Abstract
The amino acid intermediate homocysteine (Hcy) is formed during the metabolism of methionine to cysteine. Hyperhomocysteinemia (HHcy) is recognized as an independent risk factor for coronary atherosclerosis. The circulating levels of total Hcy (tHcy) can increase due to intake of foods rich in methionine or deficiencies of vitamins such as folate, pyridoxine and cyanocobalamin, which are required for the metabolism of Hcy. In addition, mutations in the genes coding for Hcy metabolizing enzymes can contribute to an increase in tHcy levels. Clinical and epidemiological studies have shown that an elevated level of tHcy measured in serum or plasma is a strong predictor of cardiovascular disease risk, which appears to be greatest in patients who have HHcy following a methionine load. Intimal hyperplasia (IH) (intima/media [I/M] ratio) is the universal response of a vessel to injury and may result in vasoconstriction when left unattended. The effect of dietary HHcy on balloon catheter-injured carotid artery and its modulation (if any) by the peroxisome proliferator-activated receptor agonist gamma rosiglitazone was evaluated in 12-week-old female Sprague-Dawley rats fed either a control diet or a diet containing 1% L-methionine. Once the rats were established on the diet, the group that was fed 1% L-methionine was further subdivided and either given an aqueous preparation of 3 mg/kg/day rosiglitazone or the vehicle via oral gavage for one week. This was followed by surgically injuring the left carotid artery using a Maverick Over-The-Wire catheter (2.0 mm × 20 mm, 3.2F; Boston Scientific, USA). The rats were continued on their respective diets and drug regimen for 21 days postsurgery. On day 22 of the procedure, the rats were sacrificed for collection of blood, the carotid arteries and liver for biochemical and histological evaluation. Compared with controls there was a significant increase in both tHcy levels and I/M ratio in the rats fed 1% L-methionine (5.4±0.28 μM versus 32.8±3.01 μM, P<0.002; and 0.175±0.05 versus 1.05±0.23, P<0.005, respectively). The effect of rosiglitazone in rats fed the control diet was not prominent. On the other hand, administration of rosiglitazone to the rats on the 1% L-methionine diet significantly reduced the levels of serum tHcy (16.6±2.1 μM versus 32.8±3.01 μM, P<0.001); however, the tHcy levels remained significantly elevated compared with animals on the control diet (P<0.002). The group receiving the L-methionine diet plus rosiglitazone had an inhibition in the development of IH compared with those receiving the L-methionine diet alone (I/M of 0.278±0.041 versus 1.05±0.23, P<0.01). Moreover, the development of IH in the group receiving the L-methionine diet plus rosiglitazone treatment was not significantly different from that observed in the group on the control diet without rosiglitazone (0.278±0.041 versus 0.175±0.05, respectively). These findings may have important implications in deciphering the molecular mechanisms involved in the augmentation of IH in HHcy and modulation of this process by rosiglitazone.
Keywords: Carotid, Hyperhomocysteinemia, Metabolism, Methionine, Vasoconstriction
Homocysteine (Hcy) is an intermediate formed during the metabolism of methionine (an essential amino acid) to cysteine. The first step in the metabolism of methionine is the formation of S-adenosyl methionine. The methyl moiety of S-adenosyl methionine is donated to methyl group acceptors, resulting in the formation of S-adenosyl Hcy, which is then deadenosylated to form Hcy. The Hcy can then be routed via the remethylation pathway to the reformation of methionine or routed via the trans-sulfuration pathway to the formation of cystathionine and then cysteine (Figure 1). An increase in total Hcy (tHcy) levels leads to the formation of an intra-molecular thioester of Hcy, namely, Hcy thiolactone.
Figure 1).
