Abstract
Using autopsy specimens and clonal technique, the authors showed that hematopoietic and stromal stem colony-forming units are present in human atheromatous vascular intima. Stromal colony-forming units were also detected in the mononuclear fraction of the blood of patients with hyperlipidemia and coronary stenosis, and were not found in the peripheral blood of normolipidemic volunteers. Using flow cytometry, the absence of stromal circulating colony-forming units in healthy volunteers and their presence in coronary patients was confirmed. It was thought that the presence of circulating stromal precursors with a certain phenotype and variations in their level in blood could serve as an informative noninvasive indicator of coronary stenosis.
Keywords: Atherosclerosis, Hematopoietic and stromal colony-forming units, Stem/progenitor cells
In the present paper, we present a brief review of our studies carried out between 1986 and 2004. Based on our observations, the concept that bone marrow stem hematopoietic and stromal colony-forming units (CFUs) are involved in vascular atherogenesis was developed.
To initially identify CFUs in the intima of atheromatous aorta and in the blood of patients with coronary atherosclerosis, the classic clonal methodology was used. In semisolid and liquid culture test systems, it was possible to demonstrate the formation of hematopoietic colonies (ie, those formed from macrophage and basophil/mast cell CFUs) and stromal colonies (ie, those formed from fibroblasts CFU [CFU-f]). Some stromal colonies synthesized extracellular reticular matrix, while others produced osteoid matrix. It was concluded that the colonies were formed by CFU-f which, when committed, were able to realize their multipotency. Using both clonal technique and flow cytometry, stromal CFU-f were shown to circulate in patients with hyperlipidemia and coronary atherosclerosis, but were absent in the blood of healthy normolipidemic volunteers. It was suggested that a correlation may exist between the progression of atherosclerosis and the circulation of multipotent stromal cells capable of penetrating into the intima and to isolate loci of inflammation/lipidosis. This could explain the presence of atherosclerotic plaques of different geneses and, in the intima, the existence of different types of sclerotic changes (fibrosis and/or chondro/osteogenesis followed by calcification). In the blood, it is thought that the appearance of circulating stromal progenitors with a certain phenotype, and the elevation of their levels in patients with vascular pathology, can be useful in monitoring the progression of coronary stenosis and the development of postsurgical restenosis.
PRESENCE OF HEMATOPOIETIC AND STROMAL STEM CFUs IN THE INTIMA OF HUMAN ATHEROMATOUS VESSELS IS THE KEY TO UNDERSTANDING HUMAN ATHEROSCLEROSIS
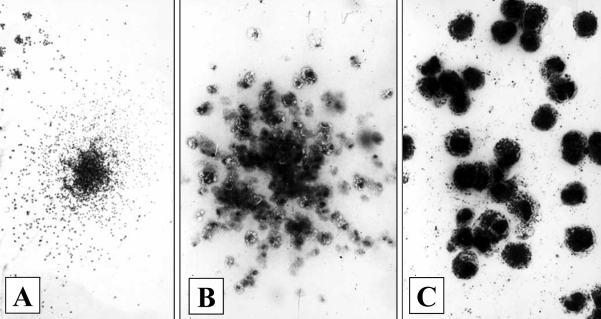
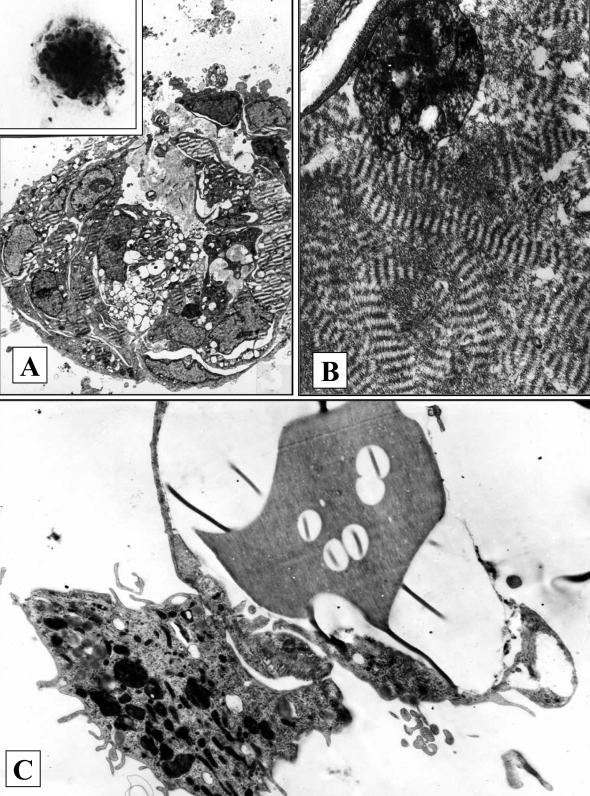
