and tocotrienols. Biochem Biophys Acta. 1993;1176:83–9. doi: 10.1016/0167-4889(93)90181-n. [DOI] [PubMed] [Google Scholar]
- 47.Sebbag L, Forrat R, Canet E, Renaud S, Delaye J, de Lorgeril M. Effects of dietary supplementation with alpha-tocopherol on myocardial infarct size and ventricular arrhythmias in a dog model of ischemia-reperfusion. J Am Coll Cardiol. 1994;24:1580–5. doi: 10.1016/0735-1097(94)90158-9. [DOI] [PubMed] [Google Scholar]