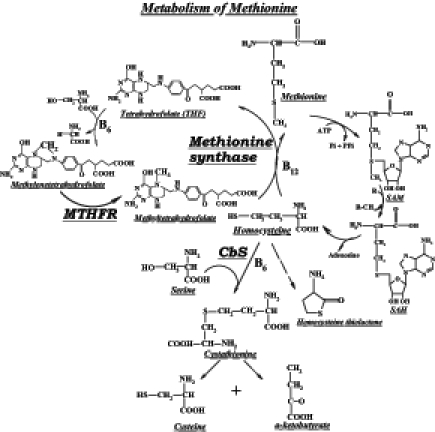
Metabolic pathway of homocysteine. CbS Cystathionine beta-synthase; MTHFR 5,10-methylenetetrahydrofolate reductase; SAH S-adenosylhomocysteine; SAM S-adenosylmethionine
Hyperhomocysteinemia (HHcy), or increased serum concentrations of Hcy, is generally recognized as an independent risk factor for coronary, cerebral and peripheral atherosclerosis (1,2). Hcy is either totally absent or present in very small quantities in various commonly consumed foods of vegetable origin (3). Consumption of foods rich in methionine can have a bearing on the tHcy concentrations. The circulating levels of tHcy can increase due to metabolic defects, usually related to alterations in the Hcy metabolizing enzyme(s). These are either acquired, as are the cofactor (vitamin) deficiencies, or are an inherited phenomenon. The inherited defects are generally due to mutations in genes coding for the enzymes of the metabolism of Hcy (4,5). The inborn errors of metabolism and related disorders are well documented in the literature (6,7). Other than the acquired and/or the inherited factors, even environmental factors and medications could contribute to variations in the levels of tHcy (8).
HHcy is associated with vascular disease in general, but particularly in subjects with significant carotid stenosis (9,10). In addition, Hcy is known to be involved in age-related diseases such as osteoporosis, Alzheimer’s disease and diabetes. The circulating levels of tHcy are contributed by cellular export mechanisms, and these are known to increase in various conditions (11). Many clinical and epidemiological studies have shown that elevated levels of tHcy measured in serum or plasma are a strong predictor of cardiovascular disease (CVD) risk (12). Mild-to-severe HHcy is known to cause harmful changes in the vascular bed, neural tube formation and kidney function, to name a few. Previous researchers have shown that the plasma tHcy level is a strong predictor of mortality in patients with angiographically confirmed coronary artery disease (13). Elevated serum tHcy is associated with sudden unexpected death in men and is especially associated with diabetes. In addition, elevated serum tHcy is associated with an increase in fibrous plaques and a relative decrease in thin-cap atheromas (14). Also, in the absence of other known risk factors, it is shown that HHcy stimulates the expression of monocyte chemotactic protein-1, vascular cell adhesion molecule-1 and E-selectin in vivo. This leads to increased monocyte adhesion to the aortic endothelium, which may contribute significantly to the development of atherosclerosis by facilitating monocyte/macrophage infiltration into the arterial wall (15). There has been a constant effort to understand the mechanistic aspects behind this.
Experimentally, HHcy can be induced by dietary manipulation with methionine (16). The progression of events involved in carotid restenosis in experimental HHcy can be studied by injuring the carotid artery. Balloon catheter-mediated injury (BCI) of the artery is a commonly used method to study intimal hyperplasia (IH). Percutaneuos transluminal coronary angioplasty catheters have been used for this purpose. Where one of the carotids can be injured, the contralateral one can serve as a reference. Studies have shown that HHcy increases IH following a carotid endarterectomy in a rat model (17,18) and, therefore, HHcy was induced experimentally in the present study.
The peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that increase transcription of target genes by binding to a specific nucleotide sequence in the gene’s promoter. These serve as receptors for two very important classes of drugs: the hypolipidemic fibrates and the insulin-sensitizing thiazolidinediones. Three different PPAR isotypes can be distinguished: alpha (α), beta (β) and gamma (γ). The beneficial effects of PPAR-γ ligands include improving insulin sensitivity, decreasing hyperinsulinemia, increasing high density lipoprotein levels, lowering blood pressure, decreasing the generation of reactive oxygen species and improving vascular reactivity. The PPAR-γ ligand rosiglitazone has been shown to have protective effects on the vessel wall (19–21). We have observed that rosiglitazone, but not fenofibrate, attenuated IH independently of insulin, glucose and triglyceride levels in the fatty Zucker rat (20) (a model of insulin resistance and mild hyperglycemia [22]). Because these effects of rosiglitazone appear to be (at least in part) independent of insulin resistance (data on lean Zucker rat [20]), we tested the hypothesis that rosiglitazone would alleviate the circulating levels of tHcy and attenuate the augmented development of IH in a model of dietary HHcy.