The analysis of the cellular composition of vascular atherosclerotic lesions in situ and in vitro allowed the finding of aortic intima (autopsy material) stem CFUs. It was suggested that because CFUs are present among low-differentiated cells, the clusters of cells with varying degrees of maturity may represent cellular clones. This was confirmed in a series of studies (1–7) where colony-forming tests in semisolid and liquid cultures were used. In populations of intimal cells isolated from the intima with varying degrees of lesions, not only hematopoietic CFUs were present (able to form colonies of monocyte/macrophages or basophils/mast cells in culture) (2–4) (Figure 1), but CFU-f were also present (4–7). The latter were able to form stromal colonies consisting of collagen-producing cells. The term CFU-f was initially introduced by Friedenstein et al (8) who showed that among reticular bone marrow cells, there existed stromal stem CFU-f. These cells were able to differentiate into fibroblasts in vitro and they also possessed the ability to differentiate into chondrocytes, osteocytes and adipocytes. Under the conditions of our experiments, stromal colonies differed from hematopoietic colonies by higher density and by ultrastructural features specific for fibroblastoid cells. The formation of osteoid matrix in liquid cultures seeded with intimal cells (4–7) pointed to the presence of osteoblast precursors in the vascular intima (Figure 2).
Figure 1).
Hematopoietic colonies in semisolid cultures of intimal cells from a lipid streak of human aorta. A A mixed granulocyte-macrophage colony (Giemsa staining; original magnification ×40). B A monocyte/macrophage colony (staining for nonspecific esterase; original magnification ×160). C A basophyl/mast cell colony (toluidine blue staining; original magnification ×400)
Figure 2).
Stromal colonies in cultures of intimal cells from a lipid streak of human aorta. A A stromal colony in semisolid culture (electron microscopy, original magnification ×1300; inset: inverted microscope, original magnification ×130). B A fragment of colony (A) in semisolid culture (electron microscopy, original magnification ×2600). C Bone matrix in liquid culture surrounded by osteoblast-like cells (electron microscopy, original magnification ×8300)
THE APPEARANCE OF CFU-f IN THE BLOOD OF CORONARY PATIENTS MAY BE A RESPONSE TO RISK FACTORS FOR ISCHEMIC HEART DISEASE
Because the CFU-f that are present in a population of vascular intimal cells are most likely of bone marrow origin and are capable of repopulating the intima, the possibility exists that CFU-f circulate in the blood of patients with atherosclerosis (9). It is worth mentioning that before 1997, it was assumed that, in contrast to hematopoietic progenitors, stromal CFUs do not circulate and only few indirect facts pointed to the presence of fibroblastoid cells in circulation (10,11).
