Abstract
After more than 15 years of intensive research in the field of functional autoantibodies (AAB) directed against G-protein-coupled receptors, there is growing evidence of a causal involvement of AAB in various cardiovascular diseases such as dilated cardiomyopathy, peripartum cardiomyopathy, malignant and essential hypertension, and preeclampsia. It has been indicated that AAB against beta-1 adrenergic receptor, alpha-1 adrenergic receptor, angiotensin-II receptor AT1 and muscarinic M2-receptors undergo agonist-like actions on the corresponding receptor and induce a permanent stimulation of G-protein-coupled signal cascades, which may cause Ca2+ overload and cardiomyocyte destruction.
Furthermore, the present review describes how G-protein-coupled receptor AAB are able to activate transcription factor nuclear factor-kappa B, which may regulate the expression of genes involved in immune and inflammatory responses.
Keywords: Autoantibodies, G-protein-coupled receptors, Hypertension, Signal cascade
Twenty-five years ago Venter et al (1) described the first autoantibodies (AAB) against beta-2-adrenergic receptors (β2-ARs) in patients with allergic asthma. Later on, several groups (2–5) identified AAB against G-protein-coupled receptors (GPCRs). AAB against β1/β2-ARs and M2-acethylcholine receptors were found in patients with Chagas’ disease (6,7) and β1-AR in dilated cardiomyopathy (DCM) (2). For identification of the AAB, Wallukat and Wollenberger (2) developed a sensitive bioassay consisting of spontaneously beating neonatal cardiomyocytes. Agonistic AAB, like AAB against the alpha (α)1-AR, β1-AR or angiotensin-II receptor 1 (AT1-R), cause an increase (positive chronotropy) of the beating rate. These AAB recognize the first or second extracellular loop of the receptors. Another group of AAB, which recognize the third extracellular loop, prevents the positive chronotropic effect caused by the corresponding agonists. These changes in beating rate can be documented by the bioassay used (2).
AAB AGAINST GPCR IN CARDIOVASCULAR DISEASES
Several cardiovascular diseases are associated with AAB directed against one or two GPCR (Table 1). AAB have been identified in the sera of patients with diagnosed heart diseases. Also, it has been hypothesized that a chronic myocarditis may develop into DCM (8). In both diseases, a virus infection and inflammation may be the primary etiological agent, and the inflammatory response may develop into an autoimmune disease (8). In patients with DCM, the prevalence of β1-AR AAB can be up to 70% (3,4). In addition, a prevalence of 36% to 39% of muscarinic M2-receptor AAB has been observed (5). These AAB undergo agonist-like actions on the receptor, but with effects contrasting those of the adrenergic system. The muscarinic M2-receptor AAB induced, like the agonist carbachol, a negative chronotropic effect. Thus, there may be an overall balance in a subpopulation of patients with DCM (9).
TABLE 1.
Autoantibodies directed against G-protein-coupled receptors in various diseases
| Receptor type | Disease | Prevalence (%) |
|---|---|---|
| Alpha1-R | Hypertension | 44 |
| β1-R | Dilated cardiomyopathy | 80 |
| β1-R | Myocarditis | 80 |
| β1-R | Chagas’ disease | 29 |
| β2-R | Chagas’ disease | 12 |
| β2-R | Allergic asthma | ND |
| AT1-R | Preeclampsia | 80 |
| AT1-R | Malignant hypertension | 14 to 33 |
| AT1-R | Vascular renal rejection | ND |
| Muscarinic M2-R | Chagas’ disease | 77 |
| Muscarinic M2-R | Dilated cardiomyopathy | 25 |
| 5HT4-R | Systemic lupus erythematosus | ND |
| Nicotinic AcCh-R | Myasthenia gravis | 90 |
| Glutamate receptor | Rasmussen’s encephalitis, noninflammatory focal epilepsy, catastrophic epilepsy | ND |
| TSH-R | Grave’s disease | >95 |
5HT4-R Serotonin receptor; β-R beta-Adrenergic receptor; AcCh-R Acetylcholine receptor; alpha-R alpha-Adrenergic receptor; AT1-R Angiotensin-II receptor 1; M2-R M2-receptor; ND No data available; TSH Thyroid stimulating hormone
AAB AND SIGNAL CASCADE
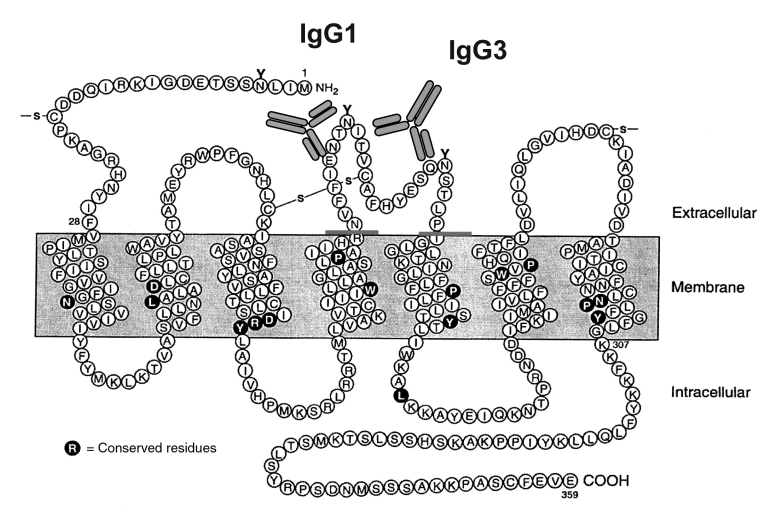
The GPCR are characterized by their seven-membrane spanning arrangement with three extra- and three intracellular loops. For epitope mapping, defined peptides of the extracellular domain of the receptors were used. These peptides, which neutralized the AAB-induced effects, characterized the epitopes on the receptor. For example, it was found that epitopes for α1- and β1-AR found in patients with essential hypertension (10,11) and DCM (3) are localized on the first or second extra-cellular loop, respectively (12). The epitopes of the AT1-R AAB from preeclamptic women and patients with malignant hypertension are localized on the second extracellular loop (Figure 1) (13,14).