In view of the association between cardiovascular effects and HHcy, it was surmised that if experimental HHcy would elicit its effects on the progression of IH then the same could be modulated by rosiglitazone. It was observed that IH (following BCI) was significantly higher in female Sprague-Dawley rats fed a 1% L-methionine diet as opposed to controls fed normal laboratory feed. The administration of rosiglitazone facilitated a reduction in tHcy levels as well as the IH due to HHcy. These findings are provocative for deciphering the molecular mechanisms involved in the augmentation of IH following BCI in experimental HHcy, and its modulation by rosiglitazone.
METHODS
The Institutional Animal Care & Use Committee of Tulane University Health Sciences Center (New Orleans, Louisiana, USA) approved the present study.
Animals and feeding schedule
Ten-week-old female Sprague-Dawley rats obtained from Harlan Laboratories (USA) were divided into two groups and fed a diet either without (control; n=7) or with methionine (experimental; n=14). In preparing the methionine diet, 10 g of L-methionine (Sigma Chemical Company, USA) was added to 1.0 kg of powdered feed (Purina Co, USA) and mixed thoroughly. After an acclimatizing period on the assigned diet, the rats on the methionine diet were further subdivided and administered either an aqueous preparation of 3 mg/kg/day rosiglitazone or the vehicle using oral gavage before the induction of BCI. The animals were continued on their respective diet and drug regimens for 21 days post-BCI. All rats had free access to the feed and water, and were maintained in a 12 h day/light cycle. The animals were weighed at weekly intervals and constantly under the supervision of the veterinarian all through the experimental period.
BCI
BCI of one of the carotid arteries was induced as previously published (23). Briefly, a 2.0 mm balloon catheter was introduced through the femoral artery to the left carotid. The balloon was inflated to 405.3 kPa and held for 20 s, and then deflated to 202.6 kPa and dragged down to the aorta. Rats were sacrificed three weeks after injury, and the carotid arteries were evaluated microscopically.
Sample collection
A baseline sample of blood (obtained for the measurement of serum tHcy) was collected under isoflurane anesthesia before the beginning of the dietary regimen. At the end of the experiment, rats were sacrificed using a CO2 chamber and from each rat, the blood, carotid arteries, liver and other tissues were excised. The blood samples were kept on ice and centrifuged immediately at 4000 rpm for 20 min (Eppendorf Centrifuge 5810-R, Brinkman Instruments, USA), and the serum was separated and stored frozen for further analysis. Liver tissue was immediately sliced, and 1.0 g portions were frozen on dry ice. These samples were stored at −70°C until analyzed. The carotid arteries (both injured and contralateral) were flushed with normal saline and transferred to cassettes for fixing and processing for paraffin embedding, sectioning and staining.
Histology and morphometric measurements
The carotid arteries were separated and flushed with normal saline for processing by conventional methods used for dehydration. They were cut from the top into four equal segments and placed sequentially for embedding in paraffin. Sections of 4 μm were cut and stained with hematoxylin and eosin for microscopy. All four segments from each artery specimen were analyzed at a magnification factor of 10, and the mean of the four segments represented the intima/media (I/M) ratio, which is an index of IH. Computerized digital microscopic software (Image-Pro Plus 5.1, Media Cybernetics, USA) was used to obtain measurements of the intimal and medial areas. The I/M ratio was used to compare treatment groups.
Measurement of Hcy levels
The tHcy levels were measured by high-pressure liquid chromatography according to an established method (24). Most methods of determination of Hcy exploit the specificity of a thiol-specific fluorogenic probe such as monobromobimane or 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate, thus facilitating the determination of cysteine, Hcy, cysteinylglycine and glutathione. Briefly, the serum samples are treated with a reducing agent, such as borohydride or tri-n-butylphosphine, to reduce the disulfides. The samples are then processed for derivatization with the fluorescent probe. After the formation of the adducts, trichloroacetic acid is added to precipitate the proteins. The samples are then centrifuged at 4000 rpm for 20 min to obtain the supernatant (containing non-protein Hcy-adduct); the protein precipitate is hydrolyzed to liberate the protein-bound Hcy-adducts. Both the protein-bound and the nonprotein-bound Hcy (from the supernatant) put together reflect the tHcy. The values of Hcy reported in the present study are that of tHcy.
Statistical analyses
All data are expressed as the mean ± SEM. Computer-assisted statistical analysis (Sigma Stat 2.0, Statistical Solutions, USA) was used for one-way ANOVA and Tukey test.