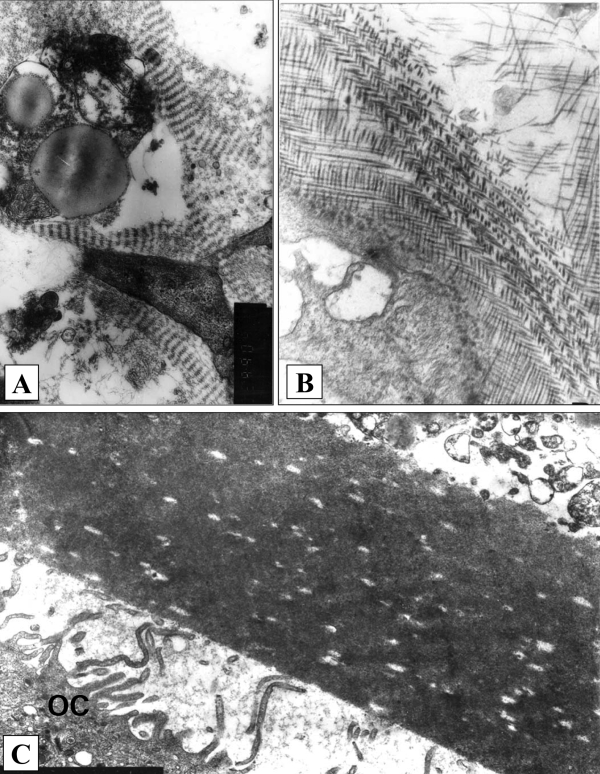
To look for circulating CFU-f, the blood of patients with primary hyperlipidemia (HLP) and frank coronary atherosclerosis was screened (9,12,13). In contrast to the gradual progression of atherosclerotic lesions during natural aging, HLP may represent a model of accelerated atherosclerosis. Culturing of mononuclears isolated from the peripheral blood of these patients in semisolid and liquid test systems (analogous to those used for vascular intimal cells) allowed for the visualization of the colonies of stromal fibroblast-like cells capable of synthesizing fibrillar and osteoid extracellular matrices. Cell colonies with osteoid matrix were stained with osteonectin (ON) as a marker for the differentiation of osteogenic cells (Figure 3). At the same time, stromal colonies did not grow from the blood mononuclears of normolipidemic donors (9,12–14).
Figure 3).
Stromal colonies in semisolid cultures of blood mononuclears from patients with primary hyperlipidemia and coronary stenosis. A,B Fibroblast-like cells synthesize organized fibrillar matrix (electron microscopy, original magnification ×8300). C Osteoid matrix with bone lamella and fragment of osteoclast (OC) (electron microscopy, original magnification ×2600)
DETERMINATION OF CIRCULATING CFU-f IN PATIENTS WITH CORONARY ATHEROSCLEROSIS MAY BE USEFUL FOR PROGNOSIS OF POSTSURGICAL AND POSTANGIOPLASTIC RESTENOSIS
It was of interest to know whether high levels of circulating stromal progenitors expressing ON are present in patients with ischemic heart disease. This was performed using fluorescence-activated cell sorting. The blood of nine patients with ischemic heart disease and six healthy subjects from the control group was analyzed. Clinical analysis of the disease, results of the loading test and 24 h electrocardiographic monitoring confirmed coronary insufficiency of various degrees. In all patients, coronary angiography demonstrated critical stenosis of at least two coronary arteries or their large branches. In all patients, surgical intervention was required.
A number of investigators believe that stromal cells are not CD34-positive (CD34+). However, in other publications, authors state that among CD34+ cells, stromal progenitors are also present (15), which express collagen types I and III, ON, fibronectin, STRO-I and some adhesion molecules (16). Mononuclear cells isolated from blood were stained with antibodies to ON. CD34+ cells were stained with antibodies to CD34+ as a marker for hematopoietic stem cells.
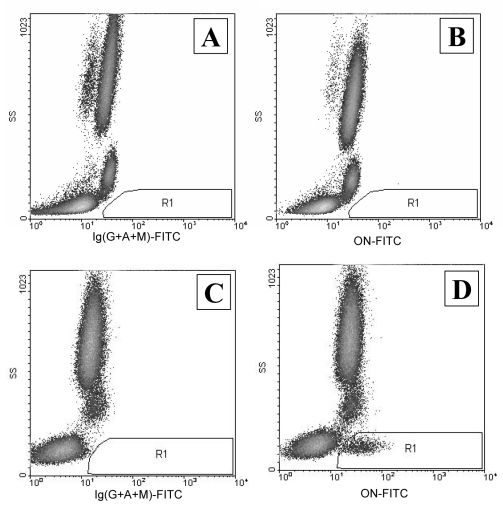
To determine the number of CD34+ stem cells using flow cytometry, the International Society of Hematotherapy and Graft Engineering protocol (17) was followed; specifically, it recommended measuring direct and side light scattering as well as the fluorescence of CD45-phycoerythrin-cyanine-5 and CD34-phycoerythrin. The number of CD34+ cells in the peripheral blood is relatively low and equals 0.01% to 0.1% of the total number of nucleated cells. It was found that the number of CD34+ stem cells in patients with coronary ischemia was not statistically different from that in healthy volunteers (0.040±0.006% and 0.031±0.004%, respectively; P<0.1). In patients with ischemia, regions with a low intensity of side light scattering and positive fluorescence of ON-fluorescein 5’-isothiocyanate (gate R1), circulating stromal cells are present which express ON (Figure 4). ON-positive cells were not found in healthy volunteers (n=6) whereas, in all patients with ischemia (n=9), their level was elevated and varied significantly (98 to 1853 ON-positive cells per 100,000 of nucleated cells). These ON-positive cells were CD45medium/low and CD34-negative.