Figure 1).
Schematic structure of the human angiotensin-II type 1 receptor as a model of G-protein-coupled receptors. The angiotensin-II type 1 receptor autoantibodies of immunoglobulin G (IgG)1 and IgG3 bind on different amino acids of the second extracellular loop
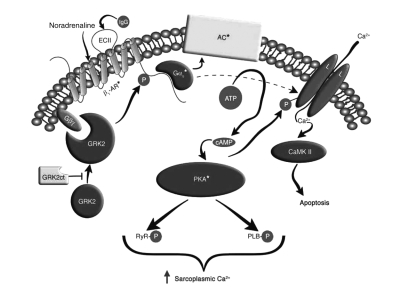
The binding of the AAB to the epitopes at the GPCR and the induction of the agonistic-like effects is not completely understood. For β1-AR and AT1-R, it has been shown that agonistic stimulation shifts the receptor to a dimeric state, stabilizes this confirmation and activates the signal cascade (15,16). We assumed that the agonistic AAB also realize their effects by a stabilization of this active dimeric conformation. In contrast to the physiologically regulated signal cascade, the AAB binding leads to a lack of receptor downregulation and a permanent over-stimulation of the GPCR (Figure 2). This results in an intracellular Ca2+ overload (as shown in the scheme of Freedman and Lefkowitz [17] for β1-AR) with severe consequences for structure and function. The same over-stimulation was observed by activation of other GPCR AAB (eg, AT1-R or α1-AR) and of their specific signal cascade.
Figure 2).
Scheme of the signal cascade with beta (β)1-adrenergic receptor (AR) autoantibodies according to Freedman and Lefkowitz (adapted with permission from reference 17). The seven-membrane-spanning receptor is stimulated by the physiological agonist noradrena-line or by the immunoglobulin G (IgG) of β1-AR autoantibodies from patients with dilated cardiomyopathy or chronic heart failure. This stimulation activates the G-protein-adenylyl cyclase (AC) system with known consequences for augmentation of Ca2+, activation of contractility and, finally, destruction of cardiomyocytes. CaMK II Calcium/calmodulin-dependent protein kinase II; cAMP Cyclic AMP; EC II Extracellular loop II; GRK2ct G-protein-coupled receptor kinase 2 C terminus; PKA Protein kinase A; PLB Phospholamban; RyR Ryanodine receptor
ACTIVATION OF NUCLEAR FACTOR-KAPPA B BY AAB
It is well known that several mediators (eg, cytokines, free radicals, bacterial or viral products such as lipopolysaccharides) rapidly activate nuclear factor-kappa B (NF-κB) by the phosphorylation and degradation of IκB. The phosphorylated form of IκB dissociates from the NF-κB complex, and the NF-κB subunits, p50 and p65, are translocated from the cytoplasm into the nucleus. There they bind to cognate DNA sequences and, thus, regulate genes involved in both innate and adaptive immunity (18,19). Our aim was to see whether AAB against GPCR are also able to activate NF-κB. Using indirect immunofluorescence with an antibody against the 65 kD subunit of NF-κB, we recognized that α1-AR AAB and AT1-R AAB from patients with essential hypertension and preeclampsia activate NF-κB and cause the translocation of their subunits into the nuclei. Not only AAB, but also specific agonists (ie, phenylephrine instead of α1-AR AAB, or angiotensin-II instead of AT1-R AAB) induce activation and translocation. Control incubations with purified immunoglobulin G from healthy controls, and the pretreatment of the AAB with peptides corresponding to the extracellular loops of the α1-AR or the AT1-R prevented translocation of the NF-κB subunits.