RESULTS
Histology
In rats fed the control diet, development of IH occurred over the three-week period and the I/M ratio was determined. There was no significant change in the medial area following catheter injury. For the rats on the methionine diet, the I/M was 1.05±0.23. The animals which were on the methionine diet but administered rosiglitazone had a significant decrease in the I/M ratio (0.278±0.041). The animals on the methionine diet exhibited augmented development of IH; however, this augmentation was attenuated by treatment with rosiglitazone.
Development of IH
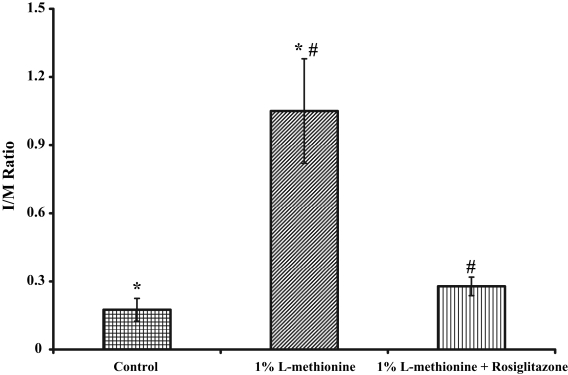
Summary data for the effect of rosiglitazone on IH are presented in Figure 2. The group of animals receiving the control diet had an I/M of 0.175±0.05. In the group on the methionine diet, however, the development of IH was markedly augmented (I/M of 1.05±0.23), which was significant compared with animals on the control diet (P<0.005). Administration of rosiglitazone to the group receiving the methionine diet inhibited the development of IH observed in the presence of the methionine diet alone (I/M of 0.278±0.041, P<0.01). Also, the development of IH in the group on the methionine diet plus rosiglitazone treatment was not statistically different from that observed in the group on the control diet without rosiglitazone treatment (0.278±0.041 versus 0.175±0.05, respectively, P<0.918).
Figure 2).
The effect of rosiglitazone on the rate of development of intimal hyperplasia (IH) in the carotid artery following balloon catheter injury. *In animals fed a high methionine diet, the development of IH was significantly augmented (P<0.005); #Administration of rosiglitazone to animals on the methionine diet inhibited the increase of IH observed in the presence of the methionine diet alone (P<0.01). Adapted from reference 26. I/M Intima/media
Hcy levels
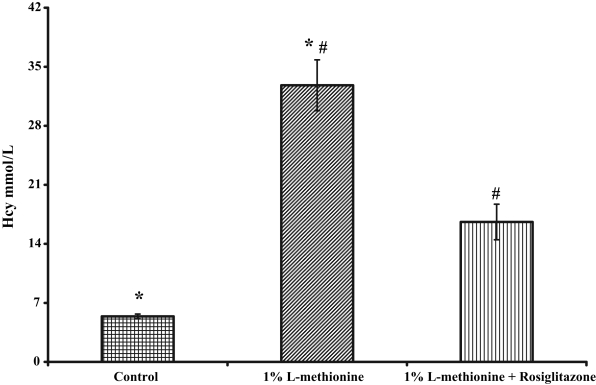
Summary data for serum Hcy are presented in Figure 3. For the control diet, the tHcy level was 5.4±0.28 μM. Administration of rosiglitazone to animals on the control diet did not affect the tHcy level. Rats receiving the methionine diet exhibited markedly enhanced levels of Hcy (32.8±3.01 μM) that were statistically significant compared with animals on the control diet (P<0.002). When rosiglitazone was administered to the animals on the methionine diet, the serum tHcy was significantly reduced (16.6±2.1 μM) compared with the methionine diet alone (P<0.001). The tHcy levels remained elevated compared with the controls and this was statistically significant (P<0.002). The cystathionine β-synthase (CβS) activity in the animals on the methionine diet and given rosiglitazone was higher than that of the animals on the methionine diet alone.
Figure 3).