Figure 4).
Fluorescence-activated cell sorting analysis of osteonectin (ON)-positive cells in peripheral blood. A A healthy volunteer with isotype-matched antibodies (control). B A healthy volunteer with anti-ON antibodies. C A patient with coronary atherosclerosis and isotype-matched antibodies (control). D A patient with coronary atherosclerosis and anti-ON antibodies. FITC Fluorescein 5′-isothio-cyanate; Ig Immunoglobulin
Flow cytometry analysis of cells from the peripheral blood demonstrated a significant elevation of circulating ON-positive stromal cells in patients with ischemic heart disease, while no stromal cells were found in the blood of healthy volunteers. These results confirmed our earlier observations made by clonal technique, which showed an absence of stromal CFUs in the blood of healthy donors and an excess in patients with coronary atherosclerosis (7,9,14). Thus, data collected by two different and independent methods suggest that a correlation may exist between the progression of atherosclerosis and the appearance of stromal stem cells in the blood that are capable of penetrating into the intima and producing various types of sclerotic changes, resulting in plaques of different geneses.
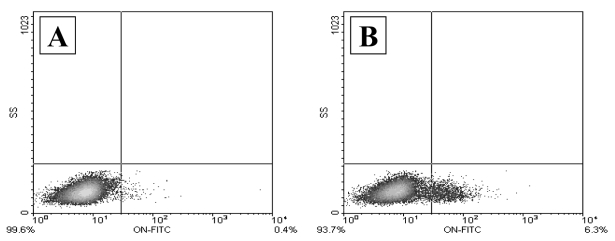
It has been further suggested that the presence of circulating stromal progenitors with a specific phenotype and a different, but elevated level of these cells in patients with ischemic heart disease can be used for the prognosis of postsurgical and/or postangioplastic restenosis (18). The comparison of two patients who had undergone bypass surgery five years previously demonstrates (Figure 5) that the level of circulating stromal progenitors of a certain type correlates with the presence of postsurgical restenosis in coronary arteries.
Figure 5).
Comparison of two patients who had undergone bypass surgery five years previously. Fluorescence-activated cell sorting analysis of the number of osteonectin (ON)-positive cells in the peripheral blood (lymphocyte-like cells CD45medium/low and CD34-negative). A Patient K, functional coronary artery grafts: 126 ON-positive cells per 100,000 white blood cells. B Patient L, critical stenosis of coronary arteries and coronary artery grafts: 1550 ON-positive cells per 100,000 white blood cells. FITC Fluorescein 5′-isothiocyanate
DISCUSSION
Although results of our studies have been discussed at many meetings and our observations have been published for a number of years, they are still not fully understood. Meanwhile, observations were recently communicated which illustrate the role of bone marrow stem cells in the development of neointimal hyperplasia in animals (19,20). However, other authors believe that the role of bone marrow stem cells in the initiation and progression of human atherosclerosis is far from being understood (21). The presence in the aortic intima of stem/progenitor cells of hematopoietic and stromal lines of differentiation, and the elevation of the stromal cell population in patients with HLP and coronary atherosclerosis, strongly support our point of view. Finally, recent transplantation studies (22) in humans show that atherosclerotic lesions of recipient arteries do indeed contain donor bone marrow cells when transplantation is performed between sex-mismatched pairs.