CONCLUSIONS
After approximately 20 years of intensive research in the field of functional AAB directed against GPCR, there is evidence of a causal involvement of AAB in various diseases. Also, some cardiovascular disorders were identified as diseases in which AAB may play a pathogenic role. In the case of DCM, essential hypertension and preeclampsia, the autoimmune background adds a causal explanation to the well-known observation of GPCR over-stimulation by the adrenergic or renin-angiotensin system.
The present study supports the hypothesis that AAB against GPCR involve not only the short-term signalling cascade regulation of contractile processes, but also gene transcription by transcription factors like NF-κB or nuclear factor of activated T cells (NFAT1) (20). This process may play an additional role in the pathogenesis of cardiovascular diseases.
REFERENCES
- 1.Venter JC, Fraser CM, Harrison LC. Autoantibodies to beta 2-adrenergic receptors: A possible cause of adrenergic hyporesponsiveness in allergic rhinitis and asthma. Science. 1980;207:1361–3. doi: 10.1126/science.6153472. [DOI] [PubMed] [Google Scholar]
- 2.Wallukat G, Wollenberger A. Effects of the serum gamma globulin fraction of patients with allergic asthma and dilated cardiomyopathy on chronotropic beta adrenoceptor function in cultured neonatal rat heart myocytes. Biomed Biochem Acta. 1987;46:S634–9. [PubMed] [Google Scholar]
- 3.Magnusson Y, Wallukat G, Waagstein F, Hjalmarson A, Hoebeke J. Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta 1-adrenoceptor with positive chronotropic effect. Circulation. 1994;89:2760–7. doi: 10.1161/01.cir.89.6.2760. [DOI] [PubMed] [Google Scholar]
- 4.Wallukat G, Nissen E, Morwinski R, Muller J. Autoantibodies against the beta- and muscarinic receptors in cardiomyopathy. Herz. 2000;25:261–6. doi: 10.1007/s000590050017. [DOI] [PubMed] [Google Scholar]
- 5.Fu LX, Magnusson Y, Bergh CH, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;91:1964–8. doi: 10.1172/JCI116416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borda E, Pascual J, Cossio P, De La Vega M, Arana R, Sterin-Borda L. A circulating IgG in Chagas’ disease which binds to beta-adrenoceptors of myocardium and modulates their activity. Clin Exp Immunol. 1984;57:679–86. [PMC free article] [PubMed] [Google Scholar]
- 7.Goin JC, Borda E, Leiros CP, Storino R, Sterin-Borda L. Identification of antibodies with muscarinic cholinergic activity in human Chagas’ disease: Pathological implications. J Auton Nerv Syst. 1994;47:45–52. doi: 10.1016/0165-1838(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 8.Liu P, Mason J. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–82. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 9.Fu ML. Anti-M2 muscarinic receptor autoantibodies and idiopathic dilated cardiomyopathy. Int J Cardiol. 1996;54:127–35. doi: 10.1016/0167-5273(96)02589-2. [DOI] [PubMed] [Google Scholar]
- 10.Luther HP, Homuth V, Wallukat G. Alpha 1-adrenergic receptor antibodies in patients with primary hypertension. Hypertension. 1997;29:678–82. doi: 10.1161/01.hyp.29.2.678. [DOI] [PubMed] [Google Scholar]
- 11.Fu ML, Herlitz H, Wallukat G, et al. Functional autoimmune epitope on alpha 1-adrenergic receptors in patients with malignant hypertension. Lancet. 1994;344:1660–3. doi: 10.1016/s0140-6736(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 12.Wallukat G, Wollenberger A, Morwinski R, Pitschner HF. Anti-beta 1-adrenoceptor autoantibodies with chronotropic activity from the serum of patients with dilated cardiomyopathy: Mapping of epitopes in the first and second extracellular loops. J Mol Cell Cardiol. 1995;27:397–406. doi: 10.1016/s0022-2828(08)80036-3. (Erratum in 1995;27:2529). [DOI] [PubMed] [Google Scholar]
- 13.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu ML, Herlitz H, Schulze W, et al. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J Hypertens. 2000;18:945–53. doi: 10.1097/00004872-200018070-00017. [DOI] [PubMed] [Google Scholar]
- 15.Hoebeke J. Structural basis of autoimmunity against G protein coupled membrane receptors. Int J Cardiol. 1996;54:103–11. doi: 10.1016/0167-5273(96)02586-7. [DOI] [PubMed] [Google Scholar]
- 16.Angers S, Salahpour A, Bouvier M. Dimerization: An emerging concept for G protein-coupled receptor ontogeny and function. Ann Rev Pharmacol Toxicol. 2002;42:409–35. doi: 10.1146/annurev.pharmtox.42.091701.082314. [DOI] [PubMed] [Google Scholar]
- 17.Freedman NJ, Lefkowitz RJ. Anti-beta(1)-adrenergic receptor antibodies and heart failure: Causation, not just correlation. J Clin Invest. 2004;113:1379–82. doi: 10.1172/JCI21748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 19.Barnes PJ, Karin M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. New Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 20.Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Sos Gynecol Investig. 2003;10:82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]