Total serum homocysteine (Hcy) levels in rats on different dietary and treatment regimens. *Animals on the methionine diet exhibited significantly enhanced levels of Hcy compared with animals on the control diet (P<0.002); #Administration of rosiglitazone to the animals on the methionine diet significantly reduced the serum Hcy (P<0.001 compared with the methionine diet alone); however, the Hcy levels remained statistically elevated compared with animals on the control diet (P<0.002). Adapted from reference 26
DISCUSSION
The present study shows that the PPARγ agonist rosiglitazone attenuates IH following arterial BCI in a model of dietary HHcy. Additionally, the demonstration of rosiglitazone decreasing serum tHcy levels in methionine diet-fed animals was very innovative. A possible mechanism for the rosiglitazone-mediated effect has been reported (25,26). High methionine intake has been shown to accelerate atherosclerosis (27). Compared with Sprague-Dawley rats fed the control diet (I/M of 0.175±0.05), a significant increase in the hyperplastic response was observed in rats on the methionine diet (I/M of 1.05±0.23). This effect was significantly reduced by rosiglitazone (I/M of 0.278±0.041). PPARγ ligands have been previously reported to decrease IH in animal models of diabetes and insulin resistance, as well as decreasing stent reocclusion in humans following angioplasty and stent implantation (20,21,28,29). We have recently demonstrated that rosiglitazone reduces IH to a much greater extent than PPARα ligands and this effect was independent of insulin, triglyceride and glucose levels, suggesting a possible direct effect on vascular smooth muscle cell proliferation and DNA synthesis (20). The present model of angioplasty-induced injury employs the catheter approach routinely used in human subjects. This approach avoids additional and more global injury to the carotid associated with both endartectomy and/or a direct approach where the catheter is inserted through an incision in the carotid.
The methionine diet resulted in an increase in serum tHcy compared with the controls (32.8±3.01 μM versus 5.4±0.28 μM, respectively). This elevation (an independent risk factor for CVD) is similar to the range observed in clinical cases. The IH following catheter injury in the group fed the methionine diet was more than fivefold higher than in controls (I/M of 1.05±0.23 versus 0.175±0.05). Treatment with rosiglitazone reduced the tHcy levels to 16.6±2.1 μM, and reduced the I/M to 0.278±0.041. The reduction in IH in response to rosiglitazone was not significantly different from the group on the control diet. The tHcy level, on the other hand, was significantly reduced and remained elevated compared with the control diet group (P<0.001). This could be due to the continuous feeding of the 1% L-methionine diet throughout the experimental period. In some respects, this observation on serum tHcy levels is not surprising because the levels following treatment are analogous to those in humans who have a normal methionine load test. We know of no treatment currently available that will remedy this. The treatment strategies for reducing tHcy levels (following a methionine load) to that observed in either animals or humans who have not been similarly loaded are limited and, therefore, we believe the post-treatment level to be the maximum possible.
The present study demonstrates that rosiglitazone stimulates the activity of CβS in a methionine-fed animal, and is in line with our earlier report on troglitazone and rosiglitazone in animals fed a normal diet with no additional methionine (25). Because CβS catalyzes the irreversible conversion of Hcy to cystathionine, these data suggest that the reduction in serum tHcy levels in the present study was due, at least in part, to the increased metabolism of Hcy in the rosiglitazone-treated group. Other data highlight the importance of CβS in increased atherosclerosis in mice genetically modified to have a deficiency of this enzyme (30). Furthermore, this enzyme is critical in lowering serum tHcy following a methionine load. High methionine intake has also been shown to accelerate atherosclerosis (27). To our knowledge, there is little activity of CβS in vascular tissue (31) and, therefore, we have only measured the activity of this enzyme in the liver where it is most abundantly expressed and active.
The PPARγ ligands have been previously reported to decrease IH in animal models of diabetes and insulin resistance, as well as decreasing stent reocclusion in humans following angioplasty and stent implantation (20,21,28,29). We have recently demonstrated that rosiglitazone reduces IH to a much greater extent than the PPARα ligand fenofibrate, and this effect was independent of insulin, triglyceride and glucose levels, suggesting a possible direct effect on vascular smooth muscle cell proliferation (20). A similar effect was seen in lean Zucker rats, demonstrating that the effect of rosiglitazone is independent of its effects on ameliorating insulin resistance (20). Our data are important in light of recent clinical trials demonstrating the lacunae in vitamin therapy (32). Hcy remains a risk factor for CVD and stroke (32,33). Alternative strategies, such as the use of a PPARγ agonist, offer a previously unrecognized therapeutic option.
CONCLUSIONS
The PPARγ agonist rosiglitazone can reduce tHcy-augmentation of catheter-induced vascular injury and also reduce the elevation of serum tHcy induced by a methionine diet. The reduction observed is due in part to the stimulation of the activity of CβS activity by the PPARγ agonist. These findings may have important implications for preventing CVD and cardiovascular events in patients with HHcy.