The presence of CFUs from macrophages (CFU-m) and CFU-f in intimal atherosclerotic lesions permits a better understanding of the paradox concerning the commonly recognized ‘fact’ that macrophages and smooth muscle cells proliferate in lesions. The increase in macrophagal mass is often explained by not only the migration of monocytes from circulation into the vascular wall, and their subsequent transformation into macrophages, but also by proliferation of macrophages in the intima (23–25). Unfortunately, many authors ignore the fact that monocytes/macrophages are really terminal stages of hematopoietic differentiation (CFU-m to promonocyte to monocyte), and proliferating monocytoid cells may be only monocyte precursors. It is worth citing the classic work on mononuclear phagocytes by van Furth et al (26), “Some authors make no distinction between promonocytes and monocytes in the bone marrow, ignoring the fact that only promonocytes divide”. This contradiction disappears when we take into account the presence in the vascular intima of CFU-m for monocyte/macrophages, and the ability to form (by these CFUs) the loci of ectopic hematopoiesis (or colonies of cells with varying proliferative potential). The efficiency of CFU-m cloning has been shown to be highest in prefibrotic lesions, low in lipid fibrotic lesions and completely absent in fibrotic plaques (2,4).
Our findings correlate with low levels of thymidine label in focal infiltrates consisting of foam cells and monocyte-like cells in vascular plaques (24,27). Studies (28) on cell proliferation in human vessels suggest that although some smooth muscle cells and macrophages were proliferating cell nuclear antigen (PCNA)-positive, high percentages of vascular PCNA-positive cells are not stained with specific antibodies to smooth muscle cells and macrophages. The same authors emphasize that the nature of PCNA-positive cell populations with undefined phenotype remains unknown.
What is the likely origin of these ‘unknown’ proliferating cells? These cells likely originate from the bone marrow and, in fact, may represent stem CFUs which repopulate the intima under conditions of lipidosis and/or vascular inflammation. As was shown by Goldstein and Brown (29), it is actively proliferating cells that incorporate the largest amounts of low density lipoproteins. A mere 1.5-fold elevation in the number of cells having the smooth muscle cell phenotype cannot explain the many-fold increase in the volume of the intima in fibrotic plaques (14,30). Hence, there should exist additional sources of cells able to synthesize the extracellular matrix components. The first suggestion was that in a plaque, there should exist a subpopulation of modified smooth muscle cells, which is different from the population of smooth muscle cells present in unchanged intima (ie, these cells have the ability to undergo hyperproduction of the extracellular matrix). Although these specific cells were indeed found in fibrotic plaque (25,30), the idea of their origin from the media was popular (23).
The presence of stromal stem CFU-f in the intima of atheromatous aorta and in the blood of patients with hyperlipidemia and coronary atherosclerosis support a bone marrow origin of matrix-secreting cells in fibrous plaques. This provides an explanation for the existence of various types of atherosclerosis in the vascular intima (fibrosis, ossification, chondrogenesis and formation of adipose tissues) and also the well-known phenomenon of bone formation in the vascular wall.
Serious arguments for intimal cell replication in a plaque were presented more than 20 years ago. In 1973, Benditt and Benditt (31) showed that atherosclerotic plaque was monoclonal in origin. These authors used glucose-6-phosphate dehydrogenase as an allotypic marker in plaques obtained from black females. However, these observations did not directly prove that the cells analyzed were indeed smooth muscle cells. Benditt and Benditt’s investigations were not continued further because, at the time, smooth muscle cells were thought to play the leading role in atherogenesis. Our data support this ‘unusual concept’ which, unfortunately, was not broadly accepted (not counting the few publications supporting Benditt and Benditt’s idea). If our data are taken into account, the monoclonality of fibrous plaque can indeed be due to pluripotent stem stromal cells and their progeny present in plaques.
Scientific interest in bone marrow stromal cells, which started in 1997, led to the general agreement on their presence in circulation (32–34). The pluripotency of these cells is indeed limitless (35), which is additionally demonstrated by their ability to repair injured tissues. Human atherosclerosis may be an example of the systemic pathology of mesenchymal tissues and, if so, intimal fibrosis leading to vascular stenosis is a mere complication of the natural ‘cell therapy’ of the vascular wall by circulating bone marrow stem/progenitor cells.
SUMMARY
Using test systems to culture intimal cells isolated from areas of the human aorta with varying degrees of atherosclerosis, it was possible to demonstrate the presence of hematopoietic CFUs and stromal CFU-f in the intima. Using clonal technique combined with immunohistochemical and ultrastructural analysis of cells grown in colonies led to a new understanding of the mechanisms of human atherogenesis. Our data suggest that during lipidosis, inflammation and the presence of other risk factors, conditions may arise in the vascular intima which facilitate the formation of loci for ectopic hematopoiesis which, in turn, serves as a starting point for plaque formation. In the progression of plaque, stromal progenitors are also involved, which explains the development of different types of atherosclerosis in the vascular wall. Experiments with blood mononuclears in culture have demonstrated the presence of CFU-f in the blood of patients with coronary atherosclerosis and hyperlipidemia, and their absence in healthy donors. The results obtained using clonal techniques were fully confirmed by flow cytometry analysis of circulating stromal cells in donors and in patients with coronary artery stenosis. It is thought that the quantitation of circulating stromal progenitors of certain phenotypes may be used for the noninvasive diagnosis of coronary stenosis, as well as for the prognosis of postsurgical restenosis. In the initiation and progression of atherosclerosis, the key target for action of risk factors and, in particular, hyperlipidemia may be bone marrow stem cells. From this point of view, human atherosclerosis may be considered a systemic disease of mesenchymal tissues. The final stage of the atherosclerotic process (the formation of plaques) looks like a byproduct of an attempt to neutralize the inflammatory process in the vascular wall. In this process, the standard mechanism of tissue regeneration is used (ie, the mobilization of mesenchymal stromal cells from bone marrow and their transport to the injured vascular loci).
REFERENCES
- 1.Soboleva EL, Popkova VM. Colony-forming cells are precursors of granulocyte-macrophages (CFU-GM) in intima of human atheromatous aorta; 8th International Symposium on Atherosclerosis; 1988. p. 872. (Abst) [Google Scholar]
- 2.Soboleva EL, Popkova VM. [Hemopoietic precursor cells in the intima of the atheromatous human aorta] Biull Exsp Biol Med. 1989;107:600–4. (Russian) [PubMed] [Google Scholar]
- 3.Soboleva EL, Popkova VM, Smirnov VN. Ectopic hemopoiesis in human aortic intima and its relation to atherosclerotic plaque formation; 59th EAS Congress in Nice; France. 1992. p. 41. (Abst) [Google Scholar]
- 4.Soboleva EL, Popkova VM, Saburova OS, Tararak EM, Tvorogova MG, Smirnov VN. Colony forming units and atherosclerosis. In: Woodford FP, Davignon I, Sniderman A, editors. Atherosclerosis X. New York: Elsevier Science; 1995. pp. 919–25. [Google Scholar]
- 5.Soboleva EL, Smirnov VN. Local hemo-and stromopoiesis in human vascular wall; Lectures, The XIIIth meeting of the International Society of Hematology; 1995. pp. 134–9. [Google Scholar]
- 6.Romanov YA, Balyasnikova IV, Bystrevskaya VB, et al. Endothelial heterogeneity and intimal blood-borne cells. Relation to human atherosclerosis. Ann N Y Acad Sci. 1995;748:12–39. doi: 10.1111/j.1749-6632.1994.tb17306.x. [DOI] [PubMed] [Google Scholar]
- 7.Soboleva EL, Saburova OS, Rozhkova TA, Tvorogova MG. Stem cells of hemopoietic and stromal differentiation lineages and human atherosclerosis. Angiology and vascular surgery. 1999;5(Suppl):190–203. [Google Scholar]
- 8.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 9.Soboleva EL, Shindler EM, Saburova OS, Tvorogova MG, Smirnov VN. Colony-forming units for fibroblasts (CFU-f) in the peripheral blood of patients with primary hypercholesterolemia; New Pathogenic Aspects of Atherosclerosis. Nordrhein-Westfalische Academie der Wissenschaften; Westdeutscher Verlag. 1994. pp. 79–93. [Google Scholar]