REFERENCES
- 1.McCully KS. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–28. [PMC free article] [PubMed] [Google Scholar]
- 2.McCully KS. Chemical pathology of homocysteine. I. Atherogenesis. Ann Clin Lab Sci. 1993;23:477–93. [PubMed] [Google Scholar]
- 3.Sakamoto A, Nishimura Y, Ono H, Sakura N. Betaine and homocysteine concentrations in foods. Pediatr Int. 2002;44:409–13. doi: 10.1046/j.1442-200x.2002.01591.x. [DOI] [PubMed] [Google Scholar]
- 4.Tsai MY, Hanson NQ, Bignell MK, Schwichtenberg KA. Simultaneous detection and screening of T833C and G919A mutations of the cystathionine beta-synthase gene by single-strand conformational polymorphism. Clin Biochem. 1996;5:473–7. doi: 10.1016/0009-9120(96)00045-8. [DOI] [PubMed] [Google Scholar]
- 5.Rothenbacher D, Fischer HG, Hoffmeister A, et al. Homocysteine and methylenetetrahydrofolate reductase genotype: Association with risk of coronary heart disease and relation to inflammatory, hemostatic, and lipid parameters. Atherosclerosis. 2002;162:193–200. doi: 10.1016/s0021-9150(01)00699-2. [DOI] [PubMed] [Google Scholar]
- 6.Scriver CR, Baudet AL, Sly WS, Valle D, editors. The Metabolic Bases of Inherited Disease. 8th edn. New York: McGraw Hill; 2001. [Google Scholar]
- 7.King RA, Rotter JI, Motulsky AG, editors. The Genetic Basis of Common Diseases. 2nd edn. New York: Oxford University Press; 2002. [Google Scholar]
- 8.Desouza C, Keebler M, McNamara DB, et al. Drugs affecting homocysteine metabolism: Impact on cardiovascular risk. Drugs. 2002;62:605–16. doi: 10.2165/00003495-200262040-00005. [DOI] [PubMed] [Google Scholar]
- 9.Selhub J, Jacques PF, Bostom AG, et al. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N Engl J Med. 1995;332:286–91. doi: 10.1056/NEJM199502023320502. [DOI] [PubMed] [Google Scholar]
- 10.Streifler JY, Rosenberg N, Chetrit A, et al. Cerebrovascular events in patients with significant stenosis of the carotid artery are associated with hyperhomocysteinemia and platelet antigen-1 (Leu33Pro) polymorphism. Stroke. 2001;32:2753–8. doi: 10.1161/hs1201.099650. [DOI] [PubMed] [Google Scholar]
- 11.Hultberg B. Modulation of extracellular homocysteine concentration in human cell lines. Clin Chim Acta. 2003;330:151–9. doi: 10.1016/s0009-8981(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 12.Nygard O, Nordrehaug JE, Refsum H, et al. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–6. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 13.Kojoglanian SA, Jorgensen MB, Wolde-Tsadik G, Burchette RJ, Aharonian VJ. Restenosis in Intervened Coronaries with Hyperhomocysteinemia (RICH) Am Heart J. 2003;146:1077–81. doi: 10.1016/S0002-8703(03)00518-0. [DOI] [PubMed] [Google Scholar]
- 14.Tyagi SC. Homocyst(e)ine and heart disease: Pathophysiology of extracellular matrix. Clin Exp Hypertens. 1999;21:181–98. doi: 10.3109/10641969909068660. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Woo CW, Sung FL, Siow YL, O K. Increased monocyte adhesion to aortic endothelium in rats with hyperhomocysteinemia: Role of chemokine and adhesion molecules. Arterioscler Thromb Vasc Biol. 2002;22:1777–83. doi: 10.1161/01.atv.0000035404.18281.37. [DOI] [PubMed] [Google Scholar]
- 16.Morita H, Kurihara H, Yoshida S, et al. Diet-induced hyperhomocysteinemia exacerbates neointima formation in rat carotid arteries after balloon injury. Circulation. 2001;103:133–9. doi: 10.1161/01.cir.103.1.133. [DOI] [PubMed] [Google Scholar]
- 17.Southern FN, Cruz N, Fink LM, et al. Hyperhomocysteinemia increases intimal hyperplasia in a rat carotid endarterectomy model. J Vasc Surg. 1998;28:909–18. doi: 10.1016/s0741-5214(98)70069-2. [DOI] [PubMed] [Google Scholar]