- 10.Keating A, Singer JW, Killen PD, et al. Donor origin of the in vitro haematopoietic microinvironment after marrow transplantation in man. Nature. 1982;298:280–3. doi: 10.1038/298280a0. [DOI] [PubMed] [Google Scholar]
- 11.Piersma AH, Ploemacher RE, Brockbank KG, Nikkels PG, Ottenheim CP. Migration of fibroblastoid stromal cells in murine blood. Cell Tissue Kinet. 1985;18:589–95. doi: 10.1111/j.1365-2184.1985.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 12.Smirnov VN, Soboleva EL, Akchurin RS, Chazov EI. Abnormal bone marrow response and blood-born stem cells in coronary patients with hyperlipidemia; 70th EAS Congress in Geneva; Switzerland. 1998. p. 60. (Abst) [Google Scholar]
- 13.Soboleva EL, Saburova OS, Smirnov VN, Akchurin RS. Circulating colony-forming units for fibroblasts (CFU-F) and hyperlipidemia. Br J Haematol. 1998;102:358. (Abst) [Google Scholar]
- 14.Romanov YA, Soboleva EL, Smirnov VN, Bobik A. Human atherosclerosis: New participants? In: Dhalla NS, Chokalingam A, Berkowitz HI, Singal PK, editors. Frontiers in Cardiovascular Health. Boston: Kluwer Academic Publishers; 2003. pp. 55–71. [Google Scholar]
- 15.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 16.Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–40. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–26. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 18.Soboleva EL, Gabbasov ZA, Agapov AA, Saburova OS, Smirnov VN. Circulating stromal stem cells and diagnostics of atherosclerosis. Atherosclerosis. 2004;5(Suppl):1. (Abst) [Google Scholar]
- 19.Han CI, Campbell GR, Campbell JH. Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res. 2001;38:113–9. doi: 10.1159/000051038. [DOI] [PubMed] [Google Scholar]
- 20.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells thet participate in the pathogenesis of atherogenesis. Nat Med. 2002;8:403–9. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 21.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: New perspectives and therapeutic strategies. Nat Med. 2002;8:1249–56. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 22.Caplice NM, Bunch TJ, Stalboerger PG, et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci USA. 2003;100:4754–9. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–8. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 24.Gordon D, Reidy MA, Benditt EP, Schwartz SM. Cell proliferation in human coronary arteries. Proc Nat Acad Sci USA. 1990;87:4600–4. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stary HC. The Evolution of Human Atherosclerotic Lesions. West Point, Pennsylvania: Merc & Co Inc; 1993. [Google Scholar]
- 26.van Furth R, Raeburn JA, van Zwet TL. Characteristics of human mononuclear phagocytes. Blood. 1979;54:485–500. [PubMed] [Google Scholar]
- 27.Spagnoli LG, Villaschi S, Neri L, et al. Autoradiographic studies of the smooth muscle cells in human arteries. Paroi Arterielle. 1981;7:107–12. [PubMed] [Google Scholar]
- 28.Schwartz SM, O’Brien ER. Absence of replication of vascular smooth muscle. In: Woodford FP, Davignon I, Sniderman A, editors. Atherosclerosis X. New York: Elsevier Science; 1995. pp. 704–20. [Google Scholar]
- 29.Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974;249:5153–62. [PubMed] [Google Scholar]
- 30.Krushinsky AV, Nestaiko GV. Morphology of smooth muscle cells from normal and atherosclerotic human aorta. Soc Med Rev A Cardiol. 1987;1:35–73. [Google Scholar]
- 31.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaque. Proc Natl Acad Sci USA. 1973;70:1753–6. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell JH, Han CL, Campbell GR. Neointimal formation by circulating bone marrow cells. Ann N Y Acad Sci. 2001;947:18–24. doi: 10.1111/j.1749-6632.2001.tb03926.x. [DOI] [PubMed] [Google Scholar]
- 33.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 34.Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–30. doi: 10.1126/science.279.5356.1528. (Erratum in 1998;281:923). [DOI] [PubMed] [Google Scholar]
- 35.Pittenger MF, Maccay AM. Multipotential human mesenchymal stem cells. Graft. 2000;3:288–94. [Google Scholar]