- 18.Smith TP, Cruz CP, Brown AT, Eidt JF, Moursi MM. Folate supplementation inhibits intimal hyperplasia induced by a high-homocysteine diet in a rat carotid endarterectomy model. J Vasc Surg. 2001;34:474–81. doi: 10.1067/mva.2001.117144. [DOI] [PubMed] [Google Scholar]
- 19.Yamakawa K, Hosoi M, Koyama H, et al. Peroxisome proliferator-activated receptor-gamma agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochem Biophys Res Commun. 2000;271:571–4. doi: 10.1006/bbrc.2000.2665. [DOI] [PubMed] [Google Scholar]
- 20.Desouza CV, Murthy SN, Diez J, et al. Differential effects of peroxisome proliferator activator receptor-alpha and gamma ligands on intimal hyperplasia after balloon catheter-induced vascular injury in Zucker rats. J Cardiovasc Pharmacol Ther. 2003;8:297–305. doi: 10.1177/107424840300800407. [DOI] [PubMed] [Google Scholar]
- 21.Choi SH, Choi DH, Ko YK, et al. Preventive effects of rosiglitazone on restenosis after coronary stenting in patients with type-2 diabetes. Diabetes. 2003;52(Suppl 1):A19. (Abst) [Google Scholar]
- 22.Berthiaume N, Mika AK, Zinker BA. Development of insulin resistance and endothelin-1 levels in the Zucker fatty rat. Metabolism. 2003;52:845–9. doi: 10.1016/s0026-0495(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 23.Schiller NK, McNamara DB. Balloon catheter vascular injury of the alloxan-induced diabetic rabbit: The role of insulin-like growth factor-1. Mol Cell Biochem. 1999;202:159–67. doi: 10.1023/a:1007005919319. [DOI] [PubMed] [Google Scholar]
- 24.Poirier LA, Wise CK, Delongchamp RR, Sinha R. Blood determinations of S-adenosylmethionine, S-adenosylhomocysteine, and homocysteine: Correlations with diet. Cancer Epidemiol Biomarkers Prev. 2001;10:649–55. [PubMed] [Google Scholar]
- 25.Murthy SN, Matta AS, Fonseca NA, et al. Rosiglitazone stimulates hepatic cystathionine beta synthase activity but does not affect plasma homocysteine levels in Zucker Fatty Rats. American Diabetes Association Meeting, Orlando. Diabetes. 2004;53:A555. (Abst) [Google Scholar]
- 26.Murthy SN, Obregon DF, Chattergoon NN, et al. Rosiglitazone reduces serum homocysteine levels, smooth muscle proliferation and intimal hyperplasia in Sprague-Dawley rats fed a high methionine diet. Metabolism. 2005;54:645–52. doi: 10.1016/j.metabol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Troen AM, Lutgens E, Smith DE, Rosenberg IH, Selhub J. The atherogenic effect of excess methionine intake. Proc Natl Acad Sci USA. 2003;100:15089–94. doi: 10.1073/pnas.2436385100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takagi T, Akasaka T, Yamamuro A, et al. Troglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with non-insulin dependent diabetes mellitus: A serial intravascular ultrasound study. J Am Coll Cardiol. 2000;36:1529–35. doi: 10.1016/s0735-1097(00)00895-0. [DOI] [PubMed] [Google Scholar]
- 29.Takagi T, Yamamuro A, Tamita K, et al. Pioglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with type 2 diabetes mellitus: An intravascular ultrasound scanning study. Am Heart J. 2003;146:E5. doi: 10.1016/S0002-8703(03)00146-7. [DOI] [PubMed] [Google Scholar]
- 30.Dayal S, Bottiglieri T, Arning E, et al. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res. 2001;88:1203–9. doi: 10.1161/hh1101.092180. [DOI] [PubMed] [Google Scholar]
- 31.Bao L, Vlcek C, Paces V, Kraus JP. Identification and tissue distribution of human cystathionine beta-synthase mRNA isoforms. Arch Biochem Biophys. 1998;350:95–103. doi: 10.1006/abbi.1997.0486. [DOI] [PubMed] [Google Scholar]
- 32.Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350:2673–81. doi: 10.1056/NEJMoa032845. [DOI] [PubMed] [Google Scholar]
- 33.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]